Abstract
Recent evidence highlighted a role for the transcription factor, Nuclear Factor of Activated T-cells (NFAT), in the transcription of the human polyomavirus JCV. Here we show that NFAT is also important in the transcriptional control of the related polyomavirus, Simian Virus 40 (SV40). Inhibition of NFAT activity reduced SV40 infection of Vero, 293A and HeLa cells, and this block occurred at the stage of viral transcription. Both NFAT3 and NFAT4 bound to the SV40 promoter through κB sites located within the 72bp repeated enhancer region. In Vero cells NFAT was involved in late transcription, but in HeLa and 293A cells both early and late viral transcription required NFAT activity. SV40 large T-Ag was found to increase NFAT activity and provided a positive feedback loop to transactivate the SV40 promoter.
Keywords: Simian Virus 40, JC virus, Nuclear Factor of Activated T-Cells, NFAT
Introduction
Simian virus 40 (SV40) was originally identified in the 1950s as a contaminant of early forms of the polio vaccine (Rollison and Shah, 2001; Shah and Nathanson, 1976). It was estimated that 30 million people may have been exposed to SV40 contaminated vaccines between 1955 and 1963 (Rollison and Shah, 2001; Stratton, Almario, and McCormick, 2002). Shortly after the contamination was discovered, SV40 was found to be an oncogenic DNA virus capable of causing a variety of neoplasias in laboratory animals (Butel and Lednicky, 1999; Diamandopoulos, 1972). Studies of those exposed to SV40 through contaminated vaccines found that there was insufficient evidence to evaluate any relationship between SV40 and cancer (Stratton, Almario, and McCormick, 2002).The current prevalence of SV40 in the human population is unknown (Vilchez, Kozinetz, and Butel, 2003), but recent evidence has detected SV40 gene products in primary brain and bone cancers, malignant mesotheliomas, and non-Hodgkin’s lymphoma (Pass et al., 1998; Vilchez et al., 2003). Many of these studies found evidence of SV40 infection in the cancers of people who had not received the contaminated polio vaccine suggesting that SV40 may be capable of circulating within the human population. Such evidence has led to concern that SV40 may pose a threat as an emerging human pathogen.
SV40 is a member of the polyomavirus family which encompasses a group of small non-enveloped double-stranded DNA viruses. The SV40 genome can be divided into two coding regions separated by a non-encoding regulatory region which contains the viral promoter and the origin of DNA replication. Like all polyomaviruses the lifecycle of SV40 is temporally regulated. Following entry into the cell the virus must traffic to the nucleus where transcription of the early viral genes occurs. Early viral gene products consist of small and large T-Antigens, both of which are regulatory proteins that aid viral transcription, DNA replication, and transformation. When enough early proteins have been produced the virus switches from early transcription to DNA replication. Following sufficient DNA replication transcription of the late viral genes then occurs, and these consist of the three viral coat proteins, VP1, VP2, and VP3, as well as another regulatory protein called agnoprotein.
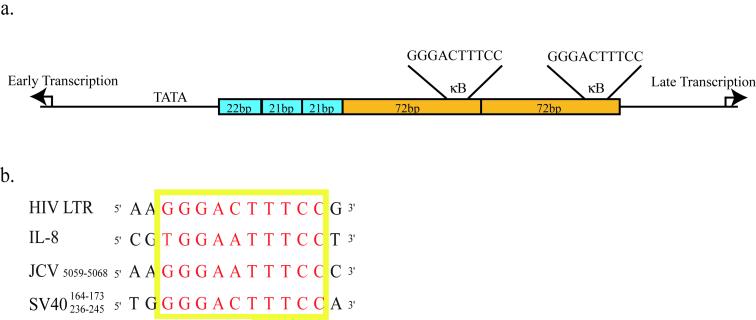
Elucidation of the regulatory components of SV40 transcription has provided significant insight into how eukaryotic cells regulate gene expression (Tjian, 1994). The SV40 promoter comprises two main elements (Fig. 1a). The first contains two 21bp repeated sequences and a 22bp sequence that differs only slightly. Each of these sequences contains multiple Sp1 binding sites and this region is particularly important to early viral transcription (Benoist and Chambon, 1981; Everett, Baty, and Chambon, 1983; Fromm and Berg, 1982). The second element of the SV40 promoter is a region that contains two perfectly repeated 72bp sequences. This region was originally characterized as the classical eukaryotic enhancer sequence and plays a role in both early and late viral transcription depending on the cell type. Numerous transcription factor binding sites have been identified within the enhancer region, including sites for AP-1 and NFκB (Macchi et al., 1989; Mermod, Williams, and Tjian, 1988).
