Abstract
PURPOSE
Age can affect the delicate physiologic balance of the internal anal sphincter diameters and pressure governed by Laplace's law. This study compares the effect of aging on the internal anal sphincter thickness and diameter in younger and older nulliparous females without symptoms of fecal incontinence undisturbed by an endoanal probe.
METHODS
Magnetic resonance images were selected from a large database of nulliparous females to form two groups: “younger” females, aged 30 years and younger (n = 32), and “older” females, aged 50 years and older (n = 32). All patients were scanned without endoanal coils to allow undistorted measurement of the internal anal sphincter diameters. Inner and outer diameters were measured from axial magnetic resonance images and used to calculate sphincter thickness and mean radius by two independent investigators blinded to patient age.
RESULTS
The mean age in the younger group was 26 ± 2.8 years, whereas that of the older group was 61.8 ± 7.6 years. Older females had a 33 percent thicker internal anal sphincter (younger vs. older: 4.5 ± 0.7 vs. 5.9 ± 1 mm; P < 0.001), a 20 percent larger inner diameter (7.1 ± 1.3 vs. 8.5 ± 1.8 mm; P = 0.001), and a 27 percent larger outer diameter (16 ± 2.1 vs. 20.3 ± 3.3 mm; P < 0.001) than younger females. Neither sphincter thickness nor inner or outer diameter correlated with body mass index.
CONCLUSIONS
There is an increase in internal anal sphincter thickness, inner diameter, and outer diameter, which correlates with age in asymptomatic nulliparous females.
Keywords: Internal anal sphincter, Magnetic resonance imaging, Fecal incontinence, Laplace's law
Fecal incontinence is a devastating clinical condition, which has social, psychologic, and economic consequences. This condition is reported to affect between 2 and 15 percent of the adult population, with approximately between 1 and 7 million Americans afflicted.1–4 As females age, the prevalence of fecal incontinence increases without a clear etiology.5 Birth, aging, and decreased function of the anal sphincters, as well as other diseases that impact bowel function, such as diabetes mellitus, have been postulated to affect bowel control.5
Anal canal closure depends on a delicate balance between the tension in the wall of the sphincters, their diameter, and the closure pressure generated by this system, which is governed by Laplace's law. Three muscular structures maintain anal canal closure: the smooth muscle of the internal anal sphincter (IAS), the striated muscle of the external anal sphincter, and the striated puborectal muscle. The IAS plays a key role in maintaining continence, providing 75 percent of the maximum resting pressure.6 Whereas this resting pressure is provided by the fatigue resistant internal anal sphincter to guarantee anal closure, the striated external anal sphincter can be volitionally used to generate additional closure pressure (maximum squeeze) during large increases in abdominal pressure, such as during coughing, sneezing, or lifting heavy objects.
The thickness of the IAS is known to increase with advancing age.7–9 Previous studies have measured IAS thickness by using ultrasound with an endoanal probe or via magnetic resonance imaging (MRI) with a coil. However, measurements performed with an endoanal probe are invasive in that they alter both the diameter and thickness of the sphincter. Sultan et al.10 illustrated the distortion of the anatomic arrangement of epithelial structures and sphincter muscles by using anal endosonography, thereby demonstrating a new approach using a vaginal ultrasound technique to visualize the sphincter.
Laplace's law describes the relationship between the muscle wall thickness, wall tension, and the squeeze pressure generated within a tubular structure, such as the anal canal. However, to determine these relationships, the radius of the tubular structure must be known. Therefore, measurements without the distortion of the canal by an endoanal probe are needed.
To better understand age-related physiologic changes in the IAS of asymptomatic nulliparous females, we compared, in younger and older females, the thickness and diameter of the IAS in MR scans undistorted by the presence of an endoanal probe.
PATIENTS AND METHODS
We selected MRI scans from our database of 676 females from four institutional review board-approved projects on pelvic floor anatomy and pelvic floor disorders. Both “cases” as well as asymptomatic “controls” were recruited between 1997 and 2006 by using newspaper advertisements, fliers, the Women's Health Registry, and the University of Michigan Urogynecology Clinic. All participants underwent pelvic MRI in the supine position. A standardized questionnaire on pelvic symptoms, including urinary and fecal incontinence, was administered.
