Abstract
A Ca2+-dependent synaptic vesicle-recycling pathway emanating from the plasma membrane adjacent to the dense body at the active zone has been demonstrated by blocking pinch-off of recycling membrane by using the Drosophila mutant, shibire. Exposure of wild-type Drosophila synapses to low Ca2+/high Mg2+ saline is shown here to block this active zone recycling pathway at the stage in which invaginations of the plasma membrane develop adjacent to the dense body. These observations, in combination with our previous demonstration that exposure to high Ca2+ causes “docked” vesicles to accumulate in the identical location where active zone endocytosis occurs, suggest the possibility that a vesicle-recycling pathway emanating from the active zone may exist that is stimulated by exposure to elevated Ca2+, thereby causing an increase in vesicle recycling, and is suppressed by exposure to low Ca2+ saline, thereby blocking newly forming vesicles at the invagination stage. The presence of a Ca2+-dependent endocytotic pathway at the active zone opens up the following possibilities: (i) electron microscopic omega-shaped images (and their equivalent, freeze fracture dimples) observed at the active zone adjacent to the dense body could represent endocytotic images (newly forming vesicles) rather than exocytotic images; (ii) vesicles observed attached to the plasma membrane adjacent to the dense body could represent newly formed vesicles rather than vesicles “docked” for release of transmitter.
The vesicle hypothesis, proposed in 1955 by del Castillo and Katz (1), has become the foundation of our present understanding of synaptic transmission. Stated in its simplest form, this hypothesis proposes that one “quantum,” or miniature end-plate potential, results from the release of transmitter stored in one synaptic vesicle and that the end plate potential represents the synchronized release of many such quanta. Transmitter substances are thought to be released from vesicles by exocytosis, i.e., fusion of the vesicle membrane with the presynaptic plasma membrane, after which an opening or pore develops, so that the vesicle contents are expelled into the synaptic cleft (for review, see ref. 2). This idea of release by exocytosis has led, in turn, to the vesicle-recycling hypothesis (3), which proposes that, after fusion and pore formation, the vesicle membrane collapses into the plasma membrane and mixes with it completely, after which it is retrieved from the plasma membrane at sites away from the active zone by clathrin-coated vesicles (for review, see ref. 4).
Electron microscopic detection of omega-shaped images at the active zone at a time when release is occurring has been adduced as evidence for exocytosis (4–6). Because of the location and time of occurrence of these omega-shaped images, there has been little resistance to the assumption that invaginations of the active zone membrane represent vesicles in various stages of collapse into the terminal plasma membrane. However, recent data from our laboratory suggest that these images might be endocytotic rather than exocytotic. Thus, we have demonstrated that there is a “fast,” Ca2+-dependent endocytotic pathway that appears to operate by direct pinch-off of presynaptic membrane adjacent to the dense body at the active zone and replenishes a small subpopulation of synaptic vesicles located at the release site (7). This is in addition to the slower, “classical” synaptic vesicle-recycling pathway (mentioned above) involving coated/uncoated vesicles and/or cisternae, which emanates from sites away from the active zone and which replenishes a larger vesicle population (nonactive zone population) dispersed throughout the terminal cytoplasm. This larger population is the one visualized by studies using fluorescent dyes such as FM1–43.
In the present paper, the transmitter release process is investigated by observing the relationship between the blockage of transmission and concomitant arrest of active zone endocytosis, which occur as a result of exposure to low Ca2+/high Mg2+ saline. Using wild-type Drosophila, it is demonstrated that exposure to low Ca2+/high Mg2+ saline causes invaginations to develop along the active zone membrane, many or all of which may be endocytotic in nature. This suggests that active zone endocytosis is blocked in reduced Ca2+ saline at the stage where invaginations are forming at the plasma membrane.
