Abstract
Copper (Cu) deficiency-induced teratogenicity is characterized by major cardiac, brain and vascular anomalies, however, the underlying mechanisms are poorly understood. Cu deficiency decreases superoxide dismutase activity, and increases superoxide anions which can interact with nitric oxide (NO), reducing the NO pool size. Given the role of NO as a developmental signaling molecule, we tested the hypothesis that low NO levels, secondary to Cu deficiency, represent a developmental challenge. Gestation day 8.5 embryos from Cu adequate (Cu+) or Cu deficient (Cu−) dams were cultured for 48 h in Cu+ or Cu− medium, respectively. We report that NO levels were low in conditioned media from Cu−/Cu− embryos and yolk sacs, compared to Cu+/Cu+ controls under basal conditions, and with NO synthase (NOS) agonists. The low NO production was associated with low endothelial NOS phosphorylation at serine 1177 and cyclic guanosine-3′,5′-monophosphate (cGMP) concentrations in the Cu−/Cu− group. The altered NO levels in Cu deficient embryos are functionally significant, as the administration of the NO donor, DETA/NONOate, increased cGMP and ameliorated embryo and yolk sac abnormalities. These data support the concept that Cu deficiency limits NO availability and alters NO-dependent signaling which contributes to abnormal embryo and yolk sac development.
Keywords: copper deficiency, nitric oxide, cyclic GMP, yolk sac, pregnancy, embryo development, nutrition, oxidative stress, superoxide dismutase
Introduction
Nitric oxide (NO), produced during the conversion of L-arginine to L-citrulline by NO synthase (NOS) isoforms, is a gaseous, free radical molecule that mediates various signaling functions, including smooth muscle relaxation, neurotransmission, and immune cell cytotoxicity [1, 2]. NO is involved in early developmental processes including ovulation, implantation and embryogenesis, and regulates the development of skeletal, lung, and cardiovascular systems [3–10]. Endothelial NOS (eNOS) knockout neonates are characterized by lower fetal and birth weight, defects in lung morphogenesis, impaired systolic and diastolic baseline contractile function, atrial septal defects, increased postnatal heart failure, abnormal limb development, and increased mortality [10–13]. NO also plays a crucial role in yolk sac vessel development [14]. Using an embryo culture system, Nath and coworkers reported that the addition of a NOS inhibitor to the embryo culture media resulted in yolk sac vasculopathy phenotypes including lack of vascularization and functional circulation, dilated vessels, and arrested vascular development [14]. These authors further showed that hyperglycemia can result in an elevated concentration of reactive oxygen species, and developmental defects that are similar to those seen with NOS inhibition [14]. Significantly, in this model system, the addition of a NO donor to the media reduces the developmental toxicity of the hyperglycemic insult.
In humans, copper (Cu) deficiency is teratogenic with adverse outcomes such as early embryonic death and structural abnormalities including cardiac, skeletal, pulmonary, vascular and neurological defects [15–18]. Similarly, in animals, Cu deficiency during pregnancy leads to numerous structural and biochemical abnormalities in the embryo and fetus, with the heart, brain, and yolk sac vascular system being most affected [19–21]. Long-term adverse effects of Cu deficiency on immune and neurobehavioral functions can also occur [15, 17, 22]. The essentiality of Cu during development is underscored by the observations that transgenic mice lacking the Cu transporters Ctr1, COX17, and Atox1 die during mid-gestation or the early postnatal period [23–25].
Excessive oxidative/nitrosative stress due, in part, to decreased Cu-zinc superoxide dismutase (Cu-Zn SOD) activity may be one mechanism contributing to Cu deficiency teratogenicity. Increased superoxide anions (O2•−), as a result of decreased Cu-Zn SOD activity, can react with NO to form peroxynitrite (ONOO−) which can exert detrimental effects in tissues/organs including blood vessels by increasing protein nitration or limiting NO bioavailability [26, 27]. We have previously shown that Cu deficient embryos have increased staining of 3-nitrotyrosine (3-NT) in the neuroepithelium region of the anterior neural tube, an indication of an environment of elevated ONOO− [19]. The increased staining of 3-NT was reversed with the addition of Cu, Cu-Zn SOD, Tiron (SOD agonist), or glutathione peroxidase [19], however, the antioxidant treatments did not rescue the Cu deficiency-induced teratogenicity. Thus, we hypothesized that a decrease in bioavailable NO may play a role in Cu deficiency teratogenicity.
That high amounts of O2•− can limit the availability of bioactive NO in vessels has been established in numerous disease conditions including atherosclerosis, diabetes, hypertension, and hypercholesterolemia [28–31]. Like hyperglycemia, Cu deficiency is an oxidative/nitrosative stress condition which may increase the requirement for NO. Dietary Cu deficiency in male weanling rats attenuates NO-mediated vessel relaxation and the response is restored in the presence of SOD, illustrating the sensitive association between Cu and NO levels [32, 33]. Cu-Zn SOD knockout mice also have higher O2•− levels, and impaired endothelium-dependent dilation of cerebral arterioles, consistent with the idea that these mice have low vascular NO levels [34, 35]. Decreased NO bioavailability can result from reduced NO synthesis due to decreased NOS activity or from accelerated breakdown, both of which can occur in oxidative stress conditions.
The present study was designed to test the hypothesis that Cu deficiency-induced changes in NO metabolism and bioavailable NO contribute to the teratogenicity of Cu deficiency. Using a mouse embryo culture model, as well as embryos taken in vivo, we report that NO concentrations in conditioned media from Cu deficient embryos and yolk sacs are low compared to Cu adequate controls, and provide data that the low NO concentrations might be secondary to low eNOS phosphorylation at serine 1177 (phospho-eNOS (Ser 1177)). NO-mediated downstream signaling is also altered in Cu deficient post-implantation staged embryos and yolk sacs as characterized by low cyclic GMP (cGMP) levels. The concept that the Cu deficiency-induced reduction in NO concentrations is functionally significant is supported by our observation that the addition of the NO donor DETA/NONOate to Cu deficient media, increases cGMP levels, and ameliorates developmental anomalies in Cu deficient embryos and yolk sacs. Collectively, these data confirm the essentiality of NO for normal embryonic development, and provide evidence for the hypothesis that altered NO availability contributes to Cu deficiency-induced teratogenicity.
Materials and methods
Animals and diets
Female virgin 5-wk-old CD-1 mice (Charles River Laboratories, Hollister, CA) were housed in pairs in suspended stainless steel wire-bottomed cages under standard conditions. Mice consumed deionized water and one of two purified diets ad libitum: Cu adequate (8.0 μg Cu/g diet, Cu+) or Cu deficient (< 0.5 μg Cu/g diet, Cu−) diet for 3 wks before breeding. The Cu content of the diets was verified by inductively coupled plasma spectroscopy (ICP-AES; Trace-Scan, ThermoElemental, Willmington, MA) after acid extraction. For the first two wks, the Cu deficient diet contained 1% (w/w) triethylenetetramine (TETA; Sigma, St. Louis, MO), a Cu chelating agent. Thereafter, the mice were fed the Cu deficient diet without TETA. After 3 wks of dietary treatment, females were bred overnight with stock diet-fed breeder males of the same strain. Females with sperm plugs the next morning were identified as gestation day (GD) 0.5. At GD 8.5 (in vitro experiments) or GD 10.5 (in vivo experiments), dams were anesthetized with CO2 and killed via abdominal aorta exsanguination. Maternal blood, liver and kidney were collected, and embryos were isolated. Depending on the assay, different numbers of embryos and yolk sacs were pooled within a litter and within a treatment group. This protocol was approved by the Animal Use and Care Administrative Advisory Committee of the University of California at Davis.
