Abstract
Nicotine at very low doses (5–30 nM) induced large amounts of luteinizing hormone-releasing hormone (LHRH) release, which was monitored as slow membrane depolarizations in the ganglionic neurons of bullfrog sympathetic ganglia. A nicotinic antagonist, d-tubocurarine chloride, completely and reversibly blocked the nicotine-induced LHRH release, but it did not block the nerve-firing-evoked LHRH release. Thus, nicotine activated nicotinic acetylcholine receptors and produced LHRH release via a mechanism that is different from the mechanism for evoked release. Moreover, this release was not caused by Ca2+ influx through either the nicotinic receptors or the voltage-gated Ca2+ channels because the release was increased moderately when the extracellular solution was changed into a Ca2+-free solution that also contained Mg2+ (4 mM) and Cd2+ (200 μM). The release did not depend on Ca2+ release from the intraterminal Ca2+ stores either because fura-2 fluorimetry showed extremely low Ca2+ elevation (≈30 nM) in response to nicotine (30 nM). Moreover, nicotine evoked LHRH release when [Ca2+] elevation in the terminals was prevented by loading the terminals with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid and fura-2. Instead, the nicotine-induced release required extracellular Na+ because substitution of extracellular NaCl with N-methyl-d-glucamine chloride completely blocked the release. The Na+-dependent mechanism was not via Na+ influx through the voltage-gated Na+ channels because the release was not affected by tetrodotoxin (1–50 μM) plus Cd2+ (200 μM). Thus, nicotine at very low concentrations induced LHRH release via a Na+-dependent, Ca2+-independent mechanism.
Nicotine augments exocytosis in rat PC12 cells (1), enhances synaptic transmission between central neurons (2, 3), and triggers exocytosis in bovine chromaffin cells (4). Nicotine also enhances the release of many neuropeptides from various systems (5–9), including the autonomic nervous systems (5–8), where release was monitored by biochemical assays of extracellular or blood levels of neuropeptides. However, the cellular mechanisms for the effect of nicotine on neuropeptide release in the autonomic nervous systems are not clear.
The plasma concentration of nicotine in habitual cigarette smokers was reported to be 16.6 nM after overnight abstinence and increased to 104.7 nM after smoking about one and one-third cigarettes (10). The plasma concentration of nicotine in healthy nonsmokers averages 4.7 nM after a 15-min exposure to passive smoking (11). In this study, we investigated the effects of nanomolar concentrations (5–30 nM) of nicotine on neuropeptide release in the bullfrog paravertebral sympathetic ganglia. In these ganglia, the presynaptic C terminals release a fast-acting neurotransmitter, acetylcholine (ACh), as well as a neuropeptide, luteinizing hormone-releasing hormone (LHRH; ref. 12). Release of ACh causes a nicotinic excitatory postsynaptic potential in the postsynaptic ganglionic C neuron, which results in an action potential in the C neuron. Release of LHRH produces a late, slow excitatory postsynaptic potential in both B and C neurons by blocking the voltage- and time-dependent M channels (13). By monitoring membrane potentials in the ganglionic neurons and by measuring [Ca2+] transients in the presynaptic C terminals, we found that nicotine triggered LHRH release. Furthermore, the nicotine-induced LHRH release was Na+ dependent and Ca2+ independent.
MATERIALS AND METHODS
Preparations of paravertebrate sympathetic ganglia 7–10 were isolated from 5- to 10-cm bullfrogs and were set up for intracellular electrophysiological recording as described (14, 15). Impaled cells were classified as B or C neurons according to the conduction velocity of their axons (14). The intraterminal [Ca2+] ([Ca2+]i) in presynaptic C terminals was measured by using fura-2 fluorimetry as described (16). The fluorimetric data were digitized at 0.1 kHz.
In some experiments, the presynaptic nerves and their terminals were filled with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) tetrapotassium salt by using a filling procedure that differed in two respects from that described previously (16). First, we used a mixture of 1 grain of fura-2 salt to 5 grains of BAPTA salt for the filling process. Second, we eliminated the cooling period between loading of the terminals and the optical and electrophysiological recordings. Images of fura-2-filled terminals were taken by a cooled digital charge-coupled device camera (Photometrics, Tucson, AZ).
