Abstract
A novel supplementation of cell growth media based on a porcine platelet lysate was developed for culture of animal-derived cells. The platelet lysate was produced from porcine blood and contained lysate of platelets and plasma components. It showed satisfactory microbiological integrity and it carried only low amount of endotoxins (<10 EU/mL). The porcine platelet lysate supported well proliferation of Vero (African green monkey transformed kidney epithelial cells), Chinese hamster ovary (CHO) and hybridoma cells comparable to fetal bovine serum (FBS). Platelet lysate shows promise as a viable choice over FBS as it can be produced in large quantities, high lot-to-lot consistency and with an attractive price structure. Furthermore it is a strong alternative to FBS for ethical reasons. It is expected that it can be used as a general supplementation for most animal cells for research studies on the proliferation of cells and their expression of products.
Keywords: Animal cell culture, Porcine platelet lysate, Fetal bovine serum, Growth supplement
Introduction
The in vitro growing and maintenance of cells, tissues and organs is of fundamental importance to biological sciences whether it is pure research in a university laboratory or production of protein pharmaceuticals in the biotechnology industry. To support cell growth and expression of product, supplementation or addition of specialized factors is needed (Whitford 2005). Typically for many years bovine serum especially fetal bovine serum was the addition of choice to media as it contained most of the vital substances required for growth and support of many cell lines (Merten 2006). However since the occurrence of the mad cow crisis there has been great concern to use animal serum or animal based components in the industry for production of biopharmaceuticals (Butler 2005). Major efforts with varying success have been undertaken to develop serum free media (SFM) for many cell lines used for production of biotherapeutics. It has not been possible to develop a SFM medium for all cell lines and applications, so developing such media has mainly been focused on industrial processes. The use of FBS among academic researchers is still very large in the range of 400 000 L per year (2005) and growing annually with 3–5% despite the current disadvantages of the product in terms of availability, cost, composition (batch-to-batch variation) and contamination profiles. In addition there have been raised ethical concerns with the FBS product as the product is mainly collected by cardiac puncture of bovine fetuses (Jochems et al. 2002). A workshop report with recommendations on how to harvest FBS was published recently (van der Valk et al. 2004).
Platelets are important in wound healing and constitute a rich source of growth factors such as PDGF and TGF-β, attachment factors such as fibronectin, enzymes and other factors. Already in the 1970s it was demonstrated that platelet components promoted cell growth in vitro (Balk 1971; Ross et al. 1974). Based on this knowledge we introduced bovine platelet lysate as an alternative source for supplementation of cell culture medium (Johansson et al. 2003). We demonstrated in this work that bovine platelet lysate can be used to supplement media for efficient animal cell culture of a number of cells. In this work we have further developed a platelet lysate based on porcine sources. Due to the large availability of porcine blood from adult animals, optimized production procedures and minimizing problems related to bovine diseases such as bovine spongiform encephalopathy (BSE), we believe that a porcine platelet lysate medium is a useful complement to bovine-based media supplementation. Platelet lysate should be of interest as an alternative to FBS and can be used as a general supplementation of media for various cells under research in academic- and industrial laboratories.
Materials and methods
Production of porcine platelet lysate
The porcine platelet lysate was produced according to Persson et al. (2006). Briefly a platelet-rich plasma was collected from whole porcine blood by centrifugation followed by ultrafiltration, lysis and coagulation. The porcine lysate was sterile filtered (0.2 μm) and then transferred to sterile tubes for storage in freezer at −18 °C.
Analyses of the platelet lysate
A clinical chemical analysis (Integra 700, Roche Diagnostica, Basel, Switzerland) was used to determine the chemical profile of porcine platelet lysate compared with FBS. The platelet lysate was tested for number of aerobic bacteria, Escherichia coli and Staphylococcus aureus by standard microbiological procedures. Endotoxins were determined with the limulus assay according to standard procedures (European Pharmacopoeia 2.6.14 v. 4) Mycoplasma was analyzed by DNA-coloration on indicator cells.
