Abstract
Background
Chronic myocardial ischemia induces endothelial dysfunction in the coronary microcirculation resulting in impaired nitric oxide signaling. This dysfunction has wide ranging effects including impaired tissue perfusion and is implicated in impairment of the angiogenic process in settings of endothelial dysfunction. We hypothesized chronic myocardial ischemia results in increased activity of Arginase I, diminishing bioavailability of L-arginine, the substrate for endothelial nitric oxide production.
Methods
Chronic myocardial ischemia was induced for 7-weeks in 6 Yucatan miniswine utilizing an ameroid constrictor placed around the left circumflex coronary artery. Ischemic and non-ischemic tissue was harvested at the 7-week timepoint. Expression of Arginase I, eNOS, and phospho-eNOS was assessed utilizing western blotting. Arginase activity was measured. Immunofluorescent staining assessed expression of Arginase I between ischemic and non-ischemic microvessels. Coronary microvascular relaxation studies were performed.
Results
Arginase I expression, activity, and staining was similar between ischemic and non-ischemic territories. Significant impairments in coronary microvascular relaxation were observed in microvessels from the ischemic territory in response to endothelial dependent agents, but remained similar between territories in response to endothelial independent agents. Regression analysis between arginase activity and degree of microvascular vasorelaxation demonstrated no significant correlation.
Conclusions
Coronary microvascular dysfunction in the setting of chronic myocardial ischemia occurs independently of Arginase I activity and expression. Alternative therapeutic strategies focusing away from arginine may be need for the treatment of this dysfunction.
Keywords: coronary, endothelial dysfunction, angiogenesis, microvascular dysfunction, arteriolar dysfunction, arginase, myocardial ischemia, coronary artery disease, arginine
Introduction
Ischemic heart disease (IHD) remains the leading cause of mortality in the United States. Key to the pathogenesis of IHD is endothelial dysfunction in the coronary microvessels, which consist of arterioles (diameter < 150 μm) functioning to match myocardial perfusion with oxygen consumption(Camici and Crea, 2007), and which play an essential role in the metabolic regulation of coronary blood flow(Lupi et al., 1997). Within this microcirculation, the endothelium plays a key role in the regulation of vascular tone by releasing nitric oxide (NO), and clinical studies have demonstrated endothelial dysfunction appears to have detrimental functional consequences such as impaired tissue perfusion, particularly during stress, contributing to myocardial ischemia(Drexler and Hornig, 1999). In addition to the immediate perfusion impairments observed, endothelial dysfunction and the resulting impairment in NO signaling in the coronary microcirculation may be central to diminished myocardial angiogenic responses, a long-term adaptation to chronic myocardial ischemia(Boodhwani et al., 2006b).
Given the importance of coronary microvascular function in acute control of myocardial perfusion, and long-term adaptation to impairments in perfusion, recent studies have attempted to elucidate the molecular mechanisms underlying this process. Although commonly seen in the global setting of IHD(Drexler and Hornig, 1999), coronary microvascular dysfunction can also occur in the setting of individual disease states such as hyperlipidemia, hypertension, and diabetes(Camici and Crea, 2007), as well in the setting of chronic myocardial ischemia alone(Sellke et al., 1990; Sellke et al., 1994; Sellke et al., 1996). Recent studies have investigated this entity in the setting of hypertension(Zhang et al., 2004), oxidative stress(Thengchaisri et al., 2006), and acute ischemia - reperfusion injury(Hein et al., 2003). These studies focused on the role of Arginase I, an enzyme expressed in the coronary microvasculature which can compete with nitric oxide synthase (NOS) for use of L-arginine as a substrate(Zhang et al., 2001) resulting in the generation of urea and ornithine, instead of NO and L-citrulline as occurs during NOS mediated metabolism of L-arginine (Figure 1). Strikingly, all three of the aforementioned studies(Hein et al., 2003; Thengchaisri et al., 2006; Zhang et al., 2004), found the coronary microvascular dysfunction observed in these pathologic states was associated with increases in Arginase I expression and activity leading to diminished bioavailability of NO. These studies seemed to be in agreement with large animal and clinical studies which have demonstrated improved endothelial function and angiogenic responses to myocardial ischemia after oral supplementation of L-arginine(Lerman et al., 1998; Nakai et al., 2005; Quyyumi et al., 1997; Voisine et al., 2005a; Voisine et al., 2005b).
Figure 1.
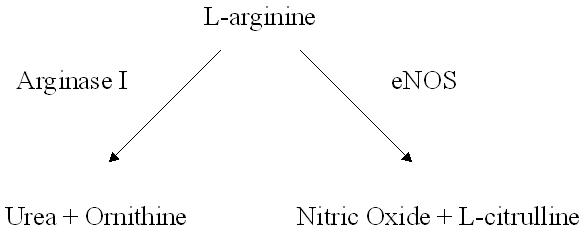
Endothelial metabolism of L-arginine. Arginase I and eNOS are both capable of metabolizing L-arginine in to urea and ornithine, or nitric oxide and L-citrulline, respectively.