MATERIALS AND METHODS
The experimental animals were 4-d-old adult Drosophila melanogaster of the wild-type strain, Oregon-R, and the single-gene mutant, shibirets1 (shi). Synapses of the sternal anterior rotator muscle of the first leg (one of six coxal muscles) were used. For the experiment using shi, see the methods described in ref. 7. For intracellular recording, the fly was anesthetized momentarily with ether and immobilized ventral side up in Tackiwax over an opening in a plastic tube and covered with saline. This situation allowed the fly to breathe through its thoracic and abdominal spiracles, which remained exposed to the air in the tube. The coxal muscle was then exposed by dissecting away the overlying cuticle (pre-episternum). A glass capillary microelectrode filled with 3 M KCl (100 MΩ resistance) was inserted into a coxal muscle fiber. Recordings were made by using a high input impedance DC amplifier, which allowed continuous monitoring of the resting potentials of the muscle fibers. Only recordings from muscle fibers with resting potentials of −80 to −90 mV were used. In some experiments, the coxal nerve was cut to prevent efferent impulses from the thoracic ganglion, whereas in other experiments the nerve was left intact. The nerve was stimulated by passing current through an electrode inserted into the thoracic ganglion or by cutting the nerve and applying current to the axon. Excitatory junction potentials (ejps) were recorded on photographic film directly from the oscilloscope. Experiments on the wild-type flies were performed at room temperature (24–26°C).
Normal saline consisted of 128 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, and 5 mM Tris-aminomethane HCl (pH 7.4). In experiments with elevated Mg2+ (20 and 35 mM) and reduced Ca2+ (1.0 mM), or elevated Ca2+ (18 mM), the salines were prepared by adjusting the concentration of NaCl by an amount so as to maintain equiosmolarity. The body cavity was perfused with the experimental solutions through a capillary inserted into the anterioventral prothorax. After the recording, the preparation was perfused with fixative (2% paraformaldehyde/2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4) for 30 min. The recording electrode was not removed from the muscle fiber during perfusion with fixative. No transmitter release, either evoked or spontaneous, was detected during application of fixative. After 30 min in fixative, the coxal muscle was dissected out of the body cavity and immersed in 4% glutaraldehyde for 2 hr. The specimen was postfixed in 2% osmium tetroxide in 0.1 mM cacodylate buffer (pH 7.4), block-stained in 1% aqueous uranyl acetate, dehydrated in alcohol, and embedded in the epoxy resin Spurr (Polysciences, Warrington, PA). Thin sections were double-stained with uranyl acetate and Millonig’s lead hydroxide and observed on a Philips CM-10 electron microscope and photographed. In other experiments, specimens were exposed to normal and low Ca2+/high Mg2+ salines without intracellular recording and then processed for EM as described above.
For the quantitative analysis of active zone invaginations, the active zone was defined as the presynaptic membrane, which was apposed to the electron dense postsynaptic membrane possessing the brush-like array. An invagination was defined as any indentation of the presynaptic plasma membrane of at least 40 nm. Various “ripples” of the active zone membrane that were <40 nm in height were not counted.
RESULTS
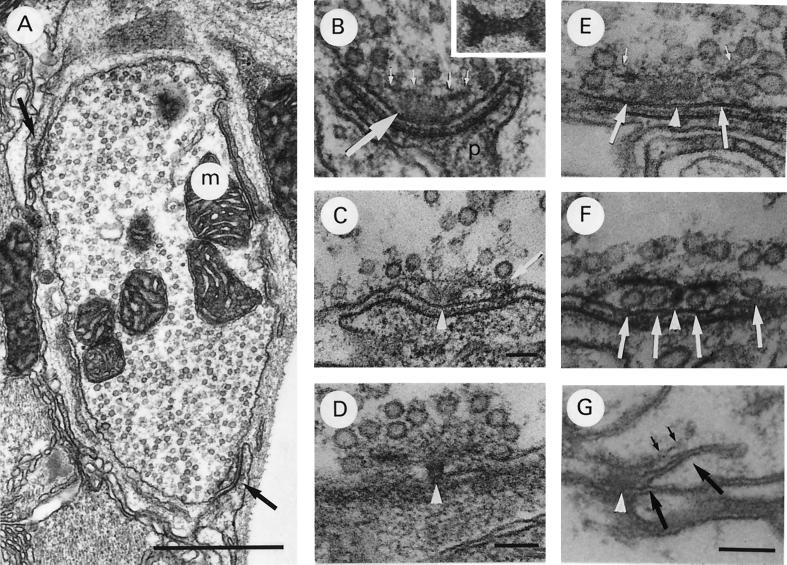
A typical example of a wild-type coxal synapse at rest, with the nerve cut to prevent efferent impulses from the ganglion, is seen in Fig. 1A. The terminal possesses many vesicles dispersed throughout the cytoplasm and two specialized release sites, or active zones. In Fig. 1B, an active zone is shown at higher magnification. The area from which release occurs can be defined as the presynaptic membrane, which is closely aligned with specialized postsynaptic membrane. The pre- and postsynaptic membranes become closely aligned only at these sites. Vesicles are observed to become attached by an electron dense substance only to this