Abstract
Background
The long-term psychological impact of pediatric sarcoma is largely unknown. As part of a cross-sectional study examining the late effects of pediatric sarcoma therapy, we examined whether psychological distress or posttraumatic stress symptoms are present in an adult cohort of pediatric sarcoma survivors.
Method
Thirty-four patients participated in the study, an average of 17 years after their treatment ended, each completing the SCID module for Posttraumatic Stress Disorder, Impact of Events Scale, Brief Symptom Inventory (BSI) and a questionnaire assessing sociodemographic variables and psychosocial issues.
Results
Significant persistent psychological distress characterized this cohort of patients. Seventy-seven percent scored in the clinical range on the BSI. Twelve percent met diagnostic criteria for PTSD. Current psychological distress was associated with intrusive thoughts and avoidant behaviors, male gender, employment, difficulty readjusting to work/school after treatment, and enduring worries about health. No differences were found based on age, presence of metastatic disease or time since diagnosis.
Conclusions
This is the first report of a clinical evaluation of psychological distress in a cohort of pediatric sarcoma survivors treated with intensive multimodal cancer therapy. The results suggest that survivors of pediatric sarcoma might be at high risk for adverse psychological outcomes. Appropriate interventions are proposed.
Keywords: psychological distress, posttraumatic stress, pediatric sarcoma, long-term survivors, intensive treatment, cancer, oncology
INTRODUCTION
The treatment of childhood cancer is one of the greatest successes of modern medicine. During the last 30 years, improved diagnosis and aggressive treatment have resulted in dramatic improvements in survival rates. Prior to 1970, when a child was diagnosed with cancer, there was little hope of long-term survival. Today, over 70% of children under 20 years of age are expected to survive the diagnosis of cancer for 5 years or more (Reis et al., 2002). Unfortunately, improved survival has resulted in a multitude of long-term treatment-related side effects, (Hudson et al., 2003). Critical attention is now being directed to the unintended consequences of this success in an attempt to improve the overall health of cancer survivors.
According to a recent report of cancer survivor-ship, as many as two-thirds of childhood cancer survivors experience at least one late effect, with one-fourth of survivors reporting a late effect that is severe or life threatening (National Cancer Policy Board (NCPB), 2003). The most common late effects reported involve neurocognitive, cardiopulmonary, endocrine and musculoskeletal difficulties, as well as second malignancies. The risk of psychological/psychiatric illness remains unclear however, as investigations designed to examine psychological outcomes in long-term survivors of childhood cancer have produced inconsistent, and at times conflicting findings (Zebrack et al., 2002; Kokkonen et al., 1997; Teta et al., 1986). Several studies suggest that childhood cancer survivors are at increased risk for maladaptive psychosocial sequalae (Koocher and O’Malley, 1981) including depression (Fritz et al., 1988; Mulhern et al., 1989), behavioral adjustment problems (Moore et al., 1987; Fritz et al., 1988; Mulhern et al., 1989; Madan-Swain and Brown, 1991), and anxiety (Zebrack and Chesler, 2002; Pendley et al., 1997; Neff and Beardslee, 1990), while others report that a significant portion of childhood cancer survivors have no higher prevalence of anxiety (Schmale et al., 1983), depression (Greenberg et al., 1989; Ross et al., 2003), overall mood disorder (Gray et al., 1992), or lowered self-concept (Anholt et al., 1993) when compared with population norms or matched controls. Moreover, a number of studies suggest that childhood cancer survivors are well-or better-adjusted compared to normative groups (Fritz et al., 1988; Barakat et al., 1997; Kazak, 1998; Cella and Tross, 1986; Kupst et al., 1995; Chesler and Zebrack, 1997). No clear pattern can sufficiently explain these disparities (Elkin, 1997) although methodological differences including sampling method, outcome variables measured, and method of survey administration as well as varied types of malignancies, treatments, and age groups have to be taken into consideration. Others have posited that severity of disease and intensity of treatment is related to the ability to cope with the illness experience (Boman and Bodegard, 2000). This is supported by the recent report from the Childhood Cancer Survivor Study which indicates that exposure to intensive chemotherapy added to the risk for depression and somatic distress among both leukemia and lymphoma survivors, and was more highly predictive than the sociodemographic characteristics most commonly recognized as risk factors for mental illness in the general population (Zebrack et al., 2002). Another study found survivors of brain tumors to have an increased risk of psychiatric hospitalization (Ross et al., 2003). While late effects of brain radiotherapy have been well described, it is possible that the intensity of even non-CNS directed therapy might impact the incidence of subsequent psychological distress. Furthermore, the relationship between sociodemographic variables and psychological distress, particularly in terms of gender differences and future employment outcomes have been studied with conflicting findings (Kazak et al., 1994; Zeltzer et al., 1997; Pui et al., 2003; Zebrack et al., 2002).
It is not surprising that distress, either immediate or delayed, occurs in response to childhood cancer. Treatment is demanding, all encompassing, often painful and frequently involves the witnessing of suffering in medical settings (NCPB, 2003). In recent years, posttraumatic stress has received clinical and research attention as a paradigm that captures the traumatic nature of childhood cancer and its treatment. Posttraumatic Stress Disorder (PTSD) is an anxiety disorder that can occur after severe life trauma, including life-threatening illness. Field trials and studies that have examined the relationship between cancer survival and PTSD have found that a substantial number of survivors report symptoms consistent with PTSD (Alter et al., 1996; Stuber et al., 1996; Pelcovitz, 1998). Rates of PTSD appear to be lower in pediatric and adolescent cancer survivors (Butler et al., 1996; Erickson and Steiner, 2002; Kazak et al., 2004) than young adult samples, with about 20% of young adult childhood cancer survivors meeting PTSD criteria (Hobbie et al., 2000). Emerging evidence that the transition to early adulthood is a time of increased psychosocial vulnerability for childhood cancer survivors (Zeltzer et al., 1997; Glover et al., 2003; Recklitis et al., 2003) supports this finding.
Recent empirical research demonstrates that a syndrome similar to PTSD exists in adolescent and adult cancer survivors, where intrusive cancer-related memories trigger intense emotional and physiological reactions similar to posttraumatic stress reactions (Hobbie, et al., 200l; Meeske et al., 2001). Considerably more survivors meet criteria for distinct PTSD stress symptoms (PTSS) (Brown et al., 2003; Erickson and Steiner, 2002; Kazak et al., 2001; Stuber et al., 1996) than meet the required criteria for a diagnosis of PTSD in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-R). Stuber et al. (1996) found that more than half of their sample of childhood cancer survivors reported posttrauma symptoms including bad dreams and feeling afraid or upset when thinking about cancer. A series of reports has documented PTSS and/or PTSD in mothers of childhood cancer survivors (Barakat et al., 1997; Brown et al., 2003; Kazak et al., 1997, 1998, 2004; Manne et al., 1998, 2002; Pelcovitz et al., 1996) as well as in their fathers (Kazak and Barakat, 1997) and siblings (Alderfer et al., 2003).