It therefore maybe possible that up-regulation of Arginase I and its competition with NOS for their common substrate L-arginine may be involved in the microvascular dysfunction induced by chronic myocardial ischemia, as NO released from the endothelium plays a major role in the determination of coronary microvascular reactivity(Kuo et al., 1992) and in the angiogenic process(Sellke et al., 1996). Thus, given the importance of Arginase I in the coronary microvascular dysfunction observed in other pathologic conditions which share endothelial dysfunction as a common entity, we hypothesized chronic myocardial ischemia would result in increased expression and activity of Arginase I, with a concomitant impairment endothelial-dependent, NO mediated coronary microvascular relaxation.
Material & Methods
Animal Protocol
Six Yucatan miniswine were utilized for the study. For all surgical procedures, anesthesia was induced with ketamine (10 mg/kg intramuscularly), thiopental (5 to10 mg/kg intravenously), and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5 to 2 L/min and isoflurane at 0.75% to 3.0%. The animals were intubated and mechanically ventilated at 12 to 20 breaths per minute. During the first procedure, the pericardium was opened through a left minithoracotomy, and a ferromagnetic ameroid constrictor (1.75 mm in internal diameter) was placed around the proximal left circumflex coronary artery. The ameroid constrictor consists of a hygroscopic core with a central open lumen surrounded by a rigid steel ring. The hygroscopic core gradually enlarges inward resulting in vessel occlusion over the course of 3 weeks to produce the onset of gradual, chronic ischemia(Hughes et al., 2003). The ameroid model is one of the most frequently utilized methods for induction of chronic myocardial ischemia for pre-clinical evaluation of angiogenic therapy(Lopez et al., 1998) (Laham et al., 1998) (Horvath et al., 1999) (Lamping et al., 2003) as it has been shown to reliably produce gradual onset chronic ischemia and endothelial dysfunction as seen in patients with cardiovascular disease. Three weeks after ameroid placement, the animals were anesthetized, and coronary angiography was performed through an 8F sheath (Cordis, Miami, FL) introduced into the right femoral artery using a catheter with the appropriate distal angulation. High atomic weight contrast was injected after selective cannulation of the right and left coronary ostia to confirm occlusion of the circumflex artery by the ameroid constrictor. Seven weeks after ameroid placement, the animals were again anesthetized and the heart exposed through a median sternotomy, and euthanasia was performed with injections of saturated potassium chloride solution. The heart was harvested, and two 1-cm thick transversal slices were cut at the midventricular level, and then sectioned into eight segments identified clockwise starting from the anterior junction of the right and left ventricles. Samples from the anterior and left lateral walls were divided and rapidly frozen in liquid nitrogen (molecular studies), and kept in 4°C Krebs solution (microvessel reactivity studies) or fixed in 10% buffered formalin (histologic studies).
To document ischemia in the circumflex territory distal to the ameroid occluder, coronary angiography (as described above) and isotope labeled microspheres (ILM) (BioPAL, Worcester, MA) using methods previously reported (Boodhwani et al., 2006a). were utilized. Isotope-labeled microspheres, 15 μm in diameter, of different isotopic mass were used at each experimental stage. Gold-labeled microspheres (1.5×107 microspheres) were injected during temporary circumflex occlusion at the time of ameroid placement to identify myocardial samples that originated from the circumflex coronary distribution (those with the lowest count of gold-labeled microspheres). Lutetium (1.5×107 microspheres) ILM were used during the second procedure to determine baseline blood flow. Samarium (1.5×107 microspheres) ILM were injected at during the third procedure. Reference blood samples were obtained from the femoral artery during the second and third procedures. Following euthanasia, ten circumferential, transmural left ventricular sections were collected for ILM assays in each animal, weighed, and dried at 60°C for 24 hours. Each sample was exposed to neutron beams and microsphere densities were measured in a gamma counter. Myocardial tissue counts of the ILMs were then compared between LAD and Cx samples to quantify the perfusion deficit in the Cx territory relative to the LAD territory.
Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and “Guide for the Care and Use of Laboratory Animals” (NIH publication 5377-3, 1996).
Microvessel Dissection / Isolation
After cardiac harvest, myocardial specimens from the non-ischemic LAD territory and the ischemic Cx territory were immersed in 4°C Krebs solution and coronary arterioles (80 to130 μm in diameter and 1 to 2 mm in length) originating from branches of the left anterior descending (LAD) non-ischemic territory, and the left circumflex (Cx) ischemic territory were dissected sharply from the surrounding tissue with a 40x magnification dissecting microscope.
Microvessel Reactivity Studies
Microvessels were mounted and examined in isolated organ chambers, as described previously(Tofukuji et al., 1998). The responses to sodium nitroprusside (SNP) (1 nM to 100 μM), an endothelium-independent cGMP-mediated vasodilator, as well as adenosine 5′-diphosphate (ADP) (1 nM to 10 μM), substance P (1 fM to 1 nM), and Vascular Endothelial Growth Factor (VEGF) (1 fM to 1 nM), three endothelium-dependent receptor-mediated vasodilators that act via bioavailable NO, were studied after pre-contraction by 20-50% of the baseline diameter with the thromboxane A2 analog U46619 (0.1-1 μM). Additionally, microvascular responses to ADP were assessed in the presense of N (G)-nitro-L- arginine methyl ester (L-NAME) (10 μM), a nitric oxide synthase inhibitor. Relaxation responses were defined as the percent relaxation of the pre-contracted diameter and 6-8 vessels were examined in each group from the LAD (non-ischemic) and the circumflex (ischemic) territories.
