Abstract
Homer, a family of scaffolding proteins originally identified in neurons, is also expressed in skeletal muscle. Previous studies showed that splice variants of Homer 1 (H1) amplify the gain of the ryanodine receptor type 1 (RyR1) channel complex. Using [3H]ryanodine ([3H]Ry) to probe the conformational state of RyR1, the actions of long- and short-forms of H1 are examined singly and in combination. At ≤200nM, H1 long-forms (H1b or H1c possessing coiled-coil (CC) domains) and short-forms (H1a or H1EVH1 lacking CC domains) enhance specific [3H]Ry-binding to RyR1. However, at a concentration >200nM, either H1 form completely inhibited [3H]Ry-binding. Importantly, the combinations of H1c + H1EVH1, or H1b + H1a acted in an additive manner to enhance or inhibit [3H]Ry-binding activity. H1a and H1c individually or in combination produced the same dynamic pattern in regulating purified RyR1 channels reconstituted in planar lipid bilayers. In combination, their net action on RyR1 channels depends on total concentrations H1. These data provide a mechanism by which constitutively and transiently expressed H1 forms can tightly regulate RyR1 channel activity in response to changing levels of expression and degradation of H1 proteins.
1. Introduction
The Homer family of proteins was originally identified in neurons where it was shown to confer important regulation of signal transduction, synaptogenesis, and receptor trafficking [1, 2]. All Homer proteins possess a conserved amino-terminal EVH1 domain, which recognizes and binds to a proline-rich sequence identified in Group 1 metabotropic glutamate receptors (mGluRs) [3], inositol-1,4,5-trisphosphate receptors (IP3R) [3, 4], ryanodine receptors (RyRs) [5, 6], transient receptor potential canonical-1 (TRPC1) ion channels [7] and the NMDA and metabotropic glutamate receptor scaffolding protein Shank [8, 9]. Most Homer proteins possess a carboxy-terminal coiled-coil (CC) structure followed by leucine zipper motifs that mediate Homer–Homer multimerization [1, 10, 11]. Homer proteins with CC-domains are recognized as “long-forms”. Homer 1a (H1a) and ania-3 which lack the CC-domain and are thus called “short-forms” [1]. Long-forms of Homer are constitutively expressed. Homer short-forms, on the other hand, can be constitutively expressed [12–14], but are also rapidly up-regulated in an immediate early gene-like fashion in response to heightened cellular activity [4]. H1a has been proposed as a natural dominant-negative that, upon up-regulation, competes with long-form Homer on target proteins thereby disrupting postsynaptic complexes and attenuating the signal gain [1].
Since the report that Homer mRNAs and their protein products are present in skeletal muscle [15, 16], Homer has been regarded as potential activity-dependent regulators of Ca2+ signaling in skeletal muscle [14]. Several studies have shown Homer proteins are capable of interacting with the RyR1 channel complex [5, 6, 17–19]. These studies have examined the actions of long-forms H1c and H1b and have consistently shown their ability to enhance Ca2+ release from junctional SR through a direct interaction with RyR1 that enhances the open probability of the channel. However among these studies there appears to be a discrepancy about the activity of short-form H1a/H1EVH1 toward the RyR1 channel function [5, 17, 18]. In one study, H1a was shown to dose-dependently attenuate the H1c-activated RyR1 channel [18]. In contrast to this observation, another study showed that functionally active H1a/H1EVH1 acted dose-dependently and additively to enhance H1c-activated spark activity mediated by RyR1 channel activity [17], suggesting a lack of competitive antagonism between short- and long-forms of Homer towards RyR1 activity, in contrast to the activity reported from neurons.
The present work addresses the gap in our understanding of how long- and short-form Homers interact with RyR1 to regulate its conformation. It shows here for the first time that both the long-and short-forms of Homer are capable of regulating RyR1 in a biphasic manner by interacting directly with the channel. The combination of H1 short and long forms act in a purely additive manner to enhance or inhibit [3H]ryanodine ([3H]Ry)-binding activity and the open probability of purified RyR1 channels reconstituted in bilayer lipid membranes (BLM), and their net effect is dependent on their combined total concentration at the receptor site. These data provide a mechanism by which constitutively and transiently expressed H1 forms could tightly regulate RyR1 channel activity in response to changing levels of expression and degradation of Homer proteins.
2. Materials and Methods
2.1 Preparation of SR Membranes and purified RyR1
Junctional SR membrane enriched in RyR1 were prepared from skeletal muscle of New Zealand White rabbits according to the method of Saito et al. [20]. The preparations were stored in 10% sucrose, 10mM HEPES, pH 7.4, at −80°C until needed. RyR1 was solubilized in CHAPS detergent as previously described [21]. RyR1 was then purified from the CHAPS-solubilized proteins by column chromatography through Sepharcyl S-300 HR (Amersham Biosciences) and the RyR1 peak further purified on a 5–20% (W/V) linear sucrose gradient [22]. The ~30S fraction containing enriched RyR1 was then concentrated on a HiTrap Heparin HP column (Amersham Biosciences) [22]. Purity of RyR1 was assessed by SDS-PAGE and silver stain. For preparation of the RyR1–FKBP12 complex, purified RyR1 was supplemented with an eightfold molar excess of recombinant FKBP12 (Sigma-Aldrich) immediately before channel reconstitution experiments.