Study 1: Effects of Cu deficiency on mouse embryonic and yolk sac vessel development and NO metabolism
Whole embryo culture
Cu adequate and Cu deficient GD 8.5 embryos having between 3–5 somite pairs were dissected from maternal decidua leaving the yolk sac and ecto-placental cone intact using standard techniques [19]. The Cu adequate and Cu deficient explanted embryos were cultured for 48 h in Cu adequate (16.96 ± 0.37 μM Cu) or Cu deficient (0.51 ± 0.08 μM Cu) culture media, respectively. Thus, there were two treatment groups (Cu+/Cu+ and Cu−/Cu−). The media contained 75 % male rat serum and 25% Tyrode’s solution. The rat serum used for the embryo culture was collected from male Sprague-Dawley rats (Charles River Laboratories) that had been fed either the Cu−adequate diet for 4 wks or the Cu−deficient diet containing TETA for 3 wks followed by one wk of TETA-free Cu deficient diet as previously described [36]. Culture bottles were incubated at 37°C and rotated at 30 rev/min for 48 h. The flasks were gassed twice daily with varying concentrations of O2, CO2, and N2 [19]. After the 48 h culture period, the embryos were staged by somite number, measured for crown-rump length, and scored according to the morphological scoring system of Brown and Fabro [37], adapted for the mouse. This scoring system allows the quantitative assessment of twelve morphological features and organ systems resulting in an overall index of embryonic growth and development. In addition to developmental staging, embryos were examined by light microscopy for gross morphology and the presence of abnormalities by an investigator who was blinded to treatment groups. After the 48 h culture period, subsets of culture media were collected for Cu analysis. Embryos and yolk sacs were then prepared for SOD activity assay, O2•− quantification, or assessment of NOS isoform protein expression. A subset of embryos and yolk sacs were further incubated for 2 h and the conditioned media analyzed for NO concentrations.
SOD activity
After the 48 h culture period, two Cu+/Cu+ or Cu−/Cu− embryos or yolk sacs were pooled within a litter and within a treatment group and homogenized in 160 μl of buffer (0.25 M sucrose, 10 mM Tris; pH 7.4, and Complete Protease Inhibitor Cocktails; Roche Applied Science, Indianapolis, IN). Total SOD activity was determined spectrophotometrically by measuring the inhibition of formazan dye formation produced from the reduction of the water-soluble tetrazolium salt, WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium, monosodium salt) by O2•− generated from the reaction of xanthine oxidase and xanthine using an SOD Assay kit-WST (Dojindo Molecular Technologies Inc., Gaithersburg, MD) [38]. Bovine erythrocyte Cu-Zn SOD (Sigma) was used to generate a standard curve. The absorbance was measured at 450 nm and SOD activity was expressed as Units/mg protein.
O2.− concentrations
Following the 48 h culture period, two Cu+/Cu+ or Cu−/Cu− embryos or yolk sacs were pooled within a litter and within a treatment group and homogenized in 200 μl of sucrose buffer (0.25 M sucrose and 10 mM Tris; pH 7.4) [20]. The homogenate (30 μl) was mixed with 10 μl of NADH (1% [w/v]), 10 μl of NADPH (1% [w/v]), 600 μl of sodium carbonate buffer (50 nM; pH 10), and nitroblue tetrazolium (NBT; 3.3 mg/ml). To determine the specificity of this assay for O2•−, each sample was also run in the presence of 50 μl of Cu-Zn SOD (800 U/ml; Sigma). Differences in the rates of NBT reduction were determined between samples with NBT alone, and samples containing NBT and Cu-Zn SOD. The change in absorbance was measured at 560 nm on a Beckman DU 640 spectrophotometer (Beckman Instruments). O2•− concentration is expressed as μmol NBT reduced/min/mg protein.
NO concentrations
To examine the effects of Cu deficiency on NO metabolism, two model systems were used. First, NO production was assessed in embryos that were collected at GD 8.5 and cultured for 48 h (in vitro experiment). Second, to complement the in vitro data, NO production was assessed in embryos and yolk sacs collected at GD 10.5 from Cu+ or Cu− dams (in vivo experiment). Individual embryo and yolk sac from the two model systems were rinsed three times in nitrate-free Krebs Ringer bicarbonate buffer (KRBB; Sigma) and placed into 96-well microplates containing 200 μl KRBB. The NOS substrate, L-arginine (200 μM; Sigma), was added to all wells except for negative control wells, which contained the NOS inhibitor, N-monomethyl-L-arginine (L-NMMA; 10 mM; Calbiochem, La Jolla, CA). To assess whether Cu deficient embryos and yolk sacs respond differently to NOS agonists compared to Cu adequate concepti, vascular endothelial growth factor (VEGF; 1 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA), acetylcholine (1 mM; Sigma), and the calcium (Ca2+) ionophore ionomycin (100 μM; Calbiochem) were added to subsets of wells. Plates were incubated for 2 h at 37°C, followed by centrifugation (2,500 g, 10 min). Conditioned KRBB supernates were collected and stored at −40°C until analyzed. Supernates (40 μl) were injected onto an NO analyzer that employs ozone-chemiluminescence technology (Sievers Nitric Oxide Analyzer 280i, Sievers Instruments, Boulder, CO). Using vanadium (III) chloride (Alfa Aesar, Ward Hill, MA) in hydrochloric acid as a reducing agent, total NO (nitrate + nitrite + nitrosothiols) was measured at 95°C. Standard curves were generated by injections of known concentrations of sodium nitrate (Sigma). Background NO levels (in KRBB alone) were subtracted from NO detected in samples. NO concentrations are expressed as nmol NO/mg of embryo or yolk sac protein.
Immunoprecipitation
Yolk sacs were collected from Cu+/Cu+ and Cu−/Cu− embryos that had been cultured for 48 h as described above. The yolk sacs were homogenized with lysis buffer supplemented with Complete Protease Inhibitor Cocktails (Roche Applied Science) and phosphatase inhibitors. The lysates were centrifuged and the supernates were either used for immunoprecipitation or resuspended in loading buffer for western blotting.
For inducible NOS (iNOS) immunoprecipitation, 1 mg protein of total yolk sac lysate in a volume of 500 μl was precleared by incubation with 0.25 μg of control IgG (Santa Cruz Biotechnology). 20 μl of GammaBind G Sepharose (Amersham Biosciences Corp., Piscataway, NJ) was added, and samples were incubated for 30 min at 4°C in a multitube rotator. The samples were then centrifuged at 1000 g for 5 min at 4°C to pellet the Sepharose beads. The supernate was transferred to a new microcentrifuge tube. 2 μg of rabbit anti-iNOS primary antibody (BD Biosciences, San Diego, CA) was added to the supernate, and incubated for 1 h at 4°C in a multitube rotator. 20 μl of GammaBind G Sepharose was added. Samples were incubated overnight at 4°C in a multitube rotator, and then centrifuged at 1000 g for 5 min at 4°C to pellet the Sepharose beads. The supernate was carefully aspirated and discarded. The pellet was washed four times by resuspension in lysis buffer, followed by centrifugation and aspiration of the supernate. After the fourth wash, the pellet was resuspended in 40 μl of 1X electrophoresis sample buffer made by the addition of 1.0 ml glycerol, 0.5 ml β-mercaptoethanol, 3.0 ml 10 % sodium dodecylsulfate (SDS), 1.25 ml Tris-HCl (pH 6.7), 2 mg bromophenol blue, and 5.75 ml of distilled H2O. The samples were then boiled for 5 min prior to loading for electrophoresis.