Western Blotting
Whole-cell lysates were isolated from mechanically homogenized samples with a RIPA buffer (Boston Bioproducts, Worcester, MA) and centrifuged at 12,000g for 10 minutes at 4°C to separate soluble from insoluble fractions. Protein concentration was measured spectrophotometrically at a 595-nm wavelength with a DC protein assay kit (Bio-Rad, Hercules, CA). Ten micrograms of total protein were fractionated by 4% to 20% gradient, sodium dodecyl sulfate polyacrylamide gel electrophoresis (Invitrogen, San Diego, CA) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Each membrane was incubated with specific antibodies as follows: anti-arginase I antibody diluted to 1:500 (BD Biosciences, San Jose, CA), anti-endothelial nitric oxide synthase (eNOS) antibody diluted to 1:2500 (BD Biosciences, San Jose, CA), and anti-phospho-eNOS antibody diluted to 1:300 (Cell Signaling, Danvers, MA). The membranes were subsequently incubated for 1 hour in diluted appropriate secondary antibody (Jackson Immunolab, West Grove, PA). Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Bands were quantified by densitometry of radioautograph films.
Arginase Activity Assay
The arginase activity assay was performed based on the protocol described by Zhang et. al.(Zhang et al., 2001{Zhang, 2004 #4). Briefly, lysate (50 μL) was added into 75 μL of Tris-HCl (50 mmol/L, pH 7.5) containing 10 mmol/L MnCl2. Heating the lysate at 55°C to 60°C for 10 minutes was used to activate arginase. The hydrolysis reaction of L-arginine by arginase was performed by incubating the mixture containing activated arginase with 50 μL of L-arginine (0.5 mol/L, pH 9.7) at 37°C for 1 hour and was stopped by adding 400 μL of the acid solution mixture (H2SO4:H3PO4:H2O=1:3:7). For calorimetric determination of urea, α-isonitrosopropiophenone (25 μL, 9% in absolute ethanol) was then added and the mixture was heated at 100°C for 45 minutes. After placing the sample in the dark for 10 minutes at room temperature, the urea concentration was determined spectrophotometrically by the absorbance at 550 nm measured with a microplate reader. The amount of urea produced, after normalization with protein, and baseline tissue urea concentration, was used as an index for arginase activity.
Urea Assay
Lysates were prepared as described above. Urea concentration was assayed utilizing the Quantichrom Urea Assay Kit (BioAssay Systems, Hayward, CA) following the manufacturer’s suggested protocol. The assay, based on the Jung method(Jung et al., 1975), utilizes a chromogenic reagent which forms a colored complex specifically with urea, the intensity of which was quantified spectrophotometrically at 520nm. Urea concentration was calculated against urea standard provided by the manufacturer.
Immunofluorescent Staining
Myocardial sample slides were obtained from 4 μm paraffin sections. Slides were deparaffinized using sequential baths in xylene, 100% ethanol, 50% ethanol, and purified water. Slides were subsequently boiled in 10mM sodium citrate pH 6.0 for 10 minutes to facilitate antigen exposure. After washing with PBS, blocking was performed with a 2% bovine serum albumin solution. Sections were then incubated with primary antibody against Arginase I diluted to 1:40 (BD Biosciences, San Jose, CA), or Platelet/Endothelial Cell Adhesion Molecule 1 (PECAM) / CD31 diluted to 1:500 (BD Biosciences, San Jose, CA) in 0.2% BSA in PBS overnight at 4° C. Sections were then washed again with PBS, followed by incubation with appropriate Alexa secondary antibody and incubated for 60 minutes. Sections were again washed followed by mounting with DAPI and analysis. Tissue was visualized using a Biorad confocal microscope system (Hercules, CA). Labeling of the tissues with either secondary alone or primary incubation with normal rabbit IgG or rabbit serum was used as a negative control.
Regression Analysis
Linear regression analysis utilizing Pearson’s correlation coefficient was performed to determine if an association existed between arginase activity and coronary microvascular relaxation to the endothelium dependent, NO mediated agents.
Data Analysis
Data are reported as means ± SEM. Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using two-way, repeated measures analysis of variance examining the relationship between vessel relaxation, log concentration of the vasoactive agent of interest, and the experimental group (SAS Version 9.1, Cary, NC). Immunoblots are expressed as a ratio of protein to loading band density and were analyzed after digitization and quantification of x-ray films with ImageJ 1.33 (National Institutes of Health, USA). Western blot and enzyme activity data were analyzed using an unpaired t-test. Probability values of less than 0.05 were considered statistically significant.
Results
Experimental Model
All animals survived the duration of the experimental protocol (7 weeks). Complete occlusion of the left circumflex coronary artery with absence of distal filling in all animals was confirmed at the 3- week time point utilizing coronary angiography.