2.2 Expression and Purification of Homer Construct
GST fusion constructs were made by PCR amplifying the H1c open reading frame and the N-terminal 360-bp fragment with in frame primers with SalI and NotI sites and inserting the PCR products into pGEX4T-2 (Pharmacia Corp). H1b (Stratagene® GeneConnectionTM clone) was expressed as a fusion protein (with C-myc and 6His tags) using supplier’s instructions. Fusion proteins were purified as described previously [23]. H1EVH1 mutant, W24A was made with the Quik-ChangeTM site-directed mutagenesis kit (Stratagene). Mena-EVH1 GST was a gift from Dr. Leahy (Johns Hopkins and Howard Hughes Medical Institute). GST fusion plasmids of H1c, H1EVH1 W24A, and Mena were transformed into BL21 cells, and positive clones were expanded. The cells were lysed by sonication, and the lysate was added to glutathione-agarose and sequentially washed [8, 9]. The slurry of glutathione-agarose beads loaded with fusion protein was then incubated with biotinylated thrombin (Novagen). Purified Homer proteins were dialyzed against PBS at 4°C overnight. Before being used for functional studies, the purity and the protein compositions of the preparations were analyzed by quantitative densitometry of silver stained SDS-PAGE; high-resolution MALDI-TOF mass spectrometry for accurate mass, and/or tandem mass spectrometry protein sequencing of in-solution digests.
2.3 Measurement of [3H]Ry Binding
Equilibrium measurement of specific high affinity [3H]Ry binding was determined according to the method of Pessah et al. [24]. SR vesicles (50µg protein/ml) were incubated with or without Homer in buffer containing 20mM PIPES or HEPES, pH7.1 or pH7.4, 140 or 250mM KCl, 15mM NaCl, 1–50µM CaCl2, and 1–5nM [3H]Ry for 3h at 37°C. The reactions were quenched by filtration through GF/B glass fiber filters and washed twice with ice-cold harvest buffer (20mM Tris-HCl, or 20mM Hepes, 250mM KCl, 15mM NaCl, 50µM CaCl2, pH 7.1 or pH7.4). Nonspecific binding was determined by incubating SR vesicles with 1000-fold excess unlabelled ryanodine.
2.4 Measurement and analysis of purified RyR1 single channel reconstituted in planar lipid bilayer
Bilayers were composed of phosphatidyl- ethanolamine: phosphatidylserine: phosphatidyl-choline (5:3:2 w/w, Avanti Polar Lipids, Inc, AL) dissolved in decane at a final concentration of 30mg/ml across a 200µm aperture on a polysulfone cup (Warner Instrument Corp. CT). The bilayer partitioned two chambers (cis and trans) with buffer solution (in mM) 500 CsCl, 7mM free Ca2+, and 20 Hepes-Tris (pH 7.4) on cis, 50 CsCl and 20 Hepes-Tris (pH 7.4) on trans. The addition of protein was made to the cis solution that was held at the virtual ground, whereas the trans solution was connected to the head stage input of an amplifier (Bilayer Clamp BC 525C, Warner Instrument, CT). After supplementation of purified RyR1 with a molar excess of FKPB12, single channels were reconstituted by introducing the FKBP12- RyR1 protein preparation in the cis chamber. Immediately after incorporation of a single RyR1 channel into BLM, the cis chamber was perfused with cis solution to prevent incorporation of additional channels into bilayer. Single channel gating was monitored and recorded at a holding potential of −40mV (applied to the trans side). The sidedness (cytosolic) of the channel was verified by the positive response to addition of micromolar Ca2+ or responding to 2µM ryanodine (at the end of the experiment). The amplified current signals, filtered at 1 kHz (Low-Pass Bessel Filter 8 Pole, Warner Instrument, CT) were digitized and acquired at a sampling rate of 10 kHz (Digidata 1320A, Axon-Molecular Devices, Union City, CA). All the recordings were made for at least 40sec to 6min under each experimental condition. The channel open probability (Po), mean open-, and mean closed-dwell times (to and tc) were obtained by using Clampfit, pClamp software 9.0 without further filteration (Axon-Molecular Devices, Union City, CA).
3. Results
3.1. H1 long- and short-forms have biphasic actions on RyR1
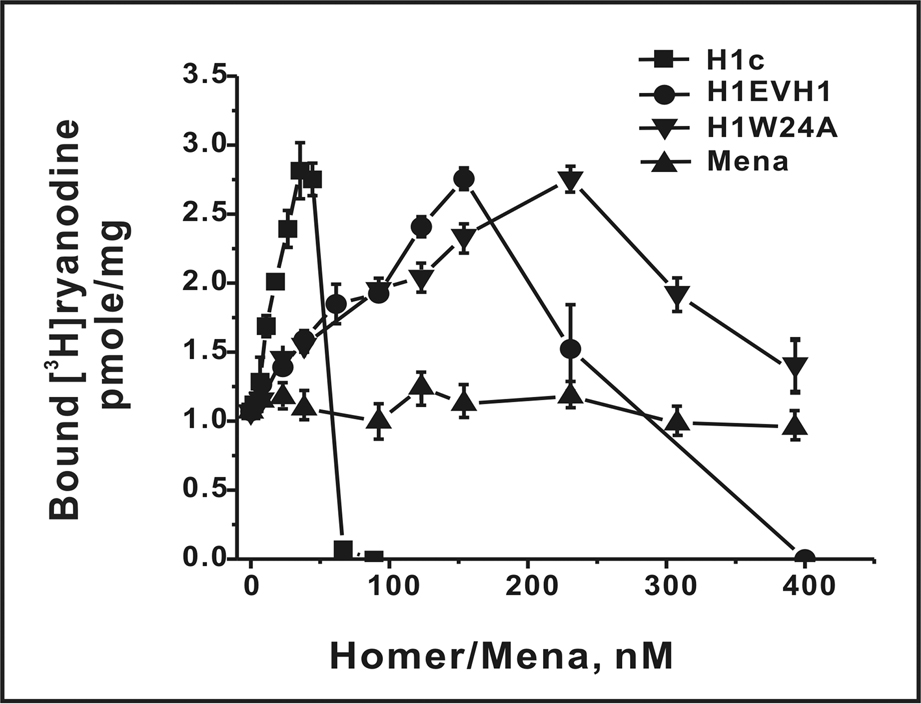
Previous reports have shown that the H1 short-forms (H1a/H1EVH1) [5, 17] and its long-forms H1c and H1b could potently enhance the activity (gain) of RyR1 channel complexes [5, 17–19]. In addition, H1c and H1-EVH1 in combination were observed to have additive actions in enhancing the binding of [3H]Ry and the frequency of Ca2+ spark in permeabilized myotubes [17]. To further understand how H1 forms modulate RyR1 channel activity, we performed an extended dose-response analysis. Figure 1 shows that both short- and long-forms of H1 dose-dependently activated RyR1 with the same maximum efficacy of [3H]ryanodine binding reaching from the control of 1.03pmole/mg to ~2.75pmole/mg. The extended dose-response curve shows that both H1 forms are capable of producing biphasic actions on RyR1. H1C is a significantly more potent activator (EC50= 9.8nM) and inhibitor (IC50= 55.5nM) than H1EVH1 (EC50= 26.2nM; IC50= 242nM). As expected, the EVH1 domain is important for the potent functional activity of H1, as demonstrated by the significantly lower potency of the H1EVH1 mutant W24A toward inhibiting RyR1 compared to H1EVH1 (EC50=26.8 nM; IC50>400nM) (Fig.1). The murine homolog of Ena (Mena) possesses an EVH1 domain that is structurally similar to Homer but binds a distinctly different proline-rich sequence [25]. Mena serves as a negative control to test the specificity of H1 activation of RyR1 [5]. Here we extended the concentration of Mena to the inhibitory range for H1 and found that Mena is inactive toward inhibiting RyR1 at a concentration of 400nM (Fig. 1).
