Abstract
The physiological importance of human placental indoleamine 2,3-dioxygenase (EC 1.13.11.42), the first and rate-limiting enzyme in tryptophan metabolism, in regulating feto-maternal immunology has been studied.
Concentrations were measured in placental villous explant conditioned media of 14 amino acids that are known to be required for lymphocyte proliferation. In the absence of interferon-γ only tryptophan and threonine were significantly lowered; in the presence of interferon-γ (known to stimulate indoleamine 2,3-dioxygenase) tryptophan but not threonine depletion was much greater.
Peripheral blood mononuclear cell proliferation determined by measuring thymidine incorporation into DNA following culture in the medium previously conditioned by culture of villous explants was markedly reduced when placental indoleamine 2,3-dioxygenase was stimulated with interferon-γ. Inhibition of placental indoleamine 2,3-dioxygenase by 1-methyl-tryptophan prevented inhibition of thymidine incorporation. Supplementation of the conditioned medium with tryptophan but no other amino acid completely reversed the inhibition of thymidine incorporation.
Flow cytometric analysis showed that CD4-positive T lymphocyte division was specifically suppressed by indoleamine 2,3-dioxygenase-mediated tryptophan depletion. This inhibition of T cell proliferation was due to arrest of cell cycle progression.
To study the mechanism of tryptophan sensing we examined the ability of 11 L-tryptophan analogues to support lymphocyte proliferation. Only L-tryptophan methyl and ethyl esters were able to stimulate proliferation in tryptophan-free media. Since both of these molecules are readily degraded to tryptophan by intracellular esterases this suggests that the tryptophan sensor is intracellular.
Our results show that mechanisms are present in the human placenta which are able to regulate cellular proliferation of the maternal immune system. This mechanism is dependent both on placental indoleamine 2,3-dioxygenase-mediated tryptophan degradation and on tryptophan sensing systems within lymphocytes.
It is a paradox that the immunologically foreign conceptus can escape the immune attack mounted by the mother throughout pregnancy. Munn et al. (1998) have proposed that in the mouse the expression of indoleamine 2,3-dioxygenase, a tryptophan catabolising enzyme, in the placenta is crucial in the prevention of immunological rejection of the fetal allograft. They suggested that T cells are inhibited by a mechanism involving indoleamine 2,3-dioxygenase-dependent localised depletion of tryptophan at the site of placentation. The role of this mechanism (discovered in mouse) in the human, and the extent to which defective activation of this process is responsible for disorders of pregnancy such as recurrent miscarriage, are currently unknown. We have recently confirmed that indoleamine 2,3-dioxygenase is expressed in human placenta (Yamazaki et al. 1985); it is a cytoplasmic enzyme and is competitively inhibited by 1-methyl-tryptophan (Cady & Sono, 1991), the critical experimental tool used by Munn et al. (1998) for inhibiting mouse placental indoleamine 2,3-dioxygenase; tryptophan transport into the trophoblast is a rate-limiting step for indoleamine 2,3-dioxygenase-mediated tryptophan degradation (Kudo & Boyd, 2001); moreover human placental indoleamine 2,3-dioxygenase activity and mRNA expression are positively regulated by cytokines such as interferon-γ (Kudo et al. 2000). It has been demonstrated that indoleamine 2,3-dioxygenase production by macrophages (Munn et al. 1999) and dendritic cells (Hwu et al. 2000) results in the inhibition of lymphocyte proliferation due to tryptophan depletion by this enzyme. To clarify the physiological potential of indoleamine 2,3-dioxygenase in the human placenta, we have now examined the relationship between human peripheral blood mononuclear cell proliferation and tryptophan concentration in medium previously conditioned by culture of term placental villous tissue.