area of the presynaptic membrane (presumed docking). The specialized postsynaptic membrane is thicker and more electron-dense than the surrounding membrane and has a brush-like substance extending from it into the synaptic cleft. This area where transmitter release occurs will be referred to as the “active zone.” Presynaptically, the active zone possesses a dense body, which consists of an electron dense base capped by a filamentous structure, the dense body plate. The base is a dumbbell-shaped structure (Fig. 1B Inset), which appears in cross section as oblong (Fig. 1B), double-lobed (Fig. 1C), or round (Fig. 1D), depending on the plane of sectioning. The plate consists of many thin filaments, which emanate from two sites on the active zone membrane and spread out over the dense body base (Fig. 1C). In cross section, these filaments appear as an electron-dense, bar-like structure above the dense body base running parallel to the presynaptic membrane (Fig. 1B, small arrows). As is demonstrated in Fig. 1A–C, vesicles in this type of synapse typically are located above and to the sides of the dense body but not under the filamentous dense body plate. With appropriate planes of sectioning, it can be seen that the vesicles in close proximity to the dense body (within ≈300 nm) are attached to it by filaments, which radiate from the dense body plate (Fig. 1D). These vesicles may represent the subpopulation that is replenished by active zone endocytosis.
Figure 1.
Typical coxal terminal. (A) Cross section of terminal in normal saline containing mitochondria (m), synaptic vesicles, and two dense bodies at active zones (arrows). (B) Active zone in normal saline demonstrating dense body, made up of the dense body base (large arrow) and filamentous cap (small arrows). Note that the postsynaptic muscle fiber (p) extends a finger-like projection to the active zone. (Inset) Dense body base sectioned horizontally, parallel to plasma membrane. (C) Active zone in normal saline demonstrating attachment of dense body plate to the presynaptic membrane (arrow). Note the double lobed appearance of dense body base (D–F, arrowhead also). (D) Active zone in normal saline sectioned slightly tangentially to the plasma membrane to demonstrate the attachment of a subpopulation of vesicles to the dense body by thin fibrils. (E and F) Active zones exposed to 18 mM Ca2+ saline for 10 min, demonstrating many vesicles “docked” at the plasma membrane under the dense body plate (large arrows). Note the attachment of the vesicles to the plate by thin fibrils in E (small arrows). (G) Active zone of shi retinula cell terminal exposed to 29°C to induce vesicle depletion, followed by exposure to 26°C for 1 min. Note the membrane emanating from the plasma membrane adjacent to the dense body base (large arrows). The dense body plate (small arrows) appears to have been displaced away from the plasma membrane by the invaginating membrane. [Bars: A, 1 μm (×30,000); C, 100 nm (×75,000); B and D, 100 nm (×90,000); and E–G, 100 nm (×135,000).]
In the coxal synapse, vesicles docked at the active zone are very rare in undissected preparations or dissected preparations exposed to normal saline. Even when a synapse is fixed during activity induced by stimulation of the nerve or by exposure to veratridine, the vast majority of active zones do not possess vesicles docked under the dense body plate or at any other location along the active zone membrane (8). This situation changes dramatically, however, if the terminals are exposed to high Ca2+ saline (l8 mM). In the latter case, most active zones possess vesicles docked on either side of the dense body base under the plate (Fig. 1 E and F). With the proper plane of sectioning, it can be seen that these vesicles are attached to the plasma membrane by an electron dense substance (8). Such vesicle docking was observed only along the active zone membrane. This effect on the number of vesicles docked at the active zone was interpreted in the aforementioned paper to indicate that Ca2+ is involved in the translocation of vesicles to the active zone from a location more interior in the terminal cytoplasm.