Fig. 1.
Biphasic influences of H1 short- and long-forms (H1EVH1 and H1c) on [3H]Ry binding activity of RyR1 channel complex. JSR membrane-bound RyR1 proteins (50µg/ml) were incubated in the binding buffer consisted of 250mM KCl, 15mM NaCl, 50µM CaCl2, 1nM [3H]Ry, 20mM PIPES, pH 7.4 at 37°C for 3hr, with indicated concentrations of H1c, H1EVH1, H1W24A or Mena. Each point represents mean±SD of n=5 samples. There were at least three independent measurements with similar results under varied experimental conditions (with 140mM KCl, 1, 5 or 50µM CaCl2 and/or 1 or 5nM[3H]Ry).
3.2. H1 long- and short-forms regulate RyR1 in a purely additive manner
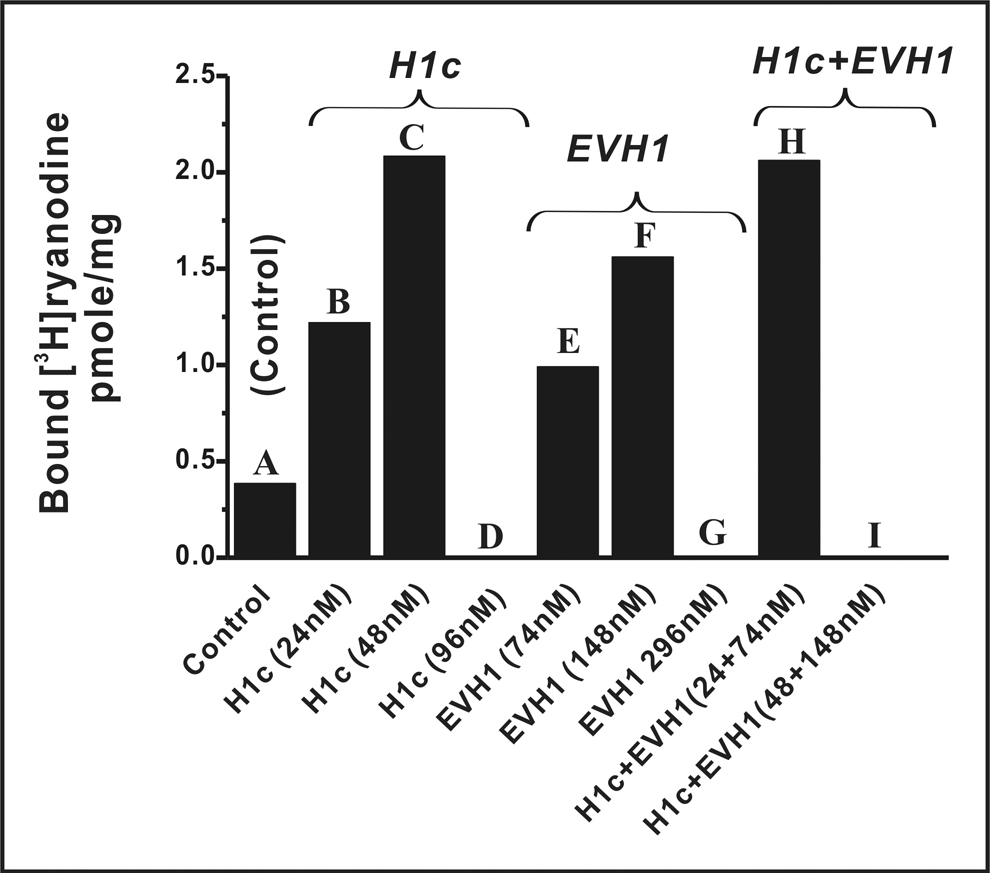
Figure 2 demonstrates that RyR1 is not only modulated in a biphasic manner by individual H1 forms, but that regulation is additive. At lower concentrations both long- (H1c at 24 and 48nM, Columns B and C) and short-forms (H1EVH1 at 74 and 148nM, Columns E and F) significantly enhance [3H]Ry binding activity in a dose-dependent manner. By contrast, higher concentrations of either H1c (96nM, Column D) or H1EVH1 (296nM, Column G) fully inhibit RyR1 function. These results serve as internal experimental controls. A combination of sub-optimal activating concentrations of H1c (24nM) + H1EVH1 (74nM) is tested and clearly shows that these long- and short-forms act in an additive manner to enhance RyR1 function (Column H). However, a combination of 48nM H1c + 148nM H1EVH1, concentrations which individually activated RyR1 optimally, fully inhibit RyR1 (Column I).
