Abstract
Objective
To compare changes in CD4+, CD8+ and total lymphocytes counts, after initiation of highly active antiretroviral therapy (HAART), between HIV-infected patients with and without a recent history of Mycobacterium avium complex (MAC) infection.
Method
Matched exposed-non exposed retrospective cohort study.
Results
51 patients with a recent history of MAC infection (MAC+) started a combination of at least three antiretroviral drugs. They were individually matched to 145 patients without any history of MAC infection (MAC−) according to CD4+ count (+/− 30 cells/mm3), previous experience of antiretroviral treatment, AIDS clinical stage at the time of HAART initiation (baseline), age (+/− 10 years) and gender. MAC+ and MAC− patients presented comparable median levels of total lymphocytes (488 vs. 688/mm3, p=0.09), CD4+ (11 vs. 16/mm3, p=0.15), CD8+ count (359 vs. 386/mm3, p=0.39) and plasma HIV RNA (5.3 vs. 5.1 log10, copies/mL, p=0.22) at baseline. After 6 months on HAART, the median increase of CD4+ count was 28 cells/mm3 (IQR 1; 63) in MAC+ and 72 cells/mm3 (IQR 34; 120) in MAC− patients (p<0.0001) while the percentage of CD4+ cells was not significantly different between the two groups (p=0.13). Comparables differences were observed for total lymphocytes and CD8+ cells (p<0.001). The six months decline of plasma HIV RNA was not significantly different according to MAC exposure (−1.6 in MAC+ vs.−1.8 log10 copies/mL in MAC− patients, p=0.65). Results were confirmed after adjustment for other characteristics than the matching variables and after taking into account potential informative bias due to unbalanced number of deaths between the two groups.
Conclusion
MAC infection at the time of HAART initiation is an important deleterious factor for immune reconstitution. A better understanding of the underlying mechanism and an evaluation of additional treatment strategies are necessary to help immune restoration in such circumstances.
Keywords: AIDS-Related Opportunistic Infections, immunology, microbiology, Adult, Antiretroviral Therapy, Highly Active, CD4 Lymphocyte Count, CD4-CD8 Ratio, Female, HIV Infections, complications, drug therapy, immunology, Humans, Lymphocyte Count, Male, Middle Aged, Mycobacterium avium Complex, Mycobacterium avium-intracellulare Infection, complications, immunology, microbiology
Introduction
Highly Active Antiretroviral Therapy (HAART) allows an immune reconstitution even in patients with advanced HIV disease [1]. Although the increase in CD4+ T-lymphocyte count does not represent the whole immune reconstitution [2], it is the main marker used in clinical practice and epidemiological studies to estimate immune reconstitution. The modifications of the CD4+ counts following HAART initiation are associated with T cell activation [3], plasma HIV RNA and proviral DNA [4] loads at the time of treatment initiation. A low nadir of CD4+ count or a sharp decline in CD4+ count before HAART initiation are important determinants of immune reconstitution [5, 6]. The other factors usually reported are an older age [7], AIDS staging [8], intravenous drug use [9], lack of adherence to HAART [10] and previous antiretroviral exposure [11].
Profound immune deficiency, in particular of the CD4+ T helper cell compartment, limits the control of specific diseases such as Cytomegalovirus infection (CMV) [12] or Mycobacterium Avium Complex (MAC) infection [13]. Conversely, restoration of immune function has led to the recommendation of discontinuation of opportunistic infections prophylaxis, including MAC infection [14, 15]. Although profound immune deficiency is an important detrimental factor for immune reconstitution with HAART, little is known on the role of specific opportunistic infections often present in this context [16]. For instance, the impact of HCV co-infection has been discussed [17–19]. A potential mechanism of the detrimental effect on immune restoration may be the activation of apoptosis through the infection of macrophage and expression of Fas-ligand [20, 21]. In the case of CMV infection, there is a known deficiency of hematopoiesis [22] due to (i) a direct cytotoxic effect of CMV which replicates inside hematopoietic progenitor cells and (ii) an inhibition of growth factors production [23]. The direct effect on CD4+ T cells regeneration may also be mediated through apoptosis [24]. Infection with MAC is also known to impair haematopoiesis [25]. However, to our knowledge, few information are available on the response to HAART of patients with history of MAC infection [26, 27]. In the ACTG protocol 393 study evaluating whether antimycobacterial therapy for MAC could be withdrawn in subjects with history of MAC, the CD4+ count rose slowly (6 cells/mm3 every 2 months) [26]. We conducted a study within the ANRS Co3 Aquitaine Cohort of HIV-infected patients [28], to compare change in CD4+, CD8+ and total lymphocytes counts between HAART treated patients with and without a recent history of MAC infection.
Methods
Design
The Aquitaine Cohort is a prospective hospital-based cohort of HIV 1-infected patients, initiated in 1987 in the Bordeaux University Hospital and four other public hospitals in Aquitaine, South-western France [28]. Clinical, biological and therapeutical information are collected prospectively at each visit under routine clinical management proceeding.