Using the temperature-sensitive mutant, shibire, in which endocytotic membrane is blocked from pinching off from the plasma membrane at 29°C, it is possible to induce an accumulation of endocytotic membrane at the active zone. In Fig. 1G, the accumulated endocytotic (recycling) membrane can be seen to emanate from the same location where vesicles are observed to be docked in high Ca2+ saline. Exposure to low Ca2+/high Mg2+ saline blocks this build-up of endocytotic membrane emanating from under the plate, demonstrating that with exposure to this saline the process of active zone endocytosis is blocked at the step where membrane is reinternalized by invagination of the plasma membrane. (The “classical” recycling pathway emanating from sites away from the active zone was not blocked by low Ca2+/high Mg2+; ref. 7).
The effect of low Ca2+/high Mg2+ saline on evoked release of wild-type coxal synapses was observed by intracellular recordings made from coxal muscle fibers. After 5 min in 20 mM Mg2+/1 mM Ca2+ saline, the ejp began to decrease in amplitude and by 10 min it stabilized at the 5–10 mV level. In 35 mM Mg2+/1 mM Ca2+ saline, the ejp reached the ≈30% failure level after 5 min, and after 10 min exposure to this saline, the response was completely blocked. This represents the typical blocking effect of low Ca2+/high Mg2+ saline on transmission, which has been observed in other neuromuscular junctions (NMJ) in Drosophila (9), as well as in other organisms. At the end of the recordings, the preparations were fixed for electron microscopy. No response was elicited from these blocked muscle fibers as a result of applying the fixative. Furthermore, it was observed that spontaneous release ceased within ≈500 msec of applying the fixative, and the muscle began to depolarize within ≈5 sec. This contrasts with the frog NMJ, in which it has been demonstrated that aldehyde fixative causes an increase in spontaneous release(10). Differences in response to fixative between these two preparations are probably related to the difference in size and location of the terminals. Thus, the grass frog cutaneous pectoris muscle is ≈20 times as thick as the Drosophila coxal muscle, and the individual nerve terminals of the frog are also ≈20 times as large as those of the fly. Furthermore, the majority of terminals of the frog NMJ are located within the muscle fiber bundle, whereas the Drosophila coxal terminals are located along the outer surface of the muscle bundle. Thus, in the case of the frog, the fixative is required to penetrate into the muscle bundle, whereas in the coxal muscle, the fixative reaches the terminals directly upon application, without the necessity of penetrating any tissue. This explains why activity can still be recorded from the frog NMJ 3 min or more after application of fixative, while activity subsides within milliseconds in the coxal NMJ.
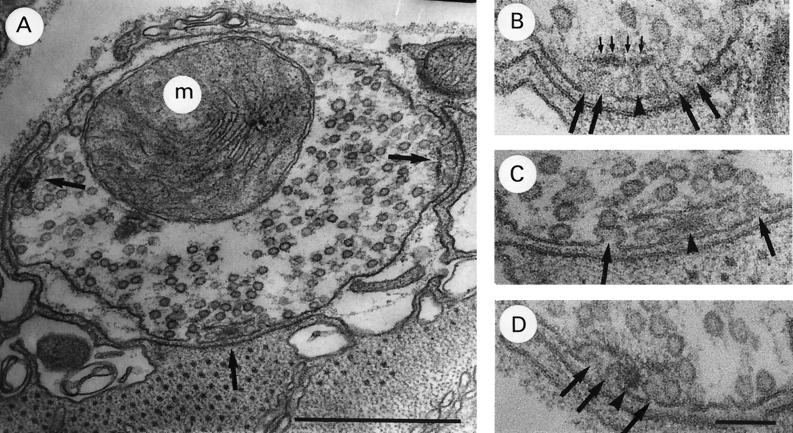
An example of a typical coxal terminal in which transmission has been completely blocked by exposure to 1 mM Ca2+/35 mM Mg2+ saline for 30 min is shown in Fig. 2A. This terminal possesses three active zones, in each of which the presynaptic membrane is abnormal in appearance, possessing a number of unusual invaginations. These zones are shown at higher magnification in Fig. 2B–D. The invaginations occur at the same location where vesicles are observed to accumulate in high Ca2+ saline. Many are similar in appearance to published reports claiming to show exocytotic images caught in the process of release (see refs. 5 and 6 for examples). However, the preparation was fixed ≈20 min after transmission had been completely blocked, and no release was observed during fixation, so that these images cannot represent exocytotic events which were occurring at the time of fixation. Furthermore, in experiments designed to observe the effect on active zone morphology of activity produced by electrical stimulation (20 Hz) and exposure to veratridine, no omega-shaped images were ever seen (175 synapses, normal Ca2+) (8). Thus, omega-shaped images are very difficult to obtain in this preparation under the conditions of mild stimulation followed by chemical fixation, which were used here.