Nicotine (3 ml of 5–30 nM) was applied by superfusion at rates between 1 and 1.5 ml/min. Because 20 or 30 nM nicotine blocked the postsynaptic ACh receptors and the blockade was reversed after a 30-min wash with normal Ringer’s solution, we typically washed for 30 min between any two drug treatments. [d-Glp1,d-Phe2,d-Trp3,6]LHRH (100 μM; Glp indicates pyroglutamic acid), a potent antagonist for LHRH receptors, chicken LHRH II (20 μM), or salmon LHRH (tLHRH; 100 μM) was applied by pressure pulses at 4 bars (1 bar = 100 kPa). The puffer pipettes had openings of ≈4 μM in diameter and were positioned 50–100 μm away from the somata of the impaled ganglion cells.
Normal Ringer’s solution contained 115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 2 mM NaHepes, pH 7.2–7.3. The Na+-free solution contained and 117 mM N-methyl-d-glucamine (NMDG) chloride, 1.8 mM CaCl2, and 2 mM KHepes, adjusted with NMDG or HCl solution to pH 7.2–7.3. For the partial Na+, partial NMDG+ solutions, [NMDG+] + [Na+] = 117 mM. Nicotine, d-tubocurarine chloride (dTC), and salts were from Sigma. [d-Glp1,d-Phe2,d-Trp3,6]LHRH, chicken LHRH II, and tLHRH were from Peninsula Laboratories. Fura-2 pentapotassium salt and BAPTA tetrapotassium salt were from Molecular Probes. Tetrodotoxin (TTX) was from Calbiochem. Group data were expressed as means ± SEM unless specified otherwise.
RESULTS
Nicotine-Induced Release of LHRH.
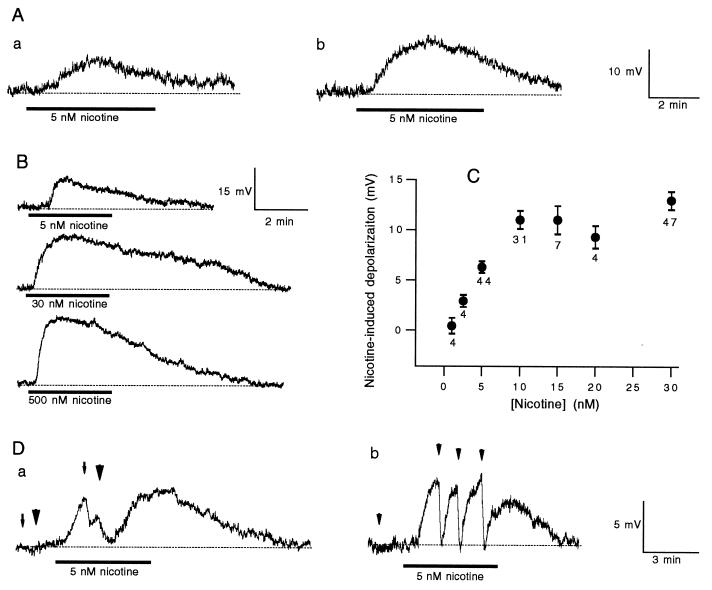
Superfusion of 3 ml of nicotine (5 nM) induced a slow depolarization in both C and B cells (Fig. 1A), whereas 1 nM nicotine produced no response (Fig. 1C). As illustrated by Fig. 1B and summarized in Fig. 1C, higher concentrations of nicotine produced larger depolarizations. Indeed, 30 nM nicotine induced depolarizations whose averaged amplitude (13.0 ± 0.9 mV) was twice of that induced by 5 nM nicotine (6.3 ± 0.6 mV) (Fig. 1C). The depolarization was produced by release of LHRH, because it was completely and reversibly blocked by pressure ejection of a potent LHRH receptor antagonist, [d-Glp1,d-Phe2,d-Trp3,6]LHRH, in both B and C neurons, as illustrated in Fig. 1D. Note that applications of the antagonist before nicotine treatments in these two cells did not produce significant membrane hyperpolarizations. Nicotine-evoked membrane depolarizations were blocked by the LHRH receptor antagonist in all tested cells (n = 29).
Figure 1.