Fig. 2.
H1EVH1 and H1c enhance or inhibit RyR1 in a dose-dependent and additive manner. In the presence of 250mM KCl, 15mM NaCl, 1µM free Ca2+, buffered by EGTA (calculated according software “Bound and Determined” [35]), 1nM [3H]Ry, 20mM PIPES, pH 7.4, JSR membrane-bound RyR1 proteins (50µg/ml) were incubated without or with indicated concentration(s) of H1c and/or H1EVH1 at 37°C for 3hr. This is a representative result performed in triplicate and is representative of four independent experiments under varied conditions (with 140mM KCl, 1, 5 or 50µM CaCl2 and/or 1 or 5nM[3H]Ry).
3.3. Long-form H1b and short-form H1a dynamically regulate RyR1
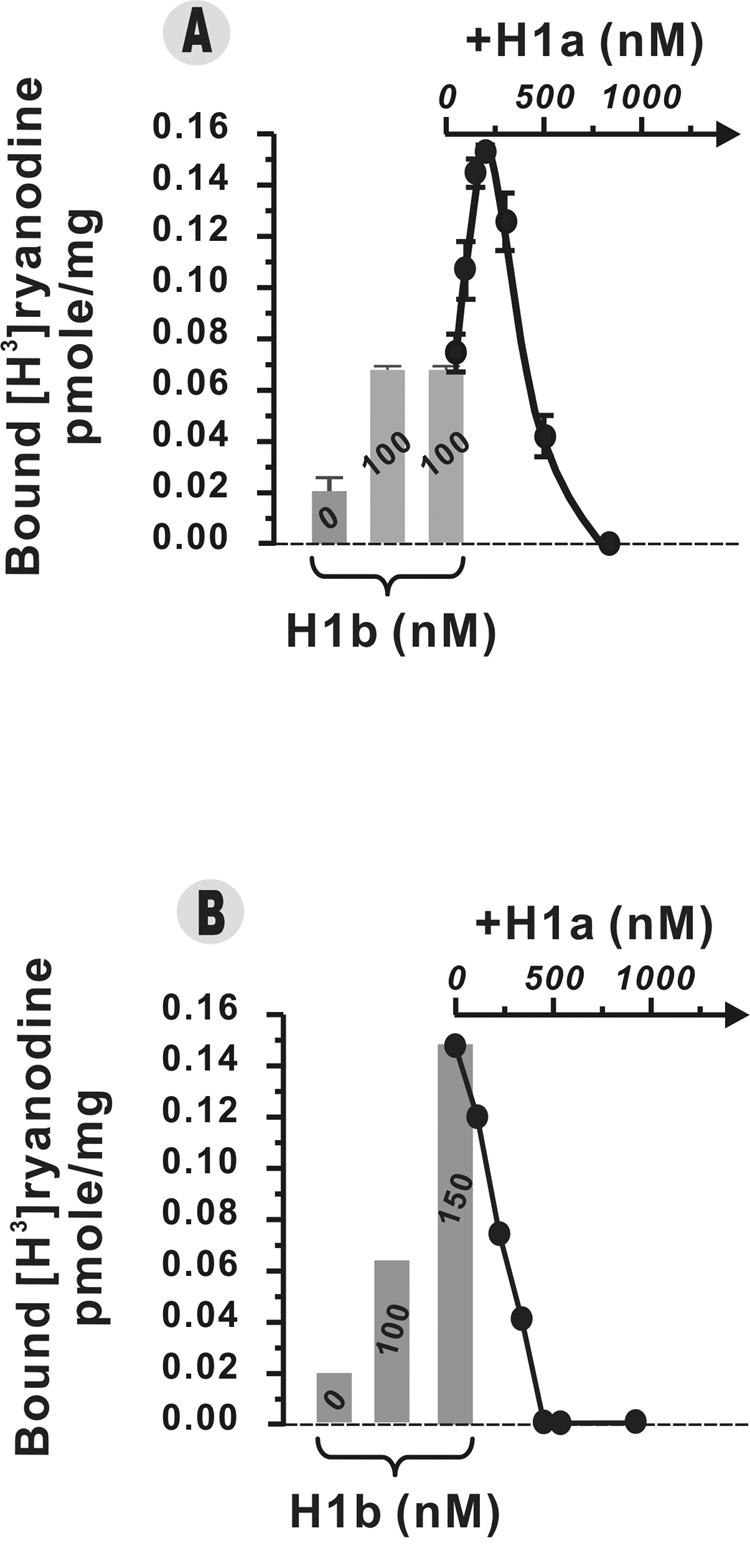
Recently H1b was reported to act as a potent agonist of the RyR1 channel [19]. Like other H1 proteins, H1b is a potent enhancer (Fig. 3) as well as an inhibitor of [3H]Ry binding to RyR1 (data not shown). At a concentration of 100nM, H1b increases [3H]Ry binding approximately 3-fold (Fig. 3A: bar labeled 100). In the presence of 100nM H1b, [3H]Ry binding levels are further enhanced by titration of H1a (between 50 and 300nM), but are inhibited by titration with higher concentrations of H1a (between 300 and 800nM), and inhibition is complete with 100nM H1b + 800nM H1a (Fig. 3A; Curve). Figure 3B shows that increasing H1b in the reaction mixture from 100nM to 150nM by itself further enhances RyR1 activity and this concentration is near its maximum efficacy for activation. However in the presence of 150nM H1b, titration of H1a within the same dose-range (50–800nM) that causes biphasic regulation of RyR1 (i.e., Fig. 3A) inhibits RyR1 activity dose-dependently (Fig. 3A; Curve).