METHODS
Culture of villous tissue
Human placental chorionic villi were cultured in vitro using a standard technique (Watson et al. 1995). Normal first trimester and term placentae were obtained (with ethical committee approval) and chilled on ice. The tissue was washed three times with ice-cold phosphate-buffered saline (PBS) containing 100 units ml−1 penicillin and 100 units ml−1 streptomycin, and chorionic villi were dissected into small pieces (a single piece was approximately 5 mg). All these procedures were carried out at 4 °C. Three pieces of chorionic villi were placed on a polyester mesh (Netwell 500 μm mesh, Costar, NY, USA) and cultured in 35 mm plastic culture dish at 37 °C in an atmosphere of 5 % CO2 and 95 % air for the indicated time. The culture medium used was RPMI medium 1640 with the addition of 5 % fetal bovine serum, 100 units ml−1 penicillin, 100 units ml−1 streptomycin and 1000 units ml−1 interferon-γ or vehicle. Other additions are described in the figure legends. Cultures were conducted in triplicate for each set of experiments to assess reproducibility. The conditioned medium collected was then used for high-performance liquid chromatography (HPLC) analysis or peripheral blood mononuclear cell culture.
Assay of indoleamine 2,3-dioxygenase
The cultured pieces of chorionic villi were washed three times to remove 1-methyl-tryptophan or 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid, suspended in ice-cold PBS and disrupted by sonication for 30 s in an ice bath at a power of 100 W. The homogenate was centrifuged at 800 g for 10 min at 4 °C to remove unbroken fragments of the tissue. The supernatant was then centrifuged at 15 000 g for 15 min at 4 °C. The resultant supernatant was used for the colorimetric assay of indoleamine 2,3-dioxygenase activity (expressed as nmol (mg protein)−1 min−1), as described previously (Kudo & Boyd, 2000).
HPLC analysis of amino acid concentration
The medium conditioned by culture with placental villous tissue was well mixed by vortexing with one-tenth volume of ice-cold 2.4 m perchloric acid. The mixture was chilled on ice for 15 min and centrifuged at 10 000 g for 3 min. The clear protein-free supernatant was used for HPLC analysis. Concentrations of l-tryptophan and l-kynurenine were analysed by a HPLC system consisting of a Kontron 420 pump, a Kontron 460 autosampler and a Kontron 432 variable wavelength detector (Watford, UK) with the Spherisorb S5-ODS1 column, 4.6 mm × 150 mm (Waters, Milford, MA, USA). The mobile phase consisted of 40 mm citrate buffer (pH 2.25), 50 % methanol and 0.4 mm sodium dodecylsulphate, which were used after filtration through a 0.45 μm membrane filter and degassed by a vacuum aspirator. A 20 μl volume of protein-free extract was injected onto the column, chromatographed at a flow rate of 2.0 ml min−1 and detected at 365 nm for l-kynurenine and at 280 nm for l-tryptophan. The minimum amount of l-kynurenine and l-tryptophan reproducibly detected was 20 pmol and calibration was linear up to 10 nmol of l-kynurenine or l-tryptophan.
Peripheral blood mononuclear cell culture and proliferation assay
Human peripheral blood mononuclear cells were isolated from healthy volunteer donors by Ficoll-Hypaque gradient centrifugation. Peripheral blood mononuclear cells (2 × 105 cells per well) were cultured in the conditioned medium of villous tissue culture and stimulated with 5 μg ml−1 phytohaemagglutinin (PHA) in 96-well flat bottom plates at 37 °C in an atmosphere of 5 % CO2 and 95 % air for 72 h. RPMI medium1640 (Select-Amine Kit) with addition of 10 μg ml−1 insulin, 5 μg ml−1 iron-saturated transferrin, 1 mg ml−1 bovine serum albumin, 100 units ml−1 penicillin and 100 units ml−1 streptomycin was used for serum- and l-tryptophan-free culture medium. Media lacking specific amino acids were made up individually. Other additions are described in the figure legends. Peripheral blood mononuclear cell proliferation was determined by pulse labelling with [3H]thymidine (1 μCi per well) for 12 h.
Flow cytometry analysis
Peripheral blood mononuclear cells were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE) in order to determine cell division (Lyons & Parish, 1994) and were cultured in the conditioned medium of villous tissue culture with or without 5 μg ml−1 PHA at 37 °C in an atmosphere of 5 % CO2 and 95 % air for 72 h. Harvested cells were labelled with anti-CD4 phycoerythrin or anti-CD8 phycoerythrin-cyanin 5.1 and were analysed on a flow cytometer (Clover et al. 2000). Ten thousand cells were analysed in each sample.
