Abstract
Although ingestion is the major route of exposure to polychlorinated biphenyls (PCBs), dietary factors altering their absorption and excretion are only poorly understood. In the present study, (±)-PCB 136 was administered orally to female C57BL/6 mice fed an unrefined (URD, 10% fat) or high fat (HFD, 40% fat) diet to investigate the effect of the dietary fat content on the disposition of PCBs. Three days after administration, PCB levels in the adipose tissue were significantly lower in HFD animals than URD animals, partly due to a higher excretion rate of PCB 136 in the HFD group. (+)-PCB 136 was enriched in all organs and in feces. In both groups, enantiomeric fractions in feces increased each day after administration. We hypothesize that low EF (enantiomeric fraction) values in feces excreted within 24 hours of exposure are due to the presence of undigested, racemic PCB. Higher EF values in feces excreted after two and three days are due to excretion of previously absorbed PCBs. Overall, our study suggests that the EF value may be a good tool to investigate the absorption and excretion of PCBs in vivo.
Keywords: polychlorinated biphenyls, enantiomeric fraction, dietary fat, absorption, bioavailability, excretion, feces
1. Introduction
Polychlorinated biphenyls (PCBs) are an important class of anthropogenic environmental contaminants (ATSDR 2000). They were manufactured as complex mixtures by batch chlorination of biphenyl. Although their production has been banned world wide, PCBs continue to be released into the environment (Breivik et al. 2002; Harrad et al. 2006). All PCB congeners are highly lipophilic compounds with octanol-water partition coefficients log Kow > 5, and many PCB congeners are resistant to chemical, biological and thermal degradation. These properties give persistent PCB congeners the tendency to bioaccumulate in organisms and to biomagnify in the food chain, resulting in high and potentially toxic levels in organisms of higher trophic levels and in humans. They are commonly found in the human food supply (Haluska et al. 1995; Hites 2004). Animal and epidemiologic studies have implicated PCB mixtures and individual PCB congeners in adverse health effects such as carcinogenesis, neurodevelopmental effects, heart disease and immunological dysfunctions (Robertson and Hansen 2001; Ludewig et al. 2007).
Several environmentally and toxicologically relevant PCB congeners are chiral (Püttmann et al. 1990; Lehmler and Robertson 2001), i.e. these congeners exist as two stable rotational isomers called atropisomers that are nonsuperimposable mirror images of each other. These chiral PCB congeners, for example PCB 136 (Figure 1), have three or four ortho chlorine substituents and an unsymmetrical chlorine substitution pattern in both phenyl rings. Although these PCBs are present as a racemate (i.e., a 1:1 mixture of both atropisomers) in technical PCB mixtures, several studies have shown an enantiomer enrichment of chiral PCBs in fish (Wong et al. 2002; Buckman et al. 2006), mammals (Püttmann et al. 1989; Kania-Korwel et al. 2006; Norström et al. 2006; Kania-Korwel et al. 2007) and humans (Glausch et al. 1995; Chu et al. 2003; Harrad et al. 2006).
Figure 1.
Chemical structure of the two PCB 136 atropisomers.
The diet, especially fish, is a predominant source of PCBs, including several chiral PCB congeners, in humans (Duarte-Davidson and Jones 1994; Chiu et al. 2004). PCBs are efficiently absorbed in the intestine in close association with dietary lipids. Several studies have shown that the type and amount of dietary fat can be an important determinant for the intestinal absorption of highly lipophilic xenobiotics (Charmann and Stella 1986; Gobas et al. 1993). A high fat diet has been shown to increase the fecal excretion of PCBs, probably due to an increased residual fat content in the feces (Gobas et al. 1993). Such an increased fecal fat content may reduce the bioavailability of PCBs and increase the excretion of PCB into the intestine over time. Similarly, undigestible fats such as Olestra® have been used to facilitate the excretion of lipophilic compounds and to significantly reduce the body burden of these compounds (Jandacek et al. 2005; Redgrave et al. 2005).
Unfortunately, it is difficult to determine the immediate source of PCBs in fecal material: PCBs may be excreted after absorption or may have never been absorbed by the body. This is a problem even in laboratory studies where the food source is controlled. Harrad et al recently reported that chiral PCBs in feces can be used to investigate current human exposure of PCBs (Harrad et al. 2006). We herein investigate if changes in the enantiomeric fraction (EF) of PCB 136 can be used to investigate the influence of the dietary fat content on the enantioselective absorption and excretion of PCB 136 atropisomers in female C57BL/6 mice. We hypothesize that non-absorbed PCBs are excreted unchanged (i.e., as a racemate), whereas previously absorbed PCBs are excreted with a significant enantiomeric enrichment. This hypothesis, if found correct, would support a new approach for monitoring the excretion of PCBs and other chiral environmental contaminants in humans.
2. Methods
2.1. Chemicals
2,2′,3,3′,6,6′-hexachlorobiphenyl (CAS 38411-22-2) was synthesized and purified as described previously (Kania-Korwel et al. 2006; Shaikh et al. 2006). Its purity was determined using GC-FID and was > 99% based on relative peak area.
2.2. Preparation of the PCB 136 containing vehicle
Vanilla Wafer cookie pieces appropriate for the body weight of each animal (7.5g/kg body weight) were selected 24 hours prior to PCB administration (Kania-Korwel et al. 2007). A defined volume of a PCB 136 solution in pesticide-grade hexanes (12.5 mg/ml) was applied to each cookie piece to allow the administration of 50 mg PCB 136/kg body weight to each animal. The solvent was allowed to evaporate under vacuum for approximately 24 hours, which is sufficient for the removal of solvent traces.