Fig. 3.
Dynamic regulation of RyR1 conformation by the combination of long-form H1b and short-form H1a. [3H]Ry binding conditions were similar as described for Fig. 2. JSR membrane-bound RyR1 proteins (50µg/ml) were incubated without Homer protein, or with 100nM H1b plus 0–800nM H1a (Fig. 3A), or with 150nM H1b plus 0–500nM H1a (Fig.3B) in the presence of 140mM KCl, 15mM NaCl, 1µM free Ca2+, 1nM [3H]Ry, 20mM Hepes, pH 7.1. The results were obtained from triplicate samples.
3.4. Short-form H1a and long-form H1c regulate RyR1 channels in a biphasic and additive manner
To further examine the action of the short-form Homer in the absence or presence of long-form we study the gating behavior of purified RyR1 channels reconstituted in BLM. H1a alone and H1a in combination with H1c are used at concentrations predicted to activate or inhibit purified RyR1 channel activities based on results from [3H]Ry -binding analysis (presented above). Consistent with our previous findings, low concentrations H1a introduced to the cytoplasmic face of the reconstituted channel is found to significantly enhance RyR1 channel activity. Figure 4A shows one minute of continuous recording representing the current traces of a RyR1 single channel before and after introducing 50nM H1a. Single channel kinetics analysis revealed that 50nM H1a increased the open probability (Po) ~13-fold (from Po=0.003 to 0.040), decreased the mean closed dwell time (τc) ~12-fold (from 142.66±210.33ms to 11.24±11.76ms).
Fig. 4.
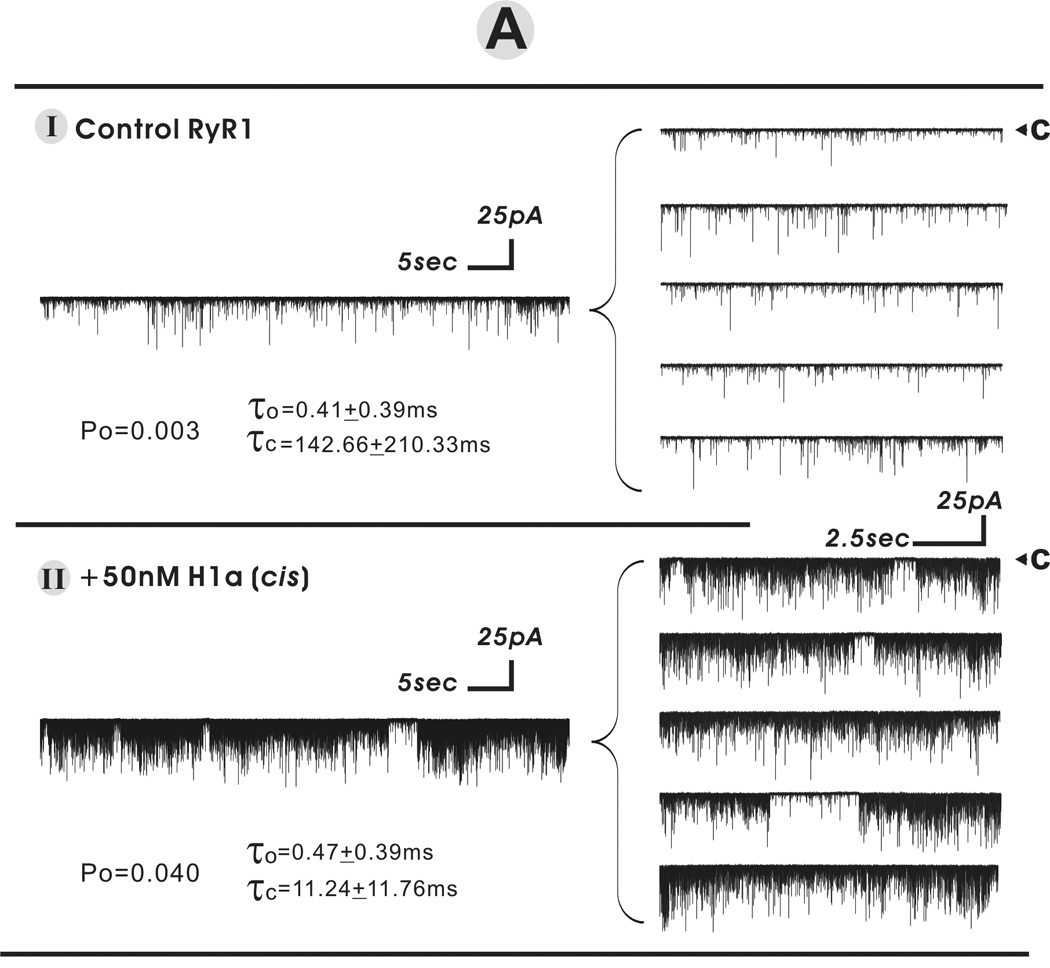
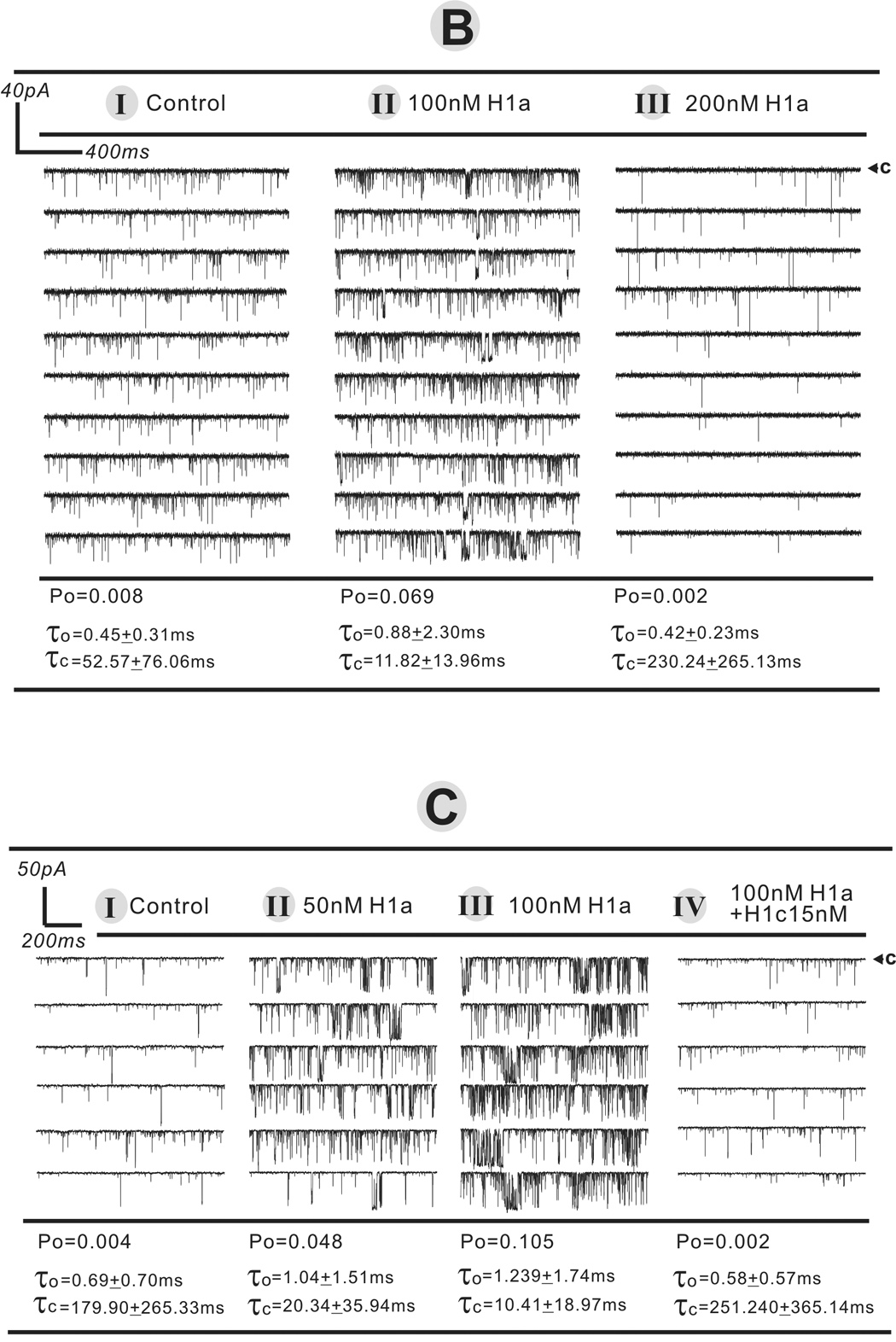
H1 short- and long-form act additively to regulate RyR1 single channel activity in a biphasic manner. The purified FKBP12/RyR1 channel activity was recorded in 7µM cis (cytoplasmic) free Ca2+ at a holding potential of −40mV applied on trans side. Under each defined condition, the single channel recordings were made for at least 40s to 5min in the total six independent bilayer experiments. The arrow and “c” indicate the current level of zero pA while the channel was in complete closed state. Panel A displays the representative current traces before and after addition of 50nM H1a in cissolution. The current trace of one minute continuous recording was expanded into 5 segments displaying on the right panels. In Panels B and C, H1a and/or H1a+H1c were sequentially introduced into the cis solution to a final concentration denoted in the figure.
Consistent with the [3H]Ry binding results, single channel studies further revealed that short-form H1a can enhance and diminish RyR1 channel activity in a dose-dependent manner as shown in figures 4B and 4C. Figure 4B shows that in the presence of 100nM short-from H1a, channel activity is enhanced; at a higher concentration of 200nM H1a is inhibitory to the channel. Figure 4C shows that once RyR1 channel activity is enhanced by an optimal concentration of H1a (100nM), subsequent addition of 15nM H1C inhibits the channel’s activity.