Western blot analysis
Embryo or yolk sac proteins were separated by electrophoresis on 5 % Tris-HCl SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Membranes were washed with Tris-buffered saline/Tween-20 (TBST; 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, and 0.05 % Tween 20), and blocked with 5 % non-fat milk in TBST for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibody for iNOS (BD Biosciences), eNOS (Santa Cruz Biotechnology), phospho-eNOS (Ser-1177) (Cell Signaling Technology, Danvers, MA), and beta-actin (Sigma), washed, and subsequently incubated with a secondary goat anti-rabbit or anti-mouse IgG antibody conjugated to horseradish peroxidase for 1 h (Promega, Madison, WI). Bands were detected by chemiluminescence using the ECL detection reagent (Amersham Biosciences Corp.). The bands were detected by chemiluminescence, scanned by a Chemidoc XRS gel scanner (Bio-Rad, Hercules, CA) with a cooled 12-bit camera, and quantified by densitometry. Positive controls included an iNOS positive control (Cayman, Ann Arbor, MI) for iNOS and human umbilical vein endothelial cells (HUVEC) induced with VEGF (Santa Cruz Biotechnology) for eNOS and phospho-eNOS (Ser-1177). Levels of phosphorylated eNOS were normalized to values for eNOS.
Study 2: Effects of NO donor supplementation to Cu deficient media on embryonic and yolk
sac vessel development
In a separate experiment, embryos were cultured in Cu adequate media (18.82 ± 0.24 μM Cu), Cu deficient media (2.42 ± 0.38 μM Cu), or Cu deficient media supplemented with the NO donor DETA/NONOate (20 μM; Calbiochem) (2.10 ± 0.60 μM Cu) to yield three treatment groups: Cu+/Cu+, Cu−/Cu−, or Cu−/Cu− + NO donor. After the 48 h culture period, embryos and yolk sacs were assessed as described above, and prepared for determination of yolk sac vessel diameter and cGMP concentrations.
Yolk Sac Vessel Diameter
Yolk sac vascularisation was assessed by determination of yolk sac vessel diameter distribution. Embryos and yolk sacs from the Cu+/Cu+, Cu−/Cu−, and Cu−/Cu− + NO donor groups were fixed overnight at 4°C in 4% paraformaldehyde in phosphate buffered saline, dehydrated in a graded series of alcohol washes, cleared in toluene, and embedded in paraffin. Using a microtome (8 μm; ‘820’ Spencer Microtome), transverse serial sections were generated, collected on slides, and stained with hematoxylin and eosin as previously described [36].
Yolk sac vessel diameters were measured in three separate slides per yolk sac using IP Lab Spectrum (Scanalytics Inc., Fairfax, VA). Photographs of 20X magnification sections were collected with an Olympus DP 71 digital camera attached to an Olympus BX 51 microscope (Olympus America Inc., San Diego, CA). The diameter of individual yolk sac vessel was calculated with the IP Lab Spectrum program and the vessel diameters were distributed into categories of 10 micron (μm) intervals. The percentage distribution of vessel diameters in each category was calculated, and then averaged to generate an average percentage vessel diameter distribution for each treatment group.
cGMP concentrations
cGMP concentrations in embryos and yolk sacs from the Cu+/Cu+, Cu−/Cu−, and Cu−/Cu− + NO donor groups were determined using a 96-well cGMP enzyme immunoassay (Assay Design, Ann Arbor, MI) according to the manufacturer’s instructions. Embryos and yolk sacs were homogenized with lysis buffer supplemented with Complete Protease Inhibitor Cocktails (Roche Applied Science) and sodium orthovanadate (Sigma). After homogenization, samples were incubated on ice for 30 min and then centrifuged at 13,000 rpm for 15 min at 4°C to remove cellular debris. Tissue lysates were acetylated with a mixture of acetic anhydride and triethylamine supplied by the manufacturer, and the absorbance was determined at 405 nm. The cGMP concentrations were calculated from a standard curve of known amounts of cGMP and are expressed as pmol cGMP/mg protein. The sensitivity of the assay is 0.088 pmol/ml. All samples were assayed in duplicate.
Statistical analysis
All statistical analyses were performed using SPSS (Version 14.0). Data are expressed as mean ± SEM unless otherwise specified. In study 1, differences between Cu adequate and Cu deficient groups were determined by t-tests. In study 2, statistical analysis was performed using one-way ANOVA. The significance of observed differences among the groups was evaluated with LSD post-hoc test. Differences in the incidence of anomalies between groups were tested for statistical significance with chi-square tests. Values of p < 0.05 were considered statistically significant.
Results
Maternal and media Cu deficiency adversely affects mouse embryonic and yolk sac vessel development
The dietary regimen resulted in suboptimal maternal Cu status as assessed by low Cu concentrations in Cu deficient vs. Cu adequate liver (55.5 ± 3.6 vs. 82.4 ± 5.00 nmol/g), kidney (62.3 ± 4.8 vs. 83.2 ± 6.6 nmol/g), and plasma (1.8 ± 0.3 vs. 10.8 ± 0.3 μM), respectively. Food intake, weight gain and the number of implantation sites per dam were similar between the groups (data not shown). The average number of resorptions per litter was higher in the Cu deficient group (1.53 ± 0.27) than in controls (0.29 ± 0.11) (p < 0.001).
Somite number and crown-rump length were similar between the two groups indicating that overall embryo growth was not adversely affected by Cu deficiency (Table 1). However, developmental score (a quantitative assessment of growth and development of major organ systems) was lower, and the incidence of developmental anomalies was markedly higher, in the Cu deficient group compared to controls. The Cu deficient embryos displayed a variety of heart (abnormal/swollen heart and pericardium effusion) and brain (forebrain hypoplasia, microcephaly, hindbrain distension, and open anterior neuropore) anomalies. The percentage of embryos with abnormal/swollen heart, forebrain hypoplasia, microcephaly, and open anterior neuroporewas significantly higher in the Cu deficient group than controls (Table 1).
Table 1.
Effects of Cu deficiency on in vitro embryo and yolk sac development
| Cu+/Cu+ | Cu−/Cu− | |
|---|---|---|
| Embryos examined (number) | 262 | 292 |
| Somite Pairs (number) | 33.0 ± 0.1 | 32.6 ± 0.1 |
| Crown-Rump length (mm) | 4.5 ± 0.0 | 4.5 ± 0.1 |
| Developmental Score | 42.2 ± 0.2 | 40.9 ±0.3* |
| Percentage of embryos with one or more embryo or yolk sac anomalies (%) | 25.2 | 60.3* |
| Percentage of embryos with one or more yolk sac anomalies (%) | 13.4 | 43.8* |
| Abnormal vascularization (%) | 10.3 | 33.6* |
| Blood pooling (%) | 4.2 | 14.4* |
| Blood islands (%) | 5.0 | 8.2 |
| Percentage of embryos with one or more heart anomalies (%) | 9.2 | 12.7 |
| Abnormal/swollen heart (%) | 2.7 | 8.6* |
| Pericardium effusion (%) | 6.5 | 6.8 |
| Percentage of embryos with one or more brain anomalies (%) | 12.6 | 35.6* |
| Forebrain hypoplasia (%) | 2.3 | 12.3* |
| Microcephaly (%) | 2.7 | 13.0* |
| Hindbrain distension (%) | 5.3 | 8.9 |
| Open anterior neuropore (%) | 1.5 | 6.2* |
GD 8.5 Cu adequate embryos were cultured for 48 h in Cu adequate medium (Cu+/Cu+) and GD 8.5 Cu deficient embryos were cultured for 48 h in Cu deficient medium (Cu−/Cu−). Values are expressed as mean ± SEM or as percentage of embryos with the specified anomaly.
p < 0.05 versus Cu+/Cu+ controls.