Inclusion and Exclusion Criteria
The “older” group in the present study was selected from the 32 continent nulliparous females aged 50 years and older in our database. The “younger” group was comprised of an equal number of continent nulliparous females randomly selected from among those aged 30 years and younger in the database. Females who reported symptoms of fecal incontinence were excluded.
Pelvic Magnetic Resonance Imaging
All females had a pelvic MR scan performed without an endoanal coil. Multiplanar, two-dimensional, fast spin, proton density MR images (echo time 15 ms, repetition time 4,000 ms) were obtained by using a 1.5 T superconducting magnet (General Electric Signa Horizon LX®, GE Medical System®, Milwaukee, WI). The axial and coronal fields of view were each 16 cm × 16 cm; the sagittal images were 20 cm × 20 cm. All three views had slice thicknesses of 4 mm with a 1-mm gap between slices.
Measurements
Axial MR images were imported into an imaging program (three-dimensional Slicer, version 2.1b1, Brigham and Women's Hospital, Boston, MA) to make measurements. These measurements were made in the lateral aspects of the internal sphincter (Fig. 1) to minimize variations in sphincter thickness caused by angulation of the longitudinal axis of the canal to the axial plane of the scan. The transverse inner and outer diameters were measured digitally from axial MR images and used to calculate IAS thickness (mean between left and right) and mean diameter by two independent investigators blinded to patient age. These measurements were made in a transverse plane so they would not be affected by variations in the anal axis with respect to the scan plane. Measurements were taken at least in three different slices in the axial plane, and the average of these values was taken.
Figure 1.
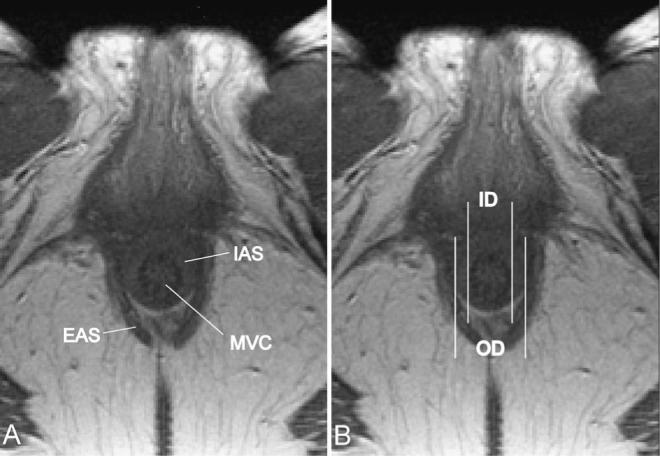
Axial MR scan. Pelvic magnetic resonance images (MRI) shown in the axial plane at the level of the sphincter complex. In all slices in which the internal anal sphincter (IAS) was clearly visible and could be distinguished from the mucosal vascular core (MVC) and the external anal sphincter (EAS) (A) measurements were taken (B). Thickness of the IAS was calculated as the difference between outer diameter of the IAS (OD) and inner diameter (ID).
Interrater reliability was r = 0.901, P < 0.001 for inner diameter, r = 0.91, P < 0.001 for outer diameter, and r = 0.776, P < 0.001 for thickness. There was a 2 percent difference between investigators in measurements of the outer diameter, which was not statistically significant, a 9.6 percent difference in inner diameter (P < 0.001), and an 11.8 percent difference in thickness (P < 0.001). To resolve these differences, we have reported the mean of the two investigators’ measurements.
Data Analysis
Statistical calculations were performed in SPSS 13.0 (SPSS Inc., Chicago, IL). Pearson correlation coefficients, Levene test for equality of variances, chi-squared test, and the two-sided Student's t-test were used to test for statistical significance. Cohen's d was calculated to characterize effect size. Cohen defined d as the appropriate way to explain the difference between the means divided by standard deviation of either group (see http://web.uccs.edu/Ibecker/Psy590/es.htm). A post hoc power calculation was performed, because preexisting data on normal anal diameters were not available. All our measurements (inner, outer diameter, and thickness) were powered at greater than 0.9 to reach a significance level of P < 0.05.