Leucine incorporation
To estimate the total protein synthesis, the degree of leucine incorporation into acid-insoluble material was measured by the incubation of peripheral blood mononuclear cells with culture medium containing l-[3H]leucine (1 μCi ml−1). After incubation with l-[3H]leucine, peripheral blood mononuclear cells were fixed to the dish and subsequently washed three times with ice-cold 10 % trichloroacetic acid. The cells were then made soluble with 0.1 m NaOH and 0.1 % SDS and an aliquot was taken for scintillation counting.
Statistical analysis
Differences between two groups were analysed using Student's t test and results were considered statistically significant at P < 0.05.
Chemicals
[methyl-3H]Thymidine (25.0 Ci mmol−1 or 925 GBq mmol−1) and l-[4,5-3H]leucine (53.0 Ci mmol−1 or 1.96 TBq mmol−1) were purchased from Amersham Life Science (Amersham, Buckinghamshire, UK). Human recombinant interferon-γ, insulin, iron-saturated transferrin, phytohaemagglutinin, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid and 1-methyl-d,l-tryptophan were obtained from Sigma-Aldrich Chemical (Poole, Dorset, UK), tissue culture supplements were from Gibco BRL (Paisley, UK) and CFSE was from Molecular Probes Europe BV (Leiden, Netherlands). All chemicals were of the highest purity commercially available.
RESULTS
Tryptophan degradation by indoleamine 2,3-dioxygenase in placental villous tissue
Table 1 (confirming the results of Kudo & Boyd (2001)) shows that after 36 h of placental villous explant culture both tryptophan degradation and kynurenine appearance are reciprocally related in either first trimester or term placenta. Additionally extracted placental indoleamine 2,3-dioxygenase activity is markedly stimulated by interferon-γ. The presence in the culture medium of either 1-methyl-tryptophan, a competitive inhibitor of human placental indoleamine 2,3-dioxygenase (Kudo & Boyd, 2000), or of 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid, a competitive inhibitor of tryptophan transport into the villous explant, inhibited tryptophan degradation. These inhibitory effects were more evident when indoleamine 2,3-dioxygenase expression was stimulated by interferon-γ. In contrast to these effects on l-tryptophan catabolism in the intact explant, neither 1-methyl-tryptophan nor 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (in contrast to interferon-γ) had any effect on the activity of indoleamine 2,3-dioxygenase (Table 1). This was demonstrated by measuring indoleamine 2,3-dioxygenase activity extracted from explants cultured in the presence and absence of interferon-γ in which the 1-methyl-tryptophan or 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid was removed by washing prior to assay.
Table 1.
Effect of interferon–γ, 1–methyl–tryptophan and 2–aminobicyclo–(2,2,1)–heptane–2–carboxylic acid on tryptophan catabolism by indoleamine 2,3–dioxygenase in placental explants
| Treatment | Tryptophan (μm) | Kynurenine (μm) | Extracted IDO activity (nmol (mg protein)−1 min−1) |
|---|---|---|---|
| Non–conditioned | 19.3 ± 0.3 | ND | – |
| First trimester placenta | |||
| Control | 17.1 ± 0.6 | 1.4 ± 0.2 | 0.08 ± 0.01 |
| IFN–γ | 5.8 ± 0.5* | 9.6 ± 1.2* | 0.80 ± 0.06* |
| Term placenta | |||
| Control | 12.0 ± 1.1 | 5.4 ± 0.5 | 0.49 ± 0.03 |
| IFN–γ | 2.3 ± 0.3* | 12.6 ± 0.5* | 1.82 ± 0.04* |
| 1–Methyl–Trp | 15.8 ± 0.2* | 2.6 ± 0.3* | 0.48 ± 0.01 |
| IFN–γ+ 1–methyl–Trp | 10.5 ± 0.8† | 6.6 ± 0.4† | 1.83 ± 0.03* |
| BCH | 14.7 ± 0.6* | 3.9 ± 0.1* | 0.49 ± 0.01 |
| IFN–γ+ BCH | 7.4 ± 0.5† | 9.2 ± 0.7† | 1.81 ± 0.03* |
ND, not detectable.
Significantly different from control.