4. Discussion
Previous studies have implicated RyR1 AAs F1777 and F1782 as being important in recognizing the conserved EVH1 domain of Homer proteins. Mutation of these residues reduces the Ca2+ amplitude attained with caffeine challenges [5, 6]. Substantial evidence from biochemical, electrophysiological and cellular studies have confirmed that nanomolar H1 physically interacts with RyR1 to enhance its Ca2+ channel activity [5, 17–19, 26]. These independent results obtained from several different research groups have indicated that both long- and short-forms of H1 are capable of enhancing RyR1 channel activity (or gain).
Important new findings identified in the present study are (1) H1 forms can individually function to enhance or diminish RyR1 activity in a graded manner; (2) the EVH1 domain is essential for both agonist and antagonist activities toward RyR1, whereas the CC domain, although not essential, strongly influences the relative potencies for both of these activities; (3) when present in combination, long- and short-forms of H1 act in a additive manner to enhance or inhibit [3H]Ry-binding activity, and their net effect is dependent on their total concentration at the receptor site.
Based on previous reports [5, 17] and the present findings, it is clear that Homer EVH1 is the essential domain necessary to mediate the physical interactions with RyR1 and modulate its conformation and channel function. This conclusion is supported by the observation that H1-EVH1 is as effective as H1a in regulating RyR1 channel activity in a biphasic manner, albeit that its potency is ~3-fold lower than long form H1c. Additional support for this conclusion comes from the fact that mutation of W24 within the EVH1 domain to “A” significantly weakens these activities towards RyR1, and the total inactivity of Mena toward RyR1. Homer has potent activity in regulating RyR1 as a ligand that is independent of the CC-domain and multimerization. Thus the adapter role of Homer is not necessary for this function. However the CC-domain does increase the potency for enhancing channel gain, and is especially important for sharply inhibiting the channel as its concentrations rise. Thus the CC-domain contributes towards stabilizing both open and closed conformations of RyR1. The enhanced potency of H1C could be the result of more productive or coordinated ligand binding between the Homer ligand sites on adjacent subunits facilitated by Homer multimers.
