Abstract
This article reviews calcium signaling in three specialized types of glial cells: Müller cells of the retina, Bergmann glial cells of the cerebellum, and radial glial cells of the developing cortex. Müller cells generate spontaneous and neuronal activity-evoked increases in Ca2+. Neuron to Müller cell signaling is mediated by neuronal release of ATP and activation of glial P2Y receptors. Müller cells, in turn, modulate neuronal excitability and mediate vasomotor responses. Bergmann glial cells also generate spontaneous and activity-evoked Ca2+ increases. Neuron to Bergmann glia signaling is mediated by neuronal release of nitric oxide, noradrenaline, and glutamate. In Bergmann glia, Ca2+ increases control the structural and functional interactions between these cells and Purkinje cell synapses. In the ventricular zone of the developing cortex, radial glial cells generate spontaneous Ca2+ increases that propagate as Ca2+ waves through clusters of neighboring glial cells. These Ca2+ increases control cell proliferation and neurogenesis.
Keywords: glia, calcium, Müller cell, Bergmann glia, radial glia
INTRODUCTION
There is a dynamic, bidirectional signaling between glial cells and neurons in the CNS. Much of what we know of this interaction has been learned by monitoring changes in Ca2+ within glial cells. Glial Ca2+ signaling was first described in astrocytes. More recently, studies of Ca2+ signaling in specialized glial cells have added to our knowledge of neuron-glia interactions.
This review summarizes research on Ca2+ signaling in three types of specialized CNS glia: Müller cells of the retina, Bergmann glial cells of the cerebellum, and radial glia in the developing cortex. We will discuss Ca2+ signaling that is generated endogenously in these glial cells as well as Ca2+ signals evoked by neuronal activity. The physiological consequences of such glial Ca2+ increases will also be addressed.
MÜLLER CELLS
Müller cells are principal glial cells of the retina. They span the entire thickness of the retina from the photoreceptors to the inner retinal surface. They are the only type of macroglial cell present in most regions of the retina and function as specialized astrocytes. Müller cells signal to retinal neurons and blood vessels, modulating neuronal activity and regulating arteriole diameter. Ca2+ signaling in Müller cells is believed to play an essential role in these intercellular interactions.
Spontaneous and Evoked Ca2+ Signaling in Müller Cells
Müller cells of the mammalian retina generate transient increases in Ca2+ in the absence of evoked neuronal activity (Newman, 2005). They occur at a frequency of 4.6 transients per cell per 1,000 s. The transients range from 2.5 to 6 s in duration and are similar to those observed in astrocytes in brain slices (Nett et al., 2002; Parri et al., 2001) and in vivo (Hirase et al., 2004).
The retina offers an important advantage over brain slice preparations in studies of Ca2+ signaling in that Ca2+ increases in Müller cells can be induced by a natural stimulus, light. Neuronal activity, evoked by light flashes, increases the frequency of Ca2+ transients in Müller cells by 28% (Fig. 1) (Newman, 2005). This increase in Ca2+ transient frequency is greatly potentiated by adenosine, which is a degradation product of ATP. Adenosine potentiation of neuron-glia signaling is of clinical interest as adenosine levels increase in certain pathological states. Both endogenous and light-evoked Ca2+ increases reflect Ca2+ release from internal stores (Newman, 2005). The increases are blocked by cyclopiazonic acid, which depletes Ca2+ stores, and are abolished by heparin, which blocks IP3 receptors (Newman and Zahs, 1997).