Myocardial Perufsion
Myocardial perfusion assays utilizing ILMs documented a 2.35-fold decrease in myocardial perfusion at 3 weeks (LAD 241900 ± 11540 vs. Cx 103600 ± 11510 dpm, p<0.001*, (Figure 2A)) and a 2.1 fold decrease in perfusion at 7 weeks (LAD 460700 ± 81100 vs. Cx 221000 ± 16270 dpm, p=0.027*, (Figure 2B)) post-ameroid insertion confirming induction of significant chronic myocardial ischemia.
Figure 2.
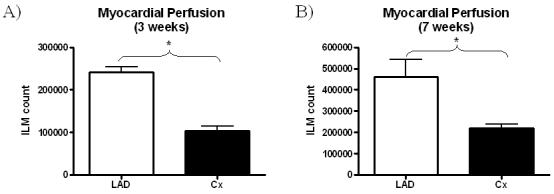
Myocardial perfusion between the LAD and Cx territories at A) the 3 week time-point, and B) the 7 week time-point. Perfusion assays were utilized to confirm induction of ischemia in the circumflex territory.
Western Blotting
Expression of Arginase I in the non-ischemic LAD territory and the ischemic Cx territory remained similar (LAD 66.8 ± 7.9 DU vs. Cx 69.5 ± 7.3 DU, p=0.81, Figure 3. eNOS expression also remained similar between territories (LAD 23.7 ± 1.3 DU vs. Cx 24.2 ± 2.2 DU, p=0.85) (Figure 4A), as did expression of phospho-eNOS (LAD 25.5 ± 1.1 DU vs. 24.3 ± 2.7 DU, p=0.70) (Figure 4B).
Figure 3.
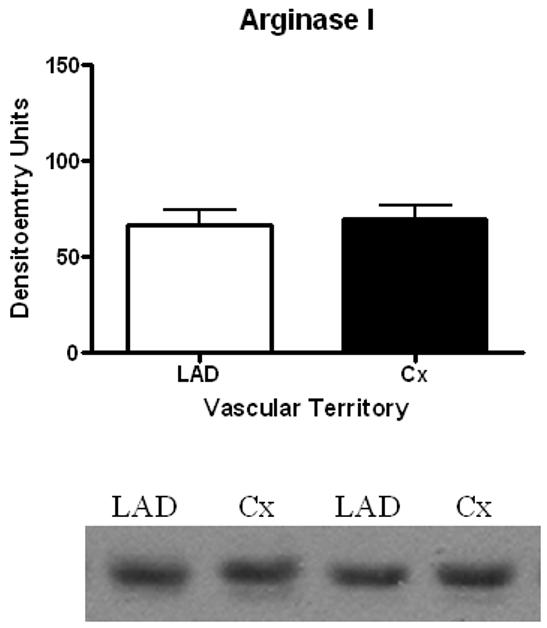
Arginase I Protein Expression. No significant difference was observed in Arginase I expression in ischemic (Cx) vs. non-ischemic (LAD) territories.
Figure 4.
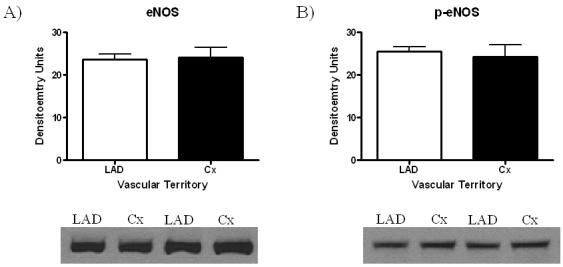
eNOS Protein Expression. No significant difference was observed in (A) eNOS expression, or (B) phospho-eNOS expression in ischemic (Cx) vs. non-ischemic (LAD) territories.
Arginase Activity
Overall arginase activity, as determined by the production of urea, remained similar between ischemic and non-ischemic territories with LAD territory arginase activity resulting in 1661 ± 155.9 mg urea / mg protein and Cx territory arginase activity resulting in 1566 ± 245.7 mg urea / mg protein (p=0.75) (Figure 5A). Baseline urea concentration was similar between samples (LAD 1.79 ± 0.19 mg/dL vs. Cx 1.67 ± 0.14 mg/dL, p=0.66), Figure 6. Similar results for arginase activity were obtained after adjustment for baseline urea concentration in both territories (LAD 0.083 ± 0.011 mg urea / mg protein vs. Cx 0.079 ± 0.016 mg urea / mg protein, p=0.84), (Figure 5B).
Figure 5.
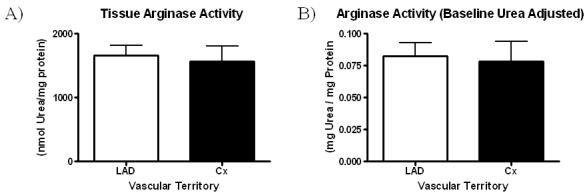
A) Arginase activity in the non-ischemic (LAD) and ischemic (Cx) territory. B) Baseline urea concentration adjusted Arginase activity.