In the present study, we constituted two groups of atients who started for the first time a combination including at least three antiretroviral drugs (defined as HAART). The exposed group (MAC+) included patients with a diagnosis of MAC infection between 12 months before and 6 months after HAART initiation. We hypothesized that diagnoses of MAC infection made in the first six months on HAART corresponded to infections that were already present at the time of treatment start but clinically silent. Robustness analyses excluding those patients gave similar results (data not shown). The unexposed group (MAC−) included patients of the Aquitaine cohort without any history of MAC or CMV infection during their entire follow-up, who were individually matched on CD4+ count (+/−30 cells/mm3), previous experience of antiretroviral treatment (naïve or not), AIDS clinical stage at the time of HAART initiation, age (+/− 10 years) and gender. The matching ratio of exposed:unexposed was 1:3, with the exception of two exposed patient for whom this ratio was 1:2 and 1:1 for three exposed patients.
Diagnosis of MAC infection
Diagnosis of disseminated MAC infection was based on the isolation of MAC from cultures of blood, bone marrow, or other normally sterile tissue or body fluids. Diagnoses based only on compatible clinical signs and symptoms were not considered in the present study.
HIV RNA load and immunophenotyping
CD4+, CD8+ and total lymphocytes counts were performed by flow cytometry in a single laboratory. Plasma HIV RNA was quantified by different laboratory kits according to the period and the hospitals. The branched DNA assays (Chiron Quantiplex RNA HIV-1, Emeryville, CA) with lower limits of detection of 2.7 log10 copies/mL (500 copies/mL) and 1.7 log10 copies/mL (50 copies/mL) were most often used. The schedule of measurements was based on clinical practice, i.e. every 3 to 6 months.
Statistical methods
The primary analyses compared the different markers values at each time point by Wilcoxon test. Time points considered were baseline (date of HAART initiation), 6 months latter (M6), M12 and M24. For each time point and each patient, the measurement that was the closer to the time point in a 3 months window was analyzed. Also, we performed multiple linear regressions of CD4+ difference from baseline to six months follow up on treatment adjusted for all potential factors influencing markers levels at a given time point including the MAC exposure as the primary factor of interest. We observed an attrition of the number of patients followed due to early deaths and lost-to-follow-up. These monotone missing data may bias estimates because patients exposed to MAC infection were more likely to die. Therefore, we conducted robustness analyses by jointly modelling repeated measurements of CD4+ count and survival time. This type of model allows taking into account these biases due to informative dropout process [29]. Briefly, the longitudinal model was a piecewise linear model explaining change in squared root of CD4+ count by a slope before HAART initiation, a slope between HAART initiation and the first 6 months and a slope after 6 months. The survival model was a lognormal survival model for time from HAART initiation to death or 24 months visit. The link between the two models was defined by the random slopes.
Results
Study population
Among 356 HAART treated patients followed in the Aquitaine Cohort and who presented a MAC infection during their follow-up, 51 patients were included in the present study because they started a combination of at least three antiretroviral drugs and they presented a MAC infection between 12 months before HAART initiation and 6 months after. Among these 51 patients, 13 MAC infections were diagnosed between 12 months and 6 months before HAART initiation, 13 MAC infections within 6 months before HAART initiation, nine at the time of HAART initiation and 16 within 6 months after HAART initiation. These MAC patients were mostly treated by a triple antibiotic combination including clarithromycin, ethambutol and rifabutin (N=17) or azithromycin, ethambutol and rifabutin (N=15). The others were treated by dual antibiotic combinations (ethambutol and rifabutin in 6 cases) or single therapy (N=6). The HAART regimen was mainly based on the combination of two nucleoside reverse transcriptase inhibitors and one protease inhibitor (88%). The most commonly used nucleosides were zidovudine (N=98) and lamivudine (N=138) and the most common PI was indinavir (N=92). Zidovudine was associated with clarithromycin in 11 cases. Two MAC patients died in the first 6 months on treatment, 10 between 6 and 12 months and six between 12 and 24 months. The 51 MAC+ exposed patients were individually matched to 145 MAC− patients. These unexposed patients, who had very low CD4+ count at HAART initiation due to the matching, had history of Kaposi sarcoma (N=21), pulmonary pneumocystosis (N=27), candidasis (but oral only) (N=22), toxoplasmosis (N=21), cryptococcosis (N=11), HIV encephalopathy (N=6), tuberculosis (N=11), lymphoma (N=5), cryptosporidiosis (N=3), recurrent bacterial pneumonia (N=1), progressive multifocal leucoencephalopathy (N=1) and salmonella septicaemia (N=l) but neither MAC nor CMV infection by design.
Characteristics of included patients are depicted in table 1. At the time of HAART initiation, there were no differences between the two groups according to matching factors as well as other characteristics.
Table 1.