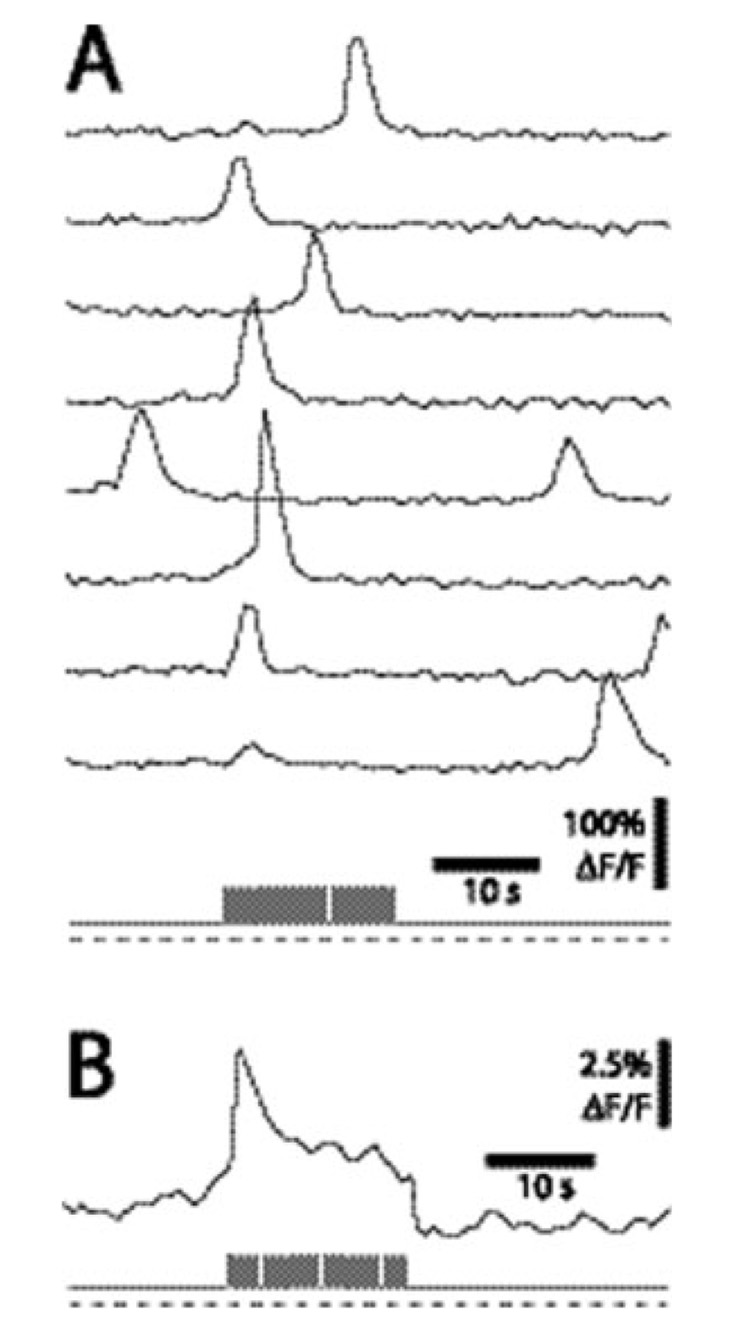
Fig. 1.
Light-evoked Ca2+ signaling in Müller cells. (A) Calcium fluorescence measured simultaneously in eight Müller cells. The retina was exposed sequentially to a dim light, a bright flickering light, and a dim light (the light stimulus is shown at the bottom in A and B). Calcium transients are more likely to be generated during the flickering light stimulus. (B) Mean Ca2+ fluorescence averaged over 84 trials. The flickering light evokes both a transient and a sustained increase in Ca2+. From (Newman, 2005).
Signaling from neurons to Müller cells is mediated by release of ATP from neurons and activation of P2Y purinergic receptors (Newman, 2005), which evoke glial Ca2+ increases. Light-evoked Ca2+ increases are blocked by suramin, a purinergic antagonist, and by apyrase, which hydrolyzes ATP, but not by antagonists to metabotropic glutamate, GABA, or muscarinic receptors. Light-evoked Müller cell Ca2+ increases are also blocked by TTX, indicating that only those retinal neurons that generate action potentials, amacrine and ganglion cells, are signaling to glial cells. Ganglion to Müller cell signaling was confirmed by antidromic activation of ganglion cells, which evokes Ca2+ increases in Müller cells.