Significantly different from IFN–γ. Villous explants were cultured with or without 1000 units ml−1 interferon–γ (IFN–γ) and/or 2mM 1–methyl–tryptophan (1–methyl–Trp), 2–aminobicyclo–(2,2,1)–heptane–2–carboxylic acid (BCH) or vehicle (control) for 36 h. Concentrations of tryptophan and kynurenine in the conditioned medium were analysed by HPLC and indoleamine 2,3–dioxygenase (IDO) activity in the tissue extract was determined colorimetrically after removing 1–methyl–tryptophan or 2–aminobicyclo–(2,2,1)–heptane–2–carboxylic acid as described in Methods. Values are the mean ± S.D. of three separate experiments with triplicate assay from five different placentae.
Effect of tryptophan concentration in the medium on PHA-stimulated peripheral blood mononuclear cell proliferation
In order to determine the effect of tryptophan in the culture medium on peripheral blood mononuclear cell proliferation activity, thymidine incorporation into DNA was determined in peripheral blood mononuclear cells that had been cultured in a modified RPMI 1640 medium containing different concentrations of tryptophan over the range of 0 to 25 μm. As shown in Fig. 1, in the absence of tryptophan there is no detectable proliferation; addition of micromolar concentrations of tryptophan stimulated thymidine incorporation into DNA in a concentration-dependent manner. The quantitative relationship between cell proliferation and tryptophan concentration is altered by pharmacological inhibition of indoleamine 2,3-dioxygenase during the proliferation assay. In the presence of indoleamine 2,3-dioxygenase inhibitor the concentration of tryptophan required for half-maximal stimulation was about one-tenth of that shown in Fig. 1.
Figure 1.
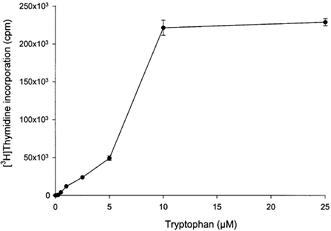
Effect of tryptophan concentration on peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cells were cultured for 72 h with the indicated concentrations of tryptophan and [3H]thymidine incorporation was then determined as described in Methods. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay.
Time course of tryptophan-dependent PHA-stimulated peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cell proliferation as a function of culture time is illustrated in Fig. 2. Thymidine incorporation into DNA was stimulated in a time-dependent manner by the presence of tryptophan and peaked at 72 h after the initiation of culture and remained elevated thereafter up to 144 h. In the absence of tryptophan, thymidine incorporation was negligible at any period. When tryptophan was added after peripheral blood mononuclear cells had been cultured without tryptophan for varying times, thymidine incorporation was initiated and reached the same maximum level.
Figure 2.
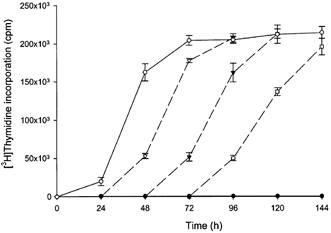
Time course of peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cells were cultured with or without 25 μm tryptophan for the time indicated and [3H]thymidine incorporation was then determined as described in Methods. ○, tryptophan; •, nil. Dashed lines, after culturing without tryptophan for the time indicated, tryptophan was added to 25 μm followed by culture for the time indicated and [3H]thymidine incorporation was then determined. ▿, 24 h; ▾, 48 h; □, 72 h. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay.
Effect of other amino acids on PHA-stimulated peripheral blood mononuclear cell proliferation
The effect of the omission of one of 20 amino acids present in RPMI medium 1640 on thymidine incorporation into DNA was determined (Fig. 3). In addition to tryptophan the omission of one of arginine, cystine, glutamine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, serine, threonine, tyrosine and valine produced marked inhibition of the proliferation of peripheral blood mononuclear cells.
Figure 3.
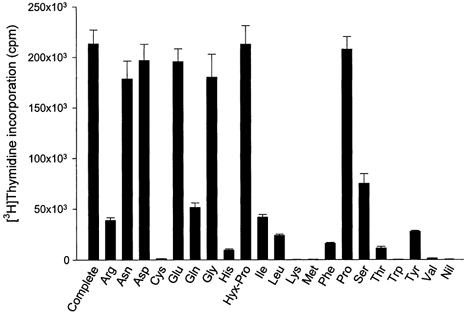
Effect of single amino acid omission from medium on peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cells were cultured for 72 h without indicated amino acid and [3H]thymidine incorporation was then determined as described in Methods. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay.