Late effects resulting from pediatric sarcomas are not well understood. Malignancies of the soft tissue and bones account for more than 10% (6.1 and 4.7%, respectively) of newly diagnosed cancers in children, adolescents, and young adults. Median age at diagnosis of rhabdomyosarcoma (RMS) is 5 years, with a male preponderance. Osteosarcomas account for approximately 60% of malignant bone tumors in the first two decades of life. Most of the remaining bone malignancies in children and adolescents are Ewings’ sarcomas and the histologically similar and genetically identical peripheral primitive neuroectodermal tumors (PNETs). Together, these tumors are often referred to as the Ewings’ family of tumors and usually occur in adolescence. Identification of specific, recurrent genetic alterations in RMS and Ewing’s sarcoma has improved diagnosis by clarifying pathogenesis. Better supportive care and systematic application of effective multimodality treatment have improved survival dramatically during the past 30 years. In a recent study by Bacci et al. (2004), the overall survival at real follow-ups of 5, 10, 15 and 20 years was 57.2, 49.3, 44.9 and 38.4%, respectively. Prognosis was poor before the advent of effective multiagent chemotherapy (5-year survivals of 10–20%, despite good local control) and continues to be dismal in patients who present with metastatic disease (one recent study reported a 3-year event-free survival of only 26.7 ±13.2%) (Ginsberg et al., 2002; Link et al., 2002; Wexler, et al., 2002). Prior published data on the late effects of survivors of pediatric sarcomas is limited and is generally found lumped together with other childhood cancer survivors. The investigations that focused exclusively on pediatric sarcomas primarily studied the incidence of second malignancies (Green et al., 1994, 1979; Strong et al., 1979; Travis et al., 1994) with a few exceptions. A study by Nicholson et al. (1992) examined the psychosocial and health outcomes of 29 long-term survivors of Ewing’s sarcoma and reports an increased risk of premature death of long-term survivors but few adverse psychosocial outcomes when compared to sibling controls. A second study by Novakovic et al. (1997) utilized a self-administered quality of life questionnaire with 89 survivors of Ewing’s sarcoma and 97 sibling controls. The findings from this study suggest employment, fertility and functional status was adversely affected in survivors. Finally, the Childhood Cancer Survivor Study (Nagarajan et al., 2004) examined the function and quality of life of survivors of pelvic and lower extremity osteosar-coma and Ewing’s sarcoma. While amputees were likely to do as well as those who underwent limb-sparing surgeries, the findings suggest female gender, lower educational attainment and older current age appear to influence function, quality of life, and disability. Despite improved survival rates, there have been no published reports specifically addressing the psychological symptom outcomes or prevalence of PTSS of pediatric sarcoma survivors.
Over the last 35 years, the Pediatric Oncology Branch (POB) of the National Cancer Institute (NCI) has been treating patients with pediatric sarcomas. The long-term survivors of this cohort represent a valuable source of information on pediatric sarcoma treatment-associated late effects. Patients received intensive, multiagent chemotherapy, radiation and surgery. Many of these patients sustained significant social disruption during their adolescent years as their treatment required months of inpatient hospitalizations away from their home. As part of a cross-sectional study examining the cardiac, endocrine, and muscular skeletal late effects of pediatric sarcoma treatment, we studied the prevalence of psychological distress and PTSS, and compared participants to published gender-matched norms for the general population.
MATERIALS AND METHODS
Study participants were identified based upon review of the POB database. Eligibility criteria included: prior enrollment in one of the POB protocols for pediatric sarcomas, greater than 2 years free from disease after completion of sarcoma therapy or greater than 5 years free from disease after recurrence, ability to travel to NCI/ POB and commit to 5 days of multidisciplinary evaluations, ability to understand and sign informed consent and if under the age of 18, accompaniment by a parent or guardian legally permitted to give consent. There were no age restrictions. The protocol and consent forms were approved by the National Cancer Institute’s institutional review board. Of 112 identified eligible patients, letters containing an outline of the study and an invitation to participate were sent to 82 patients whose address was available from the most recent time of follow-up. Among those who received letters of invitation to participate, 34 participants (41%) enrolled in the study. Nineteen declined and the remaining 29 patients were lost to follow-up since their last visit or failed to respond. Efforts were made to locate each potential participant via alternative names given during their treatment and when this was unsuccessful, through internet-wide tracking.
There were no significant differences between those who declined or were lost to follow-up and those who participated in the study with regard to diagnosis, age at diagnosis, receipt of total body irradiation or cranial radiation therapy, history of metastases or relapse, gender or race. A clinical PhD social worker (L.W.) administered the SCID, self-report measures and demographic and psychosocial instrument to each participant.
Measures
Brief symptom inventory (BSI) (Derogatis, 1993)
The BSI is a 53-item paper and pencil self-report inventory that yields severity scores for nine symptom dimensions of psychiatric symptoms (somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism) and three global indices of distress (Global Severity Index (GSI), Positive Symptom Distress Index, Positive Symptom Total (PST) (Boulet and Boss, 1991)). The GSI is a weighted frequency score based on the sum of the ratings the subject has assigned to each symptom and is the most sensitive single indicator of the respondent’s distress level as it combines information about numbers of symptoms and intensity of distress. For the purposes of this analysis, only the global indices of distress calculated from the GSI and PST will be reported. A score for ‘caseness’, indicating that a person is in the clinical range for psychological distress, was also computed. ‘Caseness’ is defined by having a t-score of 63 or above on the GSI or on any two primary subscales of the BSI. Internal consistency coefficients for the nine primary symptom dimensions ranged from 0.65 to 0.89 indicating acceptable levels of reliability.
Structured clinical interview for DSM-IV (SCID)
The SCID is a well-established semi-structured interview used to establish diagnoses consistent with the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders DSM-IV-R (American Psychiatric Association, 1994). The SCID has been demonstrated to have good reliability and validity (First et al., 1997; Segal et al., 1993, 1994, 1995). Participants were administered the PTSD section of the SCID which includes items assessing each of the DSM-IV diagnostic criteria for PTSD. Most of the questions can be answered with a ‘yes’ or ‘no’ response. Data collected using the SCID was compiled to create a categorical variable reflecting whether or not a person met diagnostic criteria for PTSD.
