Abstract
The mahogany (mg) locus originally was identified as a recessive suppressor of agouti, a locus encoding a skin peptide that modifies coat color by antagonizing the melanocyte-stimulating hormone receptor or MC1-R. Certain dominant alleles of agouti cause an obesity syndrome when ectopic expression of the peptide aberrantly antagonizes the MC4-R, a related melanocyte-stimulating hormone receptor expressed in hypothalamic circuitry and involved in the regulation of feeding behavior and metabolism. Recent work has demonstrated that mg, when homozygous, blocks not only the ability of agouti to induce a yellow coat color when expressed in the skin of the lethal yellow mouse (AY), but also the obesity resulting from ectopic expression of agouti in the brain. Detailed analysis of mg/mg AY/a animals, presented here, demonstrates that mg/mg blocks the obesity, hyperinsulinemia, and increased linear growth induced by ectopic expression of the agouti peptide. Remarkably, however, mg/mg did not reduce hyperphagia in the AY/a mouse. Furthermore, mg/mg induced hyperphagia and an increase in basal metabolic rate in the C57BL/6J mouse in the absence of AY. Consequently, although mahogany is broadly required for agouti peptide action, it also appears to be involved in the control of metabolic rate and feeding behavior independent of its suppression of agouti.
The 131-aa agouti peptide is synthesized in the follicular cells of the dermal papilla and acts in a paracrine fashion on melanocytes where it antagonizes the binding of α-melanocyte-stimulating hormone (MSH) to melanocortin-1 receptor or MC1-R (1, 2). The transcription of the wild-type mouse agouti is temporally and spatially regulated, the gene being expressed solely in the skin during part of the hair-growth cycle where it gives rise to a subapical yellow pigment band in the growing hair shaft (3). Several dominant alleles at the mouse agouti locus (e.g., lethal yellow, AY, and viable yellow, AVY), however, have an ectopic pattern of expression caused by promoter gene rearrangement (4), and are associated phenotypically with yellow fur, late-onset obesity, hyperphagia, increased linear growth, and noninsulin-dependent diabetes (3, 4). Recent studies have provided an explanation for the development of the lethal yellow obesity syndrome. In vitro experiments using recombinant agouti protein in a human embryonic kidney 293 cell system have identified the peptide not only as a MC1-R antagonist but also as a high affinity, competitive antagonist of a melanocortin receptor subtype, MC4 (1), widely expressed in regions of the hypothalamus known to be involved in the regulation of feeding behavior and metabolism (5, 6). Genetic and pharmacological studies have concluded that disruption of MC4-R signaling is the primary cause of the AY-induced obesity syndrome (7, 8). The MC4-R knockout (MC4-R KO) mouse recapitulates all phenotypic aspects of the agouti obesity syndrome (8). Moreover, intracerebroventricular administration of a melanocortin analogue, MTII, a potent agonist of the MC3 and MC4 receptors, suppresses feeding behavior in rodents, whereas injection of SHU9119, an antagonist of the same receptors, has the opposite effect (7). The pharmacological and genetic evidence point out two major conclusions: first, neurons expressing MC4-R exert a tonic inhibition on feeding behavior, and second, development of the agouti obesity syndrome is caused by chronic antagonism of MC4-R signaling by agouti. The recent discovery in the hypothalamus of another antagonist of MC3 and MC4 receptors, the agouti-related protein (Agrp) (9), or agouti-related transcript (ART) (10), raises the intriguing possibility that ectopic expression of agouti mimics the function of endogenous Agrp in the central control of energy homeostasis. Overexpression of Agrp in the mouse animal model results in an obesity and diabetes phenotype very much like that seen in the AY and MC4-R KO mice (9, 11), further demonstrating that MC4-R, activated by α-MSH or α-MSH analogues and endogenously antagonized by Agrp, is an important modulator of metabolic state. Future studies will be required to determine whether the MC3-R also is involved in the control of energy homeostasis.