2.3. Animal experiment
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa. Fourteen female C57BL/6 mice were obtained from Harlan (Indianapolis, IN). The animals were 8 weeks old with an average weight of 17.6±1.0 g. The animals were allowed to acclimatize for 7 days and then randomly assigned to three treatment groups. The mice were given ad libitum access to the respective diet and water. The unrefined diet group (URD; n=6) and the control group (n=4) were fed with a typical unrefined rodent diet (NIH-31 modified 6% mouse sterilizable diet, Harlan, Madison, WI), whereas the high fat diet group (HFD; n=4) received a high fat diet (F3282, Bio-Serv, Frenchtown, NY). Previous studies from our laboratory showed that at least four animals are needed to provide sufficient statistical power for the detection of meaningful differences in the EF values among treatment groups (assuming a difference of 0.1 and a standard deviation of 0.03: p = 0.97). In this study we used six animals in the URD treatment group to increase the statistical power to p = 0.99.
The HFD group was switched to the HFD approximately 30 minutes before PCB administration. Each animal from the URD and HFD groups received a single oral dose of PCB 136 (50 mg/kg body weight) administered with a PCB 136-treated piece of a Vanilla Wafer cookie. A group of animals serving as control group (n=4) received the vehicle alone (Kania-Korwel et al. 2007). Animals were closely monitored to ensure that the vehicle was consumed completely by the animals. All animals were then transferred into metabolism cages, and feces and urine where collected daily. It was not possible to collect feces and urine sample at the zero time point due to the route of administration (i.e., the PCB-treated cookie could not be administered in metabolism cages because crumbs would fall through the wire mesh bottom).
Mice were euthanatized three days after PCB administration by asphyxiation with carbon dioxide followed by cervical dislocation. Tissues (abdominal adipose tissue, brain, heart, intestines, kidneys, liver, lungs, right thigh muscle, abdominal skin, spleen and uterus) were excised en bloc and their wet weight was determined. All tissues were stored in aluminum foil at -20°C prior to PCB extraction and analysis.
2.4. Extraction of PCBs and lipids from tissue and whole blood samples
A pressurized solvent extraction system (ASE 200, Dionex, Sunnyvale, CA) was used for the PCB extraction and clean-up (Kania-Korwel et al. 2007). The lipid content was determined gravimetrically in adipose tissue, kidney, liver, skin and the respective rodent diets (Kania-Korwel et al. 2007). The non-volatile residue in solvent blanks was 0.42±0.31 mg (n=7) and was significantly lower than lipid amount in tissues (adipose = 67±32 mg; feces = 34±10 mg; kidney 9±1 mg; liver = 23±6 mg).
2.5. Gas chromatographic analysis
PCB 136 levels and enantiomeric fractions were determined using a HP6890 gas chromatograph equipped with a 63Ni μ-ECD detector and a Chirasil-Dex column (β-cyclodextrin chemically bonded with dimethylpolysiloxane, 25 m × 0.25 mm ID × 0.25μm film thickness, Varian, Palo Alto, CA) (Kania-Korwel et al. 2007). The resolution of PCB 136 atropisomers was calculated using the formula
where RT1 and RT2 are the retention times of the first and second eluting atropisomer peaks and BW1 and BW2 are the base widths of peaks 1 and 2, and was on average 0.64. The enantiomeric fraction was calculated using the following formula (Harner et al. 2000):
The enantiomeric elution order was determined based on the published elution order of PCB 136 on a Chirasil-Dex column (Haglund and Wiberg 1996). The racemic standard mixture of PCB 136 had an EF of 0.50 ± 0.01 (average of 58 measurements, 95% confidence interval 0.498 to 0.504).
2.6. Quality assurance
The limit of detection (LOD) for PCB 136 was calculated based on the method blanks (Kania-Korwel et al. 2007) and equals 6.3 ng. The limit of quantification, calculated as 10 times LOD, equals 63 ng. Tissue levels in untreated control animals (i.e., tissue blanks) were ≤ 2 ng/g tissue. The mean recovery rate of the surrogate standard (2,3,4,4′,5,6-hexachlorobiphenyl, PCB 166) was 92±14 %. PCB 204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl) was used as internal standard for the quantification of PCB 136.
2.7. Statistical analyses
All statistics were preformed using SAS version 9.1 (SAS institute, Cary, NC). Data are presented as mean ± standard deviation. Differences in body and organ weight between both treatment groups and control were analyzed with ANOVA with Bonferroni multiple comparison test. ANOVA with Tukey multiple comparison test was employed to determine significant differences between tissues within the same treatment group. Differences between PCB 136 levels and enantiomeric fraction in tissues of from mice from the two treatment groups were analyzed with t-test (α=0.05). The one sample t-test (α=0.05) was used to test for significant differences between the tissue EF values and the EF value of the racemic standard (EF=0.50). For all comparisons, p=0.05 was considered to be statistical significant.
3. Results and discussion
3.1. Extractable dietary fat content
To investigate if dietary fat content alters the enantioselective disposition of PCB 136, female C57BL/6 mice receiving a single oral dose of PCB 136 were fed either a unrefined (URD) or a high fat rodent diet (HFD). The extractable lipid content in the unrefined diet was 10.1±0.6 %. The high fat diet - which is frequently used to study obesity in rodents (BioServ Inc. 2007) - had an extractable fat content of 36.8±4.5 %. This fat content is in good agreement with manufacturer data (35.5%). The extractable fat content of the Keebeler Vanilla Wafer cookie used for the administration of PCB 136 was 28.4±5.7 %, which is comparable to the fat content of the high fat diet.