Figure 1. The SV40 promoter contains two κB sites situated in the 72bp repeated enhancer region.
(a) The SV40 promoter contains two major regions. The first is a series of three 21bp repeats that contain multiple Sp1 binding sites. The second region consists of two 72bp repeated sequences that contain numerous transcription factor binding sites, including a κB site in each 72bp sequence. (b) Alignment of the κB sequence from SV40, JCV, HIV-LTR and the IL-8 promoter.
Recent evidence from our lab has identified a role for the transcription factor Nuclear Factor of T-cells (NFAT) in infection by a human polyomavirus, JC virus (JCV) (Manley et al., 2006). In the case of JCV infection NFAT is important for both early and late viral gene expression, and a loss of NFAT activity results in a block to viral infection. We were able to show that NFAT binds directly to the JCV promoter via a site that is also capable of binding a related transcription factor, NFκB. In addition to a role in viral transcription, NFAT activity is enhanced by early viral gene products resulting in an amplification of viral gene transcription. The κB site in JCV is similar to sites found in other promoters, such as the Interleukin-8 promoter and the HIV-LTR, that are capable of binding either NFκB or NFAT depending on the cellular context. The κB sites of SV40 are also similar to that of JCV (Fig. 1b). We tested to see if NFAT was also capable of binding to the κB sites in SV40 and mediating viral transcription. We found that inhibition of NFAT activity in a variety of cell types was capable of inhibiting SV40 infection, and that NFAT was indeed capable of binding to and acting through the two κB sites situated in the SV40 enhancer region. We also found that SV40 large T-Ag was capable of activating NFAT transcription.
Results and Discussion
The inhibition of NFAT activity reduces SV40 infection
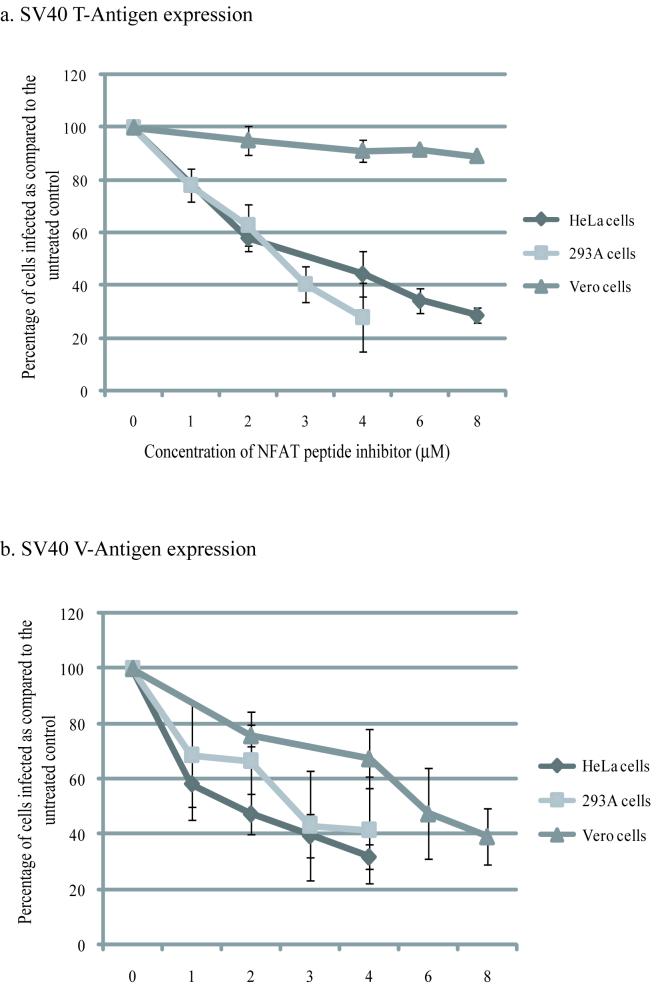
To test if SV40 infection of Vero, 293A and HeLa cells requires NFAT activity, we pretreated cells for 6hrs with a peptide inhibitor of NFAT activation (Aramburu et al., 1999) and then infected for 1hr with SV40. Following infection the cells were maintained in the inhibitor and the number of infected cells was counted 72hrs p.i. by using indirect immunofloresence to detect early and late viral proteins. Both T-Antigen and V-Antigen expression were blocked in 293A and HeLa cells (Fig. 2), indicating a role for NFAT in viral infection of these cells. In Vero cells there was no effect on T-Antigen expression (Fig. 2), but V-Antigen expression was inhibited to similar levels as those seen with HeLa and 293A cells, suggesting that NFAT is involved in the later stages of SV40 infection in Vero cells.
