Abstract
Aim
To use the superior spatial resolution of magnetic resonance imaging (MRI) to examine differences in cerebral perfusion between young alcohol dependent and normal women.
Methods
Eight alcohol dependent women and 8 controls (all ages 18–25) received single-slice resting perfusion-weighted MRI (directly proportional to brain blood flow), with slices located above the corpus callosum.
Results
Alcohol-dependent women had decreased perfusion in prefrontal and left parietal regions.
Conclusions
Reduced perfusion has not previously been reported in young, physically healthy alcohol dependent females, yet is consistent with previously reported decreased cerebral activity in alcohol dependence.
Keywords: Alcohol abuse, alcoholism, perfusion magnetic resonance imaging
INTRODUCTION
Alcohol dependence is relatively common from adolescence onwards. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies of alcohol dependent adults have shown decreased cerebral blood flow (CBF) (1) and glucose metabolic rate (2). However, most previous studies excluded females, who may be somewhat more vulnerable to the effects of chronic drinking on the brain (3). Because baseline CBF can influence the magnitude of the blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) signal (4), understanding resting perfusion differences is important for the interpretation of fMRI studies. fMRI perfusion offers finer spatial resolution that PET and SPECT methods. We hypothesized that resting fMRI perfusion would be reduced in frontal regions among young women with histories of alcohol dependence.
METHODS
Participants
Eight alcohol dependent women (18–25 years) were recruited from a study of adolescent substance abuse, and 8 control women were recruited from the community. All participants were right-handed, age 18–25, and had no history of any DSM-IV disorder except alcohol-induced syndromes, neurological illness or significant head injury, or current use of a medication which might affect cerebral circulation.
At the time of scanning, 4 women with histories of alcohol dependence engaged in heavy weekend binge drinking; one had not used alcohol in one year; 2 had not used alcohol in 6 months; and one had not used alcohol in one month. No control had any history of substance use disorder. Groups were matched for ethnicity, age, education, family history, smoking, and menstrual cycle phase. Participants voluntarily signed consent forms approved by the UCSD Institutional Review Board.
Measures
The Customary Drinking and Drug Use Record (CDDR) (5) obtained detailed alcohol, drug, and nicotine use history, including symptoms of abuse and dependence criteria and withdrawal symptoms. Participants were excluded for metallic implants or claustrophobia. General health data were obtained, along with a brief neuropsychological battery. Participants completed mood and sleepiness scales at the time of scanning (6).
Procedures
Participants were asked to abstain from alcohol (2) and other drugs (7) for at least 48 hours prior to imaging. The minimum time since last drink was 3 days. Breathalyzer (AlcoSensor IV, St. Louis, MO) and urine toxicology screens were negative for all participants. Portions of the CDDR were repeated just prior to scanning to verify absence of acute alcohol withdrawal (8).
Participants were scanned on a 1.5-Tesla Siemens scanner using a standard head coil. A high-resolution MRI image (MPRAGE, voxels 1 × 1 × 1) was obtained. Perfusion-weighted images were obtained during resting conditions (eyes open) using a pulsed arterial spin labeling sequence (9). For this sequence, the difference between tagged and control images is directly proportional to brain blood flow in ml blood/ml tissue/minute (9). A single axial slice 8 mm thick (voxels 2.81 mm × 2.81 mm) was obtained (TR: 3000 ms; TE: 15 ms; flip angle: 90°; FOV: 360 mm; 90 repetitions; total time: 4 minutes and 30 seconds). For each participant, the slice was prescribed by obtaining the first slice consisting grossly of gray matter above the corpus callosum.
Data Processing and Analysis
Image data were processed with Analysis of Functional NeuroImages (AFNI) 2.56g (10). Perfusion images were coregistered to the middle repetition to minimize movement artifact. For each repetition, images of the running difference between tagged and nontagged imageswere calculated. The first three repetitions and outlier repetitions were omitted, and the remaining repetitions were averaged. Perfusion data for individual scans were spatially smoothed (11, 12) then transformed into Talairach coordinates. Because we considered our analyses exploratory, we used 2-tailed tests and did not correct for multiple comparisons in region of interest (ROI) analyses.
Talairach Daemon-based templates (13) were created bilaterally for the cuneus and all frontal Brodmann areas (BA) expected to intersect the perfusion slice (BA 6, 7, 8, 9, 10, 24, 32, and 46). For each participant and each ROI, voxels outside the participant’s perfusion slice were omitted. Participants whose perfusion slice did not include a given ROI were excluded from analysis of that ROI. Nonparametric statistics were used where appropriate based on non-normal distributions or decreased participant numbers.