The murine mahogany (mg) and mahoganoid (md) loci were identified in the sixties as recessive suppressors of AY-induced pigmentation, which were able to shift melanogenesis from the phaeomelanin (red/yellow pigment) pathway toward eumelanin (black/brown pigment) production (12, 13). Early genetic studies positioned mg and md on chromosomes 2 and 16, respectively (14); however, several decades later the mg and md gene products remain unidentified. The murine mahogany locus is characterized by two mutations, mg, which originated in the LDJ/Le background, and mg3J in the C3HeB/FeJ background (15). The md coat color mutation originated in the C3H/HeJ genetic background (15). Recently, mg and md were found to suppress not only the yellow coat color but also the AY-induced increase in body weight (15), suggesting that they are required for agouti action both in the skin as well as in the brain. These studies also indicate that md and mg are partially semidominant, because both genes show some ability to suppress phaeomelanin synthesis as well as obesity in the AY mouse (md only) when heterozygous (15). Two possible mechanisms proposed for mg action by the recent studies include interference with agouti peptide processing or secretion, or disruption of its interaction with melanocortin receptors (15).
Although mg has been demonstrated to decrease weight gain in the AY/− mouse, no detailed analysis of the physiological effects of mg in the presence or absence of the AY gene has been published. Analysis of weight, body composition, linear growth, serum insulin, leptin, glucose, and corticosterone, feeding behavior, circadian rhythms, and basal metabolic rate (BMR) in mg/mg AY/a and normal mg/mg a/a mice is presented here. Data on the effect of md/md on feeding behavior also is presented.
MATERIALS AND METHODS
Mice.
To study the action of mg, AY, and ob genes in a uniform genetic background we recovered the mg mutation from the LDJ/Le background. All of our studies were performed on mice homozygous for the mg mutation backcrossed by one of us (M.L.L.) for 6–8 generations to the C57BL/6J background. The AY and ob alleles also were derived from congenic C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Animals homozygous for the md mutation and their controls originated in the C3H/HeJ background (Jackson Laboratories). Mice were housed as siblings, maintained at 23° ± 1°C on a 12-h/12-h light/dark cycle (7:00–19:00 h), and allowed free access to water and food (Purina, St. Louis, MO). All studies were conducted under guidelines provided by the Animal Care and Use Committee of Oregon Health Sciences University.
Body and Organ Weight.
To study the effect of mg/mg on AY-induced weight gain, C57BL/6J mice of four different genotypes (+/+ a/a, +/+ AY/a, mg/mg a/a, and mg/mg AY/a), were regularly weighed as littermates. For organ weight measurements different white fat pads (epididymal, visceral, and dorsal), brown fat, heart, and liver were dissected and weighed. Weight growth curves also were determined for +/+ a/a, +/+ ob/ob, mg/mg a/a, and mg/mg ob/ob mice.
Body Length Measurements.
Animals were lightly anesthetized with 2% avertin and extended to their maximal length to determine the nose-to-anus distance, a measure of their linear growth.
Food Intake.
For measurement of food consumption, animals of four genotypes (+/+ a/a, +/+ AY/a, mg/mg a/a, and mg/mg AY/a) were housed individually for at least 2 weeks before beginning the experiment. Preweighed standard chow pellets (4.5% fat) were provided to mice ad libitum in Petri dishes, and measurements of the remaining food were taken every 24 h for a 7-day period. To minimize food spillage mice were housed in cages with no bedding, and they were given only 2–3 food pellets at one time. The effect of md/md on food intake was analyzed by measuring weekly food consumption (9% fat diet) for md/md A/A and +/+ A/A controls. Data from both experiments are presented as g/mouse per day for comparative purposes.
Blood Analysis.
Animals were sacrificed by cervical dislocation between 16:00 and 18:00 h, and trunk blood was collected. Serum insulin and leptin concentrations were determined in duplicate by using radioimmunoassay kits with rat insulin and recombinant leptin, respectively, as standards (Linco Research Immunoassay, St. Charles, MO). For serum corticosterone measurements, blood was collected from the tail vein within 1 min of handling, and corticosterone levels were measured in the serum by using a radioimmunoassay kit (ICN). Blood glucose concentrations were measured by using a One-Touch Profile glucose meter (Lifescan, Milpitas, CA).
Motor Activity.
Animals were housed individually in metabolic cages equipped with a running wheel (Mini-Mitter, Sunriver, OR). The metabolic cages allowed telemetric monitoring of circadian rhythms as assessed via two physiological parameters: temperature and number of wheel turns. The wheel revolutions were quantified by recording the magnetic switch closures of a magnet placed on the revolving wheel. For temperature recordings mice were implanted with a radiotransmitter in the peritoneal cavity and allowed to recover for 1 week. Temperature was monitored by sampling series of digital pulses from the implanted transmitter.
BMR.