Figure 2.
Typical coxal terminal after 30 min exposure to 1 mM Ca2+/35 mM Mg2+ saline. (A) Terminal with three abnormal active zones (arrows) with invaginating plasma membrane under dense body plate. (B–D) Active zones in A shown at higher magnification. Note omega-shaped images (large arrows) under dense body plate (small arrows) Dense body base–arrowhead. [Bars: A, 0.5 μm (×68,000); and B–D, 100 nm (×135,000).]
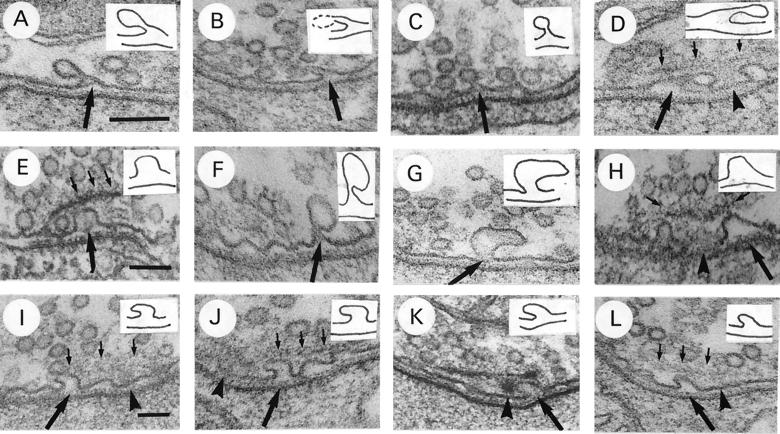
The images shown here might be interpreted to represent vesicles arrested in the process of release during the initial stages of Mg2+ block, i.e., during the first few minutes of exposure to the low Ca2+/high Mg2+ saline. This suggests that such exposure prevents the vesicle membrane from collapsing into the presynaptic plasma membrane after release, an interpretation congruent with the hypothesis that Ca2+ is necessary for the release process. However, the shape and size of many of the invaginations suggest that they represent endocytotic images rather than exocytotic images. For example, they often have long, narrow necks as if they are in the process of pulling away from the membrane (Fig. 3A–D) or are much larger than the usual vesicle (Fig. 3E–H). At times, their shape gives the impression that the invagination has been deflected in its inward growth by the dense body plate, causing it to veer sideways (Fig. 3I–L). Thus, another interpretation of these images is that they represent endocytotic images arrested in the process of membrane internalization, which is consistent with our previous observations using the shi mutant that one type of vesicle recycling, or endocytosis, occurs from under the dense body plate at the active zone and is blocked by low Ca2+/high Mg2+ saline (7).
Figure 3.
Further examples of active zones of coxal terminals exposed to 1 mM Ca2+/20 mM Mg2+ saline for 10 min. The pre- and postsynaptic membranes at the invagination have been traced and are presented as Insets in each figure. All invaginations presented are from active zone membrane aligned to the specialized postsynaptic membrane. (A–D) Note long, thin necks of invaginations (arrows). The dense body is out of the plane of sectioning in A–C. In D, dense body (arrowhead) is only lightly stained. (E–H) Note large size of invaginations (large arrows). In E–G, dense body is out of plane of sectioning. In H, the dense body is sectioned slightly tangentially, demonstrating attachment of vesicles to it by thin fibrils (small arrows). (I–L) Note apparent displacement of invagination by dense body plate (large arrows). In I, J, and L, dense body base (arrowhead) and plate (small arrows) are lightly stained. [Bars: A, D, F and J, 100 nm (×135,000); B, C, E, G, and H, 100 nm (×90,000); and I, K, and L, 100 nm (×75,000).]