Figure 6.
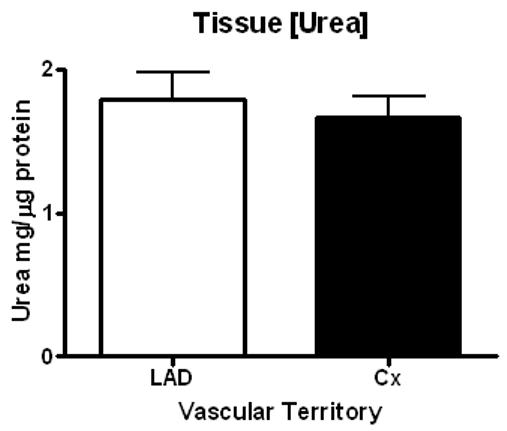
Baseline tissue urea concentration.
Immunofluorescent Staining
Immunofluorescent staining demonstrates localization of Arginase I to the endothelium as demonstrated by anti-PECAM / CD31 staining, consistent with prior reports(Zhang et al., 2001), Figure 7 & 8. No qualitative differences were observed between immunofluorescent staining for Arginase I between the LAD and Cx territories, Figure 9.
Figure 7.
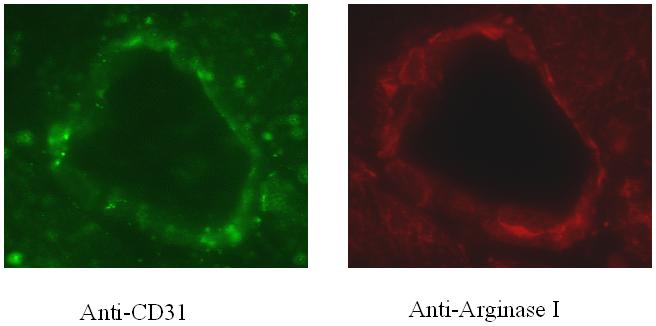
Immunofluorescent staining of coronary microvessels from the non-ischemic LAD territory for A) CD31 for the endothelium, B) Arginase I.
Figure 8.
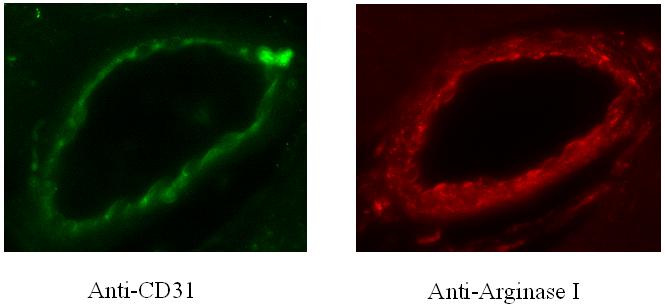
Immunofluorescent staining of coronary microvessels from the ischemic Cx territory are shown in A) CD31 for the endothelium, and B) Arginase I.
Figure 9.
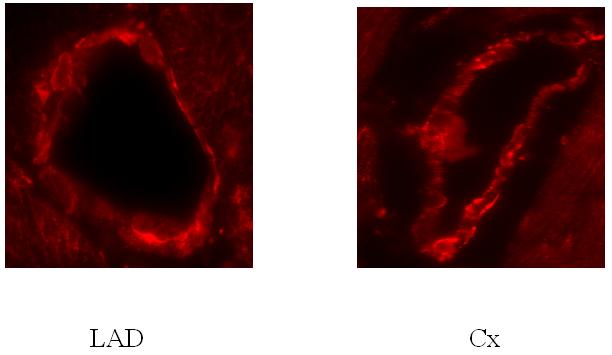
Comparison on ischemic (Cx) and non-ischemic (LAD) microvessel expression of Arginase I with immunofluorescent staining. No qualitative differences in staining intensity were observed.
Coronary Microvascular Reactivity
Microvascular vasorelaxation was significantly impaired in the ischemic circumflex territory in response to ADP (Figure 10A) and Substance P (Figure 10B), both endothelium-dependent, NO mediated agents. With the addition of L-NAME, microvascular relaxation to ADP was markedly reduced in both LAD and Cx territory microvessels (Figure 10A). Vasorelaxation in response to VEGF (Figure 10C), a partially endothelium-dependent, NO mediated agent was enhanced in the ischemic circumflex territory. Vasorelaxation responses to SNP (Figure 10D), and endothelial-independent agent acting primarily on the smooth muscle remained similar in both ischemic and non-ischemic territories, indicating bioavailable NO may be reduced in the ischemic territory.
Figure 10.