Impact of Events Scale (IES)
The 15-item IES (Horowitz et al., 1979) measures subjective stress (intrusive thoughts and avoidance) related to a specific event. In this study, the participants were asked to focus on their pediatric sarcoma experience as the stressful event. The scale yields a total score as well as two subscores for intrusive thoughts and avoidant behaviors. The IES has high internal consistency and good test–retest reliability. It discriminates between different populations and symptom levels (Schwardwald et al., 1987) in medical and nonmedical samples (Horowitz et al., 1979; Epping-Jordan et al., 2003). The scale was normed on both physical therapy students who had recently begun dissection of a cadaver as part of their education (normative sample) and on patients referred to a clinic for specialized treatment of stress response syndromes.
Demographic and psychosocial questionnaire
This questionnaire was designed by the investigators and completed during a face-to-face interview. Socio-demographic variables considered in the analysis included age at time of interview, gender, education level and employment. The youngest participant was 17 but indicated being both employed and in school. The remainder of the sample was 20 or over and so we deemed employment status to be a relevant indicator of healthy functioning. Psychosocial issues measured included use of psychological counseling, and whether or not respondents experienced difficulty readjusting to school/work after treatment ended. Cancer-related variables included in the analysis were radiation therapy, chemotherapy, surgery, age at time of cancer diagnosis, and interval from cancer diagnosis to interview. As described in an earlier CCSS publication (Zebrack et al., 2002), cumulative doses of specific chemother-apeutic agents were used to classify whether this group of survivors were intensely treated. All survivors within this cohort of pediatric sarcoma survivors were considered to have received intensive treatment.
Data analysis
Due to the small sample size, an assessment was done to determine if non-parametric tests would be more appropriate than parametric tests for this analysis, however, the data did not meet the recommended requirements for non-parametric testing (Pett, 1997). All analyses for the GSI of the BSI were conducted using independent samples t-tests or F-tests, depending on the number of categories in the independent variable. When age or years since diagnosis was analyzed as a continuous variable, Pearson product–moment correlations were used. Those tests comparing the study sample to published comparison samples utilized one-sample t-tests, using the published mean scores as the test value. As only three people met criteria for PTSD via the SCIP, data from the SCID are reported descriptively.
RESULTS
Sociodemographic factors and psychological distress
The demographic characteristics of the sample (N = 34) are listed in Table 1. Disease and treatment characteristics are listed in Table 2.
Table 1.
Demographic information
| Demographic variable (n = 34) | % (n) |
|---|---|
| Male gender | 53% (18) |
| Race | |
| White/Caucasian | 88% (30) |
| Black/African American | 3% (1) |
| Hispanic/Latino | 3% (1) |
| Other | 6% (2) |
| Mean age at diagnosis | 16 years (range 7–34) |
| Mean # of years since diagnosis | 18 years (range 4–33) |
| Mean current age | 34 years (range 17–54) |
| Level of education | |
| Completed high school | 24% (8) |
| Some college | 41% (14) |
| Completed 4 years of college | 15% (5) |
| Some postgraduate work/degree | 18% (6) |
| Other | 3% (1) |
| Marital status | |
| Single | 47% (16) |
| Married | 38% (13) |
| Divorced | 9% (3) |
| Widowed | 3% (1) |
| Other | 3% (1) |
| Have children | 38% (13) |
| Currently employed | 65% (22) |
Table 2.
Disease and treatment characteristics
| Characteristic (n = 34 unless otherwise indicated) | % (n) |
|---|---|
| Type of sarcoma | |
| Ewing’s Sarcoma | 65 (22) |
| PNET | 15 (5) |
| Rhabdomyosarcoma | 9 (3) |
| Undifferentiated sarcoma and | 12 (4) |
| Osteosarcoma | |
| Type of treatmenta | |
| Cranial radiation | 24 (8) |
| Total body irradiation | 21 (7) |
| Bone marrow transplant (n = 24) | 25 (6) |
| Surgical resection | 62 (21) |
| Amputation | 13 (4) |
| Treatment drug (n = 32) | |
| Vincristine (average 17 mg) | 100 (32) |
| CTX (average 12 700 mg/m2) | 100 (32) |
| Adriamycin (average 389 mg/m2) | 33 (28) |
| Etoposide (average 4224 mg/m2) | 41 (13) |
| Ifosfamide (average 79012 (mg/m2) | 41 (13) |
| Actinomycin D (average 127 mg/kg) | 22 (7) |
Participants may have received none, one or a combination of these treatments.
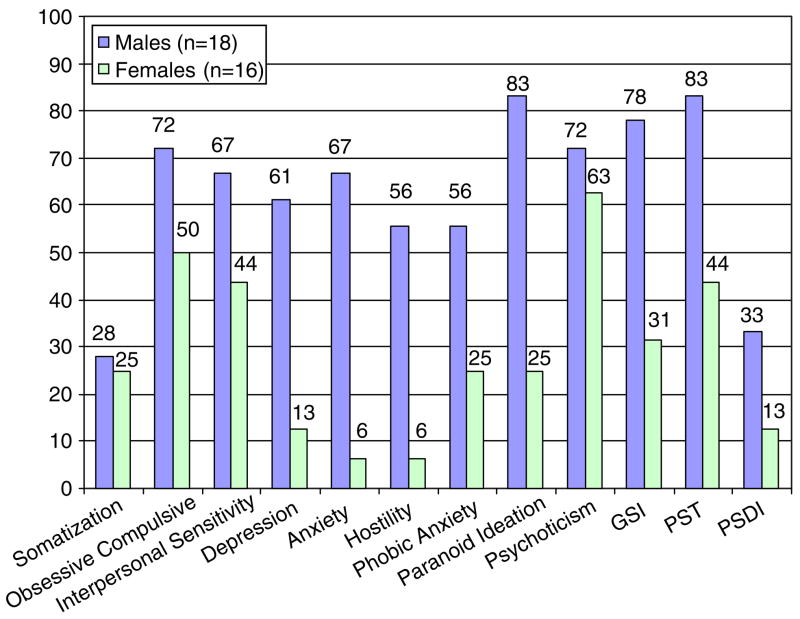
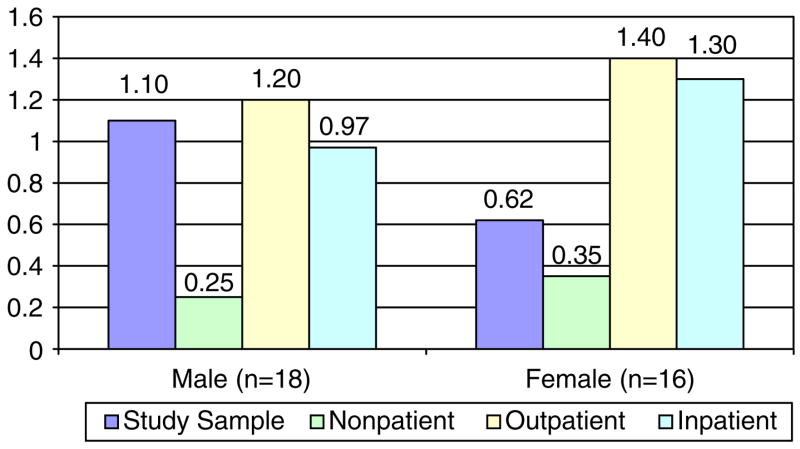
Seventy-seven percent of the survivors met criteria for ‘caseness’ on the BSI indicating significant psychological distress relative to test norms for the general population. Men had significantly higher scores than women on the GSI of the BSI (t = 4.8, p<0.001). Figure 1 displays the patterns between men and women on the symptom subscales of the BSI. Compared to published normative data on non-patient males (Derogatis, 1993), men in the study sample scored higher on the GSI (p<0.05) and scored at levels comparable to male psychiatric outpatients (Figure 2). Men were also comparable to male psychiatric inpatients on the GSI (t = 0.68, n.s.). Women in the study sample scored higher than female non-patients on the GSI (t = 3.1, p<0.01) and lower than female psychiatric out-patients and inpatients (t = 8.8, p<0.001; t = 7.9, p<0.001).