Figure 2. NFAT activity is required for SV40 infection.
Vero, HeLa and 293A cells were treated with a cell permeable NFAT inhibitor peptide (Calbiochem) 6hrs prior to infection with SV40. Cells were infected for 1hr in the presence of the inhibitor. Following infection the cells were maintained in the inhibitor. Due to the half-life of the peptide, at 24hrs and 48hrs p.i. cells were treated with fresh peptide. At 72hrs p.i. cells were fixed and stained for either T-Ag (a) or V-Ag (b). Approximately 40%, 20%, and 25% of untreated cells were infected in Vero, HeLa, and 293A cells respectively. All values are given as percentage of cells infected as compared to the untreated control. Each experiment was repeated in triplicate and used to calculate the mean and standard deviation.
NFAT is present and active in Vero, HeLa, and 293A cells
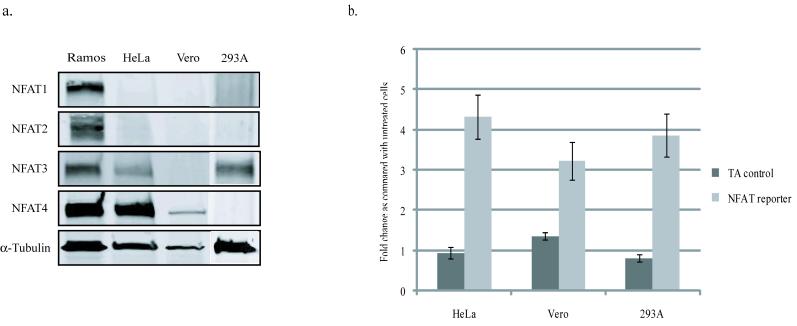
Next we looked to see which members of the NFAT family are expressed in Vero, HeLa and 293A cells. There are five members of the NFAT family (NFAT1-5), but only NFATs 1-4 are responsive to calcium signaling. Western blots probing for NFATs 1-4 reveal that Vero cells express only NFAT4 (Fig. 3a), 293A cells express only NFAT3, and HeLa cells express both NFAT3 and NFAT4.
Figure 3. NFAT expression and activation in Vero, HeLa, and 293A cells.
(a) Vero and HeLa cells express NFAT4, while HeLa and 293A cells express NFAT3 as determined by western blot analysis. Ramos cell lysates were used as a positive control. (b) NFAT activity was measured in HeLa, Vero and 293A cells using an NFAT responsive luciferase reporter construct and comparing it with a luciferase reporter construct that lacks an NFAT responsive element (TA-control). Cells were transfected with either the NFAT reporter or the TA-control and NFAT was activated by treating cells with 80ng/ml of PMA and 2μM Ionomycin. The fold-change in luciferase activity was calculated by comparing treated to untreated samples. The control construct did not show any increased in activity when treated, while the NFAT reporter showed that in all three cell types NFAT activity was triggered by treatment with PMA and Ionomycin. All values were normalized to a TK-renilla control, and each experiment was conducted in triplicate.
Treatment of cells with a combination of Phorbal 12-myristate 13-acetate (PMA) and Ionomycin is the established method of activating NFAT in a variety of cell-types. To confirm that NFAT in Vero cells, HeLa cells, and 293A cells is capable of being activated under these conditions, we transiently transfected cells with a reporter construct that expresses luciferase under the control of an NFAT responsive promoter (pNFAT-luc) and treated cells with 80ng/ml of PMA and 2μM Ionomycin. NFAT transcriptional activity was generated in all three cell types under these conditions (Fig. 3b). All subsequent experiments that required NFAT activity were carried out under these conditions.