RESULTS
Demographics
Demographics of the study population are presented in Table 1. Older females had a 20 percent higher body mass index (BMI). However, measurements of IAS thickness and diameter did not correlate with BMI in either age group (young vs. old) or within the entire study population. The majority of the study population was white. Unfortunately the group sample sizes were not large enough to address racial differences.
Table 1.
Demographic Data
| Young Group ≤30 years (n=32) | Old Group ≥50 years (n=32) | P Value | |
|---|---|---|---|
| Age (yr) | 26±2.8 | 61.8±7.6 | |
| BMI (kg/m2) | 22.7±3 | 27.1±6.1 | 0.001 |
| White (%) | 78.1 | 90.6 | 0.168 |
BMI = body mass index.
Data are means±standard deviations unless otherwise indicated (Student's t-test, chi-squared test).
Statistical analysis was not performed for age (study design). Although the chi-squared test showed that there was no significant difference in comparing white and non-white females, the study's sample size did not allow for detailed racial differences to be addressed.
Measurements
Older females had a 33 percent thicker IAS, a 20 percent larger inner diameter, and a 27 percent larger outer diameter than younger females. The mean diameter and thickness data as well as statistical evaluation of the two groups are shown in Table 2 and displayed graphically in Figure 2.
Table 2.
Results: Internal Anal Sphincter Measurements
| Young Group ≤30 years (n=32) | Old Group ≥50 years (n=32) | P Value | Effect-size Cohen's d | |
|---|---|---|---|---|
| Inner | 7.1±1.3 | 8.5±1.8 | 0.001 | 0.89 |
| diameter | ||||
| (mm) | ||||
| Outer | 16±2.1 | 20.3±3.3 | 0.001 | 1.55 |
| diameter | ||||
| (mm) | ||||
| Mean | 4.5±0.7 | 5.9±1 | <0.001 | 1.62 |
| thickness | ||||
| (mm) |
Data are means±standard deviations unless otherwise indicated.
These results are averages from two blinded investigators taken from consecutive axial magnetic resonance imaging (MRI) slices (3−5). Student's t-test was performed to test for statistical significance as well as Cohen's d for effect size. Note the larger inner and outer diameter as well as larger mean thickness in the elderly.
Figure 2.
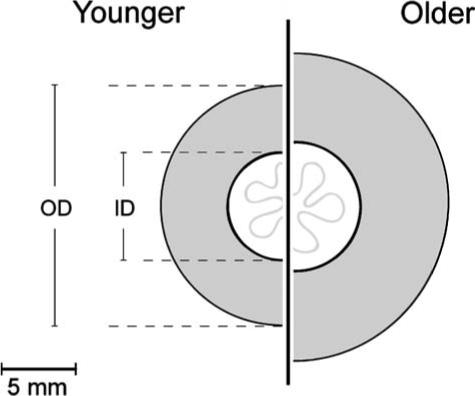
Graphical illustration of age effects. The effect of age on the inner and outer diameter of the internal anal sphincter drawn to scale. Both inner and outer diameter of the IAS increase with age. Note the thicker internal anal sphincter in older females because of a higher increase of the outer diameter compared with the inner diameter of the sphincter.
Correlation Between Thickness and Diameter
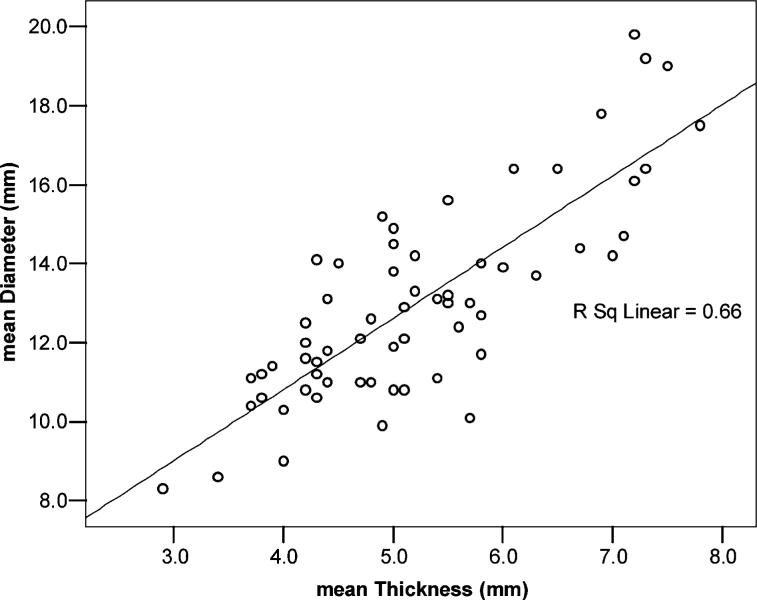
There was significant positive correlation between IAS thickness and mean IAS diameter within each group; the patients who had thicker sphincters had larger diameters. This correlation could be found in each age group (young, r = 0.599, P < 0.001; old, r = 0.747, P < 0.001) as well as within the complete study population (r = 0.813, P < 0.001, Fig. 3).