Nicotine-induced LHRH release in bullfrog sympathetic ganglia. (A) The voltage traces were recorded from a C (a) and a B (b) cell. Superfusion of nicotine is indicated by the heavy bar. (B) Depolarizations were recorded in a B neuron in response to increasing concentrations of nicotine. For each application, 3 ml of nicotine was used. (C) Dose responses of nicotine-induced depolarizations. The number of cells tested for each dose of nicotine is given under each point. The error bars are SE. (D) The arrow (a) and arrowheads (b and a) indicate pressure–ejection of [d-Glp1,d-Phe2,d-Trp3,6]LHRH (100 μM) at 4 bars for 2, 5, and 20 s, respectively. The dotted lines indicate the resting potentials.
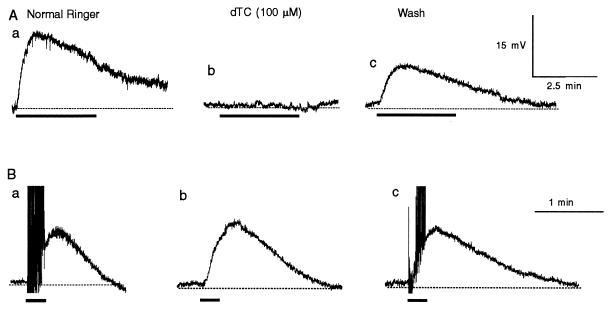
The nicotine-induced slow depolarizations were completely and reversibly blocked by a nicotinic receptor antagonist, dTC (n = 6; Fig. 2A). Thus, nicotine produced LHRH release via activation of nicotinic ACh receptors (nAChRs).
Figure 2.
Effects of dTC on nicotine-induced (A) and electrical stimulation-evoked (B) LHRH release. The records were taken in normal Ringer’s solution (Aa and Ba), at 3 (Ab) and 4 (Bb) min in dTC, and at 17 (Ac) and 6 (Bc) min of washing in normal Ringer’s solution. Each long thick bar indicates superfusion of nicotine (30 nM); each short thick bar indicates 200 stimuli delivered at 20 Hz to presynaptic C nerves. The action potentials in normal Ringer’s solution evoked by release of ACh from the C terminals were truncated (Ba and Bc). The dotted lines indicate the resting potentials. Records in A were taken from a C neuron, and records in B were taken from another C neuron.
In sharp contrast, nerve-firing-evoked release of LHRH was not blocked by dTC (n = 4; Fig. 2B), whereas the nicotinic excitatory postsynaptic potential and the orthodromic action potentials in the postsynaptic C neurons were completely blocked (Fig. 2Bb). In dTC-treated samples, the averaged peak amplitude of the LHRH excitatory postsynaptic potential was 1.2 ± 0.5 (mean ± SD) times that recorded in normal Ringer’s solution. These results suggest that nicotine activated nAChRs that were not located on the postsynaptic neurons. Moreover, a large proportion of the nerve-firing-evoked LHRH release was not caused by feedback of ACh released from the presynaptic nerve terminals.
Nicotine-Induced Release of LHRH Was Ca2+ Independent.
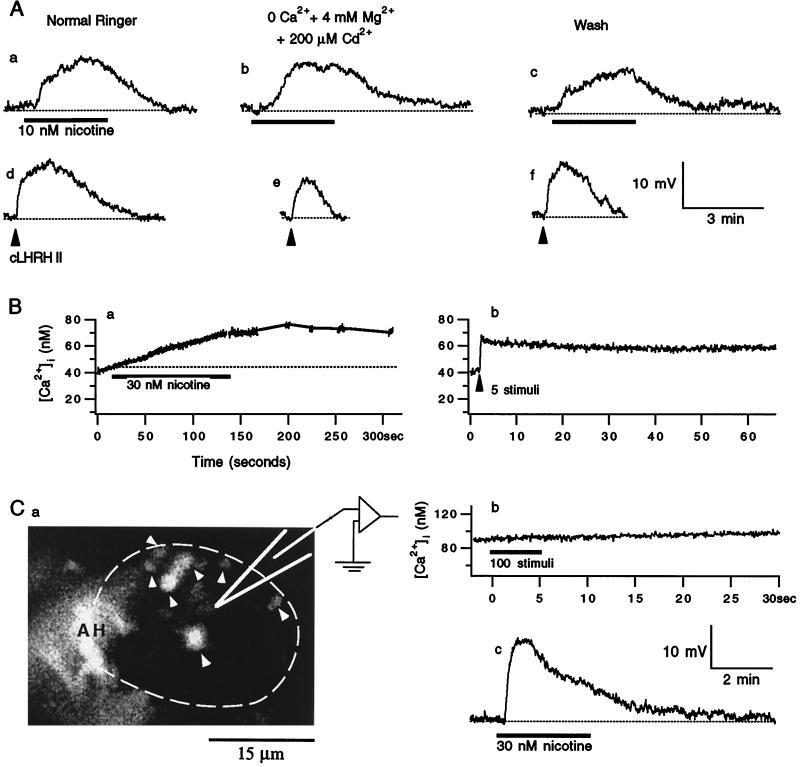
The nerve-evoked LHRH release from these terminals is known to depend on Ca2+ influx (12, 15, 16). Activation of the nAChRs could produce Ca2+ influx via direct Ca2+ entry through the nAChRs. Alternatively, the Ca2+ and Na+ influxes through the nAChRs could cause membrane depolarization, which in turn could open the voltage-gated Ca2+ channels that admitted Ca2+. To test whether nicotine-induced LHRH depends on Ca2+ entry via either of these pathways, we used a Ca2+-free Ringer’s solution that contained 4 