Amino acid concentrations in placental conditioned media
Amino acid concentrations in the medium conditioned by culture of villous explants in the presence or absence of interferon-γ were therefore analysed. Table 2 summarises the results of analysis of those amino acids for which omission had an inhibitory effect on the proliferation activity of peripheral blood mononuclear cells. The concentrations of both tryptophan and threonine were decreased after conditioning, but only the decrease in tryptophan concentration was interferon-γ dependent.
Table 2.
Amino acid concentrations as a percentage of control in the conditioned medium of villous explants
| Amino acid | Without interferon–γ | With interferon–γ |
|---|---|---|
| Arginine | 119.1 | 131.8 |
| Cystine | 164.3 | 114.4 |
| Glutamine | 98.9 | 110.5 |
| Histidine | 184.2 | 250.0 |
| Isoleucine | 97.9 | 113.2 |
| Leucine | 109.7 | 126.0 |
| Lysine | 214.8 | 216.2 |
| Methionine | 99.5 | 170.4 |
| Phenylalanine | 105.9 | 117.0 |
| Serine | ND | ND |
| Threonine | 55.4 | 54.1 |
| Tryptophan | 62.2 | 11.9 |
| Tyrosine | 117.8 | 124.2 |
| Valine | 113.3 | 129.1 |
Villous explants were cultured with or without 1000 units ml−1 interferon–γ for 36 h. Concentrations of amino acid in the conditioned medium were analysed by HPLC. Values are the mean of percentage of control (i.e. values without conditioning). ND, not determined.
Inhibitory effect of placental conditioned medium on PHA-stimulated peripheral blood mononuclear cell proliferation is mediated by tryptophan depletion
Based on these results we then examined whether placental indoleamine 2,3-dioxygenase-mediated tryptophan degradation regulates peripheral blood mononuclear cell proliferation. For this, peripheral blood mononuclear cells were cultured in the same medium as that previously conditioned by culture of villous explants. Thymidine incorporation into DNA was then determined (Fig. 4). The findings are that proliferation is inhibited by conditioned medium, that this inhibition is greater with medium conditioned in the presence of interferon-γ and that these effects are markedly blunted when 1-methyl-tryptophan or less markedly when 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid was present during the previous conditioning with villous explants. Figure 5 shows unambiguously that this effect is mediated by depletion of tryptophan since return (to the maximally conditioned medium) of a physiological concentration of l-tryptophan, but not of any of the other amino acids tested (including d-tryptophan) returns peripheral blood mononuclear cell proliferation rate to normal. In keeping with this conclusion, addition to the proliferation assay of kynurenine, of kynurenic acid or of quinolinic acid, the major indoleamine 2,3-dioxygenase-mediated metabolites of tryptophan, had no effect on peripheral blood mononuclear cell proliferation (Fig. 6).
Figure 4.
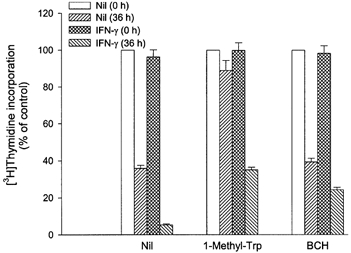
Peripheral blood mononuclear cell proliferation in medium previously conditioned by culture with villous explants
Peripheral blood mononuclear cells were cultured for 72 h either in non-conditioned medium or in medium previously conditioned by culture of villous explants with 1000 units ml−1 interferon-γ (IFN-γ) and/or 2 mm 1-methyl-tryptophan (1-Methyl-Trp) or 2 mm 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH). [3H]Thymidine incorporation was then determined as described in Methods. Culture was performed by adding 100 μl of peripheral blood mononuclear cells suspended in tryptophan-free medium to 100 μl of non-conditioned or conditioned medium, and therefore tryptophan concentration in peripheral blood mononuclear cell culture was half of that in each medium. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay, expressed as a percentage of control (i.e. values cultured in non-conditioned medium with or without 1-methyl-tryptophan or 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid).
Figure 5.