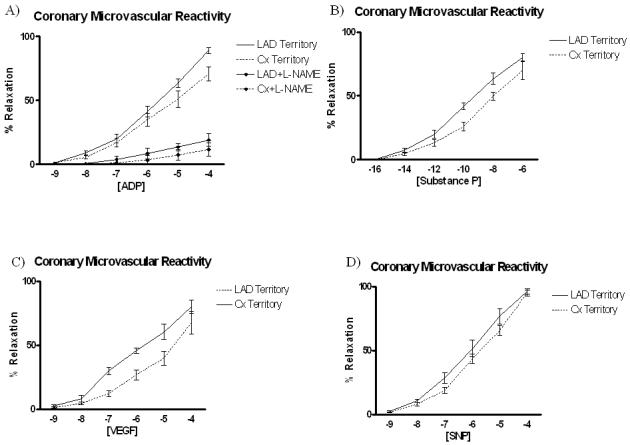
Coronary microvascular relaxation in response to A) ADP and ADP+ L-NAME, a nitric oxide synthase inhibitor B) Substance P, C) VEGF, and D) SNP. Microvascular relaxation was significantly impaired in the ischemic territory in response to the endothelial dependent / NO-mediated agents ADP and Substance P, whereas responses to SNP, an endothelial-independent agent remained similar between ischemic and non-ischemic territories.
Regression Analysis
A linear regression analysis was performed utilizing individual levels of arginase activity vs. their corresponding degrees of microvascular relaxation to the endothelium dependent vasodilators ADP, Substance P, and VEGF at four different concentrations. Statistically significant correlations were observed relaxation in response to ADP (10-4) in the LAD territory (R=0.855, p=0.03*) and in response to VEGF (10-5) in the Cx territory (R=0.820, p=0.046*). The remainder of the correlations demonstrated no significant association between arginase activity and microvascular relaxation. See Table 1.
Table 1.
Linear Regression Analysis of Coronary Microvascular Relaxation vs. Arginase Activity
| Territory | Agent | R2 | R | p-value |
|---|---|---|---|---|
| LAD | ADP-7 | 0.0338 | 0.184 | 0.7271 |
| ADP-6 | 0.0152 | 0.123 | 0.8164 | |
| ADP-5 | 0.0198 | 0.141 | 0.7899 | |
| ADP-4 | 0.7303 | 0.855 | 0.03* | |
| Cx | ADP-7 | 0.0513 | 0.227 | 0.6653 |
| ADP-6 | 0.0257 | 0.160 | 0.762 | |
| ADP-5 | 0.2371 | 0.487 | 0.3273 | |
| ADP-4 | 0.0578 | 0.240 | 0.6469 | |
| LAD | VEGF-7 | 0.1321 | 0.363 | 0.4794 |
| VEGF-6 | 0.0059 | 0.077 | 0.8847 | |
| VEGF-5 | 0.0024 | 0.049 | 0.9266 | |
| VEGF-4 | 0.2292 | 0.479 | 0.3365 | |
| Cx | VEGF-7 | 0.0941 | 0.307 | 0.554 |
| VEGF-6 | 0.2284 | 0.478 | 0.3376 | |
| VEGF-5 | 0.6719 | 0.820 | 0.0457* | |
| VEGF-4 | 0.2316 | 0.481 | 0.3341 | |
| LAD | Sub P -12 | 0.1935 | 0.440 | 0.3826 |
| Sub P -10 | 0.1862 | 0.432 | 0.3923 | |
| Sub P -8 | 0.3474 | 0.590 | 0.2177 | |
| Sub P -6 | 0.1074 | 0.328 | 0.5256 | |
| Cx | Sub P -12 | 0.3364 | 0.580 | 0.2276 |
| Sub P -10 | 0.0991 | 0.315 | 0.5431 | |
| Sub P -8 | 0.0158 | 0.126 | 0.812 | |
| Sub P -6 | 0.0841 | 0.290 | 0.5772 |
Discussion
The findings of this study demonstrate chronic myocardial ischemia results in impairment of endothelial mediated nitric oxide signaling in the coronary microcirculation, but endothelial-independent signaling for vasorelaxation in the microvasculature is relatively preserved in this setting, indicating bioavailable NO may be reduced in the ischemic territory. The diminished NO signaling observed does not appear to be secondary to increases in competition for L-arginine metabolism by Arginase I, nor is there evidence it is secondary to down-regulation of enzymes responsible for NO production - eNOS / phospho-eNOS, thus it is likely that impaired NO signaling in chronic myocardial ischemia is not dependent upon the balance between arginase I and eNOS. Downstream signaling pathways in the smooth muscle appear to be relatively intact as vasorelaxation in response to an NO donor acting beyond the endothelium (sodium nitroprusside) remained similar between ischemic and non-ischemic microvessels. Interestingly, VEGF induced vasorelaxation, which is only partially endothelial dependent, was actually improved in the setting of chronic ischemia, indicating that impairments in endothelial signaling may have been compensated for through alternate endothelial independent signaling pathways for VEGF. Taken in sum, these findings suggest the signaling derangement induced by chronic ischemia may be at the level of impaired NO production or increased NO degradation. Increased degradation of NO may occur in response to elevations in oxidative stress as can be seen in chronically ischemic tissue(Aikawa et al., 2004; Rossi et al., 2004; Valgimigli et al., 2003). These elevations in oxidative stress / free radicals can lead to the production of peroxynitrite from NO, thereby reducing its bioavailability. Relating to the production of NO, reduced levels of tetrahydrobiopterin, whether secondary to increased oxidative stress or independent of it, may be responsible for the impairments observed. Tetrahydrobiopterin is an essential cofactor required for the enzymatic activity of eNOS, and reduced concentrations of tetrahydrobiopterin can lead to impaired NO synthesis in favor of free radical production(Katusic, 2001). The role of these two areas in microvascular dysfunction in response to chronic ischemia merit future study