Oxygen consumption was simultaneously determined for multiple animals by indirect calorimetry using an Oxymax System (Columbus Instruments, Columbus, OH). For this purpose animals were housed in separate chambers maintained at 24° ± 1°C. Before beginning the experiment animals were acclimatized to the chambers and then measurements were taken for 4–5 h during the middle of the light cycle (11:00–16:00 h). Samples were recorded every 3 min with the room air reference taken every 30 min and the air flow to chambers 500 ml/min. Basal oxygen consumption was determined for individual curves as the average at the lowest plateau regions that corresponded to resting periods. Total oxygen consumption was the result of all samples recorded corresponding to periods of movement as well as inactivity.
Statistical Analysis.
All data are presented as mean ± SEM. Growth curves were analyzed by ANOVA with multiple measurements. All other statistical comparisons between different strains were performed by Student’s two-tailed t test with P < 0.05 considered significant.
RESULTS
mg Suppresses AY-Induced Obesity.
The main focus of our physiological characterization was to compare and contrast the phenotypes of mg/mg AY/a and +/+ AY/a mice, the first step being to determine which of the AY-mediated obesity parameters were suppressed by mg. We determined growth curves for +/+ AY/a, mg/mg AY/a, mg/mg a/a, and +/+ a/a female mice housed as siblings and maintained on a low-fat rodent chow (Fig. 1A). The weight gain of the animals was monitored regularly between 4 and 24 weeks of age. All weight curves, with the exception of mg/mg a/a versus +/+ a/a wild-type controls, were significantly different as analyzed by ANOVA with multiple measurements (P < 0.0001). As previously reported (16, 17), +/+ AY/a mice are characterized by a progressive increase in their body weight starting at approximately 2 months for female mice as compared with wild-type controls. By 6 months of age there is an approximate 24% increase in +/+ AY/a body weight versus mg/mg AY/a (P < 0.01), which indicates that mg is able to suppress the AY-induced weight gain. Similar data on the suppression of AY-induced weight increase by mg/mg were found in LDJ/Le/C57BL/6J hybrids and in non-littermates in the C57BL/6J background (15). In contrast, homozygosity of mg was not found to suppress the weight gain in the ob/ob mouse during the 20-week period studied (Fig. 1B). The apparent decrease in body weight of mg/mg ob/ob versus +/+ ob/ob seen at 16 weeks of age was caused by incomplete sampling of weights during this time period (n = 1–3), and was not significant, as can be seen by examination of the mean weights at 19 or 20 weeks (n = 6). To investigate whether the AY-induced gain in body weight is caused by an increase in body fat mass, various fat pads, heart, and liver from 23-week-old animals were dissected and measured. The results presented in Table 1 further show the absence of obesity in mg/mg AY/a mice. The weights of the different tissues we examined, with the exception of heart, were significantly decreased in mg/mg AY/a mice, presumably because of less triglyceride accumulation, and were equivalent to those found in wild-type controls (data not shown).
Figure 1.
(A) mg suppresses AY-induced weight gain. Data are collected from female animals (n = 4–5 for each genotype). (B) mg has no effect on ob-induced weight gain. Weight growth curves were determined for female mg/mg ob/ob (n = 6), mg/mg a/a (n = 8), +/+ ob/ob (n = 3), and +/+ a/a control mice (n = 8). All data are presented as mean ± SEM.
Table 1.
Body and organ weight measurements
| Genotype | Body weight | VF | EPI | DF | BAT | L | H |
|---|---|---|---|---|---|---|---|
| +/+ AY/a | 35.26 ± 1.72 | 0.87 ± 0.15 | 1.49 ± 0.14 | 0.85 ± 0.09 | 0.16 ± 0.02 | 1.55 ± 0.1 | 0.18 ± 0.01 |
| mg/mg AY/a | 26.99 ± 0.87 | 0.33 ± 0.06 | 0.27 ± 0.03 | 0.57 ± 0.05 | 0.08 ± 0.01 | 1.16 ± 0.1 | 0.23 ± 0.02 |
| P value | P < 0.05 | P < 0.05 | P < 0.001 | P < 0.05 | P < 0.05 | P < 0.05 | P > 0.05 |
mg suppresses AY-induced increase in adiposity. White fat pads: epididymal (EPI), visceral = mesenteric + retroperitoneal (VF), dorsal (DF), brown fat (BAT), heart (H), and liver (L) from 23-week-old male +/+ AY/a (n = 6) and mg/mg AY/a animals (n = 3) were dissected and weighed. All data are reported as mean ± SEM.
mg Suppresses AY-Induced Increased Linear Growth.