mM Mg2+ and 200 μM Cd2+. The inclusion of Mg2+ was to maintain the surface charge of the plasma membrane in Ca2+-free solution, whereas the inclusion of Cd2+ was to block all of the voltage-gated Ca2+ channels (17). As illustrated in Fig. 3A, nicotine-induced depolarization persisted in the Ca2+-free solution. Thus, nicotine-induced LHRH release did not depend on Ca2+ influx through either the nAChRs or the voltage-gated Ca2+ channels. Elimination of extracellular Ca2+ itself decreased the amplitude of the LHRH depolarization postsynaptically (Fig. 3Ae) because M channels are inhibited by lack of extracellular Ca2+ (18, 19). To compensate for the postsynaptic effects of the Ca2+-free solution on the assay system for LHRH release, we pressure ejected exogenous tLHRH or chicken LHRH II immediately after the response to nicotine under both control and experimental conditions. The nicotine-induced LHRH release then was expressed as the ratio of the depolarization induced by nicotine to that induced by exogenous LHRH. On average, the nicotine-induced LHRH release in the Ca2+-free solution was 1.3 ± 0.2 of that in normal Ringer’s solution (n = 20, P < 0.001; paired Student’s t test). Thus, nicotine-induced LHRH release did not require extracellular [Ca2+] ([Ca2+]o); in fact, it was inhibited moderately by the presence of [Ca2+]o.
Figure 3.
Nicotine-induced LHRH release did not require either extra- or intracellular Ca2+. (A) The voltage records were obtained from a single B cell in normal Ringer’s solution (a, c, d, and f) and 5 min in Ca2+-free Ringer’s solution (b and e). Each thick bar indicates superfusion of nicotine. The arrowhead indicates pressure–ejection of chicken LHRH II (cLHRH II; 20 μM) at 4 bars for 2 ms. The dotted lines indicate the resting potentials. (B) [Ca2+]i transients were recorded from a set of presynaptic terminals opposed to a single C neuron. The transients were evoked by nicotine (a) and by 20 Hz electrical stimuli to presynaptic nerves (b). (Ba) The line segments on the record are the extrapolated [Ca2+]i levels during the [Ca2+]i plateau to avoid unnecessary photobleaching of fura-2; the dotted line indicates the resting [Ca2+]i. (Ca) An image of fura-2-filled presynaptic axon(s) and their terminals that oppose a single C neuron. Fura-2 molecules were excited at 380 nm, and their emission greater than 510 nm was imaged. The postsynaptic C neuron is outlined by the dashed white curve and the filled terminal boutons are indicated by arrowheads. AH indicates the axon hillock region of postsynaptic neuron. The intense fluorescence in this region was caused by the high density of terminals in this region (16). (b) The [Ca2+]i within the filled AH and terminals as shown in a was recorded by monitoring fura-2 fluorescence in response to 20 Hz of electrical stimulation to the presynaptic nerve. (c) Nicotine-induced depolarization was recorded from the C neuron outlined in a.