Figure 1.
Percent of males and females scoring in the clinical range on the BSI.
Figure 2.
GSI Scores for men and women in study and comparison groups.
Participants were grouped into three age categories (7–12 (n = 5), 13–19 (n = 24), and 20 and older (n = 5)) to assess whether those diagnosed with cancer during adolescence would have greater levels of psychological distress than those diagnosed earlier. No significant differences were observed in the participant’s current level of psychological distress based on age at diagnosis. Twenty-four percent of participants reported having had difficulty keeping up with school or job requirements after finishing treatment. Those with difficulties scored significantly higher on the GSI (t = 2.6, p<0.05) and the PST (t = 2.6, p<0.05).
Sixty-five percent of participants were currently employed. Those who were not employed reported significantly higher scores on the GSI (t = 2.1, p<0.05) than those who were employed.
Medical and treatment-related factors and psychological distress
Participants had been out of treatment for an average of 17.4 years. No significant associations were found between number of years since initial diagnosis and psychological distress. There were also no significant associations between those who presented with metastatic disease, received brain irradiation (all participants who received brain irradiation had Ewing’s sarcoma (ESFT)), experienced a relapse since initial treatment and degree of psychological distress. It is noteworthy, however, that while the difference was not significant, 86% of those who had experienced a relapse scored in the clinical range on the BSI met criteria for ‘caseness’ while only 56% of those who did experience a relapse scored in this range.
Sixty-five percent of respondents indicated, on a dichotomous (yes/no) question, that they currently worry often about their health or about getting sick. This worry was associated with significantly increased scores on the GSI (t = 2.2, p<0.05). Eighty-five percent endorsed being distressed or bothered about trouble remembering things in the past week, but this independent finding or the fact that 94% of participants report being a better person today as a result of having had cancer was not significantly associated with GSI or any psychological measures.
Post-traumatic stress disorder and related symptoms
Twelve percent (n = 3) of participants met diagnostic criteria for PTSD. Because it is difficult to conduct statistical analysis on such a small number of people, results related to PTSD diagnoses will be reported descriptively. The individuals who met criteria of PTSD were all male, unemployed, and reported worrying more than their peers about their health or about getting sick. Symptoms measured on the IES include intrusive thoughts and memories that trigger intense emotional and physiological reactions similar to posttraumatic stress reactions and avoidant behaviors associated with the cancer experience. Table 3 contains information on the most and least frequently endorsed items from the IES.
Table 3.
Most and least frequently endorsed posttraumatic stress symptoms from the IES
| IES item | Never/ rarely (%) | Sometimes/ always (%) |
|---|---|---|
| Pictures about my illness popped into my mind | 44.1 | 55.9 |
| I thought about my cancer when I didn’t mean to | 47.1 | 52.9 |
| I avoided letting myself get upset when I thought about it or was reminded of it | 50.0 | 50.0 |
| Any reminder brought back feelings about living with cancer | 50.0 | 50.0 |
| I stayed away from reminders about cancer | 73.5 | 26.5 |
| I had dreams about my illness | 79.4 | 20.6 |
| I had trouble falling asleep or staying asleep, because of pictures or thoughts about my disease that came into my mind | 82.4 | 17.6 |
| I felt as if my cancer hadn’t happened or it wasn’t real | 88.2 | 11.8 |
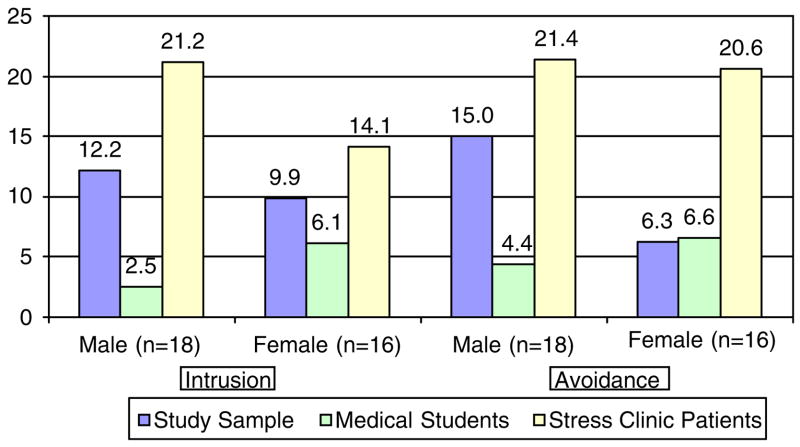
Gender was significantly associated with post-traumatic stress-related symptoms. Men reported significantly higher total and avoidant behavior scores than women in the study sample (t = 2.4, p<0.05 and t = 3.2, p<0.01, respectively) and reported avoidant behavior at levels comparable to male stress clinic patients (t = 0.46, n.s.). Men reported significantly more intrusive thoughts than a published comparison sample of physical therapy students (t = 4.1, p<0.001) (Figure 3).
Figure 3.
IES Intrusion and avoidance scores for men and women in study and comparison groups.
Those who had brain irradiation (24%) reported greater levels of intrusive thoughts about their disease than those who had not (t = 2.2, p<0.05). As all participants who received brain irradiation had ESFT, no dependence of this finding upon the sarcoma diagnosis could be determined. No other demographic or medical variables demonstrated significant associations with PTSS.