3.2. General toxicity of PCB 136 in mice
The PCB-treatment had no effect on body and organ weight compared to control animals (data not shown), which is in agreement with previous studies (Lehmler et al. 2003; Kania-Korwel et al. 2007). However, the diet had an effect on the appearance of the animals and their feces production. All HFD mice had a shiny, oily looking fur, which is typical for animals fed a high fat diet. These mice had also a reduced feces production compared to URD animals (0.05 to 0.10 g animal-1 day-1 versus 1.27 g animal-1 day-1). The feces from HFD mice also had a different appearance, i.e. was harder compared to feces from URD animals. Similar differences in the feces production between a unrefined and a high fat diet have been reported in other studies (Han et al. 2002). Despite the difference in the fat content of the diet, no significant differences in the extractable fat content was noted between the liver and kidneys of URD and HFD animals after three days.
3.3. PCB 136 levels in tissue and blood
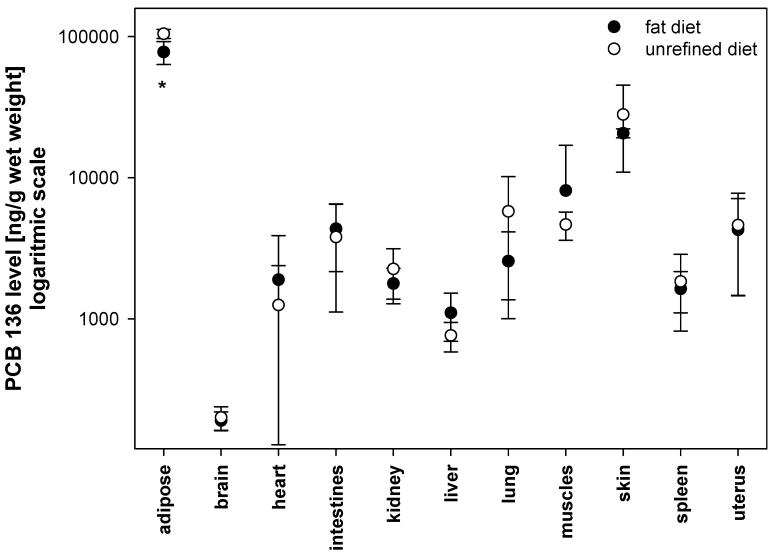
The estimated sum of the total PCB 136 content in tissues, blood and feces accounts for 23.2% and 22.8% of the total PCB dose (i.e., 50 mg/kg body weight) in mice fed the unrefined or the high fat diet, respectively (Table 1). This drastic reduction of the PCB body burden is due to the short half life of PCB 136 in mice (Mizutani et al. 1980). In agreement with similar studies from our laboratory (Lehmler et al. 2003; Kania-Korwel et al. 2007), PCB 136 accumulated mainly in tissues with a high fat content, i.e. the adipose tissue and the skin, independent of the diet (Figure 2; Tables 1 and 2).
Table 1.
PCB 136 tissue levels expressed as percent of total dose in mice fed high fat or unrefined diet.$
| Diet | Fat diet | Unrefined diet |
|---|---|---|
| Tissue | ||
| Adipose | 8.6 ± 1.4* | 11.5 ± 0.7 |
| Brain | 0.007 ± 0.002 | 0.008 ± 0.001 |
| Heart | 0.02 ± 0.02 | 0.01 ± 0.01 |
| Intestines | 0.31 ± 0.17 | 0.3 ± 0.2 |
| Kidney | 0.04 ± 0.01 | 0.05 ± 0.02 |
| Liver | 0.09 ± 0.04 | 0.07 ± 0.02 |
| Lung | 0.04 ± 0.03 | 0.09 ± 0.07 |
| Muscles | 5.6 ± 5.9 | 3.3 ± 0.8 |
| Skin | 6.5 ± 4.7 | 8.7 ± 5.4 |
| Spleen | 0.008 ± 0.003 | 0.009 ± 0.005 |
| Uterus | 0.02 ± 0.01 | 0.03 ± 0.03 |
| Sum % total dose | 21.2 ± 6.6 | 23.2 ± 4.9 |
| % total dose in feces# | 1.6 | 0.04 (0.71, 0.02) |
The percent of body weight in C57BL/6 mice was assumed to be 5.9% for adipose tissue, 38.4% for muscles, and 16.5% for skin (Brown et al. 1997). The percent of body weight for all other organs was calculated based on the respective wet weight.
Excreted over a 3 day period
different from mice fed unrefined diet at α = 0.05, p=0.02.
Figure 2.
The PCB 136 levels in mice after a single oral dose, and fed with the high fat or the unrefined rodent diet (* different from unrefined diet fed mice; t-test at α = 0.05, p=0.02).
Table 2.
Lipid adjusted levels of PCB 136 [μg/g lipid] in adipose tissue, kidney, liver and skin in mice fed with high fat and unrefined diet.
| diet | Fat diet [μg PCB 136/g lipid] | Unrefined diet [μg PCB 136/g lipid] |
|---|---|---|
| tissue | ||
| Adipose tissue | 104 ± 23* | 134 ± 9 |
| Kidney | 18 ± 6 | 25 ± 11 |
| Liver | 14 ± 4 | 13 ± 4 |
| Skin | 86 ± 66 | 122 ± 75 |
Different from mice fed unrefined diet at α = 0.05, p=0.02.