Voxelwise analyses used an independent samples between-groups t-test (14). We used a cluster threshold to protect against Type I error, requiring a minimum volume of 5 adjacent voxels (based on Monte Carlo simulations), yielding an overall α of p = .025 (15). Clusters of significant between-group differences were used as masks, with individual values examined for each participant. As with the a priori ROIs, participants whose perfusion slice did not include the cluster were excluded.
RESULTS
The alcohol-dependent group reported more drinks per month consumed (143.6 ± 212.9 vs. 8.5 ± 7.1, p < .05), more alcohol withdrawal symptoms during the previous 2 years (2.9 ± 2.7 vs. .3 ± .8, p < .05), and fewer days since last episode of drinking ≥4 drinks (106.4 ± 141.7 vs. 880.8 ± 331.6, p < .05) than controls.
Whole slice perfusion did not differ between groups (p = .44). One predefined ROI differed significantly between groups: the right middle frontal gyrus (BA 9), where alcohol dependent participants’ perfusion was less than that of controls (Mann-Whitney U, p = .004, η2 = .60). Although right BA 46 did not significantly differ between groups (based on 3 controls and 2 alcohol-dependent participants), its η2 of .75 suggests that a difference may have been apparent with adequate participant numbers. There were no ROIs or clusters in which perfusion was greater for alcohol dependent participants than for healthy controls.
Voxelwise analyses revealed 6 clusters in which perfusion was significantly less in alcohol dependent participants than in nondrinkers (see Table 1): bilateral middle frontal gyri, left precuneus, right cingulate, and bilateral inferior parietal lobules. Using each of these clusters as ROIs, we tested participants’ individual perfusion values for between-group differences. The left precuneus and bilateral inferior parietal lobule clusters remained significantly different between the groups (p < .30, Mann-Whitney U); the bilateral middle frontal gyrus clusters were marginally significant (e.g., p < .10, Mann-Whitney U). However, effect sizes (η2) for all 6 clusters ranged from .47 to .83, in the predicted direction, and were greatest for clusters in right- and left-middle frontal gyri (BA 9), particularly on the right (see Figure 1).
Table 1.
Clusters in which alcohol dependent participants had less perfusion than controls
| Talairach coordinates (mm)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Region/Brodmann’s Area | (Volume μl) | x | y | z | Effect size η2 | Mann –Whitney U p | #Participants control/AlcDep | |
| Right middle frontal Gyrus | 9 | 1408 | −43 | −15 | 32 | .75 | .057 | 4/3 |
| Left middle frontal Gyrus | 9 | 1286 | 37 | −25 | 30 | .75 | .057 | 4/3 |
| Left precuneus | 7 | 1082 | 9 | 51 | 46 | .44 | .030 | 7/4 |
| Right cingulate | 32 | 921 | −1 | −25 | 30 | .69 | ns | 4/2 |
| Left inferior parietal lobule | 40 | 354 | 56 | 40 | 46 | .50 | .093 | 8/5 |
| Right inferior parietal lobule | 40 | 351 | −55 | 51 | 45 | .55 | .059 | 8/6 |
Notes: There were no clusters in which alcohol dependent participants had greater perfusion than nondrinkers; p-values are based only on participants whose perfusion slice included the cluster (n shown in far right column).
Figure 1.
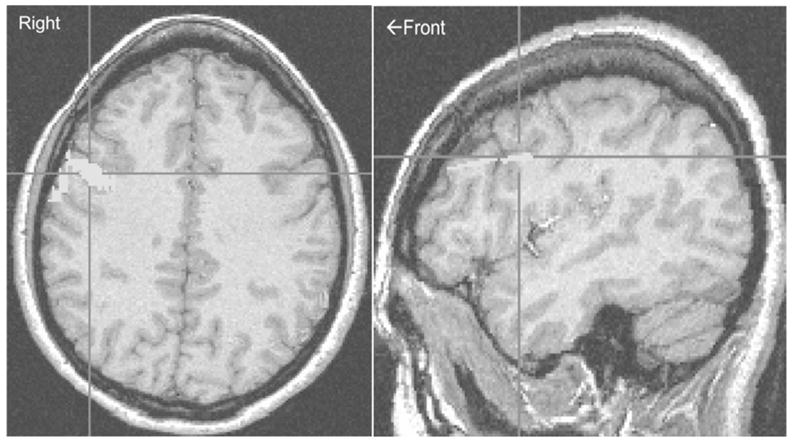
Location of the largest cluster in which alcohol-dependent young women had less perfusion than control women: right middle frontal gyrus (BA 9, Talairach coordinates x = −43, y = −15, z = 32).