Figure 3.
Correlation between thickness and diameter of the IAS. Significant correlation between mean diameter and mean thickness within the whole study population. The thicker the internal anal sphincter gets, the larger is the mean diameter. The correlation coefficient is r = 0.813, P < 0.001. This can be only shown by use of imaging techniques that do not distort the diameters. With endoanal techniques, these measurements cannot be performed.
DISCUSSION
Both the thickness and the diameter of the IAS were found to increase significantly with age. Using MRI without an endoanal probe allows measurement of the IAS geometry without distortion. An increase in IAS thickness with advancing age has been well established in the literature.7–9 However, previous studies have focused primarily on internal sphincter thickness because this is clearly visible with endoanal ultrasound. We included only nulliparous females in our study to avoid the confounding effects of vaginal delivery, which have previously been shown to effect IAS morphology.11
One limitation of the endoanal technique is that by attempting to measure IAS diameter, the probe's diameter rather than the sphincter's diameter has been evaluated. To illustrate the distortion that occurs with endoanal techniques, let us consider the inner diameter of the IAS measured with and without an endoanal apparatus. In our Urogynecologic Clinic, for example, an endoprobe is covered with a 17-mm diameter hard plastic cone. Compared with the undistorted inner diameter of the IAS (mean = 7.8 mm) measured by using MR scans, the cone causes an almost 120 percent increase in the inner diameter of the inner sphincter, with an associated architectural distortion of the sphincter. Therefore, the present study circumvents these artifacts by measuring the undisturbed sphincter diameter, a measurement that is needed to understand the effects of age, such as altered thickness and wall tension, on the delicate anal sphincter complex.
The internal anal sphincter has a cylindrical structure. The tension in its circularly oriented smooth muscle produces a high-pressure zone in the anal canal. Failure of this system to produce high pressure increases the chance that fecal incontinence will occur. The importance of knowing both sphincter thickness and diameter becomes apparent when considering Laplace's law because it relates pressure, thickness, diameter, and stress (“wall tension” per unit area) in hollow organs, such as the heart and vessels12–14 or the gastrointestinal tract. Although stress is the biomechanically correct term, we will use the word “tone” of the sphincter to denote wall tension to avoid confusion.
The following examples illustrate the importance of considering how these parameters are interrelated. According to Laplace's law (Fig. 4), the inward pressure of the IAS depends on the diameter and thickness as well as the radius and tone (more accurately stress or wall tension). More precisely, the ratio of diameter (or radius) to thickness affects the relationship between tone and pressure. For example, a 25 percent increase in radius (e.g., from 6 mm to 7.5 mm) would result in a 25 percent decrease in internal pressure, assuming constant tone and thickness. However, a 25 percent increase in thickness (e.g., from 4 mm to 5 mm) at the same time would compensate for the increased diameter and result in the original pressure. In our sample size, we reported a 25 percent increase in mean radius and a 33 percent increase in thickness with age. In the next paragraph, we shall explore the functional significance of these results.
Figure 4.