The incidence of yolk sac vessel anomalies was higher in the Cu deficient group (43.8 %) compared to controls (13.4 %) (Table 1). The types of vessel anomalies ranged in severity from less vascularization, thin or hemorrhagic blood vessels, the presence of blood pooling, blood islands, and blebbing, to in a few cases, the absence of blood vessels.
Cu deficiency increases oxidative stress and decreases NO concentrations
Cu is a required cofactor for the oxidant defence enzyme, Cu-Zn SOD, and a decrease in its activity with Cu deficiency has been reported in rats. In mouse embryonic tissues, Cu deficiency lowered SOD activities in embryos (1.24 ± 0.13 Units/mg protein) and yolk sacs (4.31 ± 0.83 Units/mg protein) compared to control embryos (4.57 ± 0.61 Units/mg protein) and yolk sacs (8.92 ± 1.49 Units/mg protein). Concomitantly, Cu deficient embryos and yolk sacs were also characterized by higher O2•− concentrations (152.3 ± 4.0 and 170.0 ± 8.8 μmol NBT reduced/min/mg protein, respectively), compared to controls (54.5 ± 1.7 and 63.7 ± 10.6 μmol NBT reduced/min/mg protein, respectively) indicating increased oxidative stress.
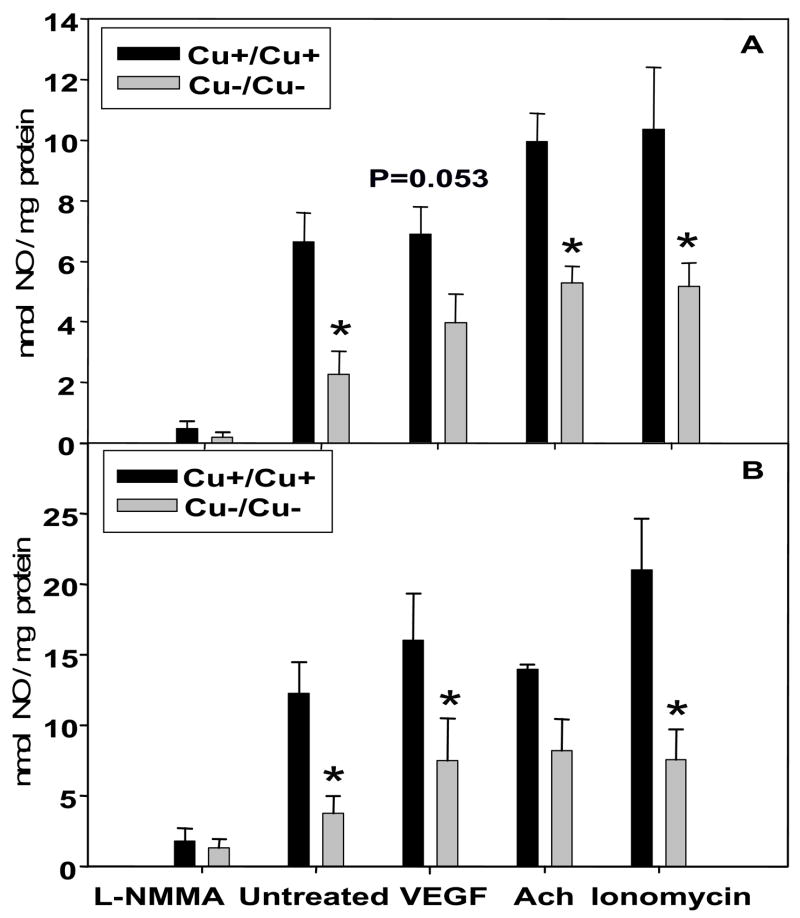
As one consequence of increased O2•− concentrations can be a decrease in bioavailable NO, we measured NO concentrations in conditioned media after 2 h incubation with NOS inhibitor or agonists. The concentrations of NO, normalized to tissue protein, were markedly lower in the conditioned media from Cu−/Cu− embryonic tissues (Figs. 1A and 1B) compared to Cu+/Cu+ tissues under basal conditions and when incubated in the presence of the calcium ionophore, ionomycin. Cu−/Cu− embryos had lower NO concentrations when incubated in the presence of VEGF (p=0.053) and acetylcholine (p=0.042), and Cu−/Cu− yolk sacs had lower NO concentrations in the presence of VEGF (p=0.017) compared to their respective controls. Addition of the NOS inhibitor, L-NMMA, resulted in low NO concentrations in all groups.
Figure 1.
Nitric oxide (NO) concentrations in conditioned medium after 2 h incubation of in vitro Cu adequate (Cu+/Cu+) and Cu deficient (Cu−/Cu−) embryo (A) and yolk sac (B) in the presence or absence of a NOS inhibitor (L-NMMA; 10 mM), or NOS agonists (VEGF; 1 μg/ml, acetylcholine (Ach); 1 mM, ionomycin; 100 μM). Values are expressed as mean ± SEM (n=6–10/group). * p < 0.05 versus Cu+/Cu+ controls.
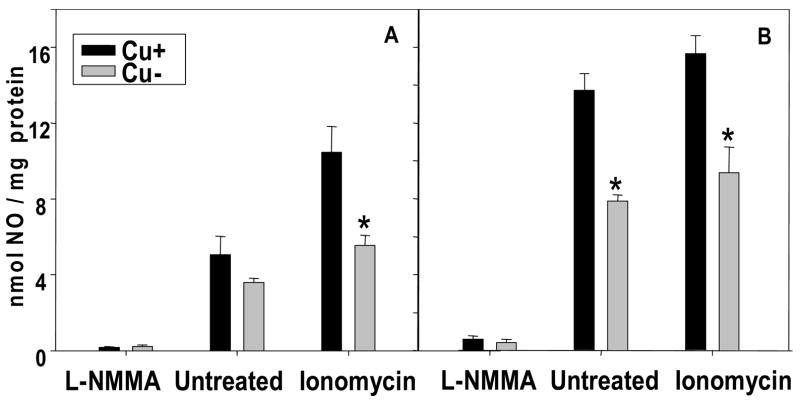
To complement the above in vitro data, Cu adequate and Cu deficient embryos and yolk sacs were collected in vivo at GD 10.5, and NO concentrations in conditioned media were measured. Under basal conditions, Cu deficient embryos tended to have lower NO than Cu adequate embryos, while Cu deficient yolk sacs had significantly lower NO than Cu adequate yolk sacs (Figs. 2A and 2B). Cu deficient embryos and yolk sacs taken in vivo were characterized by lower levels of NO compared to controls when incubated in the presence of ionomycin. The magnitude of the NO reduction in Cu deficient embryos and yolk sacs obtained in vivo was comparable to that observed in Cu deficient tissues after 48 h of culture in vitro (Figs. 1A and 1B).
Figure 2.