Scores on the IES were compared with GSI scores from the BSI. Intrusion and avoidance and total scores were all significantly positively correlated with the GSI (see Table 3). Additional psychosocial factors associated with persistent intrusive thoughts were identified. Participants who reported difficulty keeping up with home friendships during treatment scored higher on both intrusion and avoidance (t = 2.6, p<0.05; t = 2.7, p<0.05) than those who did not endorse that item. Those who reported difficulty keeping up with school/job requirements (t = 2.5, p<0.05) scored significantly higher on avoidance.
DISCUSSION
Previous reports have found equivocal results relating to the prevalence of psychological distress among pediatric cancer survivors. Our study found a relatively high prevalence of psychological distress among a cohort of adults surviving pediatric sarcomas. Sixty-five percent of the study participants indicated that they continue to worry often about their health or about getting sick. This persistent worry was associated with significantly greater levels of psychological distress. Gender, treatment intensity, difficulty transitioning from extensive medical treatment back to home routine, and being unemployed were identified as probable high-risk indicators of persistent distress.
Prior research has found conflicting results regarding the relationship between sociodemographic variables and psychological distress, particularly in terms of gender differences. While females are often at higher risk of psychiatric disturbances, negative outcomes have been reported for both male (Teta et al., 1986; Kazak et al., 1994) and female survivors (Zeltzer et al., 1997). Women survivors are reported to be less likely to be married (Pui et al., 2003) and have greater vulnerability to the adverse effects of cranial irradiation on the central nervous system (Waber and Tarbell, 1997; von der Weid et al., 2003). Our study found poorer psychological outcomes for males, both compared to females in the study and compared to population norms. It is not known whether the gender differences demonstrated here truly reflect a difference between how male and female survivors experience their disease, selection bias, or whether the differences reflect how men and women express themselves. These are questions that have been identified as warranting future investigation (Zebrack et al., 2002).
The prevalence of a clinical diagnosis of PTSD in the general population of adults (ages 15–54) is estimated to be 7.8% (Kessler et al., 1995). Prevalence among pediatric cancer survivors is reported to range from rates of 5 to 10% both for children on treatment (Butler et al., 1996) and for healthy controls (Barakat et al., 1997). Higher rates have been reported in young adult survivors ––a finding consistent with ours (Stuber et al., 1996). While the prevalence of a diagnosis of PTSD found in our study is not alarmingly high (12%), the degree of endorsed posttraumatic stress responses on the Impact of Events scale such as, ‘I thought about my cancer when I didn’t mean to’ (52.9%) and ‘Pictures of my illness popped into my mind’ (55.9%) provides evidence of persistent distressing symptoms consistent with a trauma response. Persistent distress is not surprising considering the emerging data on the risk for second malignancies and other late effects of treatment (Friedman and Meadows, 2002) however it does speak to the need for routine screening in follow-up clinics for intrusive thoughts and avoidant behaviors as indicators of increased psychological vulnerability. In fact, the relationship between psychological distress and PTSD symptoms found in our study and elsewhere (Stuber, 1996; Deimling et al., 2002) supports applicability of a posttraumatic stress model for investigating the psychological impact of cancer survivorship on older adolescents and young adults. Without intervention, intrusive thoughts or the re-experiencing of symptoms when reminded of cancer treatment may result in survivors avoiding medical care (Hobbie et al., 2000) on the one hand, or hypervigilance and higher health care utilization and associated cost on the other (Kazak et al., 2004).
Treatment intensity has been identified as a variable that can affect long-term adjustment (Hobbie et al., 2000). Patients who underwent brain irradiation were more likely to experience intrusive thoughts about their cancer experience. Whether this is related to the intensity of treatment or the widely recognized neuropsychological deficits associated with irradiation is not known. Interestingly, the most widely endorsed question on the BSI, being distressed over the past week by ‘trouble remembering things’, suggests neurocognitive sequalae not previously recognized. While a baseline neuropsychological evaluation is strongly recommended, obtaining one can be particularly challenging in most institutions due to the expense and the fact that most insurance companies do not reimburse for this service.
In addition to treatment intensity, the number of stressors experienced by children during hospitalization has been found to be a significant predictor of a child’s adjustment (Saylor et al., 1986). During the interviews, participants recalled many stressful events that they endured throughout their cancer treatment. One such stress was the required lengthy periods of time away from home and extended disruptions in school and peer activities. The correlation between difficulty adjusting back to school or work after treatment ended and current symptoms of psychological distress in a subgroup of survivors raises concerns. Perhaps with increasing emphasis on outpatient rather than lengthy inpatient care, more attention can be paid to continuing strong ties to the child’s home environment in order to assist with the transition back to school or their community once treatment ends. Furthermore, the association between emotional disconnect from home supports and school with current symptoms of posttraumatic stress suggests that social disruption and loss of social support during treatment may be an additional predictor of long-term traumatic stress effects. This supports published reports citing difficulties in social competence and peer interactions amongst pediatric cancer survivors (Reiter-Purtill and Noll, 2003; Vannatta et al., 1998). The question of what was most traumatic, the nature of the treatment (e.g. frequent IVs, becoming physically ill, loss of hair, having parents condone what was perceived to be physical assaults on their bodies) or the severing of interpersonal ties is not known and deserves further investigation.
The majority of participants reported that they are a better person today because of the cancer diagnosis and treatment that they lived through. This sentiment was also identified in a recent study where survivors of childhood cancer rated themselves high on life satisfaction, feeling useful, and being able to cope as a result of having had cancer (Zebrack and Chesler, 2002). For the pediatric sarcoma survivors who participated in this investigation, this sense of resilience is tempered by concern and uncertainty about future health problems as the majority of participants expressed continued interest in survivor-related services.
Limitations
The main limitation of this study is the self-selection of participants. The low response rate of 41% is less than ideal although the accuracy of this number may be incorrect as many of those lost to follow-up may be deceased. Without psychological or current demographic information available on the patients who did not respond to recruitment efforts, we are unable to determine how the subjects who did not participate in this study differed in psychological outcomes from those who did. There is a greater incidence of Ewing’s sarcoma in Caucasians than in all other ethnic and racial groups (Ozaki et al., 2002), a factor that can limit generalizability of the findings to minority populations of childhood cancer.
Early assessments of pediatric sarcoma patients did not obtain baseline information on psychological functioning. Therefore, the percentage of participants who presented with psychiatric distress initially is not known. As care for childhood cancer becomes more sophisticated, each treatment facility should obtain demographic data and baseline clinical assessments at the onset of cancer treatment to identify distress and to learn about the psychosocial strengths and limitations of each child. In fact, recent practice guidelines published by the National Comprehensive Cancer Network recommend that all patients be screened at appropriate intervals to ascertain their levels of distress, including at their initial visit, when changes occur in disease status, and as clinically indicated (National Comprehensive Cancer Network (NCCN), 2005). Understanding the level and nature of distress can help guide care throughout treatment and also has the potential to provide important information when investigating the long-term social, psychological, economic, and quality of life consequences of cancer on survivors and their families. Assessments of psychological distress can also help to differentiate biological factors, such as late health effects from chemotherapy agents and irradiation from influences such as missed school on poor employment and adverse psychological sequalae (Zebrack et al., 2002).