To test whether Ca2+ release from the intraterminal Ca2+ stores contributed to nicotine-induced LHRH release, we measured [Ca2+] within the presynaptic C terminals. To our surprise, 30 nM nicotine produced such small Ca2+ elevations that they were similar to Δ[Ca2+] produced by five action potentials (delivered at 20 Hz) in the same set of terminals (n = 3; Fig. 3B). Repetitive action potentials in the presynaptic C terminals generate [Ca2+] elevation, which leads to LHRH release if it exceeds a threshold [Ca2+]i level (186 nM) for LHRH release (16). Because five action potentials never produced detectable LHRH release (Y.-Y.P., unpublished data) and because the elevation of ≈30 nM in [Ca2+]i produced by either the five action potentials or 30 nM nicotine was below the threshold Ca2+ level for LHRH release, the small increase of [Ca2+]i caused by nicotine could not be responsible for the nicotine-induced LHRH release.
To further rule out the possibility of microdomains of high [Ca2+] within the presynaptic terminals that might escape detection, we filled the presynaptic terminals with BAPTA salt and fura-2 salt. Although both compounds chelate intraterminal Ca2+, fura-2 was used to identify the filled terminals and to monitor [Ca2+]i. An example of filled terminals synapsing onto a C cell is shown in Fig. 3Ca. When terminals were filled by fura-2 alone, 100 electrical stimuli delivered at 20 Hz typically increased the [Ca2+]i by several hundred nanomolar (16, 20). Because this stimulation did not produce any [Ca2+] elevation in the BAPTA- and fura-2-filled terminals (Fig. 3Cb), there must be a sufficient amount of BAPTA within these terminals to buffer hundreds of nanomolar Ca2+. Yet, nicotine-induced LHRH release persisted (Fig. 3Cc). Similar results were obtained in another two cells. Thus, Ca2+ release from intraterminal stores was not likely to cause the nicotine-induced LHRH release.
The sharp contrast in the Ca2+ dependency between the nicotine- and the nerve firing-induced LHRH release suggests that these two release processes occurred via independent mechanisms.
Nicotine-Induced LHRH Release Was Na+ Dependent.
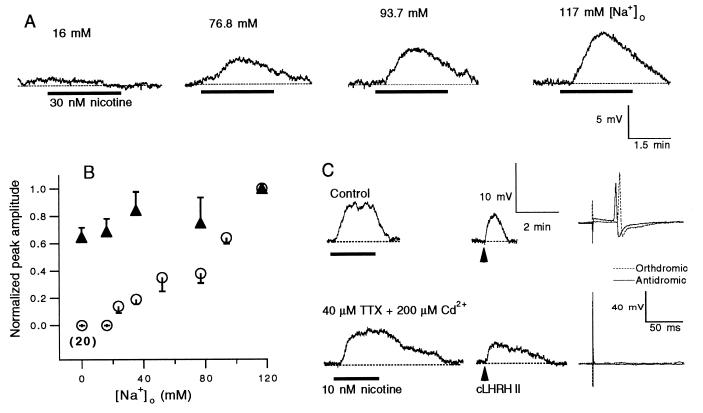
We tested whether the nicotine-induced release depended on Na+ influx. Like the situation for Ca2+ influx, Na+ influx could occur either through the nAChRs and/or indirectly through the voltage-gated Na+ channels. The latter could be activated by membrane depolarization caused by the ion fluxes through the nAChRs. We first substituted Na+ in the bath solution with NMDG+. Equimolar substitution of Na+ with NMDG+ completely abolished the nicotine-induced LHRH release in 20 cells tested (including 13 C cells and 7 B cells). In fact, [Na+]o regulated nicotine-induced LHRH release in a dose-dependent manner (Fig. 4 A and B). Removal or reduction of extracellular Na+ also decreased the postsynaptic cells’ responses to exogenous LHRH by at most 35% (Fig. 4B). But this reduction in postsynaptic response to LHRH could not account for the fact that there was no nicotine response when [Na+]o was 0 and 16 mM (Fig. 4 A and B), nor could it explain the dose dependence of nicotinic responses on [Na+]o. Thus, nicotine-induced LHRH release depended on [Na+]o.
Figure 4.