Nitric oxide (NO) concentrations in conditioned medium after 2 h incubation of in vivo Cu adequate (Cu+) or Cu deficient (Cu−) GD 10.5 embryo (A) and yolk sac (B) in the presence or absence of a NOS inhibitor (L-NMMA; 10 mM), or the NOS agonist, ionomycin (100 μM). Values are expressed as mean ± SEM (n= 3/group). * p < 0.05 versus Cu+ controls.
eNOS phosphorylation status is decreased with Cu deficiency
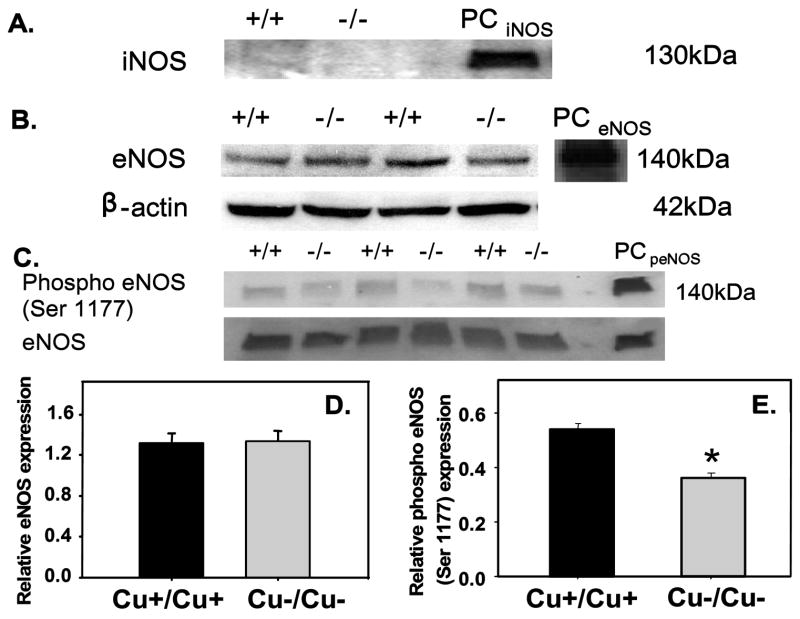
Oxidative stress such as that induced by hyperglycemia, can alter the protein expression of iNOS and eNOS and increase the requirement for NO [14]. Results from immunoprecipitation and western blotting show that iNOS protein was not detected in yolk sacs from either group at this stage of development (Fig. 3A). The eNOS protein expression normalized to beta-actin was similar between the two groups (Figs. 3B and 3D). As NOS activity can be affected by multiple factors, we determined the extent of eNOS phosphorylation at serine 1177 using western blot. Phospho-eNOS (Ser 1177) normalized to eNOS protein, was lower in Cu deficient yolk sacs compared to controls (Figs. 3C and 3E).
Figure 3.
iNOS, eNOS and phospho-eNOS (Ser 1177) protein expression. (A) Immunoprecipitation/western blot shows no detectable iNOS protein in Cu adequate (Cu+/Cu+) or Cu deficient (Cu−/Cu−) yolk sacs, only in iNOS positive control (PCiNOS; murine recombinant iNOS). (B) eNOS and beta-actin western blots in Cu+/Cu+ and Cu−/Cu− yolk sacs and positive control (PCeNOS; HUVEC induced with VEGF). (C) Phospho-eNOS (Ser 1177) and eNOS western blots in Cu+/Cu+ and Cu−/Cu− yolk sacs and positive control (PCpeNOS; HUVEC induced with VEGF). D) eNOS normalized to beta-actin was similar between the groups. Values are mean ± SEM (n= 6/group). E) Phospho-eNOS (Ser 1177) relative to eNOS expression is lower in u−/Cu− yolk sacs. Values are mean ± SEM (n=3/group). * p < 0.05 vs. Cu+/Cu+ controls.
Addition of an NO donor to Cu deficient embryo culture media ameliorates embryonic and yolk sac vessel abnormalities
To assess whether the low NO was functionally significant, Cu deficient medium was supplemented with the NO donor, DETA/NONOate. Exogenous NO administration ameliorated yolk sac and embryo abnormalities such that the incidences were similar to control levels (Table 2). The improvement was noted in developmental score as well as the incidences of yolk sac, heart, and brain anomalies.
Table 2.
Effects of NO donor supplementation to Cu deficient media on in vitro embryo and yolk sac development
| Cu+/Cu+ | Cu−/Cu− | Cu−/Cu− + NO donor | |
|---|---|---|---|
| Embryos examined (number) | 86 | 36 | 33 |
| Somite Pairs (number) | 32.3 ± 0.1 | 32.0 ± 0.2 | 32.1 ± 0.1 |
| Crown-Rump length (mm) | 4.4 ± 0.0 | 4.3 ± 0.1 | 4.4 ± 0.0 |
| Developmental Score | 41.9 ± 0.4a | 39.8 ± 0.9b | 42.3 ± 0.3a |
| Percentage of embryos with one or more embryo or yolk sac anomalies (%) | 24.4a | 77.8b | 36.4a |
| Percentage of embryos with one or more yolk sac anomalies (%) | 14.0a | 63.9b | 15.2a |
| Abnormal vascularization (%) | 12.8a | 58.3b | 15.2a |
| Blood pooling (%) | 9.3a | 36.1b | 12.1a |
| Blood islands (%) | 3.5a | 25.0b | 0a |
| Percentage of embryos with one or more heart anomalies (%) | 4.7a | 22.2b | 3.0a |
| Abnormal/swollen heart (%) | 0a | 8.3b | 0ab |
| Pericardium effusion (%) | 4.7a | 16.7b | 3.0ab |
| Percentage of embryos with one or more brain anomalies (%) | 10.5a | 41.7b | 21.2ab |
| Forebrain hypoplasia (%) | 1.2 | 2.8 | 3.0 |
| Microcephaly (%) | 2.3 | 8.3 | 3.0 |
| Hindbrain distension (%) | 4.7 | 11.1 | 9.1 |
| Open anterior neuropore (%) | 1.2a | 13.9b | 3.0ab |
Gestation day (GD) 8.5 Cu adequate embryos were cultured for 48 h in Cu adequate media (Cu+/Cu+) and GD 8.5 Cu deficient embryos were cultured for 48 h in Cu deficient media (Cu−/Cu−), or in Cu deficient media supplemented with NO donor (Cu−/Cu− + NO donor). Values with different superscripts within a parameter are significantly different at p < 0.05
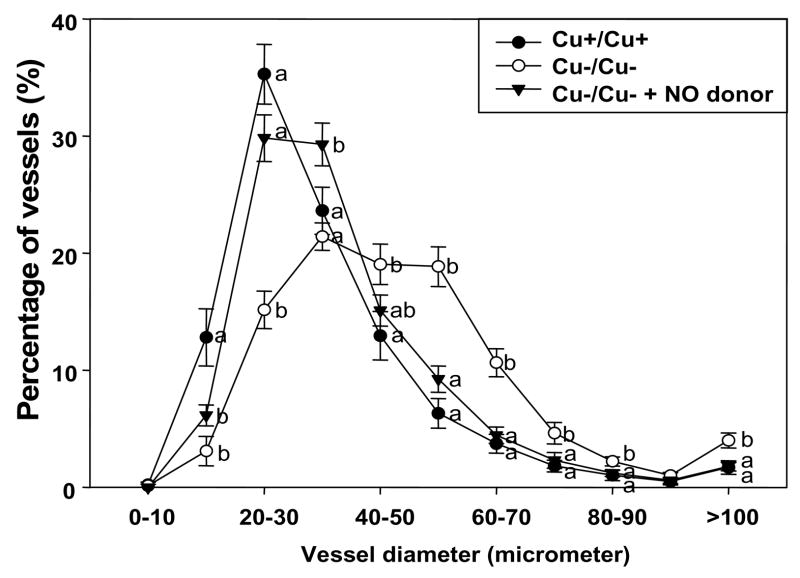
Vasculature development was assessed by determining the percentage distribution of yolk sac vessel diameters (Fig. 4). In contrast to Cu+/Cu+ yolk sac vessels which had a peak (35.3%) in the 20–30 μm range, vessel diameters in Cu−/Cu− yolk sacs were broadly distributed over the 30–60 μm range, indicating that Cu−/Cu− yolk sac vessels had enlarged vessel diameters compared to controls. NO donor supplementation to Cu deficient media resulted in similar histograms of vessel diameter distribution as that of Cu+/Cu+ yolk sac vessels.