Baseline demographic and clinical characteristics at the time of initiation of HAART of patients exposed to Mycobacterium avium complex infection (MAC+) and matched patients unexposed to MAC infection (MAC−). ANRS Co3 Aquitaine Cohort.
| MAC+(N=51) | MAC− (N=145) | ||||
|---|---|---|---|---|---|
| N or median | % or IQR | N or median | % or IQR | p value | |
| Age (years) | 37 | 32; 49 | 37 | 33; 43 | 0.74 |
| Male gender | 39 | 76.5 | 115 | 79.3 | 0.67 |
| HIV transmission group | |||||
| Intravenous Drug Users | 14 | 27.4 | 43 | 29.7 | 0.91 |
| Men who have sex with men | 21 | 41.2 | 47 | 32.4 | |
| Heterosexuals | 11 | 21.6 | 39 | 26.9 | |
| Others | 5 | 9.8 | 16 | 11.0 | |
| Baseline calendar period | |||||
| [06/03/1995–31/12/1999] | 42 | 82.4 | 118 | 81.4 | 0.88 |
| [01/01/2000–13/05/2003] | 9 | 17.6 | 27 | 18.6 | |
| Clinical stage | |||||
| A | 2 | 3.9 | 4 | 2.7 | 0.88 |
| B | 7 | 13.7 | 20 | 13.8 | |
| C | 42 | 82.4 | 121 | 83.4 | |
| Treatments | |||||
| Number of treatment regimens before HAART initiation* | |||||
| None (naïve patients) | 17 | 33.3 | 46 | 31.7 | 0.98 |
| Between 1 and 4 regimens | 19 | 37.3 | 57 | 39.3 | |
| More than 4 regimens | 15 | 29.4 | 42 | 29.0 | |
| Treatment regimen at HAART initiation | |||||
| 2 NRTIs and 1 PI | 45 | 88.2 | 134 | 91.8 | 0.13 |
| 2 NRTIs and 1 NNRTI | 2 | 3.9 | 8 | 6.2 | |
| 3 NRTIs | 4 | 7.9 | 2 | 1.3 | |
| 2 NRTIs and 1 PI and 1 NNRTI | 0 | 0.0 | 1 | 0.7 | |
| MAC prophylaxis | |||||
| Clarithromycin | 0 | 0.0 | 1 | 0.7 | 0.53 |
| Azithromycin | 0 | 0.0 | 4 | 2.8 | |
| Rifabutin | 9 | 17.6 | 35 | 24.1 | |
| Biological characteristics at HAART initiation | |||||
| Plasma HIV RNA (log10 copies/mL) | 5.3 | 4.4; 5.6 | 5.1 | 4.2; 5.5 | 0.22 |
| T Lymphocytes CD4+ count (cells/mm3) | 11 | 3; 38 | 16 | 8; 39 | 0.15 |
| T Lymphocytes CD8+ count (cells/mm3) | 359 | 206; 576 | 386 | 215; 634 | 0.39 |
| Total lymphocytes (cells/mm3) | 488 | 396; 858 | 688 | 410; 1065 | 0.09 |
| Haemoglobinemia (g/dl) | 11.5 | 9.8; 13.3 | 12.0 | 10.6; 13.0 | 0.23 |
| Platelets (103/mm3) | 177 | 143; 230 | 157 | 119; 214 | 0.09 |
IQR: interquartile range; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor
A modification of at least one drug defined a new regimen
Markers evolution
At the time of treatment initiation, MAC+ and MAC− patients presented comparable median levels of total lymphocytes (488 vs. 688/mm3, p=0.09), CD4+ count (11 vs. 16/mm3, p=0.15) and CD8+ count (359 vs. 386/mm3, p=0.39) (Table 1). During follow-up, the two groups presented a significant increase in total lymphocytes, CD4+ and CD8+ counts. However, these increases were significantly more pronounced in MAC− than in MAC+ groups (Figure 1, panels a–c). After six months on HAART, the median increase of CD4+ count was +28 cells/mm3 (IQR +1; +63) in MAC+ and +72 cells/mm3 (IQR +34; +120) in MAC− groups (p<0.0001), while the percentage of CD4+ cells was not significantly different between the two groups (p=0.13) (Figure 1, panel d). Interestingly, plasma HIV RNA decreased significantly in the two groups and the decline was not significantly different according to MAC exposure (−1.6 in MAC+ group vs. −1.8 log10 copies/mL in MAC− group at 6 months, p=0.65) (Figure 1, panel e). The proportions of patients reaching 500 copies/mL or less at 6 months were similar (48.8% and 49.5% in the MAC+ and MAC−, respectively, p=0.93). The effect of MAC exposure on the change of CD4+ count at 6 months was also confirmed after adjustment on other determinants (Table 2). Indeed, the factors significantly associated with a poorer CD4+ count increase were: MAC exposure, CD4 count nadir < 50 cells/mm3, higher baseline plasma HIV RNA and previous experience of antiretroviral treatment. Treatments including zidovudine and clarithromycin were associated with poorer CD4+ count increase at 6 months (p=0.03) but this effect did not remain after adjustment for other characteristics (p=0.51). The independent effect of MAC exposure was also confirmed at 12 and 24 months (data not shown). The effect of MAC exposure was also confirmed when modelling all repeated measures of CD4+ count taking into account potential informative drop-out due to death and adjusting for potential confounding factors. Thus, estimated CD4+ counts levels were significantly higher in the MAC− group compared to MAC+ patients at 6 months (p=<0.0001), 12 months (p=0.0002) and 24 months (p=0.0047) and not at M0 (p=0.17). The estimated slopes within the first 6 months were +19.4 cells/mm3/month ([15.9; 22.8]) in the MAC− group and +9.5 cells/mm3/month ([3.8; 15.1]) in the MAC+ group (p=0.003). After 6 months, the estimated slopes were +2.9 cells/mm3/month ([2.2; 3.5]) and +2.7 cells/mm3/month ([1.5; 3.8]), respectively (p=0.75).