These invaginations occurred primarily under the dense body plate on either side of the dense body base but also were observed emanating from active zone membrane not covered by the dense body plate. No particular difference in the size or shape of the invaginations was observed between those which emanated from under the plate adjacent to the dense body base and those which emanated from active zone membrane not covered by the plate. Thus, there was no suggestion by size or shape that the invaginations under the plate were exocytotic, whereas those away from the plate were endocytotic, or vice versa. Furthermore, invaginations such as these were never observed along nonactive zone membrane. Of 324 active zones (20 animals) exposed to 1 mM Ca2+/35 mM Mg2+ saline for 30 min, 100% possessed invaginations. On the other hand, of 217 active zones (15 animals) of control synapses exposed to normal saline for 30 min, only one possessed an invagination of active zone membrane. Because both experimental and control animals were fixed in an identical manner, there is no possibility that these invaginations resulted from exocytotic events, which were arrested in the process of release as a result of the fixation process.
These unusual invaginations at the active zone may represent arrested endocytotic events, arrested exocytotic events, or both. To clarify this point, the time of appearance of these invaginations was observed relative to transmitter release. For this experiment, coxal preparations were fixed after exposure to concentrations of low Ca2+/high Mg2+ saline, which induce either partial (20 mM Mg2+/1 mM Ca2+) or complete (35 mM Mg2+/1 mM Ca2+) transmission block after 10 min, and the number of invaginations observed at the active zones was counted. The amplitude of the ejp at the time of fixation was monitored by intracellular recording, while stimulating at 2 Hz. The data are expressed as invaginations/active zone/section, which represents the number of invaginations in a single plane of sectioning through the active zone. Because an active zone is four to six sections thick, the total number of invaginations per active zone is considerably higher than what is expressed here. Some serial sectioning was performed, and it was observed that these invaginations are present in all sections through the active zone. The average number of invaginations/active zone/section in coxal synapses exposed to the 20 mM Mg2+ saline for 5 min (at a time when the ejp amplitude is just beginning to decrease below threshold level) was 1.1, and 78% of the 212 active zones (12 animals) possessed at least one invagination. After 10 min exposure to this saline (at a time when the ejp had stabilized at ≈10 mV in amplitude), the average number of invaginations had increased to 1.9, and 96% of the 197 active zones (11 animals) had at least one invagination. Thus, the invaginations begin to develop while transmitter release is still occurring, indicating that they could be arrested exocytotic events. Of course, vesicle recycling also is assumed to occur during transmitter release, so this does not exclude the possibility that they are endocytotic images. Furthermore, the invaginations that were observed in specimens that were fixed while transmitter release was only partially blocked, often had shapes, which suggested that they were endocytotic, such as those shown in Fig. 3, suggesting that at least some of them represent arrested endocytosis. The average number of invaginations/active zone/section in coxal synapses exposed to the 35 mM Mg2+ saline for 10 min (at a time when transmission is completely blocked) was 2.4, and 100% of the 216 active zones (14 animals) had at least one invagination. Thus, during the period of time when the block in transmission is becoming more pronounced, the number of invaginations/active zone/section is increasing and also the number of active zones which possess invaginations is increasing. To determine whether invaginations continue to develop after transmission is completely blocked, specimens were fixed after exposing the coxal synapses to 35 mM Mg2+ saline for 20 additional minutes after complete blockage occurred (30 min of total exposure). The average number of invaginations had increased to 3.5 (242 active zones, 14 animals). Thus, the number of invaginations/active zone continued to increase during the period after release had been completely blocked. This demonstrates that at least some of the invaginations are endocytotic because they develop at a time when no transmitter release is occurring. No difference in the size, shape, or location of the invaginations observed after 10 min or 30 min in the low Ca2+/high Mg2+ saline could be detected. Thus, there was no indication that the invaginations that occurred after release was blocked were any different from the ones which occurred while release was still going on.
DISCUSSION
The data presented here demonstrate that exposure of the coxal synapse to low Ca2+/high Mg2+ saline causes unusual invaginations to develop along the plasma membrane adjacent to the dense body at the active zone. The number of these invaginations/active zone increases with longer exposure to the low Ca2+/high Mg2+ saline. Furthermore, the number increases both during release and after release has been blocked completely. Control synapses exposed to normal saline and fixed in an identical manner do not demonstrate any invaginations, eliminating the possibility that the aldehyde fixation itself may have caused exocytosis during the fixation process, thereby producing these images.