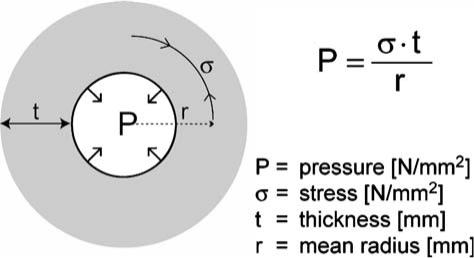
Laplace's law shown with a definition of terms. The radial inward pressure responsible for anal closure (P) depends on the smooth muscle stress (σ), the amount of force the internal anal sphincter can produce. However, thickness (t) and diameter (or radius, r) influence this delicate balanced system. Note the linear relationship between all four variables with their possible effects on anal closure.
In our study, we demonstrated a significant increase of both IAS diameter and thickness in the elderly. We can use Laplace's law to predict the tone of the IAS (smooth muscle tensile stress σ) based on our measurements of IAS thickness and radius, and the extrapolated maximal anal resting pressure from the literature.15 In our study, we reported a 25 percent difference in mean radius and a 33 percent difference in thickness (Fig. 2). According to Laplace's law, this should result in a small (6 percent) increase in anal canal pressure in the elderly, assuming constant tone. However, this is not consistent with resting anal pressures established in the literature.15 If we extrapolate values from Ryhammer's data for our two study groups, the mean maximal anal resting pressures would be 100 cm H20 in the younger group and 75 cm H2O in the older group. This represents a 25 percent decrease compared with the 6 percent increase predicted by Laplace's law. The most reasonable explanation is that σ, the tone of the IAS (smooth muscle tensile stress or “wall tension per unit area”), decreases with advancing age. We applied Laplace's law (using our own measurements of radius and thickness, and Ryhammer's data on pressure) and found a significant difference in the sphincter's tone that was 30 percent lower in older females (1.28 ± 0.16 N/mm2 vs. 0.9 ± 0.12 N/mm2 (mean ± standard deviation); P < 0.001). This is consistent with histologic studies that show increased collagen deposition (sclerosis) and decreased smooth muscle with increasing age.16
In other words, in trying to explain why a thicker internal anal sphincter is weaker, we need to emphasize changes in all four variables of Laplace's law. Whereas increasing diameter and decreasing tone (wall tension or stress) of the IAS lead to lower anal pressures, thickening of the IAS leads to increased pressures. However, it remains speculative whether an increase in IAS thickness in the elderly is an attempt at compensation, which ultimately fails because of a loss of tone (wall tension).
We could find only one other study17 in which Laplace's law was used to characterize anal sphincter function. That study focused on diameter, wall tension, and anal closure but assumed constant thickness and did not consider the effect of age as a variable.
One limitation of our study was the possible confounding effect of a significant difference in BMI between the two study groups. However, when we examined data for the whole study population, we were unable to find a significant correlation between BMI and thickness or diameter or the IAS. Because of practical limitations in our study design, we could not provide data on endoanal ultrasound or anal manometry. Therefore, we needed to use published data that described anatomic distortion caused by endoanal imaging techniques10 as well as reported data on anal manometry15 to strengthen our findings. At this time, we are recruiting patients for a new study in which MRI, endoanal ultrasound, and anal manometry will all be performed.
This study corroborates earlier findings that IAS thickness increases with age. We have shown for the first time that IAS diameter (undistorted by an endoanal apparatus) also increases with age. By using Laplace's law, we have predicted that internal anal sphincter's tone probably decreases with increasing age. This prediction awaits experimental confirmation. It is known that the maximum anal resting pressure decreases with age, therefore, alterations in thickness and diameter are crucial physiologic implications of age as well. Thus, any pathologic condition may be expected to exacerbate this loss in tonic closure and increase the risk of incontinence.
In considering the disease processes underlying anal and rectal disorders, it is crucial to distinguish between pathologic and physiologic (e.g., age-related) changes—with the latter addressed in this study. Improved treatments for fecal incontinence should result from a better understanding of the mechanisms underlying this debilitating disease.
Acknowledgments
Supported by the National Institutes of Health, ORWH & NICHD Sex & Gender Factors Affecting Women's Health SCOR: P50, and NICHD R01 HD 044406: NICHD R01 DK 051405, R01 HD 038665; German Research Foundation (DFG, HU1502/1–1).
Footnotes
Presented as a poster at the annual meeting of the American Urogynecologic Society, October 19 to 21, 2006, Palm Springs, Florida.
Presented as an oral poster at the annual meeting of the International Urogynecological Association, September 6 to 9, 2006, Athens, Greece.