Studies on amphibian and mammalian Müller cells have identified receptors linked to Ca2+ increases. Responses to ATP analogs indicate that P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors can mediate Ca2+ increases (Fries et al., 2005; Reifel Saltzberg et al., 2003). Activation of A2B adenosine receptors greatly potentiates ATP-evoked responses (Newman, 2005), while activation of receptor tyrosine kinases causes a resensitization of P2Y receptors previously desensitized by agonist application (Weick et al., 2005). In addition to ATP analogs, Müller cell Ca2+ increases are evoked by dopamine, thrombin, and lysophosphatidic acid (Newman, 2003; Puro and Stuenkel, 1995). Calcium is released from internal stores by NAD+ (Esguerra and Miller, 2002), by ryanodine, and caffeine (Keirstead and Miller, 1995) and following activation of metabotropic glutamate receptors (Keirstead and Miller, 1997). Interestingly, in the mammalian retina, glutamate is largely ineffective in evoking Ca2+ increases in Müller cells (Bringmann et al., 2002; Newman, 2005; Newman and Zahs, 1997). In contrast, glutamate evokes large Ca2+ increases in astrocytes, in brain slices, and in culture (Schipke and Kettenmann, 2004). The insensitivity of Müller cells to glutamate may be a specialization to conditions in the retina, where glutamate is released continuously from neurons. Mechanical stimulation of Müller cells also evokes Ca2+ increases mediated by release from internal stores (Newman, 2001; Newman and Zahs, 1997).
Stimulation of Müller cells and retinal astrocytes often evokes Ca2+ increases that propagate from the activated cell to adjacent glial cells as intercellular waves (Newman, 2001; Newman and Zahs, 1997). Agonist ejection or mechanical stimulation of Müller cells results in propagated intercellular Ca2+ increases that travel outwards from the point of stimulation through neighboring glia (Newman, 2001). Müller cell to Müller cell signaling occurs via release of ATP from one cell and activation of purinergic receptors on adjacent cells. These waves are blocked by the purinergic antagonist, suramin. It is not known whether intercellular Ca2+ waves occur in vivo. However, experimentally evoked waves result in the modulation of neuronal activity and in vasomotor responses (see below).
Physiological Consequences of Ca2+ Increases in Müller Cells
Selective stimulation of Müller cells by agonist ejection evokes large Ca2+ increases in these cells and subsequent hyperpolarization of adjacent ganglion cells. The ganglion cell hyperpolarization is mediated by ATP release from the Müller cells (Newman, 2003). Released ATP is rapidly converted to adenosine by ecto-ATPases and ecto-nucleotidases. Adenosine, in turn, activates A1 adenosine receptors on the ganglion cells, leading to the opening of K+ channels and to cell hyperpolarization. It should be noted that although glial stimulation results in glial Ca2+ increases, it has not been demonstrated directly that ATP release from Müller cells is Ca2+ dependent (Newman, 2003).
Müller cell stimulation may also lead to the release of d-serine and to potentiation of NMDA receptor neurotransmission in the retina. Müller cells contain d-serine, an endogenous NMDA receptor co-agonist, and serine racemase, the synthetic enzyme for d-serine (Stevens et al., 2003). Stimulation of astrocytes results in d-serine release (Schell et al., 1995), and a similar release of d-serine may occur in Müller cells.
Müller cell stimulation by photolysis of caged-IP3 or caged-Ca2+ results in vasomotor responses in neighboring arterioles (Metea and Newman, 2006). Glial stimulation can evoke either vasodilation or vasoconstriction. Both responses are mediated by metabolites of arachidonic acid. Vasodilation is mediated by production of EETs, while vasoconstriction by production of 20-HETE. It is not known whether signaling from Müller cells to arterioles is Ca2+-dependent, although stimulation of Müller cells in this study did evoke intracellular Ca2+ increases.
BERGMANN GLIAL CELLS
Bergmann glial cells are radial glia of the cerebellum and serve as specialized astrocytes. Their somata lie in the Purkinje cell layer and their processes extend through the molecular layer to the pial surface. Their processes are aligned with Purkinje cell dendrites and completely surround Purkinje cell synapses in the molecular layer. Calcium signaling in Bergmann glial cells plays a key role in regulating structural and functional interactions between these cells and Purkinje cell synapses.