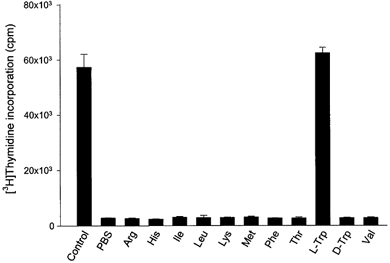
Effect of amino acids supplementation of the medium previously conditioned by culture with villous explants on peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cells were cultured for 72 h in medium previously conditioned with 1000 units ml−1 interferon-γ and supplemented with indicated amino acids to the concentrations normally present in RPMI 1640 or vehicle (phosphate-buffered saline (PBS)). [3H]Thymidine incorporation was then determined as described in Methods. Culture was performed by adding 100 μl of peripheral blood mononuclear cells suspended in tryptophan-free medium to 100 μl of supplemented conditioned medium, and therefore tryptophan concentration in peripheral blood mononuclear cell culture was half of that in each medium. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay.
Figure 6.
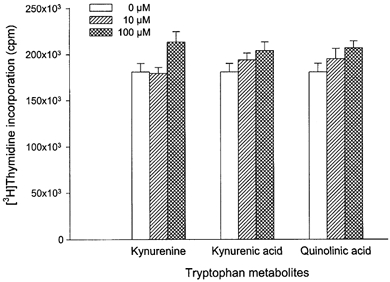
Effect of the tryptophan metabolites on peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cells were cultured for 72 h with the indicated concentrations of kynurenine, kynurenic acid, quinolinic acid or vehicle and [3H]thymidine incorporation was then determined as described in Methods. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay.
Flow cytometry analysis of lymphocyte division
In order to analyse which subpopulation of peripheral blood mononuclear cells are specifically inhibited by tryptophan depletion, the percentage of CD4-positive and CD8-positive T lymphocytes was determined using flow cytometry. Peripheral blood mononuclear cell division, determined by labelling with CFSE, was markedly stimulated by the presence of PHA; this stimulatory effect was less when peripheral blood mononuclear cells had been cultured in the conditioned medium (data not shown). As shown in Table 3, the percentage of CD4-positive T cells was markedly reduced when cells had been cultured with PHA in the conditioned medium. In contrast the percentage of CD8-positive cells, which was also increased (though less markedly) by PHA, was not affected by tryptophan depletion. For the CD4-positive T cells CFSE labelling showed that there was inhibition of cell division by conditioned medium.
Table 3.
Percentage of CD4–positive and CD8–positive cells
| CD4 (% of total) | CD8 (% of total) | |||
|---|---|---|---|---|
| Non–conditioned | Conditioned | Non–conditioned | Conditioned | |
| PHA (−) | 4.57 | 3.90 | 11.57 | 8.95 |
| PHA (+) | 49.33 | 28.90 | 19.32 | 17.90 |
Peripheral blood mononuclear cells were cultured for 72h either in non–conditioned medium or in medium previously conditioned by culture of villous explants. Percentage of CD4–positive and CD8–positive cells was then determined as described in Methods.
The effect of tryptophan analogues on PHA-stimulated peripheral blood mononuclear cell proliferation
In order to investigate the site of tryptophan sensing by peripheral blood mononuclear cells, we carried out experiments similar to those shown in Fig. 1 but with 11 analogues of tryptophan replacing the amino acid (Fig. 7). As expected at 25 μm tryptophan a maximal stimulation of proliferation was found. With 1 mmd-tryptophan proliferation was less than 10 % of that found with l-tryptophan. Of the other amino acids tested only l-tryptophan esters were able to stimulate proliferation at low concentration. (Indeed at high concentration the esters are inhibitory.) This strongly suggests that the tryptophan sensor is within the peripheral blood mononuclear cells since HPLC analysis shows these molecules to be hydrolysed to tryptophan during incubation, and since esterase activity is known to be intracellular.
Figure 7.
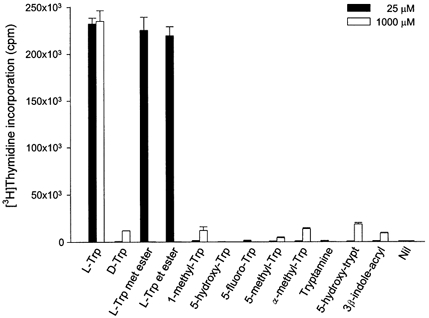
Effect of tryptophan analogues on peripheral blood mononuclear cell proliferation
Peripheral blood mononuclear cells were cultured for 72 h with the indicated concentrations of each of the tryptophan analogues or vehicle and [3H]thymidine incorporation was then determined as described in Methods. Data represent the mean ±s.d. of three separate experiments with quadruplicate assay. l-Trp met ester, l-tryptophan methyl ester; l-Trp et ester, l-tryptophan ethyl ester; α-methyl-Trp, α-methyl-tryptophan; 5-hydroxy-trypt, 5-hydroxy-tryptamine; 3β-indole-acryl, 3-β-indole-acrylic acid.