Presented as an oral poster and oral presentation at the annual meeting of the German Association of Gynecology and Obstetrics, September 19 to 22, 2006, Berlin, Germany.
REFERENCES
- 1.Nelson R, Norton N, Cautley E, Furner S. Community-based prevalence of anal incontinence. JAMA. 1995;274:559–61. [PubMed] [Google Scholar]
- 2.Reilly WT, Talley JH, Pemberton CD. Fecal incontinence: prevalence and risk factors in the community. Gastroenterology. 1995;108:A32. [meeting abstract] [Google Scholar]
- 3.Talley NJ, O'Keefe EA, Zinsmeister AR, Melton LJ., 3rd Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895–901. doi: 10.1016/0016-5085(92)90175-x. [DOI] [PubMed] [Google Scholar]
- 4.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–4. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in U.S. females: a population-based study. Am J Obstet Gynecol. 2005;193:2071–6. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Frenckner B, Euler CV. Influence of pudendal block on the function of the anal sphincters. Gut. 1975;16:482–9. doi: 10.1136/gut.16.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett SJ, Bartram CI. Endosonographic variations in the normal internal anal sphincter. Int J Colorectal Dis. 1991;6:2–4. doi: 10.1007/BF00703951. [DOI] [PubMed] [Google Scholar]
- 8.Frudinger A, Halligan S, Bartram CI, Price AB, Kamm MA, Winter R. Female anal sphincter: age-related differences in asymptomatic volunteers with high-frequency endoanal US. Radiology. 2002;224:417–23. doi: 10.1148/radiol.2242010985. [DOI] [PubMed] [Google Scholar]
- 9.Beets-Tan RG, Morren GL, Beets GL, et al. Measurement of anal sphincter muscles: endoanal US, endoanal MR imaging, or phased-array MR imaging? A study with healthy volunteers. Radiology. 2001;220:81–9. doi: 10.1148/radiology.220.1.r01jn1481. [DOI] [PubMed] [Google Scholar]
- 10.Sultan AH, Loder PB, Bartram CI, Kamm MA, Hudson CN. Vaginal endosonography. New approach to image the undisturbed anal sphincter. Dis Colon Rectum. 1994;37:1296–9. doi: 10.1007/BF02257800. [DOI] [PubMed] [Google Scholar]
- 11.Frudinger A, Halligan S, Bartram CI, Spencer JA, Kamm MA. Changes in anal anatomy following vaginal delivery revealed by anal endosonography. BJOG. 1999;106:233–7. doi: 10.1111/j.1471-0528.1999.tb08236.x. [DOI] [PubMed] [Google Scholar]
- 12.Fung YC. Biomechanics: circulation. Springer-Verlag; New York: 1996. [Google Scholar]
- 13.Hall AJ, Busse EF, McCarville DJ, Burgess JJ. Aortic wall tension as a predictive factor for abdominal aortic aneurysm rupture: improving the selection of patients for abdominal aortic aneurysm repair. Ann Vasc Surg. 2000;14:152–7. doi: 10.1007/s100169910027. [DOI] [PubMed] [Google Scholar]
- 14.Hassan N, Escanye JM, Juilliere Y, et al. 201Tl SPECT abnormalities, documented at rest in dilated cardiomyopathy, are related to a lower than normal myocardial thickness but not to an excess in myocardial wall stress. J Nucl Med. 2002;43:451–7. [PubMed] [Google Scholar]
- 15.Ryhammer AM, Laurberg S, Sorensen FH. Effects of age on anal function in normal women. Int J Colorectal Dis. 1997;12:225–9. doi: 10.1007/s003840050094. [DOI] [PubMed] [Google Scholar]
- 16.Klosterhalfen B, Offner F, Topf N, Vogel P, Mittermayer C. Sclerosis of the internal anal sphincter: a process of aging. Dis Colon Rectum. 1990;33:606–9. doi: 10.1007/BF02052217. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons CP, Trowbridge EA, Bannister JJ, Read NW. Role of anal cushions in maintaining continence. Lancet. 1986;1:886–8. doi: 10.1016/s0140-6736(86)90990-6. [DOI] [PubMed] [Google Scholar]