Spontaneous Ca2+ Signaling in Bergmann Glial Cells
Spontaneous Ca2+ increases occur in restricted regions of Bergmann glial cell processes (Grosche et al., 1999). These restricted regions are termed microdomains and have been shown in EM studies to be thin membrane sheets that completely envelop Purkinje cell synapses. Each Bergmann glial cell microdomain contacts only a few synapses (Grosche et al., 2002). Microdomains are isolated from the remainder of the cell by a narrow neck and are electrotonically isolated (Grosche et al., 2002). Spontaneous Ca2+ signaling is not synchronized between microdomain regions.
Evoked Ca2+ Increases in Bergmann Glial Cells
Stimulation of parallel fibers evokes Ca2+ increases in Bergmann glial cells (Fig. 2) (Grosche et al., 1999). Single pulses (Matyash et al., 2001) and brief trains of pulses (Grosche et al., 1999) evoke Ca2+ increases in only a few Bergmann glial cell microdomains, while more intense stimulation results in widespread Ca2+ increases in cell processes and somata (Matyash et al., 2001).
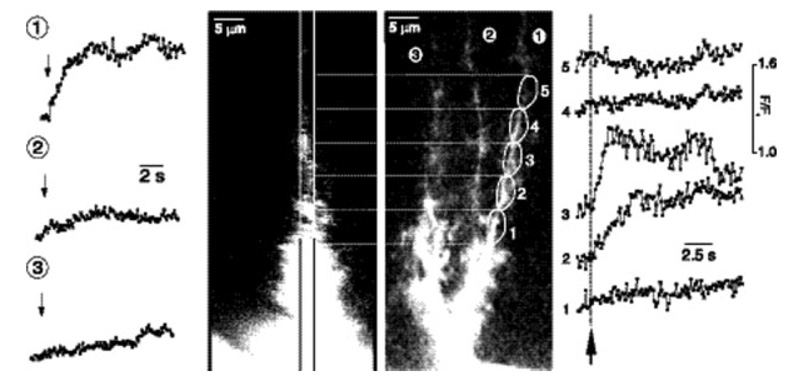
Fig. 2.
Activity-evoked Ca2+ signaling in Bergmann glial cells. Calcium fluorescence measured in three cell processes of a Bergmann glial cell following electrical stimulation of parallel fibers. Stimulation evokes a Ca2+ increase in process 1 but not in processes 2 and 3 (traces on the left). Within process 1, microdomains 2 and 3, but not 1, 4 or 5, display stimulus-evoked Ca2+ increases (traces on the right). The right hand image shows the Bergmann glial cell from which the measurements were made. From (Grosche et al., 1999).
Electrical stimulation within the granule cell layer also evokes Ca2+ increases in the somata and processes of Bergmann glial cells (Kulik et al., 1999). In contrast to Ca2+ responses evoked by parallel fiber activity, the Ca2+ responses evoked by granule cell layer stimulation are not confined to small microdomains in Bergmann glial cell processes.
Parallel fiber to Bergmann glial cell signaling is mediated by nitric oxide (NO) (Matyash et al., 2001), which is released from parallel fibers during stimulation (Kimura et al., 1998). Calcium signaling is blocked by the NO synthase inhibitor N-ω-nitro-l-arginine, but not by antagonists to α1 adrenergic, AMPA or metabotropic glutamatergic or H1 histaminergic receptors, which are expressed in Bergmann glial cells (Matyash et al., 2001). This activity-dependent Ca2+ increase is generated by Ca2+ influx rather than by release from internal stores. It is blocked by removal of external Ca2+ but not by depletion of internal Ca2+ stores with thapsigargin.
In contrast to parallel fiber-Bergmann glial cell signaling, Ca2+ increases evoked by stimulation within the granule cell layer is mediated by release of noradrenaline (Kulik et al., 1999). This Ca2+ response is blocked by the α1 adrenergic receptor antagonist prazosin as well as by cyclopiazonic acid. Similar Ca2+ increases are evoked by bath-applied noradrenaline. The evoked response is not blocked by β receptor antagonists or by ionotropic or metabotropic glutamate receptor antagonists. The Bergmann glial cell responses evoked by granule cell layer stimulation are thought to be due to activation of noradrenaline-containing locus coeruleus fibers that project to the cerebellum.