The current data is consistent with most previous reports, although there is one published study indicating that the lack of a CC-domain renders a Homer short-form inactive towards RyR1 by itself, while retaining its ability to inhibit the activation of RyR1 by a Homer long-form [18]. Although the study by Hwang et al. (18) did not examine the dose-response relationship of Homer long- and short-forms, examination of their single channel analysis indicates subtle dynamic aspects to Homer regulation of RyR1 channel function that we describe here. For example RyR1 single channel was highly active in the presence of 100nM H1c + 50nM H1a, but channel activity was significantly inhibited when H1a was further increased to 100nM (Fig. 3 in reference 18). In the present study we examined the possibility that the inhibitory effects of H1a were not the consequence of competition with H1c, but rather an additive mechanism whose net effect on RyR1 channel activity (activation or inhibition) depends the total Homer concentration at the cytoplasmic face (cis) of the channel. This mechanism was addressed by first enhancing channel activity with H1a followed by subsequent addition of long-form H1c. As shown in Fig. 4B, the open probability (Po) of RyR1 channels was significantly enhanced by increasing cis H1a from 50 to 100nM. As predicted from results obtained from [3H]Ry binding analysis, the channel’s Po was reduced upon subsequent addition of H1c (15nM). Importantly this concentration (15nM) of H1c alone consistently enhanced RyR1 channel activity (Fig. 1 and [5, 17]). In other single channel experiments, RyR1 channels initially activated by the addition of 50nM H1a responded to addition of 30nM H1c with a rapid decrease in open probability (not shown). Collectively the present results show that H1a and H1c can interact with purified RyR1 in an additive concentration-dependent manner to tightly regulate channel open probability.
Originally identified as an immediate-early gene product [4], H1a use-dependent induction of expression in neurons is believed to disrupt long-form Homer cross-linked protein complexes and thus H1a is regarded as a natural dominant-negative form [3]. The original observations of transient expression of H1a have implied that H1a expression may correlate with the dysfunction of intracellular Ca2+ stores [27–30]. In an attempt to see if experimental manipulation of the level of ER Ca2+ stores could be used to control H1a expression and hence to modify calcium signaling, Paschen & Mengesdorf, (2003) investigated the effect of changes in the functional state of the ER compartment on H1a expression [31]. They found a close relationship between H1a expression and alteration of stored Ca2+, whereby ER Ca2+ store depletion elicited by 1mM EGTA, or by blocking the SERCA pump with thapsigargin, or by exposing cells to a proteasome inhibitor known to induce ER dysfunction (MG132), resulted in significant up-regulation of H1a expression [31]. Given the fact that activity-dependent expression of H1a can act in concert with constitutively expressed Homer proteins, our results provide a novel mechanism by which Homer forms can tightly regulate the absolute activity of RyR1-sensitive Ca2+ stores in a manner dependent of the level of expression of H1 forms that rely only on the EVH1 domain for RyR1 activity, and whose CC domain defines their relative potencies (Figs. 2, 3 and 4C). The net effect of expression of H1a on the magnitude of Ca2+ release by RyR1 would be expected to depend not only on a cell’s recent history of activity but also on the cadre of other Homer proteins being expressed and bound to RyR1. The current data provides evidence that Homer proteins are dynamic regulators of long-term homeostatic maintenance and contribute an element of hysteresis to Ca2+ homeostasis and signaling. In this regard, H1a could be implicated in a variety of cellular functions that require activity-dependent regulation. In support for this hypothesis, the transient expression of H1a was recently reported to be involved in myoblast differentiation and muscle regeneration [14]. In one experimental model of short-term adaptation of skeletal muscle H1a was not found to be significantly up-regulated [14]. However, the question of whether other forms of agonist-mediated activity in skeletal muscle can influence levels of H1a needs further investigation. In a recent study, H1a was found to be significantly up-regulated in cardiac myocytes by several agonists, including endothelin-1, phenylephrine, isoprotenerol or angiotensin-II [32]. In addition to the dynamic regulation of RyR1 activity conferred by Homer proteins, long- and short-forms could contribute to other two distinct cellular processes. Constitutively expressed Homers are likely to influence the gain of Ca2+ release during E-C Coupling and reflect their steady-state level of expression over a longer time frame and more generalized aspects of the cell’s context. On the other hand, immediate early and transient expression of H1a provides a means to alter RyR1 gain in response to recent activity; for example to prevent Ca2+ store depletion during bouts of intense activity. In this model, instead of competing with Homer already bound to RyR1, H1a can directly and additively bind to unoccupied EVH1 ligand sites on RyR1 and confer either positive or negative regulation to release of Ca2+ depending on the current context of Homer-RyR1 interactions. It is known that all members of Homer family contain PEST sequences that are thought to be ubiquitin-proteasome-dependent degradation signals [33]. However, evidence of rapid ubiquitination and degradation has been reported for H1a, whereas evidence ubiquitin-dependent processing of Homer long-forms is lacking [34]. Therefore, the use-dependent expression and rapid degradation of H1a could provide a metabolic means by which the concentration of Homer protein near the surface of RyR1 is regulated.