The hemoglobin content was measured according to spectrophotometric methods measuring the absorbance at three different wavelengths (415, 450 and 700 nm) according to standard procedures. The immunoglobulin (IgG) content was measured on a protein G column, 5 mL (GE Health Care, Uppsala, Sweden) according to Johansson et al. (2003).
Cell culture
Three cell lines have been used in this study, Vero (African green monkey transformed kidney epithelial cells), CHO (Chinese hamster ovary cells) and 39.5 (hybridoma cells). All cells were obtained from ATCC except 39.5 which were provided by Dr A. Lundblad, Linköping University, Sweden. The cells were cultured at 37 °C with 7.5% CO2. All cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), high glucose (4.5 g L−1) (Sigma, St Louis, USA) with addition of 0.1% Penicillin–Streptomycin (Sigma), 4 mM L-glutamine (Sigma) and FBS (5%) (HyClone, Logan, USA) or porcine platelet lysate (5–10%) in T-flasks (Sarstedt, Newton, USA), 25-, 75- and 175 cm2, 5-, 15- and 35 mL, respectively. A typical growth study lasted for 4–7 days. Before starting a growth study on a new lysate the cells were adapted to that media for at least two passages. Cells were seeded in triplicates at a concentration of 1–2 × 105 cells mL−1 in 25 cm2 T-flasks. Vero and CHO cells were counted on day 1, 3, 4 and 6 or 7 and suspension cells were counted on day 1, 2, 3 and 4. Adherent cells were harvested by trypsination (trypsin-EDTA (0.5 g L−1porcine trypsin, 0.2 g L−1 EDTA) (Sigma) 2 × 1 mL at 20 °C and 37 °C for 1 and 2 min).
Cell counting
On the seeding day the cells were counted in a Bürker chamber with Trypan blue as a viability indicator. On the following days the cells were counted with a flow cytometer (FACS Calibur, Becton Dickinson Immunocytometry Systems, San Jose, USA). The use of a flow cytometer gives more reliable data since it is not dependent on the operator and the counting is much faster and more effective (Al-Rubeai et al. 1997). The cell population was gated in a dot plot with side scattered light versus forward scatter. Viability was determined with a fluorescent stain, Sytox green nucleic acid stain (Molecular probes, Eugene, USA). Sytox green shows dead or damaged cells by penetrating cell membranes. This uptake is seen as a green fluorescence when excited from an argon laser beam at 488 nm. The viability was detected in a dot plot with FL1-H versus forward scatter.
Results and discussion
Characterization of the porcine platelet lysate
A chemical profile was performed on platelet lysate and compared with FBS (Table 1). It can be seen that the lysate contains plasma components of for example proteins in the same range as FBS. The Immunoglobulin G content is considerable in the lysate and has to be reduced or removed for applications when producing monoclonal antibodies with hybridoma cells. Attempts to remove the IgG in platelet lysate with chromatography have been successful (<0.1 μg/mL) (data not shown) but it remains to be seen how this IgG-free medium supports growth of hybridoma cells. In order to achieve a platelet-rich plasma during the first centrifugation of whole porcine blood it is a risk that some contamination will occur with red blood cells which upon hemolysis will increase the hemoglobin content of the lysate. The production process was optimized to reduce hemoglobin to a level where it will not affect cell growth. A microbiology analysis of different lots of porcine platelet lysate showed no presence of bacteria (E. coli and S. aureus), no mycoplasma and only low levels of endotoxins (8.4 EU mL−1 in one tested batch). As the platelet lysate is of porcine origin, the biological hazard profile is distinctively different from that of bovine species. For example bovine serum can carry viruses/prions such as bovine viral diarrhea, encephalitis and bovine spongiform encephalopathy (BSE) whereas porcine platelet lysate may have a different zoonotic contamination profile such as of porcine parvovirus, circo virus and hepatitis. For any applications with cell culture with either bovine or porcine sources, ways to mitigate the risks for microbiological contamination such as using filtration have to be carefully considered.
Table 1.