Figure 1.
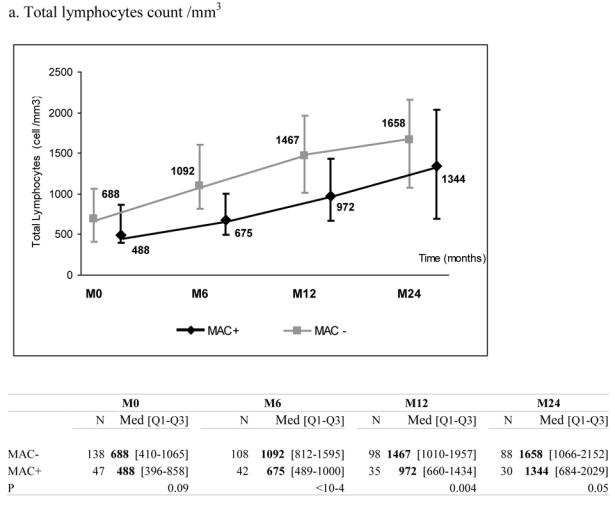
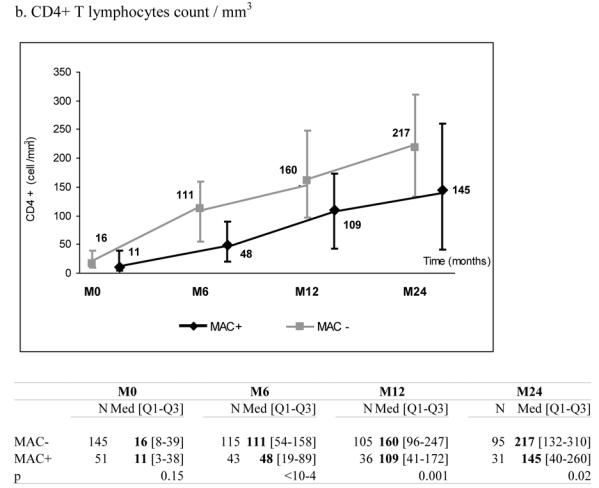
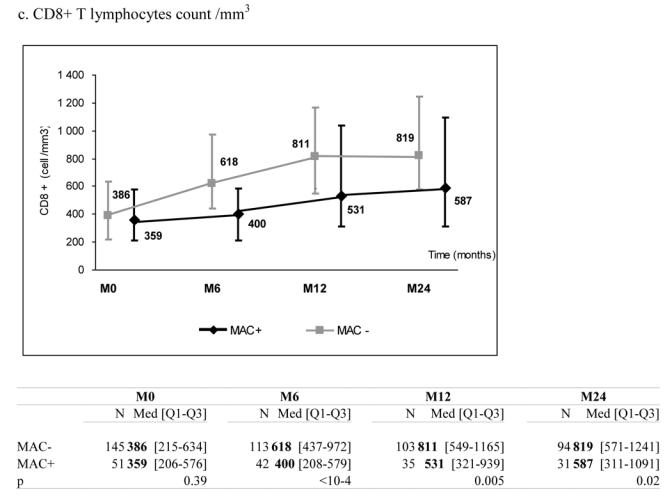
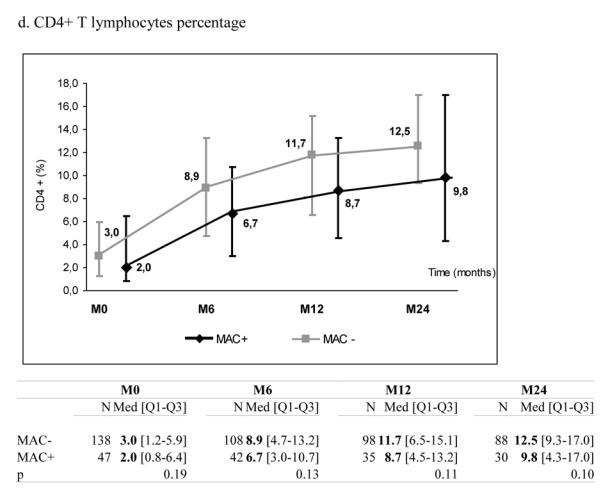
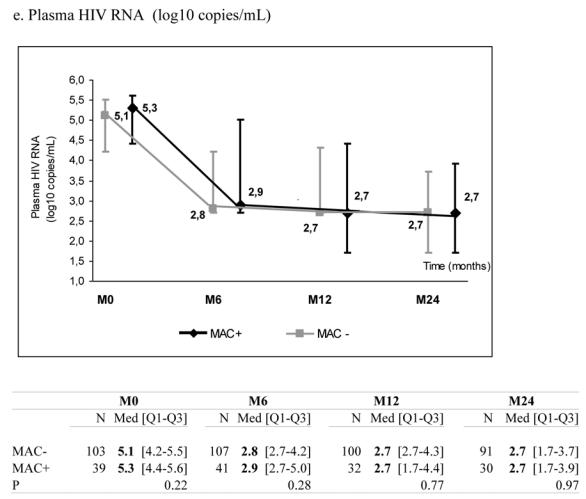
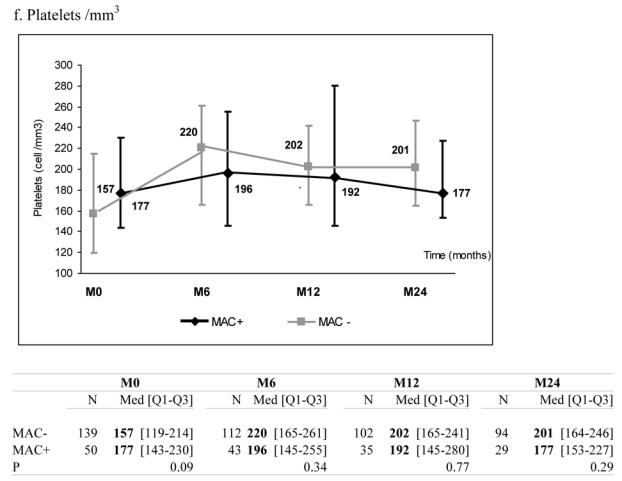
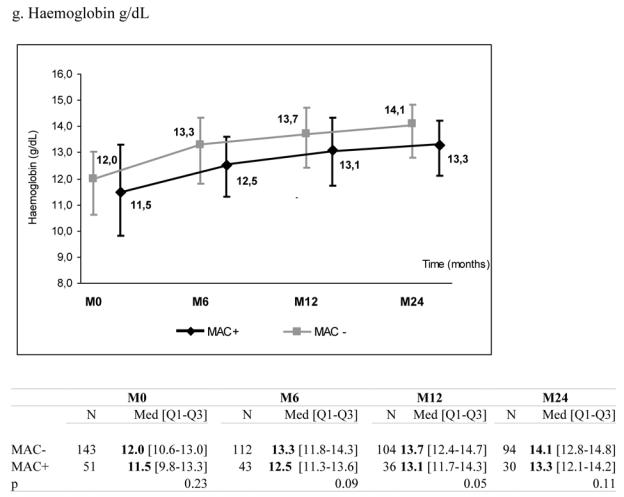
Median marker values at M0 (baseline), M6 (6 months later), M12 and M24 according to exposure group: patients with Mycobacterium avium complex (MAC+) or without (MAC−). Tables show number of available subjects at each time point, interquartile range (Q1–Q3) and p value for statistical difference of marker level between MAC+ and MAC− groups. ANRS Co3 Aquitaine Cohort.
Table 2.
Univariable and multivariable analyses of factors associated with change in CD4+ level between baseline and 6 months after HAART initiation, adjusted for baseline CD4+. ANRS Co3 Aquitaine Cohort.
| Univariable | Multivariable* | |||
|---|---|---|---|---|
| Characteristic | β | P | β | P |
| MAC exposure (vs. no exposure) | −25.5 | <0.0001 | −24.7 | <0.0001 |
| Age (for one year older) | 0.3 | 0.53 | – | – |
| Women (vs. men) | −5.8 | 0.64 | – | – |
| Intravenous Drug Users (vs. other transmission groups) | −4.5 | 0.69 | – | – |
| Naive of antiretroviral treatment (vs. experienced) | 27.7 | 0.01 | 30.5 | 0.03 |
| AIDS clinical stage (vs. other clinical stages) | 13.6 | 0.32 | – | – |
| Nadir of CD4+ count <50 cells/mm3 (vs. ≥50) | −16.6 | 0.25 | −55.8 | 0.007 |
| Baseline calendar period > 2000 (vs ≤2000) | 22.3 | 0.11 | – | – |
| Baseline HIV RNA (for 1 log copies/mL higher) | 21.2 | 0.0003 | 17.8 | 0.002 |
coefficient of the liner regression
MAC: Mycobacterium avium complex
All listed characteristics included
There was no significant effect of MAC exposure on haemoglobin and platelets levels during follow-up although haemoglobin levels tended to be lower in patients exposed to MAC infection (Figure 1, panels f–g).