Nicotine-induced LHRH release depended on [Na+]o. (A) The voltage records were obtained from a B cell. The concentration of [Na+]o is indicated on the top of each trace. (B) Relationships between the amplitudes of the nicotine- and exogenous LHRH-induced depolarizations and [Na+]o were studied in three neurons (the averaged nicotine-induced response at 0 [Na+]o was calculated from data in 20 cells). Each nicotine response was evoked by 30 nM nicotine. Each LHRH response was evoked by pressure–ejection of tLHRH at four bars for 0.5–1 s. For each neuron, the peak amplitudes of responses were normalized to the peak amplitude of the response in normal Ringer’s solution. The data are expressed as means ± SEM. (C) The voltage records were obtained from a C cell. The action potentials failed after 2 min of pretreatment with TTX and Cd2+ (Right). (Lower) The nicotine- and LHRH-induced responses were taken after the action potentials had failed. The dotted lines indicate the resting potentials.
We used TTX (1–50 μM) to test whether the effect of [Na+]o on the nicotine-sensitive LHRH release was caused by Na+ influx through the voltage-gated Na+ channels. In five C neurons, when TTX completely blocked both the orthodromic and the antidromic action potentials, it had little effect on nicotine-induced responses. Although a TTX-insensitive Na+ current has been found in ganglionic B neurons, accounting for 25% of the Na+ current elicited by membrane depolarization (21), the fact that TTX alone abolished both the orthodromic and the antidromic action potentials in the C neurons suggested that C neurons and the presynaptic C terminals do not have TTX-insensitive channels. To further support this conclusion, we added 200 μM Cd2+ to a TTX solution. If in the C neurons and in the presynaptic C terminals nicotine induced either a TTX-insensitive current or any voltage-activated Ca2+ currents because of Na+ influxes, both currents should be blocked by 200 μM Cd2+ (17, 21). As illustrated in Fig. 4C, under these conditions, the antidromic action potentials were completely blocked in the C neurons. Yet the nicotinic responses remained. Similar results were obtained in two other cells. Although this treatment reduced the amplitude of the nicotinic response slightly, i.e., the peak amplitude in TTX plus Cd2+ was 0.86 ± 0.11 (mean ± SD) of that in normal Ringer’s solution, and increased the duration of the response, these changes were postsynaptic in nature because the response to exogenous LHRH was similarly altered (Fig. 3C). In fact, the ratio of peak amplitudes of the nicotine and LHRH responses in TTX plus Cd2+ was 1.0 ± 0.3 (mean ± SD) of that in normal Ringer’s solution. In summary, the effect of [Na+]o on the nicotine-sensitive LHRH release did not appear to be caused by Na+ influx through the voltage-gated Na+ channels.
DISCUSSION
Our results showed that nicotine at very low concentrations triggered release of LHRH via a Na+-dependent and Ca2+-independent mechanism in the bullfrog sympathetic ganglia. In sharp contrast to our study, earlier studies of nicotine-enhanced release of neurotransmitters and nicotine-induced exocytosis of dense-cored vesicles both depend on the elevation of intraterminal Ca2+ via either Ca2+ influx through the nicotinic receptors (1–4) or Ca2+ release from intraterminal Ca2+ stores (4, 9). Another finding of nicotinic effects in the bullfrog sympathetic ganglia is that the nicotine-induced Na+-dependent LHRH release coexisted with the well documented nerve-firing-induced Ca2+-dependent LHRH release.
Nicotine-induced LHRH release depended on [Na+]o, and yet it was insensitive to TTX plus Cd2+. Thus, if influx of Na+ directly triggered peptide release via some unknown mechanism(s), the influx was through the nAChRs, which in autonomic ganglia are permeable to Na+ (22). The presence of [Na+]o also produced TTX-insensitive release of neuropeptides in rat isolated neurohypophysial nerve endings, where release was monitored by biochemical methods (23).
