Abstract
Diabetic foot ulcers are known to have a biomechanical etiology. Among the mechanical factors that cause foot lesions, shear stresses have been either neglected or underestimated. The purpose of this study was to determine various plantar pressure and shear variables in the diabetic and control groups and compare them. Fifteen diabetic patients with neuropathy and twenty non-diabetic subjects without foot symptoms were recruited. Subjects walked on a custom built platform capable of measuring local normal and tangential forces simultaneously. Pressure-time integral quantities were increased by 54% (p=0.013) in the diabetic group. Peak AP and resultant shear magnitudes were found to be 32% larger (p<0.05) even though diabetic subjects walked at a slower velocity. Lower AP and ML stress range (peak-to-peak) values were observed in the control subjects (p<0.05). Shear-time integral values were increased in the diabetic group by 61% and 132% for AP and resultant shear cases, respectively (p<0.05). Plantar shear is known to be effective in callus formation which has previously been associated with higher ulcer incidence. During gait shear forces are induced with twice the frequency of pressure characteristically. Therefore, plantar shear should be investigated further from a broader perspective including the temporal specifics and fatigue failure characteristics of the affected plantar tissue.
Keywords: Foot biomechanics, diabetes, diabetic ulcers, plantar stresses, plantar shear, diabetic neuropathy, diabetic foot lesions
Introduction
Diabetic foot complications continue to impair patients' lives and burden the healthcare system. In the U.S.A. alone, in 2002 there were at least 82,000 diabetes related lower extremity amputations. The initiating factor in more than 85% of these amputations was a foot ulcer (Apelqvist and Larsson, 2000). The etiology of diabetic foot ulcers is still being investigated; however the major contributing factors are believed to be the presence of peripheral neuropathy and mechanical stresses acting on the sole of the foot. In fact, Brand has held “repetitive moderate stresses” responsible for tissue breakdown (Brand, 1979).
Despite the fact that the plantar foot experiences three dimensional force vectors during gait, only the vertical component has been studied thoroughly. This may be attributable to the fact that measurement of the horizontal components, anteroposterior (AP) and mediolateral (ML) shear, is technically challenging (Perry et al, 2002).
Studies that have tried to associate the only measurable stress component with ulcer occurrence did not yield promising outcomes (Veves et al, 1992; Armstrong et al, 1998; Lavery et al, 2003). Even though elevated pressure levels have been shown to increase ulceration risk, only a relatively low correlation was seen between the maximal pressure sites and the prospective ulcer sites (Veves et al, 1992; Murray et al, 1996). As a result, foot pressure by itself has been labeled as a “poor tool” in the prediction of diabetic ulcers and where they occur (Lavery et al, 2003).
In the previous plantar shear studies, peak shear stress values were chosen as the major indicative parameter similar to the pressure studies (Lord and Hosein, 2000; Laing et al, 1992). The results reported by these studies, however, probably yielded an underestimation of plantar shear since maximal stress values do not reveal the application duration or frequency content of the ground reaction forces. To evaluate shear appropriately, it is essential to focus on its temporal characteristics. During a single stance the same local area under the foot can experience stresses in opposite directions, such as due to braking forces in the contact phase and propulsive forces in the push-off phase. This clearly indicates that for a single step the frequency of both AP and ML shear is twice that of pressure. This phenomenon can also be observed in ground reaction force plots.
In order to eliminate the underestimation of plantar shear this study has focused on the stress range, which will be noted as the “peak-to-peak” shear (Figure 1). As a secondary parameter, for the same purpose, shear-time integral has been examined. The aim of this work was to investigate whether these two parameters differ between diabetic and control subjects.
Figure 1.
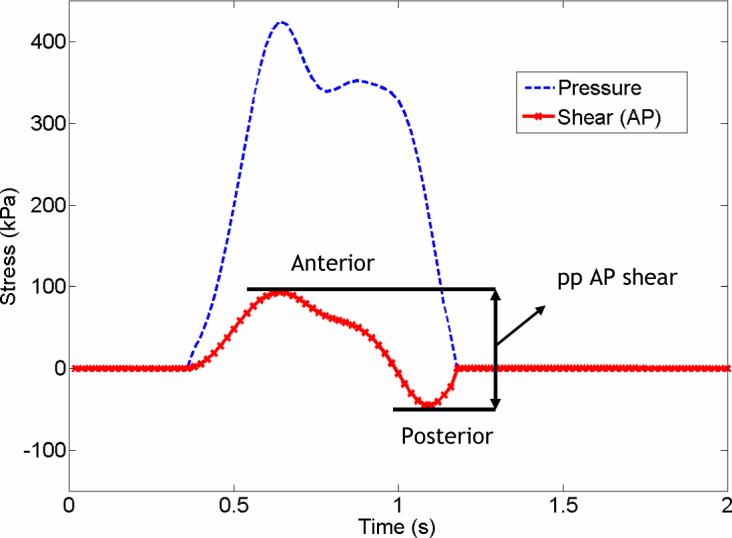
Pressure and shear (AP) curves of a representative diabetic subject obtained by a single transducer. ppAP shear was determined by adding the absolute values of maximum anterior and posterior shear magnitudes for each transducer.