Bergmann glial cells express AMPA receptors, which, unlike most neuronal AMPA receptors, are permeable to Ca2+. The Ca2+-permeability of these receptors arises because Bergmann glial cells lack the AMPA receptor subunit GluR2 (Burnashev et al., 1992; Iino et al., 2001). As a consequence, Ca2+ increases are evoked in Bergmann glia by AMPA receptor agonists. These responses are mediated by Ca2+ influx through the receptors and are blocked by the AMPA receptor antagonist CNQX and by the removal of external Ca2+ (Burnashev et al., 1992; Muller et al., 1992).
Stimulation of parallel fibers (Bellamy and Ogden, 2005; Clark and Barbour, 1997) as well as climbing fibers (Matsui and Jahr, 2004) activates Bergmann glial AMPA receptors. AMPA receptor activation by climbing fibers is mediated by ectopic vesicular release of glutamate from climbing fiber terminals (Matsui and Jahr, 2004). Bath application of glutamate can also evoke Ca2+ increases in Bergmann glial cells by release from internal stores (Kirischuk et al., 1999). This response is primarily responsible for generating slow glutamate-evoked Ca2+ responses.
Activation of both α1 adrenergic receptors and H1 histaminergic receptors can evoke Ca2+ release from internal stores in Bergmann glial cells (Kirischuk et al., 1996). The response is blocked by thapsigargin but not by removal of external Ca2+. Activation of endothelinB receptors also evokes Ca2+ increases, which are mediated by release from internal stores (Tuschick et al., 1997).
In addition, ATP activation of metabotropic purinergic receptors can evoke Ca2+ increases in Bergmann glial cells. The response is blocked by the purinergic antagonist suramin, the IP3 receptor blocker heparin, and by thapsigargin (Kirischuk et al., 1995). P2Y receptors can also be activated by nicotinic acid adenine dinucleotide phosphate, resulting in Bergmann glial cell Ca2+ increases (Singaravelu and Deitmer, 2006).
Physiological Consequences of Ca2+ Increases in Bergmann Glial Cells
As discussed above, Bergmann glial cells express Ca2+-permeable AMPA receptors. Genetic manipulation, leading to a reduction in the Ca2+ permeability of these receptors, results in profound changes in glia-neuron interactions in the cerebellum. In an in vivo study, the AMPA receptors of Bergmann glial cells were converted from Ca2+-permeable to Ca2+-impermeable by adenoviral-mediated delivery of the GluR2 gene (Iino et al., 2001). The conversion of the glial AMPA receptors to a Ca2+-impermeable type resulted in retraction of the glial cell processes ensheathing Purkinje cell synapses, which are normally completely surrounded by Bergmann glial processes. Glial process retraction resulted in decreased removal of synaptically released glutamate. It also led to multiple innervation of Purkinje cells by climbing fibers. Thus, Ca2+ permeable AMPA receptors in Bergmann glia play an essential role in determining the structural and functional relation between these cells and Purkinje cell glutamatergic synapses. In an additional study on cultured Bergmann glial cells, conversion of AMPA receptors to the Ca2+-impermeable type resulted in retraction of glial processes, while overexpression of Ca2+ permeable receptors resulted in process extension (Ishiuchi et al., 2001).
Ca2+ permeable AMPA receptors may also regulate the expression of the glial glutamate transporter GLAST. In cultured Bergmann glial cells, prolonged glutamate exposure results in a decrease in GLAST transcription (Lopez-Bayghen et al., 2003). This response is blocked by the AMPA receptor antagonist DNQX and by removal of external Ca2+. (The high affinity glycine transporter, GLY1, in Bergmann glial cells is also regulated by Ca2+; Lopez et al., 2005.) Together, these experiments suggest that Ca2+-permeable AMPA receptors play an essential role in enabling Bergmann glial cells to detect the presence of glutamatergic synapses and to extend processes to surround them.