Comparison of PCB 136 tissue levels among the URD and the HFD group revealed that HFD mice had significantly lower PCB level in the adipose tissue than URD mice. This difference was significant for PCB levels expressed as percentage of the total dose (Table 1), PCB levels adjusted for lipid content (Table 2) and PCB levels per gram wet weight (Figure 2). This difference may be due to the following reasons: First, feces from HFD animals is likely to contain more undigested fat than feces from URD animals. A higher fecal fat content is expected to increase the retention of PCB 136 in the feces and/or to reduce the bioavailability of PCB 136 in the HFD group (also see Section 3.5. below). Unfortunately, we could not determine the HFD fecal fat content because of the small amount of feces collected from HFD animals. Second, the lower PCB levels may be due to differences in the expansion of the fat compartment in animals receiving diets with different fat content and, thus, a dilution of the dose. Although we were unable to detect a difference in fat content in liver and kidney, it is possible that the overall fat content in the adipose tissue is larger in HFD than in URD animals. This explanation of the lower PCB 136 levels in the adipose tissues of HFD animals is consistent with previous studies with laboratory animals (Hansen et al. 1977; Drouillard 2003).
3.4. Enantiomeric enrichment of PCB 136 in tissues
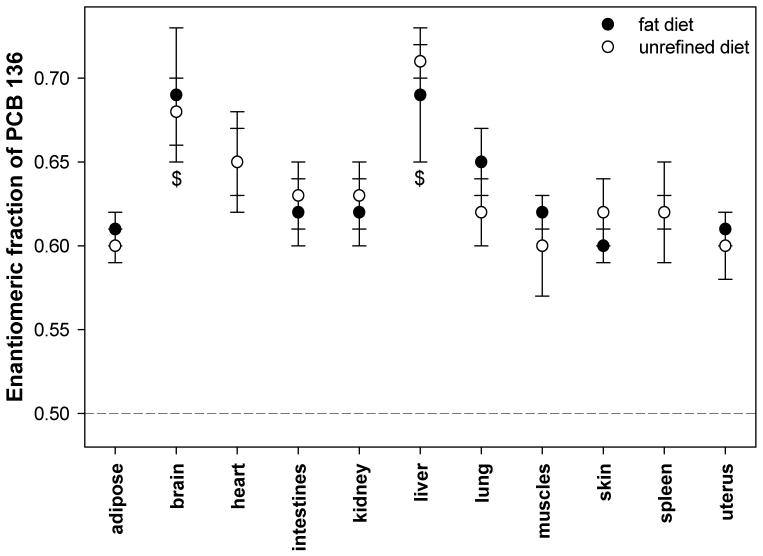
The EF value in all tissues from the URD and the HFD group is > 0.50, i.e. (+)-PCB 136 is enriched in these tissues (Figure 3). Similarly, (+)-PCB 136 was enriched in earlier studies in female and male mice (Kania-Korwel et al. 2007). As shown in Figure 3, the EF values in all tissues are significantly different from the EF value of 0.50±0.01 of the racemic PCB 136 (t-test at α=0.05, p<0.01). The highest EFs values were observed in the brain (0.68±0.02 and 0.69±0.04 in URD and HFD mice, respectively) and the liver (0.71±0.01 and 0.69±0.04 in URD and HFD mice, respectively), with both tissues having similar EF values. In both diet groups, the EF values in the brain and the liver were typically significantly higher than in other tissues. Otherwise, the overall distribution of PCB 136 atropisomers between tissues was essentially identical between URD and HFD animals, i.e., the dietary fat content did not alter the disposition of PCB 136 in an enantioselective manner.
Figure 3.
The enantiomeric fraction of PCB 136 in mice after a single oral dose, and fed with the high fat or the unrefined rodent diet. The EF values for heart and spleen overlap. The EF of the racemic mixture is EF=0.50±0.01 (shown as a dotted line) and is significantly different from the EF values of all tissues (p < 0.05). $ EF value is significantly different from all other tissues (α = 0.05, p < 0.05).
In a separate study with similar design (Kania-Korwel et al. 2007), the liver also exhibited the most pronounced enrichment of (+)-PCB 136 (EF = 0.75±0.01), with the liver EF value being significantly different from all other tissues investigated. However, in this earlier study the enantiomeric enrichment in the brain was not as clearly different from most other tissues. Overall, the observation that PCBs can be enantiomerically enriched in the brain of mice is important from a human health perspective because several chiral PCB congeners are neurotoxic (Pessah et al. 2006) and may have enantiospecific toxicities (Püttmann et al. 1989; Lehmler et al. 2005).
3.5. PCB 136 levels in feces
Feces and urine were collected every 24 hours for three days, and PCB levels and enantiomeric fractions in both feces and urine were determined. Overall, urinary excretion of parent PCB 136 appeared to be negligible (i.e., urinary PCB 136 levels above the limit of detection were always due to a contamination with fecal matter). Similarly, several animal studies have shown that urinary excretion is a minor route of excretion of parent PCB 136 in rats (Matthews and Tuey 1980; Birnbaum 1983), dogs and monkeys (Sipes et al. 1982). In rats, < 2% of the total dose PCB 136 dose are excreted with the urine, mostly due to the excretion of metabolites and not of parent PCB 136 (Matthews and Tuey 1980; Birnbaum 1983). In the present study, fecal excretion is also a relatively minor route of excretion of the parent compound, with < 2% of the total dose of PCB 136 being excreted over a three day period in both diet groups.