Figure 4.
Percentage distribution of vessel diameter in hematoxylin and eosin-stained yolk sacs from Cu adequate (Cu+/Cu+), Cu deficient (Cu−/Cu−), and Cu deficient with NO donor supplementation (Cu−/Cu− + NO donor) groups. Values are expressed as mean ± SEM (n= 5/group). Different letters within a parameter are significantly different at p < 0.05.
NO-mediated downstream signaling is altered with Cu deficiency
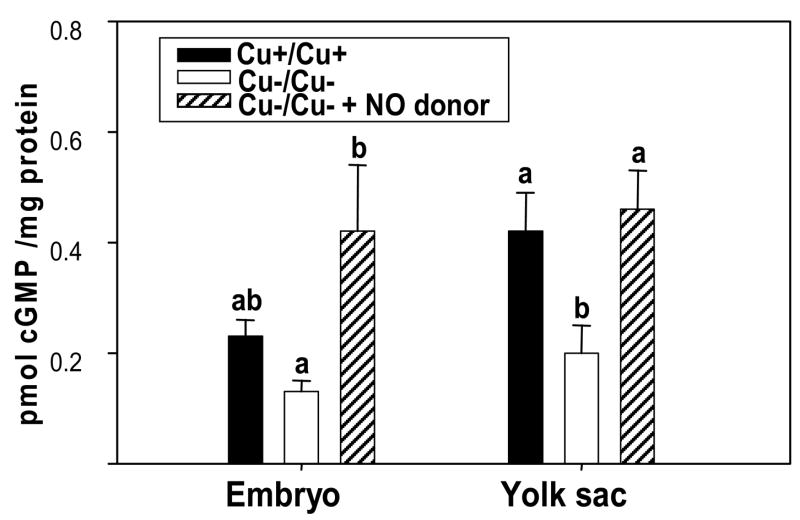
The cGMP concentrations in Cu−/Cu− embryos tended to be lower (43% decrease) than in Cu+/Cu+ embryos (Fig. 5). Cu−/Cu− yolk sacs had significantly lower cGMP levels than Cu+/Cu+ yolk sacs (p=0.022). Addition of the NO donor to the Cu deficient media increased cGMP levels in the Cu−/Cu− embryos and yolk sacs, to levels that were similar to, or above, those of controls.
Figure 5.
cyclic GMP (cGMP) concentrations in Cu adequate (Cu+/Cu+), Cu deficient (Cu−/Cu−), and Cu deficient with NO donor supplementation (Cu−/Cu− + NO donor) embryos and yolk sacs. Values are expressed as mean ± SEM (n= 5–7/group). Different letters within a parameter are significantly different at p < 0.05.
Discussion
There was a high incidence of embryo and yolk sac anomalies in the Cu−/Cu− group compared to the controls. Several mechanisms have been suggested to underlie the teratogenicity induced by Cu deficiency including high oxidative/nitrosative stress, altered extracellular matrix integrity, compromised energy production, and altered angiogenesis [39]. With regard to oxidative/nitrosative stress, Cu deficiency can result in low Cu-Zn SOD activity, and high O2•−, in Cu deficient rat embryos [20] as well as in adult Cu deficient rats [40]; these effects have not been documented in Cu deficient mouse embryos or yolk sacs. Our data show that Cu deficient mouse embryos and yolk sacs were characterized by low SOD activities and high O2•− levels, indicating similar species-dependent susceptibility to Cu deficiency-induced oxidative stress. One consequence of increased O2•− levels is the formation of ONOO− which can decrease bioavailable NO and NO-dependent effects. With regard to the vasculature, it is well established that oxidative stress from increased O2•−, lipid peroxidation, oxidized lipoproteins and ONOO− can impair NO bioactivity, and that treatment with antioxidants including SOD, glutathione peroxidase, ascorbic acid, glutathione, vitamin E and tetrahydrobiopterin (BH4) can improve endothelium–derived NO bioactivity [41–43]. Increasing evidence supports the concept that Cu deficiency can affect NO metabolism in vessels. Schuschke and coworkers [32, 33] reported that dietary Cu deficiency in male weanling rats depressed NO-mediated vascular smooth muscle relaxation, which was restored with Cu-Zn SOD addition. Similarly, other groups have reported that inhibition of Cu-Zn SOD by dietary Cu restriction, or Cu chelation, attenuates vessel relaxation [40, 44]. The dilation response of cerebral arterioles to acetylcholine is reduced in heterozygous and homozygous Cu-Zn SOD deficient mice compared to wild type mice [34] indicating the importance of adequate SOD activity. Although these studies did not directly assess NO production, the impaired endothelium-dependent relaxation, which is hypothesized to be mediated by NO, implies that there is decreased NO bioavailability in Cu deficient vessels.
With regard to embryonic tissues, our data are the first to show that NO levels from Cu deficient embryos and yolk sacs under stimulated and non-stimulated conditions, are lower compared to control tissues. The finding that Cu deficient concepti have low NO levels in response to acetylcholine or the Ca2+ ionophore, ionomycin, support the finding by Schuschke and coworkers that dietary Cu deficiency can significantly depress cellular Ca2+ mobilization and vasodilation to acetylcholine [45]. An important observation of the current paper is that the effects of Cu deficiency on NO production were remarkably similar in Cu deficient embryos collected and analyzed at GD 10.5, and Cu deficient embryos that had been explanted on GD 8.5 and cultured for 48 h in Cu deficient media and then analyzed. These data argue against the idea that low NO production observed in the cultured embryos and yolk sacs might be an artifact of the in vitro culture model. The above data also support the utility of the embryo culture model as a system to study, in a well-defined manner, the effects of nutritional insults on embryonic development.
At this time, it is not clear whether the low NO levels in conditioned media from Cu deficient tissues represent decreased NO production, increased NO consumption, or increased reuptake of NO back into tissue. It is notable that NO levels in conditioned media, normalized to tissue protein, were higher from yolk sacs compared to embryos. Several possibilities may explain this phenomenon; 1) yolk sac may have more NO-producing capacity relative to total tissue protein than embryo or 2) due to the relatively simple structure of the yolk sac (two-cell layers at this stage of development), the NO produced from the yolk sac may be easily released and accumulated in the media while some of the NO produced from the embryo may be trapped internally.
During mouse yolk sac vascular development, there are different temporal and spatial patterns of NOS isoform expression which suggests that each developmental stage requires appropriate levels of NO [14]. eNOS and iNOS protein expression are inversely related from embryonic day (E) 7.5 to E 9.5. eNOS protein is weakly expressed in the E 8 mesoderm, but strongly expressed in the inner endothelial layer at the primary capillary plexus stage and through the vessel maturation stage (E 9.5) [14]. In contrast, iNOS protein is highly expressed in E 8 yolk sac endoderm, but is absent by the vessel maturation stage. In our study, iNOS was not detected in the E 10.5-equivalent Cu adequate or deficient yolk sacs. While eNOS protein was expressed in these tissues, there was no effect of Cu deficiency; a finding that has been observed in adult vessels [45]. However, phosphorylation of eNOS at serine 1177 is decreased in Cu deficient yolk sacs indicating that NOS activation may be impaired. eNOS phosphorylation at serine 1177 enhances electron flux through the reductase domain, and is a key factor affecting NOS activity [46]. One suggested mechanism is a Cu deficiency-induced alteration in the activation of kinases that phosphorylate eNOS at serine 1177 such as serine kinase Akt and protein kinase A. Oxidative stress also inhibits NOS activation through caveolin-1 inhibition, altered eNOS phosphorylation, or deficiency of BH4 which leads to eNOS uncoupling and further O2•− production [47, 48]. As Cu deficiency is an oxidative stress condition, several of these mechanisms could contribute to Cu deficiency-induced low NO availability and embryo/yolk sac abnormalities. In addition, Schuschke et al. has shown that dietary Cu deficiency depresses cellular Ca2+ mobilization which may underlie the reduced vasodilation in response to acetylcholine [45]. Moreover, a recent provocative paper reports that the cuproenzyme ceruloplasmin (Cp) can function as a NO oxidase that converts NO to nitrite, a bioactive endocrine form of NO [49]. Cu is a required cofactor for ceruloplasmin activity; it is unknown whether a Cu deficiency-induced decrease in ceruloplasmin activity contributes to altered NO metabolism. It is important to note that Cu status seems to modulate NO metabolism differently depending on the tissue. In contrast to Cu deficient yolk sacs, hearts from Cu deficient adult rats have increased expression of iNOS and eNOS and produce large amounts of NO [50, 51] which may contribute to Cu deficiency-induced cardiomyopathy and heart failure.