RADIAL GLIA IN THE DEVELOPING CORTEX
Radial glial cells extend from the ventricular surface to the pial surface in the developing cortex. Their somata lie in the ventricular zone. Recent work has elucidated three important functions of these cells (Anthony et al., 2004; Gotz and Huttner, 2005; Noctor et al., 2001, 2004; Yokota and Anton, 2004). First, their elongated, vertically oriented processes serve as scaffolding for neurons as they migrate away from the ventricular zone. Second, through asymmetric division, radial glia give rise to neurons and neuronal progenitor cells. Third, as the brain matures, radial glia differentiate into astrocytes. Calcium signaling in radial glial cells plays an important role in these processes.
Spontaneous and Evoked Ca2+ Signaling in Radial Glial Cells
In cortical slices of the embryonic brain, spontaneous Ca2+ signaling occurs in individual radial glial cells, in pairs of cells where signaling is synchronized, and in large cell clusters through which Ca2+ waves propagate (Fig. 3) (Owens and Kriegstein, 1998; Weissman et al., 2004). The spontaneous Ca2+ waves traveling through cell clusters occur at a frequency of 1.1 event per min per mm of tissue and are largely restricted to cells in the ventricular zone (Weissman et al., 2004).
Fig. 3.
Calcium signaling in radial glial cells. The nine pseudocolor Ca2+ fluorescence images show a Ca2+ wave propagating through a cluster of radial glial cells in the ventricular zone in embryonic cortical tissue. The spontaneous wave was likely initiated in the cell indicated by the arrow and propagated to adjacent cells by release of ATP. The images were acquired at 4 s intervals. From (Owens and Kriegstein, 1998).
Calcium wave propagation between cells is mediated by release of ATP. Waves are blocked by P2Y receptor antagonists but not by TTX or by glutamate, GABA or glycine receptor antagonists (Owens and Kriegstein, 1998; Weissman et al., 2004). ATP appears to be released from radial glial cells through gap junctional hemichannels. Waves are abolished by gap junction blockers while the frequency of spontaneous waves varies as a function of external Ca2+ levels, which regulate hemichannels (Weissman et al., 2004). (These results are also consistent with ATP release through P2X7 receptors, which are blocked by gap junctional antagonists; Suadicani et al., 2006.) In addition, ATP application evokes Ca2+ increases in radial glial cells in the ventricular zone but not in neurons in more superficial cortical layers. Responses to ATP analogs indicate that ATP sensitivity is mediated by P2Y1 receptors (Weissman et al., 2004). Ca2+ increases in radial glia are generated by Ca2+ release from internal stores rather than by influx from extracellular space. Calcium waves are blocked by thapsigargin and cyclopiazonic acid but not by removal of Ca2+ from extracellular space (Weissman et al., 2004). Calcium increases in radial glial cells are evoked by mechanical and electrical stimulation as well as by purinergic agonists (Weissman et al., 2004).
Physiological Consequences of Ca2+ Increases in Radial Glial Cells
The spontaneous Ca2+ waves observed in radial glial cells may be involved in cell proliferation and neurogenesis in the developing cortex (Weissman et al., 2004). The radial glial cells that initiate Ca2+ waves, identified by Lucifer yellow uptake through open hemichannels, are often in the upper third of the ventricular zone (Weissman et al., 2004). These cells are in the S phase of cell division and are undergoing active DNA synthesis, indicating a link between Ca2+ increases and cell division. In addition, there is a correlation between Ca2+ wave generation and neuronal proliferation (Weissman et al., 2004). From day E12 through E17 there is an increase, both in the frequency of Ca2+ wave generation and in the distance that the waves travel. During this time there is a corresponding increase in neurogenesis. The link between radial glial cell Ca2+ waves and neurogenesis is strengthened by the observation that blocking Ca2+ waves with a purinergic antagonist also reduces neurogenesis (Weissman et al., 2004).