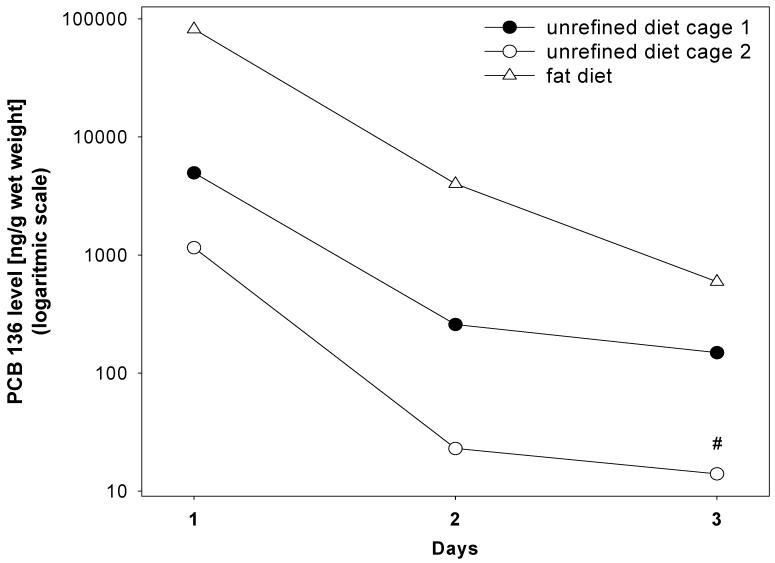
Figure 4 shows the time course of the fecal PCB 136 excretion for the three metabolism cages used in this study. The highest levels of PCB 136 were excreted in the first 24 hours following the PCB administration. PCB 136 levels in feces from HFD animals show a steady decrease on day 2 and 3. Similarly, PCB 136 levels in feces from URD animals decrease on day 2, but remain almost constant on day 3. Mice have a short average gastrointestinal transit time of 14 hours (Harkness and Wagner 1983), which suggests that the high fecal PCB levels at the 24 hour time point are, in part, due to the excretion of non-absorbed PCB 136. Fecal PCB 136 levels at the two later time points are due to the excretion of previously absorbed PCB 136 via the bile or intestinal excretion pathways into the gastrointestinal tract.
Figure 4.
The PCB 136 levels in feces of mice fed with the high fat or the unrefined diet during a 3 day period. All data points are for feces collected from cages housing three (unrefined diet) or four animals (high fat diet). # PCB 136 level is below the detection limit.
Although fecal excretion is only a minor route excretion of PCB 136 in mice, Figure 4 and Table 1 reveal small but distinct differences in its excretion due to the diet (1.6% versus 0.7% of the total dose in HFD and URD animals, respectively). These differences in the excretion of PCB 136 are due to the lower digestibility of the high fat diet, which not only explains the higher excretion rate in HFD animals but also, in part, the lower PCB 136 levels in the adipose tissue of HFD animals (see Section 3.3.). Analogously, the fecal excretion rate of PCBs in goldfish was higher in fish fed a high fat diet than in fish fed a low fat diet (Gobas et al. 1993). Unabsorbable fats such as Olestra® have also been shown to increase the fecal excretion of PCBs and other lipophilic xenobiotics in laboratory animals (Jandacek et al. 2005) and in humans (Redgrave et al. 2005). However, Olestra® was not tested in this study.
We have recently reported results from a study with a similar design, where the oral versus intraperitoneal administration of PCB 136 resulted in significantly lower PCB 136 tissue levels in the oral treatment group at day 3 (Kania-Korwel et al. 2007). In that study we could not exclude a more limited bioavailability of PCB 136 after oral administration (compared to intraperitoneal administration) as the cause of the lower tissue levels because fecal PCB levels were not determined. However, our current study does not suggest a drastic effect of oral administration with a cookie on the bioavailability of PCB 136. On the contrary, only <2% of the total dose were excreted in the feces, which suggests an excellent oral bioavailability of PCB 136. This finding is in agreement with other several oral bioavailability studies (ATSDR 2000),
3.6. Enantiomeric enrichment of PCB 136 in feces
We have reported the enantiomeric enrichment of chiral PCB congeners in tissues from mice by a currently unknown mechanism (Lehmler et al. 2003; Kania-Korwel et al. 2007). One possible explanation for the enrichment of one PCB atropisomer (e.g., (+)-PCB 136) in tissues, the selective excretion of the other PCB atropisomer (e.g., (-)-PCB 136) into the feces or urine, could not be discounted by these earlier studies as no feces EF values were determined. The time course of the fecal excretion of the PCB 136 in this study shows an enrichment of (+)-PCB 136 at all time points in both URD and HFD mice (Figure 5) and, thus, does not support the hypothesis of a selective excretion of (-)-PCB 136 in mice. Thus, the enantiomeric enrichment of (+)-PCB 136 in mice is more likely due to the selective metabolism of (-)-PCB 136. Similarly, the enantiomeric enrichment of chiral PCBs in trout (Wong et al. 2002), rats (Püttmann et al. 1989; Norström et al. 2006) and humans (Harrad et al. 2006) has been attributed to their enantioselective metabolism.