Body length (nose-to-anus distance) was determined for 23-week-old +/+ AY/a, mg/mg AY/a, and mg/mg a/a males, respectively. Our results indicate that +/+ AY/a are approximately 6.1% (P < 0.001) longer than mg/mg AY/a animals and 5.9% (P < 0.001) longer than mg/mg a/a age-matched controls (Fig. 2).
Figure 2.
mg suppresses AY-induced increased linear growth. Body length (naso-anal) measurements were taken from 24- to 28-week-old male +/+ AY/a (n = 4), mg/mg AY/a (n = 3), and mg/mg a/a animals (n = 3). Data are presented as mean ± SEM. ∗∗∗, P < 0.001.
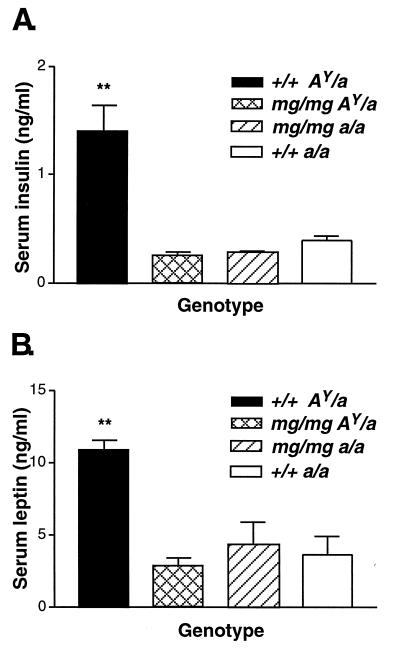
mg Blocks the Endocrine Effects of AY-Induced Obesity: Serum Insulin, Leptin, Glucose, and Corticosterone Measurements.
Blood was collected from 22-week-old +/+ AY/a, mg/mg AY/a, mg/mg a/a, and +/+ a/a male animals and assayed for insulin and leptin concentrations. Our analysis indicates that +/+ AY/a mice exhibit elevated insulin (Fig. 3A) and leptin (Fig. 3B) levels, a 5.6-fold (P < 0.01) and a 2.5-fold (P < 0.01) increase, respectively, over their mg/mg AY/a, or +/+ a/a counterparts. No significant differences were detected among the other groups. The high serum leptin and insulin concentrations found in +/+ AY/a animals are correlated with their progressive increase in body fat and lipid deposition. The AY–induced hyperglycemia also was found to be suppressed by mg as 26-week-old mg/mg AY/a male animals (n = 6) had blood glucose concentrations (114 ± 2.0 mg/dl) significantly lower (P = 0.0001) than +/+ AY/a (131 ± 3.0 mg/dl, n = 7). No differences in basal corticosterone levels were found between genotypes (3-month-old males, n = 5, data not shown).
Figure 3.
mg suppresses the endocrine effects of the agouti obesity syndrome: hyperinsulinemia and hyperleptinemia. (A) Serum insulin. (B) Serum leptin. Males (22 weeks old, n = 5 for each genotype) were used for serum insulin and leptin measurements. All data are presented as mean ± SEM. ∗∗, P < 0.01.
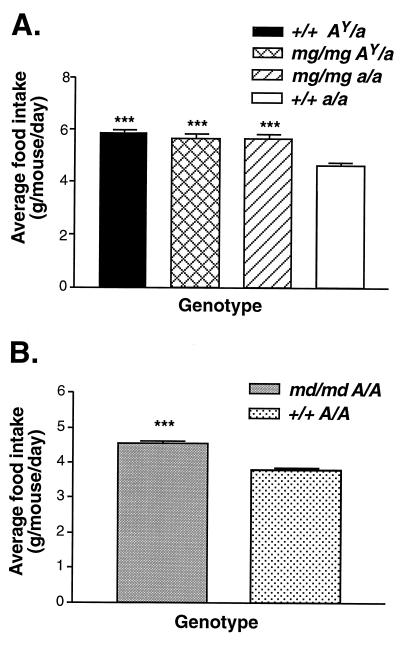
mg/mg AY/a Mice Remain Hyperphagic, and Both mg and md Independently Induce Hyperphagia.