NFAT activates the SV40 promoter via two κB sites
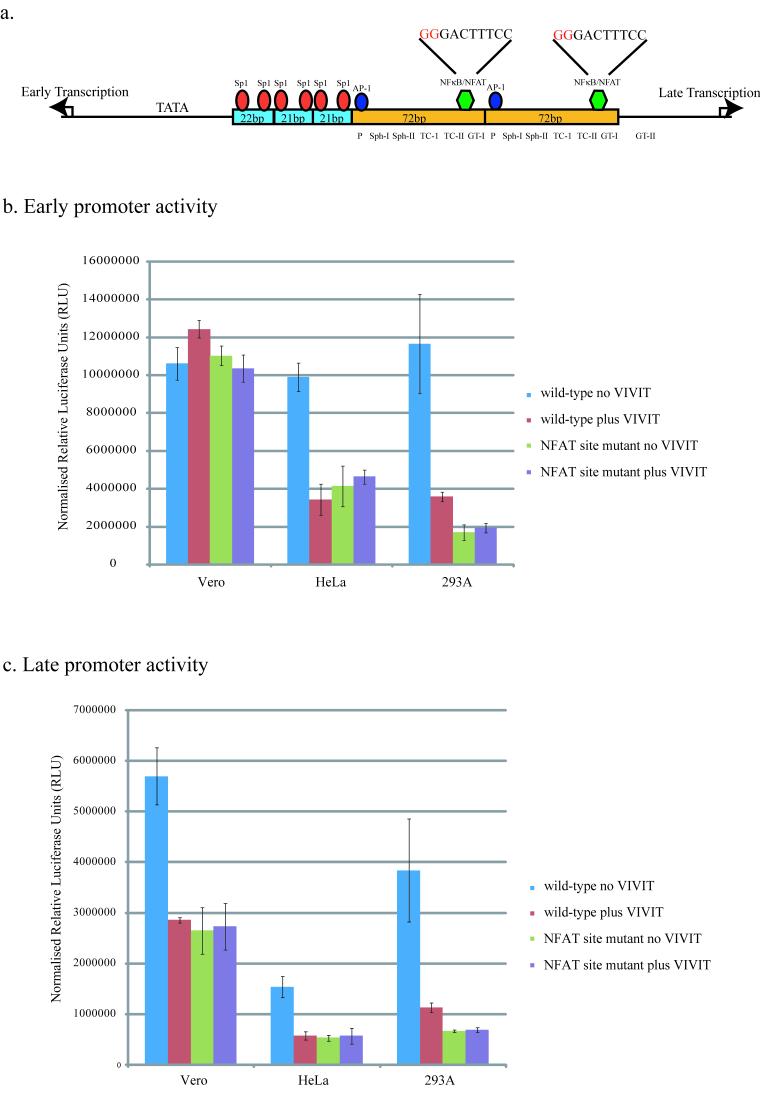
As NFAT is a transcription factor we examined the SV40 promoter for potential NFAT binding sites. JCV infection of glial cells relies upon the direct binding of NFAT4 to a κB site situated in the viral untranslated control region. This site had previously been shown to be capable of binding another transcription factor family, the NFκB family. SV40 has two NFκB sites situated in the 72bp repeated sequences of the promoter (Fig. 4a). NFκB has previously been shown to bind to these sites and be important for viral transcription, although other transcription factors have also been implicated as capable of binding to these sites in a cell-type dependent manner (Espel et al., 1990; Lattion et al., 1992; Macchi et al., 1989).
Figure 4. NFAT activates both the early and late SV40 transcription via the κ B sites in the SV40 promoter.
The SV40 promoter contains two κB sites situated in the 72bp repeats (a). The NFκB/NFAT sites were mutated in the early and late promoter luciferase reporter constructs (pluc2/SV40E and pluc2/SV40L). The nucleotides that were mutated (G→A) are in red. shown Cells were co-transfected with either the wild-type or mutated promoter reporter constructs and a construct that expresses either the NFAT peptide inhibitor (pGFP-VIVIT) or a construct that only expresses GFP as a control (phMGFP). Luciferase activity was measured 48hrs following transfection. These experiments were carried out in the presence of 80ng/ml of PMA and 2μM Ionomycin. All values were normalized to TK-renilla. Each experiment was carried out in triplicate and the averages and standard deviations were calculated.