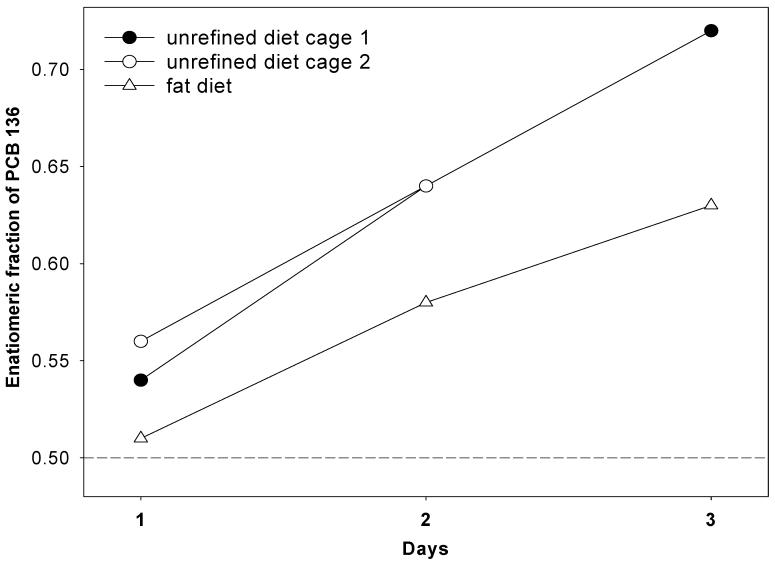
Figure 5.
The enantiomeric fraction of PCB 136 in feces of mice fed with the high fat or the unrefined diet during a 3 day period. All data points are for feces collected from cages housing three (unrefined diet) or four (high fat diet) animals. The enantiomeric fraction of PCB 136 in feces from one cage (white circle) was not determined at day 3 because the PCB 136 levels are below the detection limit.
The changes in the EF values were inversely related to the changes in the PCB levels over time, with a Spearman correlation coefficient of -0.93 (p=0.007). The EF values in feces from HFD animals after 24 hours are almost racemic and increase with time to EF = 0.63. This EF value is similar to the EF values observed in most tissues in the HFD group, which range from 0.60 to 0.65. The only exceptions are the liver and the brain, which show an even more significant enrichment of (+)-PCB 136 (0.69±0.04 and 0.69±0.04, respectively). In addition, the EF values in feces from URD animals shows a more pronounced enrichment of (+)-PCB 136 after 24 hours, which continuously increases with time to EF = 0.72. This EF values is identical to the EF value observed in the liver and brain of URD animals.
Enrichment of (+)-PCB 136 in feces requires several steps. First, PCB 136 is absorbed by the enterocyte of the small intestine, a process that is closely associated with the absorption of dietary fats (Kelly et al. 2004). Subsequently, (+)-PCB 136 is enriched by a currently unknown, enantioselective mechanism. Finally, the enantiomerically enriched PCB 136 is excreted into the gastrointestinal tract. In contrast, non-absorbed PCB 136 is excreted as a racemate. The total amount of PCB 136 excreted with the feces, Etotal, can be described by the following equation:
where Enon-absorbed is the total amount of non-absorbed, racemic PCB 136 (EF = 0.50) and Eabsorbed is the total amount of enantiomerically enriched PCB 136 (EF > 0.50) that was previously absorbed in the gastrointestinal tract and subsequently re-excreted into the intestines by the bile or other, intestinal routes. Based on this equation, the extent of the enantiomeric enrichment of (+)-PCB 136 can be used to assess if the fecal PCB 136 content is due to non-absorbed PCB 136 or PCB 136 excreted into the intestine.
The fact that the PCB 136 in the feces of HFD animals on day 1 is almost racemic suggests that most of PCB 136 excreted by HFD animals within 24 hours of administration is due to non-absorbed PCB 136 (i.e., Enon-absorbed ≫ Eabsrobed). Although the enrichment of (+)-PCB 136 in the URD group is more pronounced at the 24 hour time point, the low EF value - compared to the later time points - suggests that a significant amount of fecal PCB 136 levels in these animals is also due to non-absorbed PCB 136; however, because fecal PCB 136 levels in the URD group are at least 16-times lower compared to the HFD group at the 24 hour time point (i.e., Enon-absorbed > Eabsrobed), Eabsorbed has a more significant impact on the EF value in the URD group than in the HFD group. Furthermore, the fact that the higher PCB levels in feces from the HFD group are due to non-absorbed PCB 136 implies that the high fat diet not only increases the excretion of PCB 136 but also reduces its bioavailability.
Very little is currently known about the enantioselective excretion of PCBs in laboratory animals (Norström et al. 2006) and in humans (Harrad et al. 2006). Norström and co-workers investigated the enantiomeric enrichment of PCB 132 in feces collected over a four day period in male Wistar rats. The enantiomeric enrichment in feces was much less pronounced compared to tissue EF values, which - similar to this study - was likely due to non-absorbed PCB 132. Interestingly, methylsulfonyl metabolites excreted with the feces appeared to be enantiomerically pure (EF ∼ 1). The EF values of PCBs 95 and 149, two environmentally relevant PCB congeners, have been studied in a few human feces samples (Harrad et al. 2006). In that study, a slight enantiomeric enrichment of one PCB 95 atropisomer was observed in only two out of ten feces samples from volunteers eating a diet containing trace levels of racemic PCB 95. PCB 149, although present in the diet, was only detected in one feces sample. These findings seemingly contradict studies showing an enantiomeric enrichment of chiral PCB congeners in human tissue samples (Glausch et al. 1995; Chu et al. 2003). Therefore, the results from our study with PCB 136, which is structurally similar to PCB 95, raise the question if the EF values in the study by Harrad and co-workers are due to non-absorbed PCB 95 or differences in the disposition of PCB 95 in mice and humans.