Calcium signaling also plays an important role in the differentiation of neuronal precursor cells. In neurosphere-derived precursor striatal cells, Ca2+ signaling controls neurite outgrowth and acquisition of a neurotransmitter phenotype (Ciccolini et al., 2003). Experimental manipulations which increase Ca2+ signaling lead to increased neurite outgrowth and branching and to expression of a GABAergic phenotype. In addition, the frequency of Ca2+ transient generation in immature neurons modulates the rate of neuronal migration (Komuro and Rakic, 1996).
SUMMARY
Calcium signals in glial cells can be generated endogenously or by transmitters released from active neurons. Spontaneously generated Ca2+ transients occur in many types of glial cells, including Müller cells, Bergmann glial cells, and radial glial cells. In radial glial cells, spontaneous Ca2+ transients trigger Ca2+ waves that propagate through clusters of glia. These Ca2+ signals are likely to be involved in neurogenesis. The function of spontaneous signaling in Müller cells and Bergmann glial cells is unclear, but these Ca2+ transients may evoke release of gliotransmitters and lead to the modulation of neuronal activity.
Neuronal activity evokes Ca2+ signaling in Müller cells and Bergmann glial cells. Neuron to glia signaling is mediated by a number of neurotransmitters, including ATP for Müller cells and NO, noradrenaline, and glutamate for Bergmann glial cells. In Müller cells, Ca2+ increases are correlated with ATP release from the glial cells and inhibition of retinal neurons. The Ca2+ increases are also correlated with the production of arachidonic acid metabolites and dilation or constriction of retinal blood vessels. In Bergmann glial cells, Ca2+ increases play a key role in establishing glial contacts with Purkinje cell synapses and with regulating glutamate uptake into the glial cells.
Studies of specialized glial cells have revealed new aspects of glial Ca2+ signaling and function. These cells offer unique advantages in elucidating glial cell function based on their morphological and physiological interactions with neurons. Müller cells in the retina respond to the activation of neurons by natural light stimulation. More so than other glia, Bergmann glial cell processes envelope neuronal synapses and can be experimentally manipulated. Radial glial cells are the progenitors of many CNS neurons, offering insights into the developmental consequences of Ca2+ signaling.
Acknowledgments
Grant sponsor: NIH; Grant number: EY004077; Grant Sponsor: NSF.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve asneuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Bellamy TC, Ogden D. Short-term plasticity of Bergmann glial cell extrasynaptic currents during parallel fiber stimulation in rat cerebellum. Glia. 2005;52:325–335. doi: 10.1002/glia.20248. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Weick M, Beidermann B, Uhlmann S, Kohen L, Wiedermann P, Reichenbach A. Activation of P2Y receptors stimulates potassium and cation currents in acutely isolated human Müller (glial) cells. Glia. 2002;37:139–152. doi: 10.1002/glia.10025. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeberg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci. 2003;23:103–111. doi: 10.1523/JNEUROSCI.23-01-00103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebral slices. J Physiol. 1997;502(Part 2):335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra M, Miller RF. CD38 expression and NAD+-induced intracellular Ca2+ mobilization in isolated retinal Müller cells. Glia. 2002;39:314–319. doi: 10.1002/glia.10115. [DOI] [PubMed] [Google Scholar]
- Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T. Identification of P2Y receptor subtypes in human Müller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest Ophthalmol Vis Sci. 2005;46:3000–3007. doi: 10.1167/iovs.05-0043. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res. 2002;68:138–149. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:494–499. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Ishiuchi S, Tsuzuki K, Yamada N, Okado H, Miwa A, Kuromi H, Yokoo H, Nakazato Y, Sasaki T, Ozawa S. Extension of glial processes by activation of Ca2+-permeable AMPA receptor channels. Neuroreport. 2001;12:745–748. doi: 10.1097/00001756-200103260-00026. [DOI] [PubMed] [Google Scholar]
- Keirstead SA, Miller RF. Calcium waves in dissociated retinal glial (Müller) cells are evoked by release of calcium from intracellular stores. Glia. 1995;14:14–22. doi: 10.1002/glia.440140104. [DOI] [PubMed] [Google Scholar]