DISCUSSION
We conducted the experiments described here to ask questions concerning the functional role of indoleamine 2,3-dioxygenase in the human placenta. Building on the original hypothesis (Munn et al. 1998) and on later mechanistic work from the same group (Munn et al. 1999) we have explored the functional consequences of indoleamine 2,3-dioxygenase expression on proliferative activity of stimulated human peripheral blood mononuclear cells. Not only do we find that media conditioned by placental villous fragments strongly inhibit cellular proliferation we also see that this effect depends upon indoleamine 2,3-dioxygenase-mediated tryptophan depletion. This conclusion follows from the observations that (a) media conditioned by villi in the presence of interferon-γ, an inducer of indoleamine 2,3-dioxygenase, are more depleted of tryptophan than control media, (b) proliferation of mononuclear cells is markedly inhibited by tryptophan depletion to the level observed in the conditioned media, (c) the inhibitory effect of the conditioned medium is reversed following the addition of tryptophan but not of other essential amino acids, (d) the ability of the conditioned medium to inhibit proliferation is markedly reduced by the presence of an inhibitor of indoleamine 2,3-dioxygenase, 1-methyl-tryptophan, during conditioning, and (e) the products of tryptophan metabolism by indoleamine 2,3-dioxygenase are without effect on proliferation. All of these data are as predicted from the original hypothesis on murine pregnancy and strongly support the relevance of the proposal to human pregnancy.
T lymphocytes require one or more rounds of cell division to acquire a variety of functions (Bird et al. 1998; Oehen & Brduscha Riem, 1998) although some immune effects are independent of proliferation. Our cytometry analysis showed that it is CD4-positive T helper lymphocytes which are specifically influenced by tryptophan depletion. T helper lymphocytes, which are a major source of cytokines and which may be subdivided into subclasses Th1 and Th2, are thus the target of indoleamine 2,3-dioxygenase-mediated regulation. Thus the mechanisms described in this paper suggest that tryptophan depletion by placental indoleamine 2,3-dioxygenase may set the pathway of T helper cell differentiation at the maternal- fetal interface during normal pregnancy.
We used PHA to stimulate peripheral blood mononuclear cells, and therefore T cells rather than B cells will have been activated. Additionally in this physiological mixed cell population there will be indoleamine 2,3-dioxygenase-expressing monocytes. Direct evidence for this comes from experiments with the indoleamine 2,3-dioxygenase inhibitor 1-methyl-tryptophan; in the presence of this molecule (removing the effect of monocyte expressing indoleamine 2,3-dioxygenase) the tryptophan concentration required for half-maximal T cell proliferation is less than 1 μm, in agreement with Munn et al. (1999) who used purified T cells for determining tryptophan-dependent proliferation. Physiologically it seems reasonable to suggest that it will be the combination of circulating monocyte and trophoblast indoleamine 2,3-dioxygenase activity that will regulate T cell function at the maternal-fetal interface. The results shown in Fig. 2 also confirm the finding of Munn et al. that T cell proliferation is inhibited reversibly; thus it is due to arrest of cell cycle progression and not due to apoptosis. Total protein synthesis determined by labelled leucine incorporation into trichloroacetic acid-precipitable material in PHA-stimulated peripheral blood mononuclear cells in tryptophan-free medium is also maintained at 45-72 % of control culture (data not shown), confirming that apoptosis is not involved in the suppression of peripheral blood mononuclear cell proliferation.