The progressive weight gain and increase in adipose tissue that characterize +/+ AY/a animals result from both increased caloric intake and decreased energy expenditure. We decided therefore to look at both aspects of energy balance. The food consumption of 28-week-old females, representing the four genotypes: mg/mg AY/a, mg/mg a/a, +/+ AY/a, and +/+ a/a, was monitored over a 7-day period. In agreement with previous reports (8, 16), +/+ AY/a mice are hyperphagic, their average daily food intake in this assay being significantly higher (5.9 ± 0.1 g/24 h, P < 0.0001) than C57BL/6J controls (4.6 ± 0.2 g/24 h). Surprisingly, mg/mg did not appear to reduce AY-induced hyperphagia as we found no significant difference between +/+ AY/a and mg/mg AY/a caloric intake (Fig. 4A), despite a 24% difference in their body weight. Furthermore, mg/mg alone induced hyperphagia, the presence of homozygous mg in the absence of AY resulting in an approximate 22% increase in food intake (5.7 ± 0.2 g/24 h versus 4.6 ± 0.2 g/24 h, P < 0.0001) over C57BL/6J controls of equal weight. As it has previously been shown that the md locus encodes a phenocopy of mg (12, 15), we examined the effect of md/md on food intake as well. Homozygosity for md also resulted in hyperphagia (Fig. 4B).
Figure 4.
mg and md independently induce hyperphagia. (A) Food intake was measured daily over a 7-day period for 28-week-old females (n = 4–5 for each group). Data are reported as mean of seven measurements ± SEM. (B) Food consumption of md/md A/A males and +/+ A/A controls (n = 4 for each genotype) was monitored weekly between 6 and 16 weeks of age. Data are presented as average of 11 measurements ± SEM. ∗∗∗, P ≤ 0.0001.
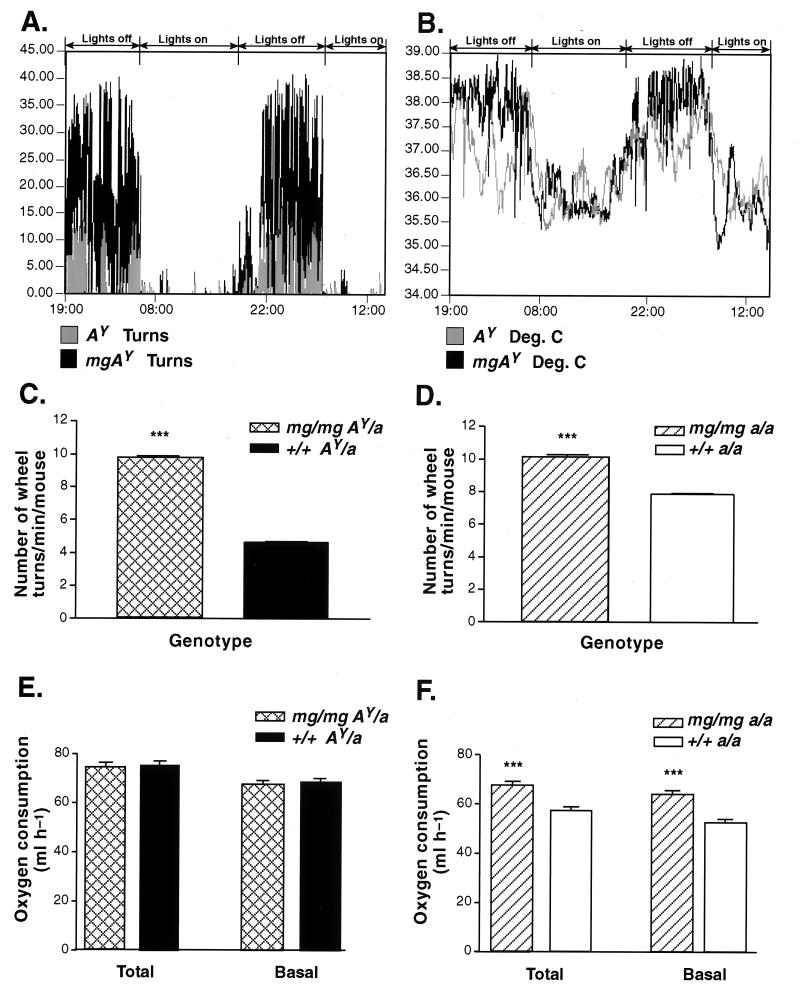
mg Increases Motor Activity and BMR.