4. Conclusions
This study for the first time investigates the effect of a high fat versus an unrefined diet on the enantioselective disposition of PCB 136 atropisomers. All tissues displayed a significant enrichment of (+)-PCB 136; however, the dietary fat content did not alter the distribution of both PCB 136 atropisomers among tissues. Overall, these findings provide evidence that the absorption of PCBs does not involve enantioselective processes - such as binding to proteins involved in the absorption and transport of dietary fats. Similar to the tissues, (+)-PCB 136 was enriched in all feces samples, independent of the diet. The fecal excretion of PCB 136 decreased with time in both the HFD and the URD group, with the excretion of PCB 136 always being higher in the HFD group due to the increased content in undigested fats. Based on the fecal EF values, the excretion of relatively high levels of PCB 136 within the first 24 hours after exposure is due to non-absorbed PCB 136, i.e., the high dietary fat content resulted in a small reduction (∼1 % of the total dose) of the bioavailability of PCB 136. The excretion of PCB 136 two and three days after exposure shows a significant enrichment of (+)-PCB 136 and, thus, is due to the excretion of previously absorbed PCB 136. These results demonstrate that EF values can be used to distinguish if fecally excreted PCBs are due to non-absorbed PCBs or to previously absorbed PCBs in mice. However, more research is needed to determine if the EF value can also be used to investigate the absorption and excretion of PCBs in humans.
Acknowledgment
We thank Dr. Regine Garcia Boy and Dr. Wei Xie for their assistance with the animal procedures; Collin Just for his help with the GC analysis; and Holly Moriarty and Allison Smith for help with the analytical work. The research was supported by grants ES05605, ES013661 and ES012475 from the National Institute of Environmental Health Sciences, NIH, and Major Research Instrumentation grant BES-0420378 form the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR A. Toxicological profile for polychlorinated biphenyls (PCBs) Department of Health and Human Services, Public Health Service; Atlanta, GA: 2000. [Google Scholar]
- BioServ Inc 2007 Retrieved February 6, 2007, from http://www.bio-serv.com/newcatalog/eeprod/rodent/highfat.html.
- Birnbaum LS. Distribution and excretion of 2,3,6,2′,3′,6′- and 2,4,5,2′,4′,5′-hexachlorobiphenyl in senescent rats. Toxicol. Appl. Pharmacol. 1983;3(4):262–272. doi: 10.1016/0041-008x(83)90102-3. [DOI] [PubMed] [Google Scholar]
- Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners - a mass balance approach 1. Global production and consumption. Sci. Total Environ. 2002;290:181–198. doi: 10.1016/s0048-9697(01)01075-0. [DOI] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health. 1997;13(4):407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol. 2006;78(2):176–185. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Charmann WNA, Stella VJ. Effects of lipid class and lipid vehicle volume on the intestinal lymphatic transport of DDT. Int. J. Pharm. 1986;33(13):165–172. [Google Scholar]
- Chiu A, Beaubier J, Chiu J, Chan L, Gerstenberger S. Epidemiologic studies of PCB congener profiles in North American fish consuming populations. J. Environ. Sci. Health. 2004;C22(1):13–36. doi: 10.1081/GNC-120038004. [DOI] [PubMed] [Google Scholar]
- Chu S, Covaci A, Schepens P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 2003;93:167–176. doi: 10.1016/s0013-9351(03)00016-1. [DOI] [PubMed] [Google Scholar]
- Drouillard KGN,RJ. The influence of diet properties and feeding rates on PCB toxicokinetics in the ring dove. Arch. Environ. Contam. Toxicol. 2003;44(1):97–106. doi: 10.1007/s00244-002-1199-y. [DOI] [PubMed] [Google Scholar]
- Duarte-Davidson R, Jones KC. Polychlorinated biphenyls (PCBs) in the UK population: estimated intake, exposure and body burden. Sci. Total Environ. 1994;151:131–152. doi: 10.1016/0048-9697(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Glausch A, Hahn J, Schurig V. Enantioselective determination of chiral 2,2′,3,3′,4,6′- hexachlorobiphenyl (PCB 132) in human milk samples by multidimensional gas chromatography/electron capture detection and by mass spectrometry. Chemosphere. 1995;30:2079–2085. doi: 10.1016/0045-6535(95)00085-m. [DOI] [PubMed] [Google Scholar]
- Gobas FAPC, McCorquodale JR, Haffner GD. Intestinal absorption and biomagnification of organochlorines. Environ. Toxicol. Chem. 1993;12:567–576. [Google Scholar]
- Haglund P, Wiberg K. Determination of the gas chromatographic elution sequences of the (+) and (-) enantiomers of stable enantiomeric PCBs on Chirasil-Dex. J. High Resol. Chromatogr. 1996;19:373–376. [Google Scholar]
- Haluska L, Barancikowa G, Balaz S, Derova K, Vrana B, Paz-Weisshaer M, Furciowa E, Bielek P. Degradation of PCB in different soils by inoculated Alcaligenes xylosoxidans. Sci. Total Environ. 1995;175:275–285. doi: 10.1016/0048-9697(95)04927-4. [DOI] [PubMed] [Google Scholar]
