Abstract
Leguminous plants in symbiosis with rhizobia form either indeterminate nodules with a persistent meristem or determinate nodules with a transient meristematic region. Sesbania rostrata was thought to possess determinate stem and root nodules. However, the nature of nodule development is hybrid, and the early stages resemble those of indeterminate nodules. Here we show that, depending on the environmental conditions, mature root nodules can be of the indeterminate type. In situ hybridizations with molecular markers for plant cell division, as well as the patterns of bacterial nod and nif gene expression, confirmed the indeterminate nature of 30-day-old functional root nodules. Experimental data provide evidence that the switch in nodule type is mediated by the plant hormone ethylene.
Keywords: Azorhizobium caulinodans/phytohormones/tropical legume/waterlogging
The interaction between rhizobia and legumes leads to the formation of nitrogen-fixing root nodules that, based on morphology, development, and physiology, have been classified into two main types, indeterminate and determinate (for a review, see ref. 1). It is generally accepted that the nodule type is fixed and depends on the host plant; the most common nodule type is indeterminate (J. Sprent, personal communication). Indeterminate nodules occur on temperate legumes and on the majority of tropical legumes; they originate from the inner cortex, and have a cylindrical shape because of a persistent apical meristem that continues to function for the full lifespan of the nodule. Determinate nodules occur on certain tropical tribes, including several important crop species (J. Sprent, personal communication); they often develop from the outer cortex, and the meristematic activity is transient, giving rise to round nodules.
Sesbania rostrata is a semiaquatic tropical legume that grows during the rainy season in immersed soils in the Sahel region of West Africa. As an adaptation to flooding, it carries adventitious root primordia along the stem. These primordia develop into roots upon submergence. Inoculation with the microsymbiont Azorhizobium caulinodans ORS571 (2) or with some Sinorhizobium spp. (3, 4) induces stem-borne nodules at the bases of the adventitious rootlets, whereas on the root system, nodules are formed at the bases of lateral roots. Both stem-borne nodules and root nodules have been described as being of the determinate type, although the development of both was found to be of a hybrid nature (5–8). Developing nodules induced by A. caulinodans show a transient coexistence of different developmental zones, and thus resemble indeterminate nodules. However, after a week, nodule meristem activity stops and mature nodules are round and determinate.
Here, we show that depending on the environmental conditions, mature root nodules of S. rostrata plants can be of the indeterminate type. Molecular markers for cell division and parenchyma formation were used to demonstrate differences between determinate S. rostrata nodules. Our observations illustrate a case of phenotypic plasticity in plant development. We also present experimental evidence for a role of the plant hormone ethylene in the determination of the nodule type on the S. rostrata roots.
MATERIALS AND METHODS
Bacteria.
Azorhizobium caulinodans ORS571 and plasmids pRG960SD-32 and pRS2002 have been described previously (9, 10). The strains were grown at 37°C in YEB medium (0.5% beef extract/0.1% yeast extract/0.5% peptone/0.5% sucrose/2 mM MgSO4) (11).
Plants.
S. rostrata Brem seeds were surface-sterilized as described by Goethals et al. (12). After 2 days of germination at 28°C in the dark, the seedlings were transferred to Leonard jars, tubes, or pots. Nutrient solution for Leonard jars and tubes was nitrogen-free Norris medium, pH 7.0 (13). The upper part of the Leonard jars was filled with vermiculite, and the seedlings were inoculated with 1 ml of an overnight culture of A. caulinodans ORS571 and covered with perlite. The tubes (20 × 200 mm) were provided with a filter paper strip for root support, an aluminum foil cap, and an aluminum foil cover. After 7 days, the plantlets were inoculated with 100 μl of an overnight culture of A. caulinodans ORS571. In assays for stem nodulation, pot-grown plants were treated according to Goormachtig et al. (14), but after inoculation the stem was covered with a cardboard cylinder of 4-cm diameter to exclude light. After inoculation, the plants were grown for approximately 1 month at 37°C during the day (16-h) and at 25°C during the night (8-h) period.