Chemical profile of porcine platelet lysate and FBS
| Substance | FBS | Porcine platelet lysate |
|---|---|---|
| Total protein (g L−1) | 36 | 42 |
| Chloride (mmol L−1) | 101 | 41 |
| Sodium (mmol L−1) | 137 | 127 |
| Potassium (mmol L−1) | 11.7 | 5.1 |
| Phosphate (mmol L−1) | 3.2 | 1.4 |
| Albumin (g L−1) | 25 | 23 |
| Iron (μmol L−1) | 37 | 17 |
| Calcium (mmol L−1) | 3.7 | >5.0 |
| Glucose (mmol L−1) | 6.5 | 3.9 |
| IgG (g L−1) | 0.2 | 7.7 |
| Hemoglobin (g L−1) | 0.3 | 1.64 |
Proliferation of Vero and CHO cells
The Vero cells were able to grow well (viabilities over 90 %) in the porcine platelet lysate and reached cell densities of approximately 3.0 × 106 cells mL−1 in 10% platelet lysate as compared to 4.0 × 106 cells mL−1 in 5% FBS (Fig. 1). The Vero cells were growing in multilayers in both the FBS and the porcine platelet lysate medium. The morphology of the cells was similar in both media.
Fig. 1.
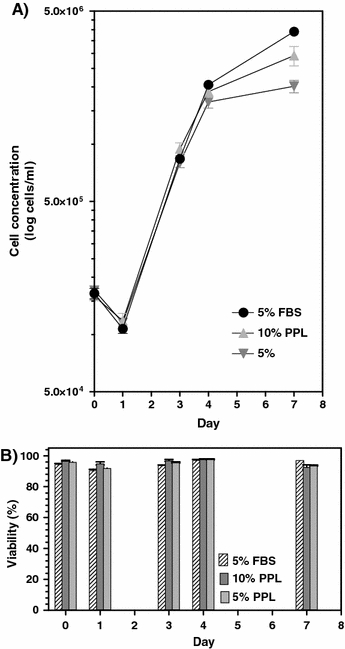
(a) Cell growth and (b) Viability of Vero cells cultured in porcine platelet lysate (PPL) and FBS supplemented media. Error bars represent the SEM value
Similar growth results were obtained with CHO cells. They were able to grow (viabilities over 90%) in the porcine platelet lysate to cell densities of 6.0 × 106 cells mL−1 in 10% platelet lysate as compared to approximately 5.0 × 106 cells ml−1 in 5% FBS (Fig. 2). The CHO cells were growing both as attached cells and in suspension. In the platelet lysate medium the CHO cells grew adherently until one layer of confluence was reached and then they continued to grow in suspension. The morphology of the cells changed after day three in the lysate supplemented medium where the cells tended to be more stretched out than in FBS supplemented medium. Higher concentrations of porcine platelet lysate resulted in more attachment of cells.
Fig. 2.
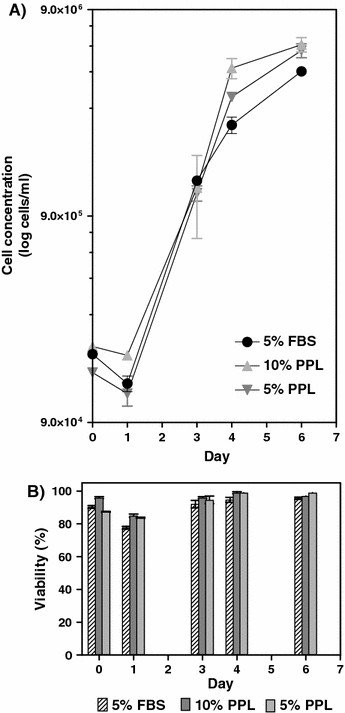
(a) Cell growth and (b) Viability of CHO cells cultured in porcine platelet lysate (PPL) and FBS supplemented media. Error bars represent the SEM value
Proliferation of 39.5 hybridoma cells
The 39.5 cells grew to approximately 2.3 × 106 cells mL−1 in both platelet lysate (10%) and FBS (5%) (Fig. 3). The viability was decreasing rapidly in both media which is typical for a hybridoma culture (showing apoptotic cell death). Although differences in growth curves are seen with 5 and 10% platelet lysate as also evidenced with Vero- and CHO cells, this can be also attributed to other factors such as variations in seeding concentration. More studies need to be performed to confirm the correlation between growth and amount of medium.