Because no release occurred during fixation in this preparation, and the number of invaginations increased with longer exposure to the low Ca2+/high Mg2+ saline, the invaginations seen here do not represent exo/endocytotic events occurring at the moment of fixation but, rather, must represent events that have been arrested at the invagination stage by exposure to the low Ca2+/high Mg2+ saline. These data suggest that at least some of them represent arrested endocytotic events for two reasons: (i) Some of the invaginations develop at a time when no release is occurring, i.e., within the 20-min period after complete blockage of transmission has taken place; and (ii) many possess shapes that are not consistent with the shape of a vesicle that has fused with the plasma membrane and is in the process of collapse. It is difficult to imagine that the images shown in Fig. 3, especially those with long, thin necks, such as Fig. 3 A–D, F, and G, could result from a vesicle that had been docked previously as shown in Fig. 1E and F. Therefore, in combination with our previous demonstration with the shi mutant showing that the recycling pathway emanating from the active zone is blocked in low Ca2+/high Mg2+ (7), it is likely that some if not all of these images represent arrested endocytotic events.
Evidence suggesting an endocytotic pathway for vesicle reformation at the active zone is not new. In 1979, Ceccarelli and coworkers (11) demonstrated that under conditions of intense stimulation, freeze fracture images (dimples/protuberances assumed to represent exo/endocytotic events) observed at the active zone during transmitter release were observed also with a similar frequency and distribution during the rest period immediately after stimulation. It was suggested that the images observed during release were exocytotic, whereas those observed during the rest period were endocytotic. This led to the proposal that vesicles do not collapse into the plasma membrane after exocytosis but, rather, immediately pinch back off (kiss and run hypothesis). Our observations on active zone endocytosis do not support the kiss and run hypothesis because the process described here involves reformation of vesicles by invagination of the active zone membrane. However, the freeze fracture images that led to that hypothesis could represent endocytosis by invagination of the active zone membrane so that these past data support the observations made here. More recently, it has been reported that the density of freeze-fracture images of exo/endocytotic pits at electric organ synapses increases after the end of a brief tetanic stimulation when transmission is over, suggesting the pits to be endocytotic (12).
The fact that the invaginations shown here (and the accumulation of recycling membrane, which occurs when membrane pinch-off is arrested using shi) occur in exactly the same location as the “docked” vesicles which accumulate with exposure to high Ca2+ saline (Fig. 1E and F), is problematical because it seems likely that the slower process of endocytosis by invagination of the plasma membrane would interfere with repeated exocytosis if the two processes were occurring in exactly the same location. One possibility is that the presynaptic membrane adjacent to the dense body is reserved for endocytosis (vesicle reformation), while transmitter release occurs along active zone membrane away from the dense body. Another line of reasoning also suggests this possibility. The deformation of the active zone membrane caused by high Mg2+ would certainly prevent the approach and docking of vesicles to this area, thereby blocking release; yet it has been demonstrated that evoked release can be restored from Mg2+ block within 10–20 msec by applying a pulse of Ca2+ (13), which seems too short a time for the membrane under the dense body to be cleared of unretrieved vesicle membrane by endocytosis. This result implies that exocytosis is not occurring from under the dense body.