Discussion
In this study, we showed that patients who experienced a MAC infection during their follow up and started a HAART regimen, presented a weaker increase in their total lymphocytes, CD4+ and CD8+ counts despite similar virological response. MAC infection is known to compromise hematopoiesis and in particular erythropoiesis and granulopoiesis. This mechanism is thought to be multi-factorial. First, during the course of MAC disease, infection massively widespreads in bone marrow which must impair hematopoiesis [30]. Second, the presence of mycobacteria induces the production of immunosuppressive cytokines by the host adaptative immune response, the secretion of soluble factors that inhibit erythroblastic progenitors [31], the deleterious effect of TNF-α on haematopoiesis [32] and Fas Ligand expression [33] by infected macrophages known to induce the apoptosis of T lymphocytes. Apoptosis is also one of the mechanisms explaining the deficiency of haematopoiesis in the course of CMV [24] or HCV infections [20, 21] as well as it impaired immune restoration in HIV-infected patients [34]. Treatment of MAC infection such as clarithromycin might also contribute to these disorders. In fact, the association of clarithromycin and zidovudine in HIV-infected patients is known to suppress lymphopoiesis and hematopoiesis [35]. Nevertheless, the impact of MAC infection and its management was not observed on haemoglobinemia and platelets, favouring the hypothesis of a specific alteration of the lymphocyte lineage.
This study presents three main limitations. First, we were not able to compare the effect of different opportunistic infections at the time of treatment initiation on the entire Aquitaine cohort because of the limited number of individual opportunistic infections in about 3000 HAART-treated patients. This calls for the need of analyses of this kind in multi-cohort collaborations [36]. Second, we do not considered the possible effects of adherence, ethnicity or social class which may vary in the two groups because it was not recorded in our database. Third, it would be useful to perform more specific measurements (apoptosis, naive and memory cells, activation…) to better characterize the mechanisms in this context [2].
With respect to clinical care, hematotoxic drugs should be avoided in patients presenting MAC infection. Furthermore, immunotherapy may be indicated in such a context. The G-CSF and GM-CSF factors activate destruction of intracellular bacteria and stimulate the granulocyte colony. These factors have been successfully proposed to treat patients with MAC infection [37]. Moreover, such treatment might help in immune restoration [38]. I1-2 may also be a good candidate because of its stimulating effect on CD4+ proliferation [39] and its inhibitory effect on cell apoptosis [40], thus increasing the survival of CD4+ T lymphocytes [41].
In conclusion, MAC infection at the time of HAART initiation seems to be an important deleterious factor for immune reconstitution. A better understanding of the underlying mechanism and an evaluation of additional treatment strategies are necessary to help immune restoration in such circumstances.
Acknowledgments
The ANRS Co3 Aquitaine Cohort and Jérémie Guedj are supported by the Agence Nationale de Recherches sur le SIDA (Action Coordonnée no.7, Cohortes and research grant for PhD).
Appendix: Composition of the GECSA
Scientific committee: Prs J. Beylot, M. Dupon, M. Longy-Boursier, JL. Pellegrin, JM. Ragnaud and R. Salamon (Chair)
Scientific coordination: Prs G. Chêne and F. Dabis (Coordinator), Dr S. Lawson-Ayayi, C. Lewden, R. Thiébaut
Medical coordination: Prs M. Dupon, JF. Moreau, P. Mercié, P. Morlat, D. Neau, JL. Pellegrin and JM. Ragnaud, Drs N. Bernard, D. Lacoste and D. Malvy
Data management and Statistical analysis: E. Balestre, L. Dequae-Merchadou, V. Lavignolle-Aurillac
Technical team: MJ. Blaizeau, M. Decoin, S. Delveaux, C. Hanappier, S. Labarrère and B. Uwamaliya, G. Palmer and D. Touchard, D. Dutoit and L. Houinou
Participating Hospital Departments (participating physicians): Bordeaux University Hospital: Pr J. Beylot (Pr P. Morlat, Drs N. Bernard, M. Bonarek, F. Bonnet, D. Lacoste and R. Vatan), Pr P. Couzigou, Pr H. Fleury (Pr ME. Lafon, Drs B. Masquelier and I. Pellegrin), Pr M. Dupon (Dr H. Dutronc, F. Bocquentin and S. Lafarie), Pr JL. Pellegrin (Pr JF. Viallard, Drs O. Caubet, E. Lazaro and C. Nouts), Pr M. Longy-Boursier (Pr P. Mercié, Dr D. Malvy, T. Pistonne and C. Receveur), Pr JF. Moreau (Dr P. Blanco), Pr JM. Ragnaud (Pr D. Neau, Drs C. Cazorla, D. Chambon, C. De La Taille, and A. Ochoa); Dax Hospital: Dr P. Loste (Dr L. Caunègre); Bayonne Hospital: Dr F. Bonnal (Drs S. Farbos and MC. Gemain); Libourne Hospital: Dr J. Ceccaldi (Dr S. Tchamgoué); Mont-de-Marsan Hospital: Dr S. de Witte
References