- Han L-K, Zheng YN, Xu B-J, Okuda H, Kimura Y. Saponins from Platycodi Radix ameliorate high fat diet-induced obesity in mice. J. Nutr. 2002;132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- Hansen LG, Welborn ME, Borchard RE, Teske RH, Metcalf RL. Tissue distribution of PCB components in swine and sheep fed three different rations containing Aroclors 1242 and 1254. Arch. Environ. Contam. Toxicol. 1977;5:257–278. doi: 10.1007/BF02220909. [DOI] [PubMed] [Google Scholar]
- Harkness J, Wagner J. The biology and medicine of rabbits and rodents. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Harner T, Wiberg K, Norstrom R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ. Sci. Technol. 2000;34(1):218–220. [Google Scholar]
- Harrad S, Ren J, Hazrati S, Robson M. Chiral signatures of PCB#s 95 and 149 in indoor air, grass, duplicate diets and human faeces. Chemosphere. 2006;63(8):1368–1376. doi: 10.1016/j.chemosphere.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Hites RAF, Jeffery A, Carpenter David O., Hamilton M. Coreen, Knuth Barbara A., Schwager Steven J. Global assessment of organic contaminants in farmed salmon. Science. 2004;303(5655):226–229. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Jandacek R, Anderson N, Liu M, Zheng S, Yang Q, Tso P. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(2):G292–9. doi: 10.1152/ajpgi.00285.2004. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Garrison AW, Avants JK, Hornbuckle KC, Robertson LW, Sulkowski WW, Lehmler H-J. Distribution of chiral PCBs in selected tissues in the laboratory rat. Environ. Sci. Technol. 2006;40:3704–3710. doi: 10.1021/es0602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobifenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Gobas FAPC, McLachlan MS. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife and human. Environ. Toxicol. Chem. 2004;23(10):2324–2336. doi: 10.1897/03-545. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Price DJ, Garrison AW, Birge WJ, Robertson LW. Distribution of PCB 84 enantiomers in C57Bl/6 mice. Fresenius’ Environ. Bull. 2003;12:254–260. [Google Scholar]
- Lehmler H-J, Robertson LW. Atropisomers of PCBs. In: Robertson LW, Hansen LG, editors. Recent Advances in the Environmental Toxicology and Health Effects of PCBs. University Press of Kentucky; Lexington: 2001. pp. 61–65. [Google Scholar]
- Lehmler H-J, Robertson LW, Garrison AW, Kodavanti PRS. Effects of PCB 84 enantiomers on [3H] phorbol ester binding in rat cerebellar granule cells and 45Ca2+-uptake in rat cerebellum. Tox. Lett. 2005;156:391–400. doi: 10.1016/j.toxlet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Esch H, Robertson LW. Polyhalogenierte Bi- und Terphenyle. In: Dunkelberg H, Gebel T, Hartwig A, editors. Handbuch der Lebensmitteltoxikologie. Wiley-VCH; Weinheim: 2007. pp. 1031–1094. [Google Scholar]
- Matthews HB, Tuey DB. The effect of chlorine position on the distribution and excretion of four hexachlorobiphenyl isomers. Toxicol. Appl. Pharma. 1980;53:377–388. doi: 10.1016/0041-008x(80)90351-8. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Hidaka K, Ohe T, Matsumoto M, Yamamoto K, Tajima K. Comparative study on accumulation and elimination of hexachlorobiphenyls and decachlorobiphenyl in mice. Bull. Environ. Contam. Toxicol. 1980;25:181–187. doi: 10.1007/BF01985508. [DOI] [PubMed] [Google Scholar]
- Norström K, Eriksson J, Haglund J, Silvari V, Bergman A. Enantioselective formation of methyl sulfone metabolites of 2,2′,3,3′,4,6′-hexachlorobiphenyl in rat. Environ. Sci. Technol. 2006;40:7649–7655. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem. Res. Toxicol. 2006;19(1):92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Püttmann M, Arand M, Oesch F, Mannschreck A, Robertson LW. Chirality and the induction of xenobiotic-metabolizing enzymes: Effects of the atropisomers of the polychlorinated biphenyl 2,2′,3,4,4′,6-hexachlorobiphenyl. In: Frank H, Holmstedt B, Testa B, editors. Chirality and Biological Activity. Alan R. Liss, Inc.; New York: 1990. pp. 177–184. [Google Scholar]
- Püttmann M, Mannschreck A, Oesch F, Robertson L. Chiral effects in the induction of drug-metabolizing enzymes using synthetic atropisomers of polychlorinated biphenyls (PCBs) Biochem. Pharmacol. 1989;38(8):1345–1352. doi: 10.1016/0006-2952(89)90342-0. [DOI] [PubMed] [Google Scholar]
- Redgrave T, Wallace P, Jandacek R, Tso P. Treatment with a dietary fat substitute decreased Arochlor 1254 contamination in an obese diabetic male. J. Nutr. Biochem. 2005;16(6):383–384. doi: 10.1016/j.jnutbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effect. University Press of Kentucky; Lexington: 2001. [Google Scholar]
- Shaikh N, Parkin S, Lehmler H-J. The Ullmann coupling reaction: A new approach to tetraarylstannanes. Organometallics. 2006;25(17):4207–4214. [Google Scholar]
- Sipes I, Slocumb M, Perry D, Carter D. 2,4,5,2′,4′,5′-Hexachlorobiphenyl: distribution, metabolism, and excretion in the dog and the monkey. Toxicol. Appl. Pharma. 1982;65(2):264–72. doi: 10.1016/0041-008x(82)90009-6. [DOI] [PubMed] [Google Scholar]
- Wong CS, Lau F, Clark M, Mabury SA, Muir DCG. Rainbow Trout (Oncorhynchus mykiss) can eliminate chiral organochlorine compound enantioselectively. Environ. Sci. Technol. 2002;36:1257–1262. doi: 10.1021/es0156791. [DOI] [PubMed] [Google Scholar]