To control for any influence of recent heavy drinking, perfusion values for all a priori ROIs, cluster-derived ROIs, and whole slice means were reanalyzed using ANOVA within days since last heavy drinking episode (≥4 drinks) as a covariate. Days since last heavy drinking did not significantly predict perfusion in any cluster, and perfusion remained significantly reduced in the alcohol dependent group compared to controls for right BA 46, right BA 32, and left BA 40 (all p < .05), with a trend toward significance for right BA 9 (p = .08).
DISCUSSION
Our findings of decreased resting perfusion in alcohol dependent participants compared with nondrinkers are consistent with previous PET and SPECT reports, particularly for frontal areas (7). Careful study execution ruled out intoxication or withdrawal as confounds. Like previous BOLD findings (6), these results document abnormal brain activity in relatively young alcohol dependent participants. However, most previous imaging studies excluded females and studied considerably older alcohol dependent participants. These studies suggest that chronic heavy drinking, even in physically healthy young adults, might be associated with altered CBF regulation.
Based on expected involvement of right dorsolateral prefrontal cortex (DLPFC) in spatial working memory, altered resting perfusion in right BA 9 and 46 is consistent with altered activation patterns during performance of a spatial working memory task (6). The right DLPFC is in the same vascular territory in which abnormal vascular response to acetazolamide improved with abstinence (1). Whether right anterior and middle cerebral artery territories are more susceptible to the effects of alcohol dependence on cerebral circulation remains unclear. Sites of decreased perfusion were not limited to DLPFC or to frontal lobe.
One limitation of this study is the use of single slice perfusion imaging, which does not reveal abnormal perfusion elsewhere in the brain. We have recently published whole-brain perfusion data using a faster pulse sequence (11, 12). Second, power and reliability are limited by sample size.
Nonetheless, the data reported show multiple sites in which resting perfusion was decreased in young alcohol dependent women. To our knowledge, they represent the first brain imaging of resting activity in females (traditionally under represented in studies of alcohol dependence), specifically in early adulthood. These areas of decreased perfusion were evident independent of days since last heavy drinking episode, suggesting that altered cerebrovascular activity may persist several months into abstinence. Further study may help clarify the relationship between BOLD and perfusion MR signal in alcohol-dependent populations and provide further clues as to the nature and duration of altered cerebral activity.
Acknowledgments
Supported by 5 K08 MH01642, R01 AA13419, R21 AA12519, and the VISN 22 Mental Illness, Research, Education, and Clinical Center.
We would like to thank Lesley Wetherell for her help with data analysis.
References
- 1.Suzuki Y, Oishi M, Mizutani T, Sato Y. Regional cerebral blood flow measured by the resting and vascular reserve (RVR) method in chronic alcoholics. Alcohol Clin Exp Res. 2002;26(8 Suppl):95S–99S. doi: 10.1097/01.ALC.0000026984.37262.82. [DOI] [PubMed] [Google Scholar]
- 2.Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzmann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24(6):822–829. [PubMed] [Google Scholar]
- 3.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcohol Clin Exp Res. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- 4.Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: Differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23(7):829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- 5.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 6.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–245. [PubMed] [Google Scholar]
- 7.Gottschalk PC, Kosten TR. Cerebral perfusion defects in combined cocaine and alcohol dependence. Drug Alcohol Depend. 2002;68(1):95–104. doi: 10.1016/s0376-8716(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 8.Tutus A, Kugu N, Sofuoglu S, Nardali M, Simsek A, Karaaslan F, Gonul AS. Transient frontal hypoperfusion in Tc-99 m hexamethylpropyleneamineoxime single photon emission computed tomography imaging during alcohol withdrawal. Biol Psychiatry. 1998;43(12):923–928. doi: 10.1016/s0006-3223(97)00322-3. [DOI] [PubMed] [Google Scholar]
- 9.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 10.Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 11.Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, Sutherland AN, Gillin JC. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Research: Neuroimaging. 2006;146(1):43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark CP, Brown GG, Frank L, Thomas L, Sutherland AN, Gillin JC. Improved anatomic delineation of the antidepressant response to partial sleep deprivation in medial frontal cortex using perfusion-weighted functional MRI. Psychiatry Research: Neuroimaging. 2006;146(1):213–222. doi: 10.1016/j.pscychresns.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapping. 1995;2:189–210. [Google Scholar]
- 15.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional Magnetic Resonance imaging (fMRI): Use of cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]