Zhang et al, have previously demonstrated Arginase I, but not Arginase II, is constitutively expressed in coronary microvascular endothelial cells and Arginase I counteracts NO-mediated vasodilatory function(Zhang et al., 2001). This finding served as an impetus for investigation in to the role Arginase I played in endothelial dysfunction and provided evidence in subsequent studies that up-regulation of the expression and activity of this enzyme was key in the coronary microcirculatory endothelial dysfunction seen in hypertension, oxidative stress, and acute ischemia-reperfusion injury(Hein et al., 2003; Thengchaisri et al., 2006; Zhang et al., 2004). While impaired responses to endothelial-dependent agents was observed in these studies and ours, we found these responses to manifest despite no significant alteration in Arginase I expression / activity. This finding is important to highlight as it indicates differing molecular mechanisms underlie the central process of endothelial dysfunction between varying pathologic states. Evidence indicates each co-morbid condition may itself induce endothelial dysfunction which is additive with other conditions, resulting in a greater degree of impairment(Rodriguez-Porcel et al., 2003a; Rodriguez-Porcel et al., 2003b). Therefore, emphasis must be placed on understanding the mechanisms underlying each process in order to develop individualized therapeutics, as monotherapy, such as L-arginine supplementation or arginase inhibition, may not be effective in ameliorating endothelial dysfunction induced in the coronary microcirculation by chronic ischemia.
Several limitations exist in the present study. The degree of endothelial dysfunction induced by chronic ischemia is less severe in our model than observed in prior reports(Sellke et al., 1994), which may be due to the shorter period of chronic ischemia employed in our experiments. But, given the clear absence of difference in arginase activity and expression, it is likely arginase does not play a role in this dysfunction. Additionally, as our tissue harvest occurred at a single time point, ephemeral changes in the phosphorylation of eNOS may not have been detected.
Understanding endothelial dysfunction in the coronary microcirculation is essential to developing new therapeutic interventions for the treatment of IHD given its role in governing acute and chronic myocardial perfusion. As multiple etiologies for endothelial dysfunction in the coronary microvasculature co-exist in patients with IHD, and that each may additively combine to produce a cumulatively greater degree of dysfunction, understanding the molecular mechanisms behind each pathologic condition is essential. This is doubly important given the molecular mechanisms behind each may differ, and therefore offer multiple targets for therapeutic intervention which may in themselves be cumulatively beneficial.
Acknowledgments
Supported by NHLBI grant HL69024-02. Drs. Sodha and Clements are supported in part by NIH grant T-32HL076130-02 and the Irving Bard Memorial Fellowship
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikawa K, Saitoh S, Muto M, Osugi T, Matsumoto K, Onogi F, Maehara K, Yaoita H, Maruyama Y. Effects of antioxidants on coronary microvascular spasm induced by epicardial coronary artery endothelial injury in pigs. Coron Artery Dis. 2004;15:21–30. doi: 10.1097/00019501-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006a;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Sodha NR, Laham RJ, Sellke FW. The future of therapeutic myocardial angiogenesis. Shock. 2006b;26:332–341. doi: 10.1097/01.shk.0000225318.08681.a7. [DOI] [PubMed] [Google Scholar]
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol. 1999;31:51–60. doi: 10.1006/jmcc.1998.0843. [DOI] [PubMed] [Google Scholar]
- Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. Faseb J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- Horvath KA, Chiu E, Maun DC, Lomasney JW, Greene R, Pearce WH, Fullerton DA. Up-regulation of vascular endothelial growth factor mRNA and angiogenesis after transmyocardial laser revascularization. Ann Thorac Surg. 1999;68:825–829. doi: 10.1016/s0003-4975(99)00842-5. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Post MJ, Simons M, Annex BH. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol. 2003;94:1689–1701. doi: 10.1152/japplphysiol.00465.2002. [DOI] [PubMed] [Google Scholar]
- Jung D, Biggs H, Erikson J, Ledyard PU. New Colorimetric reaction for end-point, continuous-flow, and kinetic measurement of urea. Clin Chem. 1975;21:1136–1140. [PubMed] [Google Scholar]
- Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol. 2001;281:H981–986. doi: 10.1152/ajpheart.2001.281.3.H981. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res. 1992;70:465–476. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- Laham RJ, Simons M, Tofukuji M, Hung D, Sellke FW. Modulation of myocardial perfusion and vascular reactivity by pericardial basic fibroblast growth factor: insight into ischemia-induced reduction in endothelium-dependent vasodilatation. J Thorac Cardiovasc Surg. 1998;116:1022–1028. doi: 10.1016/S0022-5223(98)70055-8. [DOI] [PubMed] [Google Scholar]