Research Design and Methods
Fifteen subjects diagnosed with diabetic neuropathy and twenty subjects with no clinical foot symptoms were recruited for the study. Peripheral neuropathy was assessed by testing the subjects' feet with a 5.07 Semmes-Weinstein monofilament and a biothesiometer according to Armstrong et al (1998b). Individuals with active foot ulcers, gross foot deformities (minor clawing of the toes was permissible), prior foot surgeries and foot pain were excluded from the study. The protocol was explained to the volunteers before their participation and each signed an informed consent form which was approved by the Institutional Review Board. There was no significant difference in the body mass index values (BMI) between the two groups (p=0.064). Detailed patient characteristics are shown in Table 1.
Table 1.
Characteristics of the subjects
| Diabetic | Control | |
|---|---|---|
| No of subjects | 15 (10 Type I, 5 Type II) | 20 |
| Gender | 3f, 12m | 8f, 12m |
| Age (years) | 60.5 ± 10.1 (44−73) | 45.8 ± 19.8 (17−82) |
| BMI | 29.2 ± 8.0 (19.6−50.7) | 24.9 ± 3.4 (17.3−33.7) |
| Weight (kg) | 90.6 ± 24.6 (60.4−140.7) | 74.6 ± 11.2 (59.0−106.7) |
Values are presented as the mean ± standard deviation, with the range in parentheses.
A custom built shear and pressure platform, 80 sensors (12.5mm × 12.5mm) arranged in an 8×10 array, was used to collect local barefoot forces acting on the foot-ground interface. The data recorded by the platform had an average error percentage of 1.0%, 4.6% and 5.0% respectively for pressure, anteroposterior and medio-lateral shear channels. The detailed specifications of the system have been described elsewhere (Yavuz et al, 2007). Forefoot of the subjects was of primary interest since diabetic ulcers most frequently occur in this area (Oyibo et al, 2001; Caselli et al, 2002). The 2-step method was preferred in data acquisition (McPoil et al, 1999; Bus et al, 2005). The tests were carried out at self selected speeds for only one foot (left or right) with 3 trials for each subject.
Resultant shear stress magnitudes were calculated by vector addition of AP and ML shear stresses. Peak pressure and shear stresses were found by picking the maximal absolute values. AP and resultant (RES) shear-time integral (STI) and pressure-time integral (PTI) values were determined for each transducer and maximal values were obtained. PTI and STI were calculated by implementation of the trapezoidal rule over the shear stress curves using 99 subdivisions. Duration of the stance was identified by the time period from initial contact on the platform until the foot was off the device.
Peak-to-peak (pp) AP stress was determined by adding the absolute values of maximum anterior stress and maximum posterior stress measured on each transducer (Figure 1). pp ML was calculated similarly by adding maximum medial and lateral stresses. Maximal pp AP and ML stress magnitudes of a subject were picked among the sensor-by-sensor calculated values.
An ANOVA test with a significance level of 0.05 was performed on all of the variables to reveal any differences between the diabetic and the control groups. Minitab™ (Minitab Inc, PA) was used to carry out statistical tests.
Results
No significant difference (p=0.083) was found between the groups with regard to peak pressure even though the diabetic group had a 23% greater mean value. Mean PTI, however, was 54% higher in the diabetic group (p=0.013).
Peak AP and resultant shear magnitudes were observed to be 33% and 31% larger in the diabetic group respectively (p=0.014 and p=0.016) even though mean walking speed was less than of the control group (p=0.008). Peak-to-peak stress variables showed a similar trend with an approximate increase of 30% in the diabetic individuals. Statistical analysis yielded significant p values both in pp AP and ML shear cases (Table 2).
Table 2.