The constant evolution of therapeutic strategies demonstrating sequential treatment eras and the different cohorts of patients representing diverse disease entities complicate the study of late effects. For example, treatment for this population required lengthy periods of time away from home. Typical admissions for chemotherapy for sarcomas today are usually up to 5-day intervals and pediatric sarcoma patients may not suffer from the same social disruptions as this cohort of patients experienced.
Compared to most psychological survivor studies whose data are derived from mailed self-administered questionnaires and chart reviews, we had the opportunity of evaluating each participant in person. While the number of pediatric sarcoma survivors is small, it is important to attempt to construct studies with a large enough sample to allow for the analysis of the differences in psychological outcomes based on disease entity and treatment modality. When this is not possible, it is recommended that consistent measures of psychological distress be used across studies in order to allow for valid comparisons. Agreement amongst researchers regarding the most appropriate measures for assessing psychological distress, coping, and physical, and posttrauma symptoms would help bring consensus to future research findings (Eiser et al., 2000).
Conclusion
This is the first study to examine psychological distress and posttraumatic stress reactions in adult long-term survivors of a pediatric sarcoma. Our findings suggest that psychological distress and posttraumatic responses may persist years after completing cancer treatment for a pediatric sarcoma. Both prospective and retrospective studies are needed to quantify the incidence and prevalence of adverse sequalae in representative cohorts of survivors. As evidence continues to emerge regarding late effects, ideally, providers of follow-up care will have systems in place to disseminate information to survivors about possible late-effects and psychological support (NCPB, 2003). This is especially important given the significant gaps that young adults have demonstrated regarding knowledge of their medical history and vulnerability (Kadan-Lottick et al., 2002). Specific informal interventions should include giving survivors information regarding their medical late effects while minimizing the anxiety that such education may provoke (Hudson et al., 2003) and providing anticipatory guidance about normative psychosocial symptoms (e.g. anxiety and worry about medical late effects, distress when reminded of cancer and late effects). More gender-specific formal programs might be useful as well.
Persistent distress and the correlation of symptoms of PTSD with psychological distress suggest that carefully designed investigations of the long-term psychological and behavioral consequences of cancer and early interventions to assess and treat psychological distress should go hand in hand with the investigation of the medical consequences of disease (Eiser et al., 2000).
References
- Alderfer M, Labay LE, Kazak AE. Brief report: Does posttraumatic stress apply to siblings of childhood cancer survivors? J Adv Nurs. 2003;28:281–286. doi: 10.1093/jpepsy/jsg016. [DOI] [PubMed] [Google Scholar]
- Alter CL, Pelcovitz D, Axelrod A, et al. Identification of PTSD in cancer survivors. Psychosomatics. 1996;37:137–143. doi: 10.1016/S0033-3182(96)71580-3. [DOI] [PubMed] [Google Scholar]
- Anholt UV, Fritz GK, Keener M. Self-concept in survivors of childhood and adolescent cancer. J Psychosoc Oncol. 1993;11:1–16. [Google Scholar]
- Bacci G, Forni C, Longhi A, et al. Long-term outcome for patients with non-metastatic Ewing’s sarcoma treated with adjuvant and neoadjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer. 2004;40(1):73–83. doi: 10.1016/j.ejca.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Kazak AE, Meadows AT, Casey R, Meeske K, Stuber ML. Families surviving childhood cancer: a comparison of posttraumatic stress symptoms with families of healthy children. J Pediatr Psychol. 1997;22:843–859. doi: 10.1093/jpepsy/22.6.843. [DOI] [PubMed] [Google Scholar]
- Boman K, Bodegard G. Long-term coping in childhood cancer survivors: influence of illness, treatment and demographic background factors. Acta Paediatr. 2000;89:105–111. doi: 10.1080/080352500750029167. [DOI] [PubMed] [Google Scholar]
- Boulet J, Boss MW. Reliability and validity of the brief symptom inventory. J Consult Clin Psychol. 1991;3:433–437. [Google Scholar]
- Brown RT, Madan-Swain A, Lambert R. Post-traumatic stress symptoms in adolescent survivors of childhood cancer and their mothers. J Trauma Stress. 2003;16:309–318. doi: 10.1023/A:1024465415620. [DOI] [PubMed] [Google Scholar]
- Butler R, Rizzi L, Handwerger B. Brief report: The assessment of posttraumatic stress disorder in pediatric cancer patients and survivors. J Pediatr Psychol. 1996;21:499–504. doi: 10.1093/jpepsy/21.4.499. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tross S. Psychological adjustment to survival from Hodgkin’s disease. J Consult Clin Psychol. 1986;54:616–622. doi: 10.1037//0022-006x.54.5.616. [DOI] [PubMed] [Google Scholar]
- Chesler MA, Zebrack BJ. An Updated Report on Our Studies of Long-Term Survivorship of Childhood Cancer and a Brief Review of the Psychosocial Literature. Center for Research on Social Organization University of Michigan; Ann Arbor, MI: 1997. [Google Scholar]
- Deimling GT, Kahana B, Bowman KF, Schaefer ML. Cancer survivorship and psychological distress in later life. Psycho-Oncology. 2002;11:479–494. doi: 10.1002/pon.614. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. BSI: Brief Symptom Inventory: Administration, Scoring, and Procedures Manual. National Computer Systems Inc.; Minneapolis, MN: 1993. [Google Scholar]
- Eiser C, Hill JJ, Vance Y. Examining the psychological consequences of surviving childhood cancer: systematic review as a research method in pediatric psychology. J Pediatr Psychol. 2000;25:449–460. doi: 10.1093/jpepsy/25.6.449. [DOI] [PubMed] [Google Scholar]
- Elkin TD, Phipps S, Mulhern RK, Fairclough D. Psychological functioning of adolescent and young adult survivors of pediatric malignancy. Med Pediatr Oncol. 1997;29:582–588. doi: 10.1002/(sici)1096-911x(199712)29:6<582::aid-mpo13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan R, Bengoa R, Yach D. Chronic conditions––the new health challenge. SAMJ. 2003;93:585–590. [PubMed] [Google Scholar]