Given that Cu deficient embryos and yolk sacs have low available NO, we hypothesized that modulating NO levels would improve Cu deficiency-induced teratogenicity. NO donor addition to the Cu deficient culture media resulted in embryos with similar developmental score as controls. Yolk sac vessel diameter distribution patterns, a marker of yolk sac vessel development [36], showed that Cu deficient yolk sacs had a greater proportion of vessels with enlarged vessel diameters compared to control yolk sacs, suggesting decreased sprouting angiogenesis, impaired vessel integrity, or arrested vessel development. A similar shift to larger diameter yolk sac vessels has been reported with another oxidative stress condition, hyperglycemia [14]. In both Cu deficiency and hyperglycemia, NO donor supplementation to the embryo culture media resulted in similar histograms as that observed in controls. It is speculated that increased availability of NO helps to rescue Cu deficiency-induced vasculopathy by stimulating angiogenic processes and by stabilizing vascular extracellular matrix. Taken together, the data indicate that NO plays an important role in vessel size determination and vascular organization.
Importantly, exogenous NO also markedly reduced Cu deficiency-induced embryonic and yolk sac anomalies. The extent of the effect of a NO donor on post-implantation embryonic development differed depending on the type of tissues/organs examined. The most significant effect of NO supplementation to Cu deficient media was on yolk sac development, reducing the incidence of yolk sac anomalies to control levels. Heart anomalies were also markedly reduced with exogenous NO while there was modest improvement in the incidence of brain anomalies. These data support the hypothesis that Cu deficiency-induced alterations in NO metabolism represent a significant developmental insult.
NO can act as a signaling molecule through the activation of soluble guanylyl cyclase (sGC) and subsequent increase in cGMP production from GTP by sGC [1]. Our results show that Cu deficient yolk sacs were characterized by low cGMP concentrations indicating altered NO-mediated signaling. cGMP is involved in early embryonic development [52, 53]. Treatment of NOS inhibitor impairs the development of two-cell stage embryos, which is rescued by addition of cGMP analogue [53]. We suggest that the increase in the NO pool that occurred in the Cu deficient embryos as a consequence of the addition of the NO donor, resulted in improved embryonic development through an upregulation of NO-dependent-cGMP signaling pathways.
Summary/Conclusions
Our data show that Cu deficient embryos and yolk sacs are characterized by a high incidence of yolk sac and embryonic anomalies and altered NO metabolism. The reduction in NO levels in Cu deficient embryonic and yolk sac tissues may be due, in part, to decreased eNOS phosphorylation at serine 1177, as well as other factors including altered Ca2+ mobilization [45], and increased oxidation of the key NOS cofactor, BH4 [54], all of which can negatively impact NOS activity and lead to decreased NO production. In addition to low NO levels, SOD activity is lower, and O2•− levels are higher in Cu deficient tissues, which can result in increased ONOO−. Cu deficient tissues are characterized by elevated 3-NT levels, suggesting that the already compromised NO pools in the Cu deficient embryo can be further reduced via ONOO− formation [19]. Thus, there are likely multiple mechanisms underlying the occurrence of low NO pools in the Cu deficient embryo. Our data demonstrate that normal development is driven by a complex balance of numerous reactive oxygen and nitrogen signaling molecules, which become unbalanced in Cu deficiency or other oxidative stress conditions. Importantly, the results obtained from the NO donor study provide strong evidence linking altered NO metabolism and altered NO-mediated signaling with Cu deficiency teratogenicity.
Acknowledgments
This work was supported by National Institutes of Health grant HD-26777, and a gift from the International Copper Association.
This work was supported by National Institutes of Health grant HD-26777, and a gift from the International Copper Association.
Abbreviations
- NO
nitric oxide
- NOS
nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- Cu+
copper adequate
- Cu−
copper deficient
- SOD
superoxide dismutase
- O2•−
superoxide anion
- ONOO−
peroxynitrite
- 3-NT
3-nitrotyrosine
- cGMP
cyclic guanosine-3′,5′-monophosphate
- TETA
triethylenetetramine
- GD
gestation day
- NBT
nitroblue tetrazolium
- L-NMMA
N-monomethyl-L-arginine
- BH4
tetrahydrobiopterin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanafy KA, Krumenacker JS, Murad F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med Sci Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 3.Fantel AG, Nekahi N, Shepard TH, Cornel LM, Unis AS, Lemire RJ. The teratogenicity of N(omega)-nitro-L-ariginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, in rats. Reprod Toxicol. 1997;11:709–717. doi: 10.1016/s0890-6238(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 4.Shukovski L, Tsafriri A. The involvement of nitric oxide in the ovulatory process in the rat. Endocrinology. 1994;135:2287–2290. doi: 10.1210/endo.135.5.7525265. [DOI] [PubMed] [Google Scholar]
- 5.Jablonka-Shariff A, Basuray R, Olson LM. Inhibitors of nitric oxide synthase influence oocyte maturation in rats. J Soc Gynecol Investig. 1999;6:95–101. doi: 10.1016/s1071-5576(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 6.Sengoku K, Takuma N, Horikawa M, Tsuchiya K, Komori H, Sharifa D, Tamate K, Ishikawa M. Requirement of nitric oxide for murine oocyte maturation, embryo development, and trophoblast outgrowth in vitro. Mol Reprod Dev. 2001;58:262–268. doi: 10.1002/1098-2795(200103)58:3<262::AID-MRD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Gouge RC, Marshburn P, Gordon BE, Nunley W, Huet-Hudson YM. Nitric oxide as a regulator of embryonic development. Biol Reprod. 1998;58:875–879. doi: 10.1095/biolreprod58.4.875. [DOI] [PubMed] [Google Scholar]
- 8.Nath AK, Madri JA. The roles of nitric oxide in murine cardiovascular development. Dev Biol. 2006;292:25–33. doi: 10.1016/j.ydbio.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol. 2002;282:L379–385. doi: 10.1152/ajplung.00462.2000. [DOI] [PubMed] [Google Scholar]
- 10.Hefler LA, Reyes CA, O'Brien WE, Gregg AR. Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol Reprod. 2001;64:666–673. doi: 10.1095/biolreprod64.2.666. [DOI] [PubMed] [Google Scholar]
- 11.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res. 2004;94:1115–1123. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 12.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 13.Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, Yee SP. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation. 2002;106:873–879. doi: 10.1161/01.cir.0000024114.82981.ea. [DOI] [PubMed] [Google Scholar]