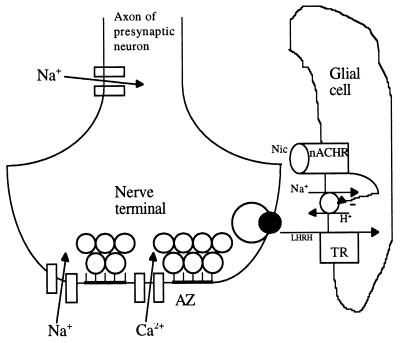
Results of our TTX experiments suggest that Na+ affected nicotine-induced release at sites close to the presynaptic nAChRs but not close to the Na+ channels. Because Na+ channels are located at the terminals as well as along the presynaptic axons, the nAChRs are likely to be in the glial cells that surround the terminals. One possible scenario that is consistent with all of our current data is depicted in Fig. 5, where we depict that nicotine induces apparent LHRH release via inhibition of LHRH transporters. The reason for putting the nAChRs in the glial cells that surround the terminals is that this would explain the extremely low intraterminal [Ca2+] elevation caused by nicotine. The postulated existence of LHRH transporters is because of the requirement for [Na+]o and the ineffectiveness of Na+ influx through the Na+ channels when release is induced by nicotine. One of the two known classes of peptide transporters is driven by a proton gradient across the plasma membrane, which is set up by the Na+/H+ exchangers (24). Thus, LHRH transporters driven similarly would require [Na+]o, but not transient Na+ influx, to function. Although we are not aware of any reported study of LHRH transporters, on principle it is not impossible for such molecules to exist because many types of peptide transporters have been found in the gut and kidney epithelial cells. We postulate that activation of nAChRs blocks the LHRH transporters via inhibition of the Na+/H+ exchangers. One such receptor for neurotransmitters, β2-adrenergic receptors, recently has been found to inhibit Na+/H+ exchangers (25). For the postulated scheme to operate in the absence of evoked LHRH release, there must be spontaneous release of LHRH. We detected spontaneous release of LHRH in most ganglionic neurons (unpublished data).
Figure 5.
A diagram of a presynaptic C terminal and an adjacent glial cell. AZ, active zones; TP, LHRH transporters. The ACh-containing synaptic vesicles are docked at the AZ, whereas exocytosis of a LHRH-containing dense-cored vesicle occurs away from any AZ.
To reiterate the hypothesis, some of the LHRH molecules that are spontaneously released from the terminals are taken up into the surrounding glial cell by proton-coupled LHRH transporters. Nicotine activation of nAChRs somehow inhibits the transporters, and the inhibited uptake of LHRH manifests itself as LHRH “release,” which is detected by the postsynaptic neuron. Many lines of research are needed to verify this hypothesis.
Activity of C neurons regulates the tension of the artery walls by releasing both epinephrine and neuropeptide Y (26–28). Moreover, neuropeptide Y greatly potentiates the tension produced by epinephrine (29). Thus, in bullfrogs, nicotine at nanomolar concentrations could increase blood pressure via enhanced release of either epinephrine and/or neuropeptide Y. Nicotine-induced LHRH release depolarized both ganglionic B and C cells and thus rendered them more excitable. This would lead to at least an increased release of epinephrine from the terminals of the C neurons. If nicotine at such a small dose could increase release of neuropeptide Y via a mechanism similar to that for release of LHRH, nicotine’s effects on blood pressure should be even more profound. On the other hand, nicotine’s effects on the B neurons would alter the activities of the target tissues of the B neurons.
The sympathetic system in humans is similar to that in bullfrogs except that, instead of epinephrine, the terminals of the ganglionic neurons release norepinephrine. Because passive smoking could increase the blood level of nicotine to ≈5 nM, our finding of the strong effects of nanomolar concentrations of nicotine on the ganglionic neurons could have implications on the effects of smoking, both active and passive, for humans.
In summary, we found that nicotine at very low concentrations produced LHRH release in bullfrog sympathetic ganglion. Moreover, this release depended on extracellular Na+ instead of extra- or intraterminal Ca2+. Neurotransmitter release from nerve terminals and/or exocytosis from the somata of many types of nonneuronal cells has been found to be triggered by intracellular [Ca2+] increases. Our results revealed a different mechanism for neuropeptide release. Moreover this release process coexisted with the Ca2+-dependent release in the same synaptic system.
Acknowledgments
We thank Ms. Diane Hall for editing the manuscript. This work was supported by National Institutes of Health Grant NS32429 and a grant from the Brain Research Foundation (to Y.-Y.P.).