Comparison of the shear stress variables among control and diabetic groups
| Control | Diabetic | p value | |
|---|---|---|---|
| Pressure (kPa) | 497.5 (135.2) | 614.2 (246.6) | 0.083 |
| AP Shear (kPa) | 62.3 (15.8) | 83.3 (31.2) | 0.014 |
| RES Shear (kPa) | 70.0 (19.8) | 92.1 (31.6) | 0.016 |
| AP STI (kPa.s) | 11.8 (4.6) | 27.4 (16.6) | 0.000 |
| RES STI (kPa.s) | 22.1 (5.8) | 35.6 (15.1) | 0.000 |
| pp AP Shear (kPa) | 70.2 (17.3) | 91.6 (32.8) | 0.018 |
| pp ML Shear (kPa) | 50.6 (15.1) | 63.6 (18.7) | 0.030 |
| PTI (kPa.s) | 167.3 (54.1) | 257.9 (141.8) | 0.013 |
| Stance duration (sec) | 0.72 (0.10) | 0.83 (0.14) | 0.008 |
Values are presented as the mean with the standard deviation in parentheses.
The increase in the shear-time integral magnitudes was more dramatic. AP and RES STI values were shown to have elevated by 132% and 61%, respectively, both of which were also significantly different.
Discussion
The plantar foot experiences cyclic loading during walking. Among the force components that the plantar aspect of the foot bears, AP and ML shear fundamentally acts twice as frequently as the normal force. This phenomenon might yield to fatigue failure in the skin and the underlying tissues. In fact, investigators have suggested fatigue failure by explaining the formation of diabetic lesions and referring to “repetitive moderate stresses” as the major factors. In this study temporal shear parameters have been the main focus, which are thought to be valuable in the prediction of ulcer formation as they disclose the characteristics of cyclic tangential loading.
Surprisingly the results of the present study did not show a significant difference between the diabetic and control groups regarding peak pressure. However mean peak pressure within the former group was 23% higher. Ctercteko et al (1981), Pitei et al (1999) and Rahman et al (2006) reported similarly lower rates of peak pressure increase (8%, 32% and 15%, respectively) in diabetic subjects.
Higher peak AP and RES shear values have been observed in the diabetic patients even though they walked about 15% slower than the control subjects. The elevated magnitudes of PTI and STI can be partly explained by the increase of contact time. Stress localizations under prominent regions of the foot might also result in increased magnitudes; however, such regions were not identified in this study.
In general, increase in pressure variables (peak pressure and PTI) was less than the increase observed in their shear counterparts (peak AP and RES shear, AP and RES STI). As foot pressure alone has been defined as a “poor” indicator for diabetic ulceration, plantar shear might be more beneficial in predicting ulcers. Moreover, the differences in the locations of peak pressure and shear stress in diabetic patients (Yavuz et al, 2007b) might explain why only moderate correlations between the locations of peak pressure and ulcer have been observed in previous studies (Armstrong et al, 1998; Lavery et al, 2003; Murray et al, 1996).
It is now well documented that diabetic ulcers frequently occur at the same locations with callus (Murray et al, 1996). Early lichenification and hyperkeratosis studies suggest that application of frictional shear forces result in callosities (Goldblum et al, 1954; Mackenzie et al, 1974). In another study by Goldstein and Sanders (1998) where constant pressure and cyclic shear has been applied to animal skin, it has been shown that tissue breakdown occurred earlier when shear forces were increased.
The current study had certain limitations related to the spatial resolution and overall size of the platform. Only barefoot locomotion was assessed and subjects were permitted to visually aim the platform. The decision was based on the report by Grabiner et al (1995) which has shown that targeting of a force plate did not produce different ground reaction force data.
The mean age of both groups differed by 14.7 years. However there was no significant correlation between the shear variables and age of the individuals in both groups. There was however a single exception to this with the RES variable in control group. In this specific case, RES decreased slightly with age in control subjects. This implicated that a control group with a higher mean age would most possibly increase the statistical significance.
In summary, this study measured shear-time integral and shear stress range on the plantar aspect of human feet. Our results indicate that diabetic subjects have significantly higher peak-to-peak AP and ML shear magnitudes and dramatic increases in shear-time integral values. In this regard, plantar shear has the potential to fill gaps in our understanding of the complex etiology of plantar ulceration.