To test if NFAT was activating the SV40 promoter through these NFκB sites, we used promoter reporter assays to compare the effect of the NFAT peptide inhibitor on both the wild-type SV40 promoter, and an SV40 promoter in which the κB sites have been mutated so they are no longer functional. The reporter constructs used contained the luciferase gene under the control of the SV40 promoter in either the early or late orientation (pluc2/SV40E and pluc2/SV40L). To generate the reporter constructs which lacked functional κB sites the first two nucleotides of each site were mutated from GG to AA (Fig. 4a). The wild-type and mutated reporter constructs were co-transfected into each of the cell-types with a construct that constitutively expresses the NFAT inhibitor peptide conjugated to GFP (pGFP-VIVIT). Compared to a control construct that only expressed GFP (phMGFP), expression of the VIVIT-GFP peptide resulted in a decrease in both early and late wild-type promoter activity in both HeLa and 293A cells (Fig. 4b and c). In contrast only late transcription was affected in Vero cells by the expression of VIVIT-GFP (Fig. 4c). These results match those seen when cells were treated with the NFAT peptide inhibitor and then challenged with virus, suggesting that in HeLa and 293A cells both early and late viral transcription utilize NFAT activity, while in Vero cells it is only necessary for late transcription.
In both HeLa and 293A cells mutation of the κB sites resulted in a significant decrease in promoter activity in both the early and late orientations as compared to the wild-type promoters (Fig. 4b and c). Consistent with NFAT only playing a role in late transcription in Vero cells, the mutations only affected transcription from the promoter in the late orientation (Fig. 4c). Early transcription was unaffected by mutation of the κB sites in Vero cells. Inhibition of NFAT by expressing VIVIT-GFP failed to further reduce either early or late promoter activity in any of the cells (Fig. 4b and c), suggesting that NFAT plays only a direct role in viral transcription and not an indirect one.
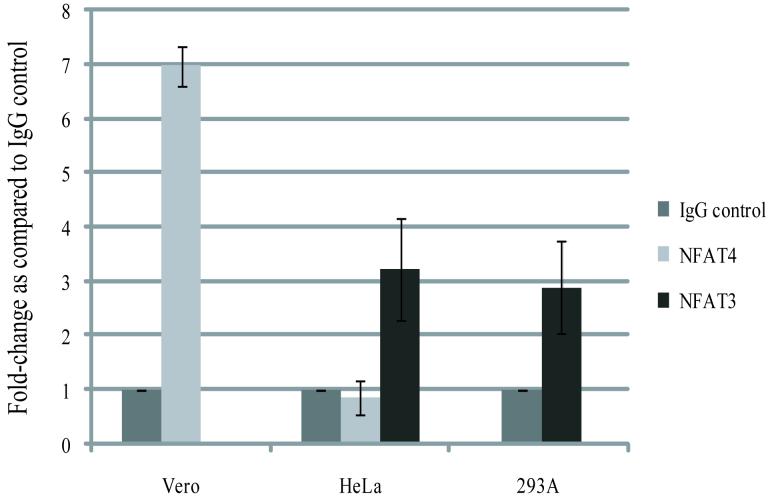
To demonstrate that NFAT binds to the SV40 promoter during infection we carried out Chromatin Immunoprecipitation assays on cells infected with SV40. When chromatin from HeLa and 293A cells was immunoprecipitated with an NFAT3 antibody there was about a 3-fold increase in the amount of SV40 promoter DNA as compared with DNA immunoprecipitated using a non-specific IgG antibody (Fig. 5). NFAT4 was also tested for its ability to bind to the SV40 promoter in HeLa and Vero cells. NFAT4 appeared to bind to the SV40 promoter in Vero cells but not HeLa cells (Fig. 5), suggesting that in cells were both NFAT3 and NFAT4 are available NFAT3 will be preferentially bound.
Figure 5. NFAT binds directly to the SV40 promoter region.
ChIP analysis was carried out on HeLa, Vero, and 293A cells infected with SV40 for 72hrs in the absence of PMA and Ionomycin. Chromatin was immunoprecipitated with either a monoclonal α-NFAT4 antibody (Santa Cruz Biotech. Inc.), a polyclonal α-NFAT3 antibody (Santa Cruz Biotech. Inc.), or a mouse IgG antibody (Santa Cruz Biotech. Inc.).