In root nodulation assays, Ag2SO4 (10 μM final concentration), 1-aminocyclopropane-1-carboxylic acid (ACC; 20 μM final concentration), and 2-chloroethylphosphonic acid (CEPA; 20 μM final concentration) were added to the Norris medium 5 days after inoculation, and then twice per week. In the stem assay, a solution of 100 μM Ag2SO4 was sprayed twice per week. After inoculation, the number of nodules was counted after 25 days in the tubes and after 30 days in the Leonard jars. Nodules used for in situ hybridization or for β-glucuronidase or β-galactosidase staining were harvested approximately 30 days after inoculation.
Zonation of Indeterminate Nodules.
For histological observations, indeterminate nodules were embedded in Technovit as described by the manufacturer (Kulzer, Wehrheim, Germany). Semithin sections (2 μm) were cut on a microtome (Reichter-Jung, Nussloch, Germany), mounted on Vectabond-coated slides (Vector Laboratories), and stained with 0.5% toluidine blue solution. After drying, the slides were mounted with Depex (BDH) and analyzed in bright field using a Diaplan microscope (Leitz).
In situ hybridizations were performed as described by Goormachtig et al. (8). Probes of H4–1Sr, Sesro;CycB1;1, and SrEnod2 have been described previously (8, 15).
Staining with β-glucuronidase and sectioning of nodules were carried out according to Van den Eede et al. (9). After staining, the nodules were embedded in 5% agarose, and 100-μm sections were made by using a vibro-cutter. Staining for β-galactosidase was performed on 100-μM sections according to Boivin et al. (16).
RESULTS
Two Types of Nodules Can Develop on S. rostrata Roots.
We have used A. caulinodans as inoculant in the nodulation system of Ndoye et al. (7), in which germinated seeds are transferred to test tubes that contain Jensen’s nitrogen-free medium on agar slants and the shoot is allowed to grow out of the tube. Irrespective of whether the roots are grown in the dark (tubes covered with aluminum foil) or in alternating light/dark conditions, we have observed only determinate nodules. Here, S. rostrata plants were grown in Leonard jars (13) on nitrogen-free Norris medium under a 16-h photoperiod. Large, cylindrical, pink nodules appeared on the dark-grown part of the root system in vermiculite, whereas green determinate nodules were formed on parts of the same root protruding out of the vermiculite in the lower compartment (Fig. 1). The seedling inoculation procedure also led to nodulation of the lower part of the stem, and these nodules were pink or green depending on the degree of light shielding, but they were always of the determinate type (Fig. 1).
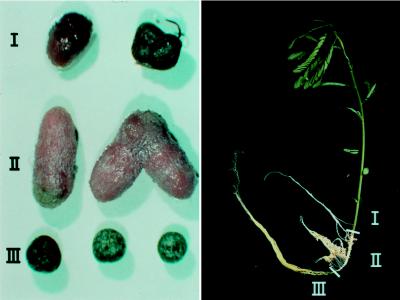
Figure 1.
Determinate and indeterminate nodules formed on the same Sesbania rostrata plant. (Left) Nodules harvested from different parts of the host plant as indicated on the Right. Region I corresponds to the lower part of the stem, region II is the part of the root grown in the dark in the vermiculite compartment of the Leonard jar, and region III is the youngest part of the root, which protrudes in the lower compartment of the Leonard jar.
Histology and Molecular Markers Confirm the Indeterminate Nodule Type.
Elongated root nodules, when stained with toluidine blue, showed a zonation pattern typical of indeterminate nodules, possessing an apical (distal) meristem, followed by an invasion zone, and a huge fixation zone of infected and uninfected plant cells (Fig. 2A).