- Erickson S, Steiner H. Trauma and personality correlates in long term pediatric cancer survivors. Child Psychiatry Hum Dev. 2002;31:195–213. doi: 10.1023/a:1026477321319. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV Axis 1 Disorders––Clinician Version (SCID-CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Friedman D, Meadows A. Late effects of childhood cancer therapy. Pediatr Clin North America. 2002;18:4060–4066. doi: 10.1016/s0031-3955(02)00032-9. [DOI] [PubMed] [Google Scholar]
- Fritz GK, Williams JR, Amylon M. After treatment ends: psychosocial sequelae in pediatric cancer survivors. Am J Orthopsychiatry. 1988;58:552–561. doi: 10.1111/j.1939-0025.1988.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Woo S, Johnson ME, Hicks MJ, Horowitz ME. Ewing’s sarcoma family of tumors. In: Poplack PAD, editor. Principles and Practice of Pediatric Oncology, Pizzo. Lippincott-Raven; Philadelphia: 2002. pp. 973–1016. [Google Scholar]
- Glover DA, Byrne J, Mills JL, et al. Impact of CNS treatment on mood in adult survivors of childhood leukemia: a report from the children’s cancer group. J Clin Oncol. 2003;21:4395–4401. doi: 10.1200/JCO.2003.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RE, Doan BD, Shermer P, et al. Surviving childhood cancer: a descriptive approach to understanding the impact of life-threatening illness. Psycho-Oncology. 1992;1:235–245. [Google Scholar]
- Green DM, Zevon MA, Reese PA, et al. Second malignant tumors following treatment during childhood and adolescence for cancer. Med Pediatr Oncol. 1994;22:1–10. doi: 10.1002/mpo.2950220102. [DOI] [PubMed] [Google Scholar]
- Green MH, Glaubiger DL, Mead GD, et al. Subsequent cancer in patients with Ewing’s sarcoma. Cancer Treatment Rep. 1979;63:2043–2046. [PubMed] [Google Scholar]
- Greenberg HS, Kazak AE, Meadows AT. Psychologic functioning in 8-to 16 year old cancer survivors and their parents. J Pediatr. 1989;114:488–493. doi: 10.1016/s0022-3476(89)80581-5. [DOI] [PubMed] [Google Scholar]
- Hobbie WL, Stuber M, Meeske K, et al. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J Clin Oncol. 2000;18:4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ, Wilner N, Alvarez W. Impact of events scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer. J Am Med Assoc. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: childhood cancer survivor study. J Am Med Assoc 10. 2002;287(4):1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- Kazak AE. Posttraumatic distress in childhood cancer survivors and their parents. Med Pediatr Oncol Suppl. 1998;1:60–68. doi: 10.1002/(sici)1096-911x(1998)30:1+<60::aid-mpo9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Alderfer M, Rourke M, Simms S, Streisand R, Grossman JP. Posttraumatic stress symptoms (PTSS) and posttraumatic stress disorder in families of adolescent childhood cancer survivors. J Pediatr Psychol. 2004;29:211–219. doi: 10.1093/jpepsy/jsh022. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Barakat L. Parenting stress and quality of life during treatment for childhood leukemia predicts child and parent adjustment after treatment ends. J Pediatr Psychol. 1997;22:749–758. doi: 10.1093/jpepsy/22.5.749. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Barakat L, Alderfer M, et al. Posttraumatic stress in survivors of childhood cancer and mothers: development and validation of the Impact of Traumatic Stressors Interview Schedule (ITSIS) J Clin Psychol Med Settings. 2001;8:307. [Google Scholar]
- Kazak AE, Christakis D, Alderfer M, Coiro MJ. Young adolescent cancer survivors and their parents: adjustment, learning problems, and gender. J Fam Psychol. 1994;8:74–84. [Google Scholar]
- Kessler R, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kokkonen J, Vainionpaa L, Winqvist S, Lanning M. Physical and psychosocial outcome for young adults with treated malignancy. Pediatr Hematol Oncol. 1997;14(3):223–232. doi: 10.3109/08880019709009492. [DOI] [PubMed] [Google Scholar]
- Koocher GP, O’Malley JE. The Damocles Syndrome: Psychosocial Consequences of Surviving Childhood Cancer. McGraw Hill; New York: 1981. [Google Scholar]
- Kupst MJ, Natta MB, Richardson CC, Schulman JL, Lavigne JV, Das L. Family coping with pediatric leukemia: ten years after treatment. J Pediatr Psychol. 1995;20:601–617. doi: 10.1093/jpepsy/20.5.601. [DOI] [PubMed] [Google Scholar]
- Link PM, Gebhardt MC, Meyers PA. Osteosarcoma. In: Poplack PAD, editor. Principles and Practice of Pediatric Oncology, Pizzo. Lippincott-Raven; Philadelphia: 2002. pp. 1051–1089. [Google Scholar]
- Madan-Swain A, Brown RT. Cognitive and psychosocial sequelae for children with acute lymphocytic leukemia survivors and their families. Clin Psychol Rev. 1991;11:267–294. [Google Scholar]
- Manne Sl, Du Hamel K, Gallelli K, Sorgen K, Redd WH. Posttraumatic stress disorder among mothers of pediatric cancer survivors: diagnosis, comorbidity, and utility of the PTSD checklist as a screening instrument. J Pediatr Psychol. 1998;23:357–366. doi: 10.1093/jpepsy/23.6.357. [DOI] [PubMed] [Google Scholar]
- Manne S, DuHamel K, Nereo N, et al. Predictors of PTSD in mothers of children undergoing bone marrow transplantation: the role of cognitive and social processes. J Pediatr Psychol. 2002;27:607–617. doi: 10.1093/jpepsy/27.7.607. [DOI] [PubMed] [Google Scholar]
- Meeske KA, Ruccione K, Globe DR, Stuber ML. Posttraumatic stress, quality of life, and psychological distress in young adult survivors of childhood cancer. Oncol Nurs Forum. 2001;28:481–489. [PubMed] [Google Scholar]
- Mulhern RK, Wasserman AL, Friedman AG, Fairclough D. Social competence and behavioral adjustment of children who are long-term survivors of cancer. Pediatric. 1989;83(1):18–25. [PubMed] [Google Scholar]
- Moore I, Glasser M, Ablin A. The late psychosocial consequences of childhood cancer. J Ped Nurs. 1987;3:150–158. [PubMed] [Google Scholar]
- Nagarajan R, Clohisy DR, Neglia JP, et al. Function and quality of life of survivors of pelvic and lower extremity osteosarcoma and Ewing’s sarcoma: the childhood cancer survivor study. Br J Cancer. 2004;91:1858–1865. doi: 10.1038/sj.bjc.6602220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Policy Board (NCPB) Childhood Cancer Survivorship: Improving Care and Quality of Life. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN) Distress management. Pract Guidelines Oncol. 2005;1:1–42. [Google Scholar]