The nature of the normal immunological and inflammatory response to pregnancy is controversial, probably reflecting multiple mechanisms in early implantation in the successful solution of Medawar's paradox (Medawar, 1953). The data that we describe here, derived from normal human tissues, show that placental indoleamine 2,3-dioxygenase activity can cause localised tryptophan depletion and that this inhibits lymphocyte proliferation in response to a standard T cell mitogen. This demonstrates that the mechanism of ‘immunosuppression by starvation’ so elegantly established by Munn and colleagues (1998) in murine pregnancy could function in human pregnancy. There is as yet no clear evidence that allogeneic rejection of human pregnancy occurs under any circumstance; nor is there a clear understanding of mechanisms of how the maternal immune system tolerates pregnancy. The nature of the interface between mother and fetus changes progressively as gestation advances. At first it is exclusively formed between trophectoderm and decidua with extravillous cytotrophoblast, bearing HLA-C, -E and -G, in direct contact with decidual large granular lymphocytes, macrophages and a small population of T cells. In late gestation it is formed mainly from syncytiotrophoblast, which is devoid of all HLA expression, in contact with moving maternal blood. One hypothesis is that tryptophan depletion will have a more functional effect in early gestation. At this stage, extravillous trophoblast has close and sustained contact with immune cells. The extent to which the T cells can react with the HLA-C, -E or -G expressed by the trophoblast is not known, although interactions with large granular lymphocytes expressing HLA receptors (CD94/NKG2, KIR and ILT) can be envisaged (King et al. 2000). Our current studies are directed to establishing the role of indoleamine 2,3-dioxygenase in the relevant tissues from first trimester human pregnancy.
Acknowledgments
We thank Dr C. M. Siman and Professor C. P. Sibley, Academic Unit of Child Health, St Mary's Hospital, University of Manchester for valuable advice on the explant assay, staff at the John Radcliffe Hospital, Oxford for assistance with obtaining placentae and Action Research for financial support.
References
- Bird J J, Brown D R, Mullen A C, Moskowitz N H, Mahowald M A, Sider J R, Gajewski T F, Wang C R, Reiner S L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Cady S G, Sono M. 1-Methyl-DL-tryptophan, β-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and β-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Archives of Biochemistry and Biophysics. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- Clover L M, Coghill E, Redman C W G, Sargent I L. A three-colour flow cytometry technique for measuring trophoblast intracellular antigens: the relative expression of TAP1 in human cytotrophoblast and decidual cells. Placenta. 2000;21:743–753. doi: 10.1053/plac.2000.0583. [DOI] [PubMed] [Google Scholar]
- Hwu P, Du M X, Lapointe R, Do M, Taylor M W, Young H A. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. Journal of Immunology. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- King A, Hiby S E, Gardner L, Joseph S, Bowen J M, Verma S, Burrows T D, Loke Y W. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors - a review. Placenta. 2000;21(suppl A):S81–S85. doi: 10.1053/plac.1999.0520. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd C A R. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochimica et Biophysica Acta. 2000;1500:119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd C A R. The role of l-tryptophan transport in l-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants. Journal of Physiology. 2001;531:417–423. doi: 10.1111/j.1469-7793.2001.0417i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd C A R, Sargent I L, Redman C W G. Modulation of indoleamine 2,3-dioxygenase by interferon-γ in human placental chorionic villi. Molecular Human Reproduction. 2000;6:369–374. doi: 10.1093/molehr/6.4.369. [DOI] [PubMed] [Google Scholar]
- Lyons A B, Parish C R. Determination of lymphocyte division by flow cytometry. Journal of Immunological Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Medawar P B. Some immunological and endocrinological problems raised by evolution of viviparity in vertebrates. Symposia of the Society for Experimental Biology. 1953;7:320–328. [Google Scholar]
- Munn D H, Shafizadeh E, Attwood J T, Bondarev I, Pashine A, Mellor A L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. Journal of Experimental Medicine. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D H, Zhou M, Attwood J T, Bondarev I, Conway S J, Marshall B, Brown C, Mellor A L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Oehen S, Brduscha Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. Journal of Immunology. 1998;161:5338–5346. [PubMed] [Google Scholar]
- Watson A L, Palmer M E, Burton G. Human chorionic gonadotrophin release and tissue viability in placental organ culture. Human Reproduction. 1995;10:2159–2164. doi: 10.1093/oxfordjournals.humrep.a136253. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochemical Journal. 1985;230:635–638. doi: 10.1042/bj2300635. [DOI] [PMC free article] [PubMed] [Google Scholar]