- Keirstead SA, Miller RF. Metabotropic glutamate receptor agonists evoke calcium waves in isolated Müller cells. Glia. 1997;21:194–203. [PubMed] [Google Scholar]
- Kimura S, Uchiyama S, Takahashi HE, Shibuki K. cAMP-dependent long-term potentiation of nitric oxide release from cerebellar parallel fibers in rats. J Neurosci. 1998;18:8551–8558. doi: 10.1523/JNEUROSCI.18-21-08551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A. Glutamate-triggered calcium signalling in mouse Bergmann glial cells in situ: Role of inositol-1, 4,5-trisphosphate-mediated intracellular calcium release. Neuroscience. 1999;92:1051–1059. doi: 10.1016/s0306-4522(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Moller T, Voitenko N, Kettenmann H, Verkhratsky A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci. 1995;15:7861–7871. doi: 10.1523/JNEUROSCI.15-12-07861.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by α1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci. 1996;6:1198–1208. doi: 10.1111/j.1460-9568.1996.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Kulik A, Haentzsch A, Luckermann M, Reichelt W, Ballanyi K. Neuron-glia signaling via α1 adrenoceptor-mediated Ca2+ release in Bergmann glial cells in situ. J Neurosci. 1999;19:8401–8408. doi: 10.1523/JNEUROSCI.19-19-08401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bayghen E, Espinoza-Rojo M, Ortega A. Glutamate down-regulates GLAST expression through AMPA receptors in Bergmann glial cells. Brain Res Mol Brain Res. 2003;115:1–9. doi: 10.1016/s0169-328x(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Lopez E, Lee-Rivera I, Lopez-Colome AM. Characteristics and regulation of glycine transport in Bergmann glia. Neurochem Res. 2005;30:1567–1577. doi: 10.1007/s11064-005-8835-7. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Differential control of synaptic and ectopic vesicular release of glutamate. J Neurosci. 2004;24:8932–8939. doi: 10.1523/JNEUROSCI.2650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Filippov V, Mohrhagen K, Kettenmann H. Nitric oxide signals parallel fiber activity to Bergmann glial cells in the mouse cerebellar slice. Mol Cell Neurosci. 2001;18:664–670. doi: 10.1006/mcne.2001.1047. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells both dilate and constrict blood vessels: A mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Moller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25:5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Puro DG, Stuenkel EL. Thrombin-induced inhibition of potassium currents in human retinal glial (Müller) cells. J Physiol. 1995;485(Part 2):337–348. doi: 10.1113/jphysiol.1995.sp020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifel Saltzberg JM, Garvey KA, Keirstead SA. Pharmacological characterization of P2Y receptor subtypes on isolated tiger salamander Müller cells. Glia. 2003;42:149–159. doi: 10.1002/glia.10198. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. d-serine, an endogenous synaptic modulator: Localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipke CG, Kettenmann H. Astrocyte responses to neuronal activity. Glia. 2004;47:226–232. doi: 10.1002/glia.20029. [DOI] [PubMed] [Google Scholar]
- Singaravelu K, Deitmer JW. Calcium mobilization by nicotinic acid adenine dinucleotide phosphate (NAADP) in rat astrocytes. Cell Calcium. 2006;39:143–153. doi: 10.1016/j.ceca.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Stevens ER, Esquerra M, Kim P, Newman EA, Snyder SH, Zahs KR, Miller RF. d-serine and serine racemase are present in the vertebrate retina and contribute to the functional expression of NMDA receptors. Proc Natl Acad Sci USA. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, Kettenmann H. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium. 1997;6:409–419. doi: 10.1016/s0143-4160(97)90052-x. [DOI] [PubMed] [Google Scholar]
- Weick M, Wiedemann P, Reichenbach A, Bringmann A. Resensitization of P2Y receptors by growth factor-mediated activation of the phosphatidylinositol-3 kinase in retinal glial cells. Invest Ophthalmol Vis Sci. 2005;46:1525–1532. doi: 10.1167/iovs.04-0417. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–671. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Anton ES. Calcium waves rule and divide radial glia. Neuron. 2004;43:599–601. doi: 10.1016/j.neuron.2004.08.030. [DOI] [PubMed] [Google Scholar]