- 1.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 2.Lederman HM, Williams PL, Wu JW, et al. Incomplete immune reconstitution after initiation of highly active antiretroviral therapy in human immunodeficiency virus-infected patients with severe CD4(+) cell depletion. The Journal of Infectious Diseases. 2003;188:1794–1803. doi: 10.1086/379900. [DOI] [PubMed] [Google Scholar]
- 3.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. The Journal of Infectious Diseases. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 4.Rouzioux C, Hubert JB, Burgard M, et al. Early levels of HIV-1 DNA in peripheral blood mononuclear cells are predictive of disease progression independently of HIV-1 RNA levels and CD4+ T cell counts. The Journal of Infectious Diseases. 2005;192:46–55. doi: 10.1086/430610. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico R, Yang YJ, Mildvan D, et al. Lower CD4+ T lymphocyte nadirs may indicate limited immune reconstitution in HIV-1 infected individuals on potent antiretroviral therapy: Analysis of immunophenotypic marker results of AACTG 5067. Journal of Clinical Immunology. 2005;25:106–115. doi: 10.1007/s10875-005-2816-0. [DOI] [PubMed] [Google Scholar]
- 6.Renaud M, Katlama C, Mallet A, et al. Determinants of paradoxical CD4 cell reconstitution after protease inhibitor-containing antiretroviral regimen. AIDS. 1999;13:669–676. doi: 10.1097/00002030-199904160-00007. [DOI] [PubMed] [Google Scholar]
- 7.Viard JP, Mocroft A, Chiesi A, et al. Influence of age on CD4 cell recovery in human immunodeficiency virus- infected patients receiving highly active antiretroviral therapy: Evidence from the EuroSIDA study. The Journal of Infectious Diseases. 2001;183:1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 8.Thiébaut R, Jacqmin-Gadda H, Walker S, et al. Determinants of response to first HAART regimen in naive patients with an estimated time since HIV seroconversion. HIV Medicine. 2006;7:1–9. doi: 10.1111/j.1468-1293.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 9.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Carrieri MP, Raffi F, Lewden C, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: a 3-year follow-up study. Antiviral Therapy. 2003;8:585–594. doi: 10.1177/135965350300800606. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita TE, Phair JP, Munoz A, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS. 2001;15:735–746. doi: 10.1097/00002030-200104130-00009. [DOI] [PubMed] [Google Scholar]
- 12.Sacre K, Carcelain G, Cassoux N, et al. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. Journal of Experimental Medicine. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havlir DV, Schrier RD, Torriani FJ, Chervenak K, Hwang JY, Boom WH. Effect of potent antiretroviral therapy on immune responses to Mycobacterium avium in human immunodeficiency virus-infected subjects. The Journal of Infectious Diseases. 2000;182:1658–1663. doi: 10.1086/317620. [DOI] [PubMed] [Google Scholar]
- 14.Zeller V, Truffot C, Agher R, et al. Discontinuation of secondary prophylaxis against disseminated Mycobacterium avium complex infection and toxoplasmic encephalitis. Clinical Infectious Diseases. 2002;34:662–667. doi: 10.1086/338816. [DOI] [PubMed] [Google Scholar]
- 15.Shafran SD, Mashinter LD, Phillips P, et al. Successful discontinuation of therapy for disseminated Mycobacterium avium complex infection after effective antiretroviral therapy. Annals of Internal Medicine. 2002;137:734–737. doi: 10.7326/0003-4819-137-9-200211050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Cassol E, Page T, Mosam A, et al. Therapeutic response of HIV-1 subtype C in African patients coinfected with either Mycobacterium tuberculosis or human herpesvirus-8. The Journal of Infectious Diseases. 2005;191:324–332. doi: 10.1086/427337. [DOI] [PubMed] [Google Scholar]
- 17.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 18.Antonucci G, Girardi E, Cozzi-Lepri A, et al. Role of hepatitis C virus (HCV) viremia and HCV genotype in the immune recovery from highly active antiretroviral therapy in a cohort of antiretroviral-naive HIV-infected individuals. Clinical Infectious Diseases. 2005;40:el01–109. doi: 10.1086/430445. [DOI] [PubMed] [Google Scholar]
- 19.Braitstein P, Zala C, Yip B, et al. Immunologic Response to Antiretroviral Therapy in Hepatitis C Virus-Coinfected Adults in a Population-Based HIV/AIDS Treatment Program. The Journal of Infectious Diseases. 2006;193:259–268. doi: 10.1086/498908. [DOI] [PubMed] [Google Scholar]
- 20.Graham C, Koziel M. Why should hepatitis C affect immune reconstitution in HIV-1-infected patients ? Lancet. 2000;356:1865–1866. doi: 10.1016/S0140-6736(00)03251-7. [DOI] [PubMed] [Google Scholar]
- 21.Laskus T, Radkowski M, Jablonska J, et al. Human immunodeficiency virus facilitates infection/replication of hepatitis C virus in native human macrophages. Blood. 2004;103:3854–3859. doi: 10.1182/blood-2003-08-2923. [DOI] [PubMed] [Google Scholar]