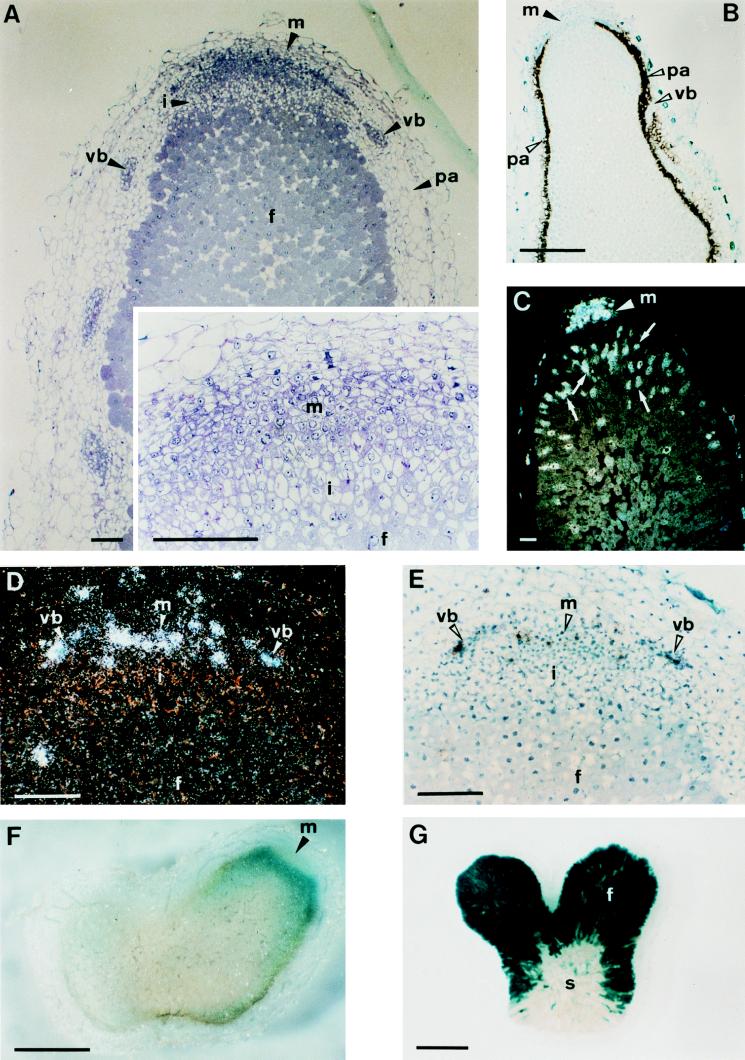
Figure 2.
Zonation of indeterminate 30-day-old Sesbania rostrata nodules. (A) Longitudinal, 2-μm section of a nodule embedded in Technovit and stained with toluidine blue. The Inset is a magnification of the apical part. (B) Bright-field image of an in situ hybridization with a SrEnod2 antisense probe in a 10-μm longitudinal section. Silver grains are seen as black dots. (C) Dark-field image of an in situ hybridization with a histone H4–1Sr antisense probe. Silver grains are seen as white dots. (D and E) Dark- and bright-field images of an in situ hybridization with a Sesro;CycB1;1 antisense probe. (F) Section (100-μm) of a β-glucuronidase-stained nodule induced by Azorhizobium caulinodans ORS571 (pRG960SD-32) harboring a plasmid with a nodA-gusA fusion. (G) β-Galactosidase-stained 100-μm section of a nodule induced by A. caulinodans ORS571 (pRG2002) harboring a plasmid with a nifH-lacZ fusion. Arrowheads indicate the different nodule tissues and hybridization signals and arrows (C) point out cells with endoreduplication. Abbreviations: f, fixation zone; i, invasion zone; m, meristem; pa, nodule parenchyma; s, senescence zone; vb, vascular bundle. [Bars = 100 μm (A and C–E) or 1 mm (B, F, and G).]
During the ontogeny of determinate root and stem nodules, the meristem disappears approximately 8 days after inoculation. The central tissue of the mature nodules does not show any zonation (7). By using molecular markers for cell division (Sesro;CycB1;1, and H4–1Sr) and parenchyma formation (SrEnod2), Goormachtig et al. (8, 15) confirmed the transient nature of the meristematic activity in determinate S. rostrata nodules and showed that SrEnod2 expression surrounded completely the fully differentiated central tissue.
The same markers were used to investigate the expression pattern in mature 30-day-old elongated root nodules. In situ hybridizations of 10-μm sections showed a patchy pattern of cyclin and histone H4 transcript accumulation in the narrow apical zone of small cells (Fig. 2 C–E). H4 transcripts, but no cyclin transcripts, were found in sporadic cells of the younger part of the fixation zone (Fig. 2C). This observation indicates the occurrence of DNA synthesis without cell division. The SrEnod2 transcript accumulation pattern was typical for indeterminate nodules with an apical region devoid of expression (Fig. 2B). This scenario is in sharp contrast with that obtained with mature determinate nodules on S. rostrata (15).