In light of the finding that mg/mg mice were hyperphagic, it became essential to determine how these animals remained lean while consuming 22% more calories. Therefore, the two major components of daily energy expenditure, BMR and energy expenditure caused by physical activity, were determined as a function of genotype. Activity on an exercise wheel was measured in mg/mg AY/a and +/+ AY/a, as well as in mg/mg a/a versus +/+ a/a mice. mg/mg AY/a animals showed increased nighttime activity, and a corresponding 0.5°C gain in body temperature correlating with the increased exercise (Fig. 5 A and B, representative 48-h traces). During nighttime mg/mg AY/a exhibited an approximate 2-fold increase (P < 0.0001) in the number of wheel turns over +/+ AY/a animals (Fig. 5C), whereas mg/mg a/a showed a 30% increase in their motor activity (P < 0.0001) when compared with wild-type mice (Fig. 5D). We concluded that presence of the mg gene was sufficient to augment physical activity and therefore caloric usage during nighttime. We further analyzed BMR in 20- to 28-week-old animals. At the time of our experiment +/+ AY/a mice were significantly heavier than their age-matched mg/mg AY/a complements (39.7 ± 2.3 g versus 29.1 ± 1.6 g, P < 0.01). On a per-animal basis, no significant difference in oxygen consumption could be detected when comparing the +/+ AY/a and mg/mg AY/a mice (Fig. 5E). Surprisingly, mg/mg a/a animals (27.4 ± 0.9 g) showed an approximate 21% gain (P < 0.0001) in their BMR over weight-matched wild-type controls (26.2 ± 0.4 g, Fig. 5F). Our results indicate therefore that mg/mg significantly elevates BMR in the normal C57BL/6J animal.
Figure 5.
mg increases motor activity and basal metabolic rate. (A) Circadian rhythm of wheel activity. (B) Circadian rhythm of body temperature. Measurements were taken every 1 min during a 48-h time period for 24- to 28-week-old mg/mg AY/a and +/+ AY/a animals (representative traces from 48 h of recording, two mice/genotype). The regions of increased activity or temperature correspond to nighttime whereas no or low activity is seen during daytime. (C and D) Quantitative comparison of average physical activity (wheel turns) as measured during nighttime in 20- to 28-week-old mg/mg AY/a (n = 7) and +/+ AY/a animals (n = 9) (C) and in 26- to 34-week-old mg/mg a/a (n = 5) versus +/+ a/a wild-type animals (n = 5) (D). (E) Oxygen consumption of 20- to 28-week-old mg/mg AY/a and +/+ AY/a mice (n = 8 for each group) as measured by indirect calorimetry. (F) Oxygen consumption of 20- to 28-week-old mg/mg a/a versus +/+ a/a wild-type animals as determined by indirect calorimetry (n = 11 for each genotype). Both male and female animals were included in all measurements. Data in C–F are reported as mean ± SEM. ∗∗∗, P < 0.0001.
DISCUSSION
Data presented here demonstrate that, with one very important exception (hyperphagia), homozygous mg suppresses the phenotypes of the lethal yellow obesity syndrome: adult-onset obesity, hyperinsulinemia, hyperleptinemia, hyperglycemia, and increased linear growth. The results show that mg/mg AY/a mice have a normal body size and adiposity, and their insulin and leptin levels are comparable to wild-type values. The ability of mg/mg to suppress the centrally mediated effects of agouti on feeding and endocrine parameters, as well as the peripherally mediated inhibition of eumelanin synthesis by agouti in the melanocyte, suggests that the mg gene product is expressed and functions in both brain and skin. In the AY mouse, in which the skin agouti peptide is ubiquitously expressed, mg is clearly required for agouti function in the hypothalamus. In the C57BL/6J animal, a potential target for mg action in the brain, where agouti is not normally expressed, could be the recently discovered hypothalamic Agrp. Furthermore, it is noteworthy that according to results presented here the effects of mg and md gene products are not limited to agouti and/or Agrp, because inhibition of the function of either of these peptides would not be expected to lead to hyperphagia, but precisely the opposite. Thus, the ability of homozygous mg to induce hyperphagia implies pathways of action of the mg gene that are independent of agouti and Agrp. Because hyperphagia in the mg/mg AY/a mice could be mg or AY-driven, it cannot be determined from the data presented here whether mg actually suppresses AY-induced hyperphagia and concurrently induces hyperphagia via a different pathway. Interestingly, the inability of mg/mg to suppress obesity in the ob/ob mouse indicates that the effectors dependent on mg do not include all peptide regulators of energy homeostasis, because if this gene was required for neuropeptide Y action (18) it might be expected to ameliorate the obesity resulting from leptin deficiency.