- 22.Maciejewski JP, St Jeor SC. Human cytomegalovirus infection of human hematopoietic progenitor cells. Leukemia & Lymphoma. 1999;33:1–13. doi: 10.3109/10428199909093720. [DOI] [PubMed] [Google Scholar]
- 23.Randolph-Habecker J, Iwata M, Torok-Storb B. Cytomegalovirus mediated myelosuppression. Journal of Clinical Virology. 2002;25 (Suppl 2):851–56. doi: 10.1016/s1386-6532(02)00092-6. [DOI] [PubMed] [Google Scholar]
- 24.Goldmacher VS. Cell death suppression by cytomegaloviruses. Apoptosis. 2005;10:251–265. doi: 10.1007/s10495-005-0800-z. [DOI] [PubMed] [Google Scholar]
- 25.Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex. Clinical microbiology reviews. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aberg JA, Williams PL, Liu T, et al. A study of discontinuing maintenance therapy in human immunodeficiency virus-infected subjects with disseminated Mycobacterium avium complex: AIDS Clinical Trial Group 393 Study Team. The Journal of Infectious Diseases. 2003;187:1046–1052. doi: 10.1086/368413. [DOI] [PubMed] [Google Scholar]
- 27.MacGregor RR, Hafner R, Wu JW, et al. Clinical, microbiological, and immunological characteristics in HIV-infected subjects at risk for disseminated Mycobacterium avium complex disease: an AACTG study. AIDS Research and Human Retroviruses. 2005;21:689–695. doi: 10.1089/aid.2005.21.689. [DOI] [PubMed] [Google Scholar]
- 28.Thiébaut R, Morlat P, Jacqmin-Gadda H, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. AIDS. 2000;14:971–978. doi: 10.1097/00002030-200005260-00008. [DOI] [PubMed] [Google Scholar]
- 29.Thiébaut R, Jacqmin-Gadda H, Babiker A, Commenges D. Joint modelling of bivariate longitudinal data with informative dropout and left-censoring, with application to the evolution of CD4+cell count and HIV RNA viral load in response to treatment of HIV infection. Statistics in Medicine. 2005;24:65–82. doi: 10.1002/sim.1923. [DOI] [PubMed] [Google Scholar]
- 30.Hafner R, Inderlied CB, Peterson DM, et al. Correlation of quantitative bone marrow and blood cultures in AIDS patients with disseminated Mycobacterium avium complex infection. The Journal of Infectious Diseases. 1999;180:438–447. doi: 10.1086/314865. [DOI] [PubMed] [Google Scholar]
- 31.Gascon P, Sathe SS, Rameshwar P. Impaired erythropoiesis in the acquired immunodeficiency syndrome with disseminated Mycobacterium avium complex. The American Journal of Medicine. 1993;94:41–48. doi: 10.1016/0002-9343(93)90118-9. [DOI] [PubMed] [Google Scholar]
- 32.Kremer L, Estaquier J, Wolowczuk I, Biet F, Ameisen JC, Locht C. Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infection and Immunity. 2000;68:4264–4273. doi: 10.1128/iai.68.7.4264-4273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isgro A, Mezzaroma I, Aiuti A, et al. Decreased apoptosis of bone marrow progenitor cells in HIV-1-infected patients during highly active antiretroviral therapy. AIDS. 2004;18:1335–1337. doi: 10.1097/00002030-200406180-00013. [DOI] [PubMed] [Google Scholar]
- 34.Benveniste O, Flahault A, Rollot F, et al. Mechanisms involved in the low-level regeneration of CD4(+) cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. The Journal of Infectious Diseases. 2005;191:1670–1679. doi: 10.1086/429670. [DOI] [PubMed] [Google Scholar]
- 35.Freund YR, Dousman L, Mohagheghpour N. Prophylactic clarithromycin to treat mycobacterium avium in HIV patients receiving zidovudine may significantly increase mortality by suppressing lymphopoiesis and hematopoiesis. International immunopharmacology. 2002;2:1465–1475. doi: 10.1016/s1567-5769(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 36.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 37.Bermudez LE, Petrofsky M, Stevens P. Treatment with recombinant granulocyte colony-stimulating factor (Filgrastin) stimulates neutrophils and tissue macrophages and induces an effective non-specific response against Mycobacterium avium in mice. Immunology. 1998;94:297–303. doi: 10.1046/j.1365-2567.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen SD, Ersboll AK, Mathiesen L, Nielsen JO, Hansen JES. Highly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus-infected patients. The Journal of Infectious Diseases. 1998;178:1299–1305. doi: 10.1086/314464. [DOI] [PubMed] [Google Scholar]
- 39.Levy Y, Durier C, Krzysiek R, et al. Effects of interleukin-2 therapy combined with highly active antiretroviral therapy on immune restoration in HIV-1 infection: a randomized controlled trial. AIDS. 2003;17:343–351. doi: 10.1097/00002030-200302140-00008. [DOI] [PubMed] [Google Scholar]
- 40.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–4966. [PubMed] [Google Scholar]
- 41.Kovacs JA, Lempicki RA, Sidorov IA, et al. Induction of prolonged survival of CD4(+) T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. The Journal of Clinical Investigation. 2005;115:2139–2148. doi: 10.1172/JCI23196. [DOI] [PMC free article] [PubMed] [Google Scholar]