- Neff EJ, Beardslee CI. Body knowledge and concerns of children with cancer as compared with the knowledge and concerns of other children. J Pediatr Nurs. 1990;5:179–189. [PubMed] [Google Scholar]
- Nicholson HS, Mulvihill JJ, Byrne J. Late effects of therapy in adult survivors of osteosarcoma and Ewing’s sarcoma. Med Pediatr Oncol. 1992;20:6–12. doi: 10.1002/mpo.2950200103. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Fears TR, Horowitz ME, Tucker MA, Wexler LH. Late effects of therapy in survivors of Ewing’s sarcoma family tumors. J Pediatr Hematol Oncol. 1997;19:220–225. doi: 10.1097/00043426-199705000-00008. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Schaefer KL, Wai D, et al. Population-based genetic alterations in Ewing’s tumors from Japanese and European Caucasian patients. Ann Oncol. 2002;13(10):1656–1664. doi: 10.1093/annonc/mdf218. [DOI] [PubMed] [Google Scholar]
- Pelcovitz D, Goldenberg B, Kaplan S, et al. Posttraumatic stress disorder on mothers of pediatric cancer survivors. Psychosomatics. 1996;37:116–126. doi: 10.1016/S0033-3182(96)71577-3. [DOI] [PubMed] [Google Scholar]
- Pelcovitz D, Libov BG, Mandel F, Kaplan S, Weinblatt M, Septimus A. Posttraumatic stress disorder and family functioning in adolescent cancer. J Trauma Stress. 1998;11(2):205–221. doi: 10.1023/A:1024442802113. [DOI] [PubMed] [Google Scholar]
- Pendley JS, Dahlquist LM, Dreyer Z. Body image and psychological adjustment in adolescent cancer survivors. J Pediatr Psychol. 1997;22:29–43. doi: 10.1093/jpepsy/22.1.29. [DOI] [PubMed] [Google Scholar]
- Pett MA. Nonparametric Statistics for Health Care Research: Statistics for Small Samples and Unusual Distributions. Sage Publications; Thousand Oaks, CA: 1997. [Google Scholar]
- Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lympoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- Recklitis C, O’Leary T, Diller L. Utility of routine psychological screening in the childhood cancer survivor clinic. J Clin Oncol. 2003;21:787–792. doi: 10.1200/JCO.2003.05.158. [DOI] [PubMed] [Google Scholar]
- Reis L, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1973–1999. National Cancer Institute; Bethesda, MD: 2002. [Google Scholar]
- Reiter-Purtill J, Noll R. Peer relationships of children with chronic illness. In: Roberts M, editor. Handbook of Pediatric Psychology. 3. Guilford; New York: 2003. [Google Scholar]
- Ross L, Johansen C, Oksbjerg Dalton S, et al. Psychiatric hospitalizations among survivors of cancer in childhood or adolescence. N Eng J Med. 2003;349:650–657. doi: 10.1056/NEJMoa022672. [DOI] [PubMed] [Google Scholar]
- Saylor C, Pallmeyer T, Finch A, Eason L, Trieber F, Folger C. Predictors of psychological distress in hospitalized pediatric patients. J Am Acad Child Adol Psychiatry. 1987;26:232–236. doi: 10.1097/00004583-198703000-00020. [DOI] [PubMed] [Google Scholar]
- Schmale AH, Morrow GR, Schmitt MH, et al. Well-being of cancer survivors. Psychosom Med. 1983;45:163–169. doi: 10.1097/00006842-198305000-00008. [DOI] [PubMed] [Google Scholar]
- Schwardwald J, Solomon Z, Weisenberg M, et al. Validation of the impact of event scale for psychological sequelae of combat. J Consult Clin Psycho-Oncol. 1987;55:251–256. doi: 10.1037//0022-006x.55.2.251. [DOI] [PubMed] [Google Scholar]
- Segal DL, Hersen M, Van Hasselt VB, et al. Reliability of diagnosis in older psychiatric patients using the structured clinical interview for DSM-III-R. J Psychopathol Behav Assess. 1993;15:347–356. [Google Scholar]
- Segal DL, Hersen M, Van Hasselt VB. Reliability of the structured interview for DSM-III-R: an evaluative review. Compr Psychiatry. 1994;35:316–327. doi: 10.1016/0010-440x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Segal DL, Kabacoff RI, Hersen M. Update on the reliability of diagnosis in older psychiatric outpatients using the structured clinical interview for DSM-IIIR. J Clin Geropsychol. 1995;1:313–321. [Google Scholar]
- Strong LC, Herson J, Osborne BM, Sutow WW. Risk of radiation-related subsequent malignant tumors in survivors of Ewing’s sarcoma. J Natl Cancer Inst. 1979;62:1401–1406. [PubMed] [Google Scholar]
- Stuber ML, Christakis DA, Houskamp B, Kazak AE. Posttrauma symptoms in childhood leukemia survivors and their parents. Psychosomatics. 1996;37:254–261. doi: 10.1016/S0033-3182(96)71564-5. [DOI] [PubMed] [Google Scholar]
- Teta MJ, Del Po MC, Kasl SV, Meigs JW, Myers MH, Mulvihill JJ. Psychosocial consequences of childhood and adolescent cancer survival. J Chronic Dis. 1986;39:751–759. doi: 10.1016/0021-9681(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Travis LB, Curtis RE, Hankey BF, Fraumeni JF. Second cancers in patients with Ewing’s sarcoma. Med Pediatr Oncol. 1994;22:296–297. doi: 10.1002/mpo.2950220417. [DOI] [PubMed] [Google Scholar]
- von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukemia treated with chemotherapy alone: age and sex related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Waber DP, Tarbell NJ. Toxicity of CNS prophylaxis for childhood leukemia. Oncology. 1997;11:259–264. [PubMed] [Google Scholar]
- Wexler LH, Christ WM, Helman LJ. Rhabdo-myosarcoma and undifferentiated sarcomas. In: Pizzo PA, Poplack D, editors. Principles and Practice of Pediatric Oncology. Lippincott-Raven; Philadelphia: 2002. pp. 939–971. [Google Scholar]
- Zebrack BJ, Chesler MA. Quality of life in childhood cancer survivors. Psycho-Oncology. 2002;11:132–141. doi: 10.1002/pon.569. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, Zeltzer LK, Whitton J, et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin’s disease, and non-Hodgkin’s lymphoma: a report from the childhood cancer survivor study. Pediatrics. 2002;110:42–52. doi: 10.1542/peds.110.1.42. [DOI] [PubMed] [Google Scholar]
- Zeltzer LK, Chen E, Weiss K, et al. Comparison of psychological outcome in adult survivors of childhood acute lymphoblastic leukemia versus Sibling Controls a cooperative children’s cancer group and National Institutes of Health Study. J Clinic Oncol. 1997;15:547–556. doi: 10.1200/JCO.1997.15.2.547. [DOI] [PubMed] [Google Scholar]