Fig. 3.
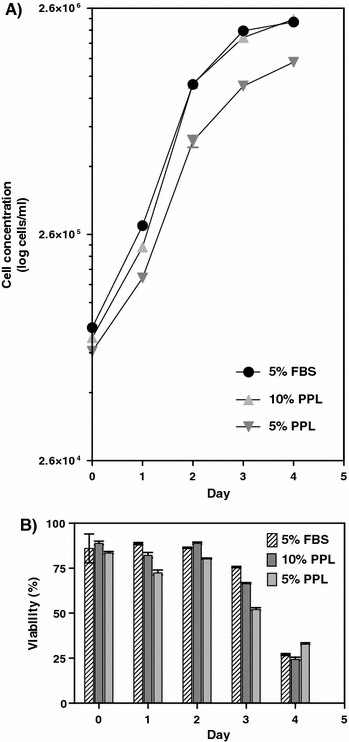
(a) Cell growth and (b) Viability of 39.5 hybridoma cells cultured in porcine platelet lysate (PPL) and FBS supplemented media. Error bars represent the SEM value
Variability of platelet lysate batches
The lot-to-lot consistency of supplements is an important quality parameter of a cell culture medium. The fluctuations in fetal bovine serum composition from batch-to-batch is a well known problem (Jochems 1997) partly due to small size of batches (500–2000 L) used. The batch variation can compromise the scientific relevance of the cell culture study and for that reason it can be difficult to achieve the same result in a repeated cell culture study.
We evaluated the variation of three porcine platelet lysate batches (batch size: 1.5 L) by establishing growth curves on Vero- and 39.5 hybridoma cells. As can be seen from Fig. 4a and b, the growth pattern was similar in all three batches indicating small variability between batches. Furthermore, the chemical profile of the three batches indicated small variations (less than 5% in variation) (data not shown). As porcine blood is in abundant supply very large batches can be produced with over 10,000 L per batch and they would be more uniform between lots.
Fig. 4.
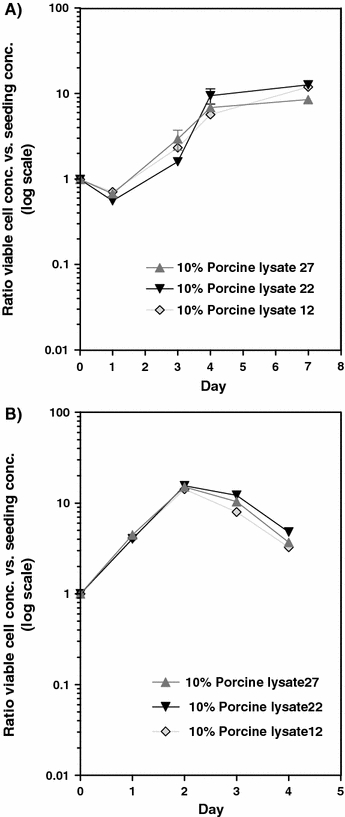
(a) Growth curves of Vero cells with the ratio between viable cell concentration and the seeding concentration. Error bars represent the SEM value. (b) Growth curves of 39.5 hybridoma cells with the ratio between viable cell concentration and the seeding concentration. Error bars represent the SEM value
Conclusions
We have demonstrated in this work as well as earlier studies (Johansson et al. 2003) that a platelet lysate both from porcine- and bovine origin can sustain the proliferation of various animal cells both anchorage dependent and growing in suspension. Other studies have shown the benefits of using platelet enriched human plasma fractions for the proliferation of corneal epithelial cells to treat ocular surface diseases (Geerling et al. 2005), in stem-cell culture for bone regeneration (Watatani et al. 2005) and in oral mucosal wound healing (Lindeboom et al. 2007).