The analysis of the mg phenotype demonstrates that homozygous mg increases BMR as well as physical activity in the C57BL/6J animal. In the AY/a mouse mg/mg also elevates physical activity; however, an increase in BMR is not detected on a per-animal basis. BMR is the major component of daily energy expenditure, accounting for up to 70% of the caloric utilization. The factors that regulate BMR include hormonal stimuli (i.e., thyroid hormone) and the sympathetic nervous system. These modulators remain to be investigated in the mg animal. The phenotype of hyperphagia present in the mg/mg mouse potentially could be secondary to elevation of its metabolic rate in an attempt to overcome a negative energy balance and maintain body weight homeostasis. This suggestion is supported by the preliminary observation that mg animals lose significantly more body weight than their counterparts after a 24-h fast (mg/mg a/a: 4.62 ± 0.29 g versus +/+ a/a: 3.38 ± 0.18 g, P < 0.01, n = 4–5). It is interesting that mice homozygous for the md mutation in the C3H/HeJ background display the same hyperphagia phenotype seen by us in C57BL/6J-mg/mg animals, demonstrating that both agouti suppressors can alter feeding behavior independent of background strain.
Based on the analysis of energy intake and expenditure in mg/mg animals we conclude that the pathways in which mg functions are largely overlapping with proopiomelanocortin-agouti-Agrp melanocortinergic neurocircuits, but also appear to include independent pathways regulating metabolic rate and feeding behavior.
Acknowledgments
We thank Ximena Opitz-Araya and Dee Horne for technical support. This work was funded by grants to R.D.C. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 51730) and M.L.L. (National Institutes of Health EY-10223).
ABBREVIATIONS
- mg
mahogany
- AY
lethal yellow
- Agrp
agouti-related protein
- md
mahoganoid
- BMR
basal metabolic rate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Lu D, Willard D, Patel I R, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik R P, Wilkison W O, et al. Nature (London) 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y K, Ollmann M M, Wilson B D, Dickinson C, Yamada T, Barsh G S, Gantz I. Mol Endocrinol. 1997;11:274–280. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- 3.Bultman S J, Michaud E J, Woychik R P. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 4.Michaud E J, Bultman S J, Klebig M L, van Vugt M J, Stubbs L J, Russell L B, Woychik R P. Proc Natl Acad Sci USA. 1994;91:2562–2566. doi: 10.1073/pnas.91.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson S J, DelValle J, Yamada T. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 6.Mountjoy K G, Mortrud M T, Low M J, Simerly R B, Cone R D. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 7.Fan W, Boston B A, Kesterson R A, Hruby V J, Cone R D. Nature (London) 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 8.Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Fang Q, Berkemeier L R, Gu W, Kesterson R A, Boston B A, Cone R D, et al. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 9.Ollmann M M, Wilson B D, Yang Y, Kerns J, Chen Y, Gantz I, Barsh G S. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 10.Shutter J R, Graham M, Kinsey A C, Scully S, Luthy R, Stark K L. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 11.Graham M, Shutter J R, Sarmiento U, Sarosi I, Stark K L. Nat Genet. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- 12.Lane P W. Mouse News Lett. 1960;22:35. [Google Scholar]
- 13.Lane P W, Green M C. J Hered. 1960;51:228–230. [Google Scholar]
- 14.Green M C. In: Genetic Variants and Strains of the Laboratory Mouse. Lyon M F, Searle A G, editors. Oxford: Oxford Univ. Press; 1989. pp. 12–403. [Google Scholar]
- 15.Miller K A, Gunn T M, Carrasquillo M M, Lamoreux M L, Galbraith D B, Barsh G S. Genetics. 1997;146:1407–1415. doi: 10.1093/genetics/146.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickerson G E, Gowen J W. Science. 1947;105:496–498. doi: 10.1126/science.105.2732.496-a. [DOI] [PubMed] [Google Scholar]
- 17.Fenton P F, Chase H B. Proc Soc Exp Biol. 1951;77:420–422. doi: 10.3181/00379727-77-18800. [DOI] [PubMed] [Google Scholar]
- 18.Erickson J C, Hollopeter G, Palmiter R D. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]