If the area under the dense body is reserved for endocytosis, and exocytosis occurs elsewhere at the active zone, then the “docked” vesicles which accumulate in high Ca2+ under the dense body plate would represent newly formed vesicles rather than vesicles positioned for release, which would suggest that extracellular Ca2+ may stimulate active zone endocytosis—the counterpart of the observation made here that reduced extracellular Ca2+ blocks endocytosis. The possibility that docked vesicles are really newly formed vesicles is corroborated by the observation that the first vesicles observed when recycling is allowed to proceed after shi-induced depletion are located in a “docked” position adjacent to the dense body (7), and at this time the amplitude of the excitatory junction potential is reduced, suggesting that these vesicles are not all readily releasable (J.H.K. and K.Y., unpublished observations). This possibility has profound implications for the well accepted assumptions that the dense body is a structure involved with exocytosis, that vesicles in close association with the active zone plasma membrane represent a readily releasable subpopulation, and that omega-shaped images (and freeze-fracture dimples) located adjacent to the dense body represent vesicles caught in the process of release. Initially, omega-shaped images were only observed 4–6 msec after a single stimulus in the presence of 4-aminopyridine, even though transmitter release occurs within 2 msec (6, 14). Later, invaginations were demonstrated 2.5 msec after the stimulus (15), but this preparation was exposed to high Ca2+ saline for 10–30 min before stimulating. Because our previous observations (8) demonstrate that exposing a synapse to high Ca2+ actually causes such images to form (along with an accumulation of “docked” vesicles), these images may have been formed even before the stimulus, as a result of stimulating endocytosis. Taking into consideration that endocytosis stimulated by Ca2+ entry might begin almost immediately after an action potential invades the terminal, these data can be interpreted as demonstrating endocytotic events just as easily as exocytotic events.
In conclusion, the data presented here demonstrate that exposure of a synapse to low Ca2+/high Mg2+ saline causes invaginations to develop adjacent to the dense body at the active zone at a time when transmitter release is completely blocked. Our previous data have demonstrated that an unusual accumulation of seemingly “docked” vesicles occurs in exactly the same location at the active zone with exposure to high Ca2+ saline. Furthermore, an accumulation of recycling membrane emanating from exactly the same location at the active zone is observed also by using the shi mutant to block pinch-off of recycling membrane after depletion, and this accumulation of recycling membrane is blocked by exposure to low Ca2+/high Mg2+ saline. These data, taken together, provide compelling evidence for a Ca2+-dependent vesicle-recycling pathway emanating from the plasma membrane adjacent to the dense body at the active zone and bring into question the interpretation of one piece of critical evidence for the vesicle hypothesis—that omega-shaped images at the active zone represent vesicles in the process of exocytosis.
Acknowledgments
This work was supported by the U.S. Public Health Service, a National Institutes of Health Grant NS 18856, and a National Science Foundation Grant BNS 8415920.
ABBREVIATIONS
- NMJ
neuromuscular junctions
- ejp
excitatory junction potentials
References
- 1.del Castillo J, Katz B. Microphysiologie Comparée des Eléments Excitables. Vol. 6. Paris: Centre National de la Recherche Scientifique Colloques Internationaux; 1955. pp. 234–258. [Google Scholar]
- 2.Zucker R S. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 3.Heuser J E, Reese T S. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heuser J E. Q J Exp Physiol. 1989;74:1051–1069. doi: 10.1113/expphysiol.1989.sp003333. [DOI] [PubMed] [Google Scholar]
- 5.Couteaux R, Pecot-Dechavassine M. Comptes Rend Acad Sci (Paris) Ser D. 1970;271:2346–2349. [PubMed] [Google Scholar]
- 6.Heuser J E, Reese T S. J Cell Biol. 1981;88:564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig J H, Ikeda K. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig J H, Yamaoka K, Ikeda K. J Neurosci. 1993;13:2313–2322. doi: 10.1523/JNEUROSCI.13-06-02313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda K. In: The Genetics and Biology of Drosophila. Ashburner M, Wright T R F, editors. Vol. 2. New York: Academic; 1980. pp. 369–405. [Google Scholar]
- 10.Smith J E, Reese T S. J Exp Biol. 1980;89:19–29. doi: 10.1242/jeb.89.1.19. [DOI] [PubMed] [Google Scholar]
- 11.Ceccarelli B, Grohovaz F, Hurlbut W P, Iezzi N. J Cell Biol. 1979;81:178–192. doi: 10.1083/jcb.81.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parducz A, Loctin F, Babel-Guérin E, Dunant Y. Neuroscience. 1994;62:93–103. doi: 10.1016/0306-4522(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 13.Katz B, Miledi R. J Neurophysiol. 1967;189:535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heuser J E, Reese T, Dennis M J, Jan Y, Evans L. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torri-Tarelli F, Grohovaz F, Fesce R, Ceccarelli B. J Cell Biol. 1985;101:1386–1399. doi: 10.1083/jcb.101.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]