The coexistence of different developmental zones was further illustrated by the patterns of bacterial nif and nod gene expression. Plants were inoculated with A. caulinodans derivatives that harbored a nifH-lacZ fusion (pRS2002) (10) or a nodA-uidA gene fusion (pRG960SD-32) (9). nodA promoter-directed β-glucuronidase activity appeared as a band in the apical part of 30-day-old nodules (Fig. 2F), and nifH promoter-directed β-galactosidase activity was present in invaded plant cells of the fixation zone (Fig. 2G). These patterns are very similar to those observed by Sharma and Signer (17) in indeterminate alfalfa nodules induced by Rhizobium meliloti.
Ethylene-Mediated Control of Nodule Plasticity.
In a first attempt to define physiological factors that mediate the switch from a transient to a persistent meristem during S. rostrata root nodule development, the putative involvement of the plant hormone ethylene was investigated. We obtained evidence that ethylene, known to be induced by light and other stresses in plant roots (18), is involved in nodule type determination. We observed that in a tube assay, the formation of indeterminate nodules on roots could be triggered by the addition of Ag+ ions, which are widely used inhibitors of ethylene action (19). A variant of the tube assay (4) was used with Norris medium and without agar slant (see Materials and Methods). The tubes were covered with aluminum foil to keep the roots in darkness. Control roots and roots grown in the presence of ACC, the immediate precursor of ethylene, or CEPA, an ethylene-releasing compound, developed determinate nodules. In the presence of silver ions (10 μM Ag2SO4; see Materials and Methods), root nodules became indeterminate (Table 1).
Table 1.
Root nodulation assays on S. rostrata
| Treatment | Tube assay
|
Leonard jar assay
|
||
|---|---|---|---|---|
| Type | Number | Type | Number | |
| Control | D | 100.0 ± 11.6 a | I | 34.2 ± 2.1 a |
| 10 μM Ag2SO4 | I | 20.1 ± 2.0 b | I | 33.0 ± 4.7 a |
| 20 μM ACC | D | 105.2 ± 10.3 a | D | 45.0 ± 6.6 a |
| 20 μM CEPA | D | ND | D | 44.8 ± 5.1 a |
Type (D, determinate; I, indeterminate) and number (average of three independent experiments with 10 plants each) of mature nodules developed on S. rostrata roots grown in tubes or in the vermiculite compartment of Leonard jars are tabulated. The same letter in a column indicates numbers that are significantly different (P > 0.05). ND, not determined.
In a complementary experiment, Ag+ ions, ACC, or CEPA was added to the Leonard jar system (see Materials and Methods). In this experiment, the dark-grown part of control and Ag2SO4-treated plant roots developed indeterminate nodules, whereas the dark-grown part of ACC and CEPA-treated roots developed determinate nodules (Table 1).
Silver ions caused a significant reduction in nodule number in the test tube assay but not in the Leonard jars (Table 1). ACC or CEPA had no statistically significant effects (Table 1). Finally, the addition of silver ions affected the size of the plants in both assay systems (data not shown). This effect of silver ions on plant size was also observed in non-nodulated plants grown in the presence of 1 mM ammonium nitrate and therefore is not related to ethylene effects on nodulation or nitrogen fixation (data not shown).
Typically elongated indeterminate nodules were not observed on stems. When plants were grown under conditions that allow indeterminate nodule formation on roots, but the stems were inoculated under both light and dark conditions, only round determinate stem nodules were obtained, even when silver ions were applied locally (data not shown).
DISCUSSION
Phenotypic plasticity is observed in many organisms, especially in plants, as an adaptation to changing environmental conditions (20, 21). Our observations illustrate plasticity in S. rostrata root nodule development. We have observed that mature root nodules on S. rostrata can be either determinate or indeterminate depending on conditions of root growth. The occurrence of two nodule types on the same host plant is interesting in light of the origin of the determinate nodule type (nodule phylogeny). Indeed, determinate nodules are thought to have originated independently from indeterminate nodules several times (22). In this scenario, indeterminate nodules represent a “ground state” (23) in the evolution of legume nodulation.
Mature, determinate nodules appear on S. rostrata as an environmentally imposed premature termination of an ontogenetic phase that persists when indeterminate nodules are formed. Perhaps this plasticity is related to the special adaptation of this tropical legume to growth under waterlogged conditions, for which it may be an advantage to keep features of plasticity that have been lost in other tropical legumes.