The abbreviations used are
- SR
sarcoplasmic reticulum
- JSR
junctional SR
- Ry
ryanodine
- RyR1
skeletal-type ryanodine receptor
- EVH1
Enabled/VASP homology 1
- Mena
murine homolog of Ena
- H1a
Homer 1a protein
- H1b
Homer 1b protein
- H1c
Homer 1c protein
- Po
open probability
- τo
mean open dwell time
- τc
mean closed dwell time
Footnotes
This work was partially supported by National Institute of Arthritis and Musculoskeletal and Skin Disease Grants AR43140 and AR52354 (to PD Allen and IN Pessah) and National Institute on Drug Abuse DA10309 (PW). We also thank Jackelynn Dao for her excellent technical assistance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 2.Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 4.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 5.Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF, Pessah IN. Homer regulates gain of ryanodine receptor type 1 channel complex. J Biol Chem. 2002;277:44722–44730. doi: 10.1074/jbc.M207675200. [DOI] [PubMed] [Google Scholar]
- 6.Westhoff JH, Hwang SY, Scott Duncan R, Ozawa F, Volpe P, Inokuchi K, Koulen P. Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell Calcium. 2003;34:261–269. doi: 10.1016/s0143-4160(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 7.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 8.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 9.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 10.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 11.Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Sci STKE. 2002;2002:RE8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- 12.Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Differential expression of Homer family proteins in the developing mouse brain. J Comp Neurol. 2004;473:582–599. doi: 10.1002/cne.20116. [DOI] [PubMed] [Google Scholar]
- 14.Bortoloso E, Pilati N, Megighian A, Tibaldo E, Sandona D, Volpe P. Transition of Homer isoforms during skeletal muscle regeneration. Am J Physiol Cell Physiol. 2006;290:C711–C718. doi: 10.1152/ajpcell.00217.2005. [DOI] [PubMed] [Google Scholar]
- 15.Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur J Biochem. 2000;267:634–639. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandona D, Tibaldo E, Volpe P. Evidence for the presence of two homer 1 transcripts in skeletal and cardiac muscles. Biochem Biophys Res Commun. 2000;279:348–353. doi: 10.1006/bbrc.2000.3948. [DOI] [PubMed] [Google Scholar]
- 17.Ward CW, Feng W, Tu J, Pessah IN, Worley PK, Schneider MF. Homer protein increases activation of Ca2+ sparks in permeabilized skeletal muscle. J Biol Chem. 2004;279:5781–5787. doi: 10.1074/jbc.M311422200. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SY, Wei J, Westhoff JH, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium. 2003;34:177–184. doi: 10.1016/s0143-4160(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 19.Pouliquin P, Pace SM, Curtis SM, Harvey PJ, Gallant EM, Zorzato F, Casarotto MG, Dulhunty AF. Effects of an alpha-helical ryanodine receptor C-terminal tail peptide on ryanodine receptor activity: modulation by Homer. Int J Biochem Cell Biol. 2006;38:1700–1715. doi: 10.1016/j.biocel.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pessah IN, Francini AO, Scales DJ, Waterhouse AL, Casida JE. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986;261:8643–8648. [PubMed] [Google Scholar]
- 22.Samso M, Wagenknecht T, Allen PD. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol. 2005;12:539–544. doi: 10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catanzariti AM, Soboleva TA, Jans DA, Board PG, Baker RT. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 2004;13:1331–1339. doi: 10.1110/ps.04618904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pessah IN, Waterhouse AL, Casida JE. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1985;128:449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- 25.Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, Leahy DJ. Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron. 2000;26:143–154. doi: 10.1016/s0896-6273(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 26.Stiber JA, Tabatabaei N, Hawkins AF, Hawke T, Worley PF, Williams RS, Rosenberg P. Homer modulates NFAT-dependent signaling during muscle differentiation. Developmental Biology. 2005;287:213–224. doi: 10.1016/j.ydbio.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Parsons JT, Churn SB, Kochan LD, DeLorenzo RJ. Pilocarpine-induced status epilepticus causes N-methyl-D-aspartate receptor-dependent inhibition of microsomal Mg(2+)/Ca(2+) ATPase-mediated Ca(2+) uptake. J Neurochem. 2000;75:1209–1218. doi: 10.1046/j.1471-4159.2000.0751209.x. [DOI] [PubMed] [Google Scholar]
- 28.Mestdagh N, Wulfert E. Bicuculline increases Ca2+ transients in rat cerebellar granule cells through non-GABA(A) receptor associated mechanisms. Neurosci Lett. 1999;265:95–98. doi: 10.1016/s0304-3940(99)00213-x. [DOI] [PubMed] [Google Scholar]
- 29.Berg M, Bruhn T, Frandsen A, Schousboe A, Diemer NH. Kainic acid-induced seizures and brain damage in the rat: role of calcium homeostasis. J Neurosci Res. 1995;40:641–646. doi: 10.1002/jnr.490400509. [DOI] [PubMed] [Google Scholar]
- 30.Niebauer M, Gruenthal M. Neuroprotective effects of early vs. late administration of dantrolene in experimental status epilepticus. Neuropharmacology. 1999;38:1343–1348. doi: 10.1016/s0028-3908(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 31.Paschen W, Mengesdorf T. Conditions associated with ER dysfunction activate homer 1a expression. J Neurochem. 2003;86:1108–1115. doi: 10.1046/j.1471-4159.2003.01884.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto T, Togi K, Yamauchi R, Yoshida Y, Nakashima Y, Kita T, Tanaka M. Endothelin-1 activates Homer 1alpha expression via mitogen-activated protein kinase in cardiac myocytes. Int J Mol Med. 2006;18:193–196. [PubMed] [Google Scholar]
- 33.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 34.Ageta H, Kato A, Fukazawa Y, Inokuchi K, Sugiyama H. Effects of proteasome inhibitors on the synaptic localization of Vesl-1S/Homer-1a proteins. Brain Res Mol Brain Res. 2001;97:186–189. doi: 10.1016/s0169-328x(01)00303-5. [DOI] [PubMed] [Google Scholar]
- 35.Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]