By optimization of production procedures of platelet lysate and the access to vast supplies of raw material, large lots can be produced with minimum batch-to-batch variation. The use of porcine medium may be a useful alternative to bovine sources when e.g. BSE is an issue for concern. These media should offer an attractive alternative to fetal bovine serum especially as a general supplement for research work on expression studies and culture of animal-derived cells. Furthermore, the platelet lysate can be produced cost-efficiently (10–25% of the cost of production of FBS) and in large batches (>10,000 L) with small variations from batch-to-batch. Also, if ethical concerns are considered the use of adult bovine or porcine blood as a provider of raw material of for example platelet lysate may present a higher ethical quality as it has a lower severity index than fetal bovine serum (Jochems 1997).
Acknowledgements
Financial support from the University of Kalmar, Ellco Food AB, ProLiff AB and the Knowledge Foundation is gratefully acknowledged.
Abbreviations
- FBS
Fetal bovine serum
- PDGF
Platelet derived growth factor
- TGF-β
Transforming growth factor-β
- BSE
Bovine spongiform encephalopathy
- DMEM
Dulbecco’s Modified Eagle’s Medium
- SEM
Standard error of the mean
- CHO
Chinese hamster ovary
- SFM
Serum free media
- EU
Endotoxins units
- PPL
Porcine platelet lysate
References
- Al-Rubeai M, Welzenbach K, Lloyd DR, Emery AN (1997) A rapid method for evaluation of cell number and viability by flow cytometry. Cytotechnology 24:161–168 [DOI] [PMC free article] [PubMed]
- Balk SD (1971) Calcium as a regulator of the proliferation of normal, but not transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci USA 68(2):271–275 [DOI] [PMC free article] [PubMed]
- Butler M (2005) Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol 68:283–291 [DOI] [PubMed]
- Geerling G, Liu L, Harloff S, Wedel T, Hartwig D (2005) Platelet releasate: a potential treatment for ocular surface disease. The Ocular Surf 3:S64
- Jochems, CEA (1997) Use, trade and harvest of livestock sera. In Department of Laboratory Animal Sciences. Utrecht University, Utrecht, pp 1–64
- Jochems CEA, van der Valk JBF, Stafleu FR, Baumans V (2002) The use of fetal bovine serum: ethical or scientific problem? ATLA- Alternatives to Laboratory Animals 30:219–227 [DOI] [PubMed]
- Johansson L, Klinth J, Holmqvist O, Ohlson S (2003) Platelet lysate: A Replacement for Fetal Bovine Serum in Animal Cell Culture. Cytotechnology 42:67–74 [DOI] [PMC free article] [PubMed]
- Lindeboom JAH, Mathura KR, Aartman IHA, Kroon FHM, Milstein DMJ, Ince C 2007. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin Oral Implant Res 18:133–139 [DOI] [PubMed]
- Merten O-W (2006) Introduction to animal cell culture technology–past, present and future. Cytotechnology 50:1–7 [DOI] [PMC free article] [PubMed]
- Persson A, Alfredsson N, Christensson K, Ohlson S, Holmqvist O (2006) Blood platelet lysate and method for producing the same. PCT application WO 2006/137778A1. Proliff AB
- Ross R, Glomset J, Kariya B, Harker L (1974) A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA 71(4):1207–1210 [DOI] [PMC free article] [PubMed]
- van dert Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, Thalen M, Baumans V (2004) The Humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro 18:1–12 [DOI] [PubMed]
- Watatani S, Terashi H, Saigo K, Yokoyama M, Yamamoto M, Yokoo S, Komori T (2005) Autologous platelet-rich plasma (PRP) is more useful than fetal calf serum (FCS) in adipose-derived stem cells (ADSCS) culture for bone regeneration. Int J Oral Maxillofac Surg 34:123 [DOI]
- Whitford WG (2005) Supplementation of animal cell culture media. BioProcess Int 3:28–36