Acknowledgements
This research was possible due to support from National Institutes of Health (Grant # 2R44DK061164). The authors would like to thank Gordon Hirschman and Lynn Bardsley of Infoscitex, Inc for their kind input during the course of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16(1):S75–83. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr139>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? Journal of Foot and Ankle Surgery. 1998;37(4):303–7. doi: 10.1016/s1067-2516(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Lavery LA, Harkless LB. Who is at risk for diabetic foot ulceration? Clin Podiatr Med Surg. 1998b;15:11–9. [PubMed] [Google Scholar]
- Brand PW, Hall OC. The etiology of the neuropathic plantar ulcer. Journal of the American Podiatry Association. 1979;69(3):173–7. doi: 10.7547/87507315-69-3-173. [DOI] [PubMed] [Google Scholar]
- Bus SA, de Lange A. A comparison of the 1-step, 2-step, and 3-step protocols for obtaining barefoot plantar pressure data in the diabetic neuropathic foot. Clinical Biomechanics (Bristol, Avon) 2005;20(9):892–9. doi: 10.1016/j.clinbiomech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Caselli A, Pham H, Giurini JM, Armstrong DG, Veves A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care. 2002;25(6):1066–1071. doi: 10.2337/diacare.25.6.1066. [DOI] [PubMed] [Google Scholar]
- Ctercteko GC, Dhanendran M, Hutton WC, Le Quesne LP. Vertical forces acting on the feet of diabetic patients with neuropathic ulceration. British Journal of Surgery. 1981;68:608–614. doi: 10.1002/bjs.1800680904. [DOI] [PubMed] [Google Scholar]
- Goldblum RW, Piper WN. Artificial lichenification produced by a scratching machine. Journal of Investigative Dermatology. 1954;22(5):405–15. doi: 10.1038/jid.1954.57. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Sanders J. Skin response to repetitive mechanical stress: a new experimental model in pig. Arch Phys Med Rehabil. 1998;79(3):265–72. doi: 10.1016/s0003-9993(98)90005-3. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Feuerbach JW, Lundin TM, Davis BL. Visual guidance to force plates does not influence ground reaction force variability. Journal of Biomechanics. 1995;28(9):1115–1117. doi: 10.1016/0021-9290(94)00175-4. [DOI] [PubMed] [Google Scholar]
- Laing P, Deogan H, Cogley D, Crerand, Hammond P, Klenerman L. The development of the low profile Liverpool shear transducer. Clin Phys Physiol Meas. 1992;13(2):115–24. doi: 10.1088/0143-0815/13/2/002. [DOI] [PubMed] [Google Scholar]
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care. 2003;26(4):1069–73. doi: 10.2337/diacare.26.4.1069. [DOI] [PubMed] [Google Scholar]
- Lord M, Hosein R. A study of in-shoe plantar shear in patients with diabetic neuropathy. Clinical Biomechanics. 2000;15:278–83. doi: 10.1016/s0268-0033(99)00076-5. [DOI] [PubMed] [Google Scholar]
- MacKenzie IC. The effects of frictional stimulation on mouse ear epidermis. Journal of Investigative Dermatology. 1974;63(2):194–8. doi: 10.1111/1523-1747.ep12679356. [DOI] [PubMed] [Google Scholar]
- McPoil TG, Cornwall MW, Dupuis L, Cornwell M. Variability of plantar pressure data. A comparison of the two-step and midgait methods. Journal of the American Podiatric Medicine Association. 1999;89(10):495–501. doi: 10.7547/87507315-89-10-495. [DOI] [PubMed] [Google Scholar]
- Murray HJ, Young MJ, Hollis S, Boulton AJM. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabetic Medicine. 1996;13:979–982. doi: 10.1002/(SICI)1096-9136(199611)13:11<979::AID-DIA267>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, Boulton AJ. The effects of ulcer size and site, patient's age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabetic Medicine. 2001;18(2):133–8. doi: 10.1046/j.1464-5491.2001.00422.x. 2001. [DOI] [PubMed] [Google Scholar]
- Perry JE, Hall JO, Davis BL. Simultaneous measurement of plantar pressure and shear forces in diabetic patients. Gait Posture. 2002;15(1):101–7. doi: 10.1016/s0966-6362(01)00176-x. [DOI] [PubMed] [Google Scholar]
- Pitei DL, Lord M, Foster A, Wilson S, Watkins PJ, Edmonds ME. Plantar pressures are elevated in the neuroischemic and the neuropathic diabetic foot. Diabetes Care. 1999;22(12):1966–70. doi: 10.2337/diacare.22.12.1966. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Aziz Z, Acharya UR, Ha TP, Kannathal N, Law C, Subramaniam T, Shuen WY, Fang SC. Analysis of plantar pressure in diabetic type 2 subjects with and without neuropathy. ITBM-RBM. 2006;27:46–55. [Google Scholar]
- Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35(7):660–3. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- Yavuz M, Botek G, Davis BL. Plantar shear stress distributions: Comparing actual and predicted frictional forces at the foot-ground interface. Journal of Biomechanics. 2007 doi: 10.1016/j.jbiomech.2007.02.006. doi:10.1016/j.jbiomech.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz M, Erdemir A, Botek G, Hirschman GB, Bardsley L, Davis BL. Peak plantar pressure and shear locations: Relevance to diabetic patients. Diabetes Care. 2007b doi: 10.2337/dc07-0862. in press. in press. [DOI] [PubMed] [Google Scholar]