- 14.Nath AK, Enciso J, Kuniyasu M, Hao XY, Madri JA, Pinter E. Nitric oxide modulates murine yolk sac vasculogenesis and rescues glucose induced vasculopathy. Development. 2004;131:2485–2496. doi: 10.1242/dev.01131. [DOI] [PubMed] [Google Scholar]
- 15.Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- 16.Klevay LM. Cardiovascular disease from copper deficiency--a history. J Nutr. 2000;130:489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 17.Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J Nutr. 2004;134:1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- 18.Keen CL, Hanna LA, Lanoue L, Uriu-Adams JY, Rucker RB, Clegg MS. Developmental consequences of trace mineral deficiencies in rodents: acute and long-term effects. J Nutr. 2003;133:1477S–1480S. doi: 10.1093/jn/133.5.1477S. [DOI] [PubMed] [Google Scholar]
- 19.Beckers-Trapp ME, Lanoue L, Keen CL, Rucker RB, Uriu-Adams JY. Abnormal development and increased 3-nitrotyrosine in copper-deficient mouse embryos. Free Radic Biol Med. 2006;40:35–44. doi: 10.1016/j.freeradbiomed.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Hawk SN, Lanoue L, Keen CL, Kwik-Uribe CL, Rucker RB, Uriu-Adams JY. Copper-deficient rat embryos are characterized by low superoxide dismutase activity and elevated superoxide anions. Biol Reprod. 2003;68:896–903. doi: 10.1095/biolreprod.102.009167. [DOI] [PubMed] [Google Scholar]
- 21.Hawk SN, Uriu-Hare JY, Daston GP, Jankowski MA, Kwik-Uribe C, Rucker RB, Keen CL. Rat embryos cultured under copper-deficient conditions develop abnormally and are characterized by an impaired oxidant defense system. Teratology. 1998;57:310–320. doi: 10.1002/(SICI)1096-9926(199806)57:6<310::AID-TERA4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Prohaska JR, Downing SW, Lukasewycz OA. Chronic dietary copper deficiency alters biochemical and morphological properties of mouse lymphoid tissues. J Nutr. 1983;113:1583–1590. doi: 10.1093/jn/113.8.1583. [DOI] [PubMed] [Google Scholar]
- 23.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi Y, Kako K, Kashiwabara S, Takehara A, Inada Y, Arai H, Nakada K, Kodama H, Hayashi J, Baba T, Munekata E. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Mol Cell Biol. 2002;22:7614–7621. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci U S A. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Zalba G, Beaumont FJ, San Jose G, Fortuno A, Fortuno MA, Diez J. Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction? Nephrol Dial Transplant. 2001;16(Suppl 1):2–5. doi: 10.1093/ndt/16.suppl_1.2. [DOI] [PubMed] [Google Scholar]
- 30.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 31.Kelm M, Rath J. Endothelial dysfunction in human coronary circulation: relevance of the L-arginine-NO pathway. Basic Res Cardiol. 2001;96:107–127. doi: 10.1007/s003950170061. [DOI] [PubMed] [Google Scholar]
- 32.Schuschke DA, Saari JT, Miller FN. A role for dietary copper in nitric oxide-mediated vasodilation. Microcirculation. 1995;2:371–376. doi: 10.3109/10739689509148281. [DOI] [PubMed] [Google Scholar]
- 33.Schuschke DA, Percival SS, Saari JT, Miller FN. Relationship between dietary copper concentration and acetylcholine-induced vasodilation in the microcirculation of rats. Biofactors. 1999;10:321–327. doi: 10.1002/biof.5520100402. [DOI] [PubMed] [Google Scholar]
- 34.Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke. 2006;37:1850–1855. doi: 10.1161/01.STR.0000227236.84546.5a. [DOI] [PubMed] [Google Scholar]
- 35.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 36.Yang SJ, Uriu-Adams JY, Keen CL, Rucker RB, Lanoue L. Effects of copper deficiency on mouse yolk sac vasculature and expression of angiogenic mediators. Birth Defects Res B Dev Reprod Toxicol. 2006;77:445–454. doi: 10.1002/bdrb.20096. [DOI] [PubMed] [Google Scholar]
- 37.Brown NA. Routine assessment of morphology and growth:scoring system and measurements of size. In: Copp AJ, Cockroft DL, editors. Postimplantation Mammalian Embryos: A Practical Approach. Oxford: IRL Press at Oxford University Press; 1990. pp. 93–108. [Google Scholar]
- 38.Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin Chim Acta. 2000;293:157–166. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- 39.Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwik-Uribe CL, Rucker RB. Effect of copper deficiency on prenatal development and pregnancy outcome. Am J Clin Nutr. 1998;67:1003S–1011S. doi: 10.1093/ajcn/67.5.1003S. [DOI] [PubMed] [Google Scholar]
- 40.Lynch SM, Frei B, Morrow JD, Roberts LJ, 2nd, Xu A, Jackson T, Reyna R, Klevay LM, Vita JA, Keaney JF., Jr Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler Thromb Vasc Biol. 1997;17:2975–2981. doi: 10.1161/01.atv.17.11.2975. [DOI] [PubMed] [Google Scholar]
- 41.Thomas SR, Chen K, Keaney JF., Jr Oxidative stress and endothelial nitric oxide bioactivity. Antioxid Redox Signal. 2003;5:181–194. doi: 10.1089/152308603764816541. [DOI] [PubMed] [Google Scholar]
- 42.Landmesser U, Harrison DG, Drexler H. Oxidant stress-a major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur J Clin Pharmacol. 2006;62(Suppl 13):13–19. [Google Scholar]
- 43.Tomasian D, Keaney JF, Vita JA. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc Res. 2000;47:426–435. doi: 10.1016/s0008-6363(00)00103-6. [DOI] [PubMed] [Google Scholar]
- 44.Omar HA, Cherry PD, Mortelliti MP, Burke-Wolin T, Wolin MS. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res. 1991;69:601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- 45.Schuschke DA, Falcone JC, Saari JT, Fleming JT, Percival SS, Young SA, Pass JM, Miller FN. Endothelial cell calcium mobilization to acetylcholine is attenuated in copper-deficient rats. Endothelium. 2000;7:83–92. doi: 10.3109/10623320009072203. [DOI] [PubMed] [Google Scholar]
- 46.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 47.Karaa A, Kamoun WS, Clemens MG. Oxidative stress disrupts nitric oxide synthase activation in liver endothelial cells. Free Radic Biol Med. 2005;39:1320–1331. doi: 10.1016/j.freeradbiomed.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Shinozaki K, Kashiwagi A, Masada M, Okamura T. Stress and vascular responses: oxidative stress and endothelial dysfunction in the insulin-resistant state. J Pharmacol Sci. 2003;91:187–191. doi: 10.1254/jphs.91.187. [DOI] [PubMed] [Google Scholar]
- 49.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 50.Saari JT, Dahlen GM. Nitric oxide and cyclic GMP are elevated in the hearts of copper-deficient rats. Med Sci Res. 1998;26:495–497. [Google Scholar]
- 51.Saari JT, Wold LE, Duan J, Ren J, Carlson HL, Bode AM, Lentsch AB, Zeng H, Schuschke DA. Cardiac nitric oxide synthases are elevated in dietary copper deficiency. J Nutr Biochem. 2006 doi: 10.1016/j.jnutbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Krumenacker JS, Murad F. NO-cGMP signaling in development and stem cells. Mol Genet Metab. 2006;87:311–314. doi: 10.1016/j.ymgme.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Chen HW, Jiang WS, Tzeng CR. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil Steril. 2001;75:1163–1171. doi: 10.1016/s0015-0282(01)01780-0. [DOI] [PubMed] [Google Scholar]
- 54.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]