ABBREVIATIONS
- NMDG
N-methyl-d-glucamine
- LHRH
luteinizing hormone-releasing hormone
- ACh
acetylcholine
- nAChR
nicotinic ACh receptor
- tLHRH
salmon LHRH
- dTC
d-tubocurarine chloride
- TTX
tetrodotoxin
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- [ ]i
intraterminal concentration
- [ ]o
extracellular concentration
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Harkins A B, Fox A P. J Gen Physiol. 1998;111:257–269. doi: 10.1085/jgp.111.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGehee D S, Heath M J S, Belber S, Devay P, Role L W. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 3.Gray R, Rajan A S, Radcliffe K A, Yakehiro M, Dani A. Nature (London) 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 4.Mollard P, Seward E P, Nowycky M C. Proc Natl Acad Sci USA. 1995;92:3065–3069. doi: 10.1073/pnas.92.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wigoda P, Netscher D T, Thornby J, Yip B, Rappaport N. J Hand Surg (Am) 1995;20:718–724. doi: 10.1016/S0363-5023(05)80420-4. [DOI] [PubMed] [Google Scholar]
- 6.Boyadjieva N I, Sarkar D K. Life Sci. 1997;61:PL59–PL66. doi: 10.1016/s0024-3205(97)00444-x. [DOI] [PubMed] [Google Scholar]
- 7.Tabassian A R, Nylen E S, Giron A E, Snider R H, Cassidy M M, Becker K L. Life Sci. 1988;42:2323–2329. doi: 10.1016/0024-3205(88)90185-3. [DOI] [PubMed] [Google Scholar]
- 8.Curro D, Preziosi P. Br J Pharmacol. 1997;121:1105–1112. doi: 10.1038/sj.bjp.0701245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnier M, Lamacz M, Tonon M C, Vaudry H. Proc Natl Acad Sci USA. 1994;91:11743–11747. doi: 10.1073/pnas.91.24.11743. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Benowitz N L, Porchet H, Sheiner L, Jacob P. Clin Pharmacol Ther. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 11.Hausberg M, Mark A L, Winniford M O, Brown R E, Somers V K. Circulation. 1997;96:282–287. [PubMed] [Google Scholar]
- 12.Jan L Y, Jan Y N. J Physiol (London) 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams P R, Brown D A, Constanti A. J Physiol (London) 1982;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd J, Horn J P. J Physiol (London) 1983;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y-Y, Horn J P. J Neurosci. 1991;11:85–95. doi: 10.1523/JNEUROSCI.11-01-00085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Y-Y, Zucker R S. Neuron. 1993;10:465–473. doi: 10.1016/0896-6273(93)90334-n. [DOI] [PubMed] [Google Scholar]
- 17.Tsien R W, Lipscombe D, Madison D V, Bley K R, Fox A P. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y-Y, Horn J P. Soc Neurosci Abstr. 1990;16:358. [Google Scholar]
- 19.Marrion N V. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y-Y. J Neurosci. 1996;16:6703–6712. doi: 10.1523/JNEUROSCI.16-21-06703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jassar B S, Pennefather P S, Smith P A. J Physiol (London) 1993;472:203–231. doi: 10.1113/jphysiol.1993.sp019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fieber L A, Adams D J. J Physiol (London) 1991;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuenkel E L, Nordmann J J. J Physiol (London) 1993;468:357–378. doi: 10.1113/jphysiol.1993.sp019776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganapathy V, Leibach F H. Curr Opin Nephrol Hypertens. 1996;5:395–400. doi: 10.1097/00041552-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hall R A, Premont R T, Chow C-W, Blitzer J T, Pitcher J A, Claing A, Stoffel R H, Barak L S, Shenolikar S, Weinman E J, et al. Nature (London) 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 26.Stofer W D, Fatherazi S, Horn J P. J Auton Nerv Syst. 1990;31:141–152. doi: 10.1016/0165-1838(90)90071-p. [DOI] [PubMed] [Google Scholar]
- 27.Thorne R, Smith M S, Horn J P. J Auton Nerv Syst. 1992;38:231–236. doi: 10.1016/0165-1838(92)90034-e. [DOI] [PubMed] [Google Scholar]
- 28.Horn, J. P. (1992) Can. J. Physiol. Pharmacol. 70, Suppl., 819–826. [DOI] [PubMed]
- 29.Thorne R, Horn J P. J Physiol (London) 1997;498:201–214. doi: 10.1113/jphysiol.1997.sp021851. [DOI] [PMC free article] [PubMed] [Google Scholar]