- Lamping KG, Christensen LP, Tomanek RJ. Estrogen therapy induces collateral and microvascular remodeling. Am J Physiol Heart Circ Physiol. 2003;285:H2039–2044. doi: 10.1152/ajpheart.00405.2003. [DOI] [PubMed] [Google Scholar]
- Lerman A, Burnett JC, Jr., Higano ST, McKinley LJ, Holmes DR., Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Thomas KA, DiSalvo J, Caputo RP, Carrozza JP, Douglas PS, et al. Angiogenic potential of perivascularly delivered aFGF in a porcine model of chronic myocardial ischemia. Am J Physiol. 1998;274:H930–936. doi: 10.1152/ajpheart.1998.274.3.H930. [DOI] [PubMed] [Google Scholar]
- Lupi A, Buffon A, Finocchiaro ML, Conti E, Maseri A, Crea F. Mechanisms of adenosine-induced epicardial coronary artery dilatation. Eur Heart J. 1997;18:614–617. doi: 10.1093/oxfordjournals.eurheartj.a015305. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Voisine P, Bianchi C, Xu SH, Feng J, Malik T, Rosinberg A, Sellke FW. Effects of L-arginine on the endogenous angiogenic response in a model of hypercholesterolemia. Surgery. 2005;138:291–298. doi: 10.1016/j.surg.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA, Dakak N, Diodati JG, Gilligan DM, Panza JA, Cannon RO., 3rd Effect of L-arginine on human coronary endothelium-dependent and physiologic vasodilation. J Am Coll Cardiol. 1997;30:1220–1227. doi: 10.1016/s0735-1097(97)00279-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Porcel M, Lerman A, Herrmann J, Schwartz RS, Sawamura T, Condorelli M, Napoli C, Lerman LO. Hypertension exacerbates the effect of hypercholesterolemia on the myocardial microvasculature. Cardiovasc Res. 2003a;58:213–221. doi: 10.1016/s0008-6363(03)00250-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Porcel M, Lerman LO, Herrmann J, Sawamura T, Napoli C, Lerman A. Hypercholesterolemia and hypertension have synergistic deleterious effects on coronary endothelial function. Arterioscler Thromb Vasc Biol. 2003b;23:885–891. doi: 10.1161/01.ATV.0000069209.26507.BF. [DOI] [PubMed] [Google Scholar]
- Rossi P, Riutta A, Kuukasjarvi P, Vehmas T, Mucha I, Salenius JP. Revascularization decreases 8-isoprostaglandin F2alpha excretion in chronic lower limb ischemia. Prostaglandins Leukot Essent Fatty Acids. 2004;71:97–101. doi: 10.1016/j.plefa.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Sellke FW, Quillen JE, Brooks LA, Harrison DG. Endothelial modulation of the coronary vasculature in vessels perfused via mature collaterals. Circulation. 1990;81:1938–1947. doi: 10.1161/01.cir.81.6.1938. [DOI] [PubMed] [Google Scholar]
- Sellke FW, Wang SY, Friedman M, Harada K, Edelman ER, Grossman W, Simons M. Basic FGF enhances endothelium-dependent relaxation of the collateral-perfused coronary microcirculation. Am J Physiol. 1994;267:H1303–1311. doi: 10.1152/ajpheart.1994.267.4.H1303. [DOI] [PubMed] [Google Scholar]
- Sellke FW, Wang SY, Stamler A, Lopez JJ, Li J, Simons M. Enhanced microvascular relaxations to VEGF and bFGF in chronically ischemic porcine myocardium. Am J Physiol. 1996;271:H713–720. doi: 10.1152/ajpheart.1996.271.2.H713. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–2042. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- Tofukuji M, Metais C, Li J, Hariawala MD, Franklin A, Vassileva C, Simons M, Sellke FW. Effects of ischemic preconditioning on myocardial perfusion, function, and microvascular regulation. Circulation. 1998;98:II197–204. discussion II204-195. [PubMed] [Google Scholar]
- Valgimigli M, Merli E, Malagutti P, Soukhomovskaia O, Cicchitelli G, Macri G, Ferrari R. Endothelial dysfunction in acute and chronic coronary syndromes: evidence for a pathogenetic role of oxidative stress. Arch Biochem Biophys. 2003;420:255–261. doi: 10.1016/j.abb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Voisine P, Bianchi C, Khan TA, Ruel M, Xu SH, Feng J, Li J, Malik T, Rosinberg A, Sellke FW. Normalization of coronary microvascular reactivity and improvement in myocardial perfusion by surgical vascular endothelial growth factor therapy combined with oral supplementation of l-arginine in a porcine model of endothelial dysfunction. J Thorac Cardiovasc Surg. 2005a;129:1414–1420. doi: 10.1016/j.jtcvs.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Voisine P, Li J, Bianchi C, Khan TA, Ruel M, Xu SH, Feng J, Rosinberg A, Malik T, Nakai Y, Sellke FW. Effects of L-arginine on fibroblast growth factor 2-induced angiogenesis in a model of endothelial dysfunction. Circulation. 2005b;112:I202–207. doi: 10.1161/CIRCULATIONAHA.104.526350. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. Faseb J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, Kuo L. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]