Which physiological factors play a role in the determination of the nodule type? Experimental data suggest a major role of the plant hormone ethylene. A first clue came from the observation that in a tube assay under conditions that normally favor the formation of determinate root nodules, indeterminate nodules were formed in the presence of silver ions, which are inhibitors of ethylene action. Further experiments showed that in a Leonard jar assay system, in which normally indeterminate nodules are formed, the addition of an ethylene precursor (ACC) or an ethylene-releasing compound (CEPA) caused the formation of determinate nodules.
Ethylene is a plant hormone that has already been implicated in phenotypic plasticity in plants. For example, it has been shown to partly control the stem elongation plasticity in Stellaria longipes (24) by the differential regulation of members of the ACC synthase gene family (25). As far as nodulation is concerned, exogenously applied ethylene is a potent inhibitor of nodulation in many systems, blocking cortical cell division and reducing the number of cortical infections (26–28). Not all legumes, however, show the same susceptibility to exogenous ethylene, e.g., ethylene-insensitive cultivars of soybean have been described (29). Also, S. rostrata is less sensitive to ethylene, because exogenous ACC or CEPA did not negatively influence root nodulation.
Recent data in the literature indicate a role for endogenous ethylene in control of nodulation e.g., by determining the position of nodule primordium formation in pea (30) and by restricting the number of persistent bacterial infections in alfalfa (31). In S. rostrata roots, differential ethylene concentrations may determine the fate of nodule development by influencing the persistence of the nodule meristem. Differential ethylene concentrations could be caused by mechanisms interfering with ethylene production, diffusion, or perception. Gas diffusion, for instance, is probably different in soil- or vermiculite-grown than in waterlogged roots. It is striking that indeterminate nodules were never observed (neither in the light nor in the dark) on stems of S. rostrata. Stem nodules develop in the air, where they are exposed to higher oxygen concentrations, and they may have a greater oxygen barrier in the nodule cortex than root nodules (32). Recently, by using O2-specific microelectrodes, James et al. (33) illustrated the presence of an enhanced gaseous diffusing barrier in the mid-inner cortices of stem nodules. Perhaps such a barrier also diminishes outward diffusion of ethylene, leading to endogenous concentrations that are incompatible with indeterminate nodule formation.
The environmental signals that influence the nodule type can be multiple and complex, but our data suggest that a major factor could be the vermiculite versus water support of roots, which may reflect conditions of aerated soil versus waterlogging in nature. The adjustment of nodule type and number could thus be related to the semiaquatic nature of S. rostrata and its capacity to grow and nodulate well under submerged conditions.
Acknowledgments
The authors thank Janet Sprent, Euan James, and William Broughton for helpful discussions and valuable suggestions, Dominique Van Der Straeten and Vladimir Mironov for critical reading of the manuscript, and Martine De Cock for help preparing it. This work was supported by a grant from the European Union Training and Mobility Network (CHRX-CT94-0656). W.D. is indebted to the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-Technologisch Onderzoek in de Industrie for a predoctoral fellowship. M.H. is a Research Director of the Fund for Scientific Research (Flanders).
ABBREVIATION
- ACC
1-aminocyclopropane-1-carboxylic acid
- CEPA
2-chloroethylphosphonic acid
References
- 1. Hirsch A M. New Phytol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfus B, Garcia J L, Gillis M. Int J Syst Bacteriol. 1988;38:89–98. [Google Scholar]
- 3.Tomekpe K, Dreyfus B, Holsters M. Can J Microbiol. 1996;42:187–190. [Google Scholar]
- 4.Boivin C, Ndoye I, Lortet G, Ndiaye A, de Lajudie P, Dreyfus B. Appl Environ Microbiol. 1997;63:1040–1047. doi: 10.1128/aem.63.3.1040-1047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsien H C, Dreyfus B L, Schmidt E L. J Bacteriol. 1983;156:888–897. doi: 10.1128/jb.156.2.888-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duhoux E. Can J Bot. 1984;62:982–994. [Google Scholar]
- 7.Ndoye I, de Billy F, Vasse J, Dreyfus B, Truchet G. J Bacteriol. 1994;176:1060–1068. doi: 10.1128/jb.176.4.1060-1068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goormachtig S, Alves-Ferreira M, Van Montagu M, Engler G, Holsters M. Mol Plant–Microbe Interact. 1997;10:316–325. doi: 10.1094/MPMI.1997.10.3.316. [DOI] [PubMed] [Google Scholar]
- 9.Van den Eede G, Deblaere R, Goethals K, Van Montagu M, Holsters M. Mol Plant–Microbe Interact. 1992;5:228–234. doi: 10.1094/mpmi-5-228. [DOI] [PubMed] [Google Scholar]
- 10.Kaminski P A, Elmerich C. Mol Microbiol. 1991;5:665–673. doi: 10.1111/j.1365-2958.1991.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 11.Geremia R A, Mergaert P, Geelen D, Van Montagu M, Holsters M. Proc Natl Acad Sci USA. 1994;91:2669–2673. doi: 10.1073/pnas.91.7.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goethals K, Gao M, Tomekpe K, Van Montagu M, Holsters M. Mol Gen Genet. 1989;219:289–298. doi: 10.1007/BF00261190. [DOI] [PubMed] [Google Scholar]
- 13.Vincent J M. A Manual for the Practical Study of the Root-Nodule Bacteria. Oxford: Blackwell; 1970. , IBP Handbook No. 15. [Google Scholar]
- 14.Goormachtig S, Valerio-Lepiniec M, Szczyglowski K, Van Montagu M, Holsters M, de Bruijn F J. Mol Plant–Microbe Interact. 1995;8:816–824. doi: 10.1094/mpmi-8-0816. [DOI] [PubMed] [Google Scholar]
- 15.Goormachtig S, Van Montagu M, Holsters M. Mol Plant–Microbe Interact. 1998;11:237–241. doi: 10.1094/MPMI.1997.10.3.316. [DOI] [PubMed] [Google Scholar]
- 16.Boivin C, Camut S, Malpica C A, Truchet G, Rosenberg C. Plant Cell. 1990;2:1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S B, Signer E R. Genes Dev. 1990;4:344–356. doi: 10.1101/gad.4.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Eliasson L, Bollmark M. Physiol Plant. 1988;72:605–609. [Google Scholar]
- 19.Beyer E M., Jr Plant Physiol. 1976;58:268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan H S, Pigliucci M, Schlichting C D. BioEssays. 1997;19:519–525. doi: 10.1002/bies.950190611. [DOI] [PubMed] [Google Scholar]
- 21.Voesenek L A C J, Blom C W P M. J Ecol. 1996;84:111–119. [Google Scholar]
- 22.Doyle J J. Annu Rev Ecol Syst. 1994;25:325–349. [Google Scholar]
- 23.Hirsch A M, LaRue T A. Crit Rev Plant Sci. 1997;16:361–392. [Google Scholar]
- 24.Emery R J N, Reid D M, Chinnappa C C. Plant Cell Environ. 1994;17:691–700. [Google Scholar]
- 25.Kathiresan A, Nagarathna K C, Moloney M M, Reid D M, Chinnappa C C. Plant Mol Biol. 1998;36:265–274. doi: 10.1023/a:1005994118535. [DOI] [PubMed] [Google Scholar]
- 26.Lee K H, LaRue T A. Plant Physiol. 1992;100:1759–1763. doi: 10.1104/pp.100.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grobbelaar, N., Clarke, B. & Hough, M. C. (1971) Plant Soil, Spec. Vol. 1971, 215–223.
- 28.Goodlass G, Smith K A. Plant Soil. 1979;51:387–395. [Google Scholar]
- 29.Xie Z-P, Staehelin C, Wiemken A, Boller T. J Plant Physiol. 1996;149:690–694. [Google Scholar]
- 30.Heidstra R, Yang W C, Yalcin Y, Peck S, Emons A, van Kammen A, Bisseling T. Development (Cambridge, UK) 1997;124:1781–1787. doi: 10.1242/dev.124.9.1781. [DOI] [PubMed] [Google Scholar]
- 31.Penmetsa R V, Cook D R. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- 32.James E K, Iannetta P P M, Nixon P J, Whiston A J, Peat L, Crawford R M M, Sprent J I, Brewin N J. Plant Cell Environ. 1996;19:895–910. [Google Scholar]
- 33.James E K, Minchin F R, Oxborough K, Cookson A, Baker N R, Witty J F, Crawford R M M, Sprent J I. Plant J. 1998;13:29–38. [Google Scholar]