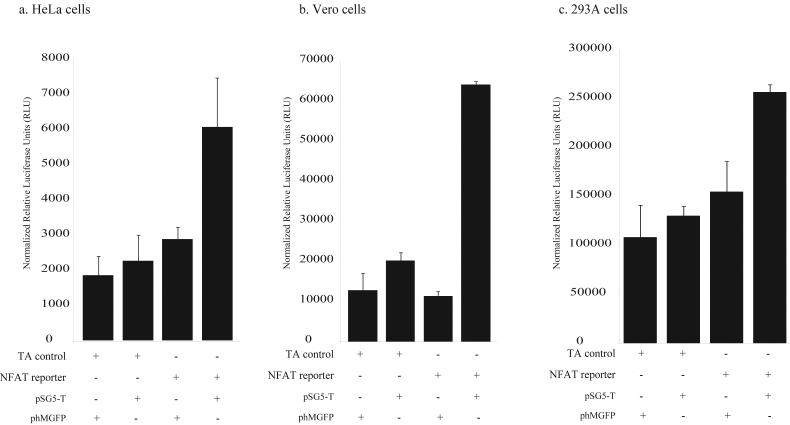
SV40 large T-Ag is capable of activating NFAT mediated transcription
Previous evidence has shown that both JCV early proteins and SV40 large T-Ag are capable of activating NFAT mediated transcription. To see if SV40 large T-Ag was capable of activating NFAT transcription in Vero, HeLa, and 293A cells we transiently transfected either a control construct (pTA-luc) or the NFAT reporter construct (pNFAT-luc) with a construct that expresses the SV40 large T-Ag protein under the control of the RSV promoter (pSG5-T). Compared with a control plasmid that expresses only GFP (phMGFP), SV40 large T-Ag was capable of activating NFAT in all three cell-types. There was a 2-fold activation of NFAT activity in HeLa and 293A cells, while there was a 5-fold increase in NFAT activity in Vero cells (Fig. 6). SV40 large T-AG failed to have any effect on a reporter plasmid that lacked the NFAT binding sites (pTA-luc).
Figure 6. NFAT is activated by the expression of SV40 large T-Ag.
Cells (in the absence of PMA and Ionomycin) were co-transfected with the NFAT reporter construct (pNFAT-luc) and either a control construct (phMGFP) or a construct expressing SV40 large T-Ag (pSG5-T). The effect of SV40 T-Ag on a control reporter construct (pTA-luc), which is missing the NFAT binding site in the promoter, was also tested. Luciferase activity was measured 48hrs following transfection.
Understanding how viruses transcribe their genes has been fundamentally important for understanding the complexity of eukaryotic transcriptional control mechanisms. Previously we demonstrated that the transcription factor, Nuclear Factor of Activated T-cell 4 (NFAT4), binds to a κB site in the promoter of the human polyomavirus JCV, and directly activates both early and late viral transcription. Here we show that SV40 also utilizes NFAT to infect a variety of cell types. In the two human cell lines tested (293A cells and HeLa cells) NFAT3 is capable of binding to the κB sites in the enhancer region of the SV40 promoter and activates both early and late transcription. In contrast, SV40 infection of a monkey kidney cell line (Vero cells) utilized NFAT4 to directly activate late transcription but not early transcription. In addition, expression of SV40 T-Ag activates NFAT mediated transcription in all three cell types, indicating a role for NFAT in T-Ag mediated transactivation of the SV40 promoter.
NFκB has previously been shown to bind to the κB sites in the SV40 promoter, but is also known that SV40 can infect cells that do not express NFκB. Other factors have been shown to bind to these κB sites but their identity is unclear. Here we identify NFAT as another transcription factor capable of binding the κB sites of the SV40 promoter. The ability of NFAT to bind to the κB site in the promoter attests to the versatility of the SV40 promoter. As both NFκB and NFAT are capable of binding and activating transcription the number of cell types that SV40 can infect is increased. The fact that both NFAT and NFκB can bind to these sites in promoters such as the IL-8 promoter and HIV-LTR depending on the signaling context, suggests that SV40 can infect cell types that contain both NFAT and NFκB under a variety of signaling conditions, again increasing the versatility of the SV40 promoter.
Whether NFAT is important for both early and late transcription or just early appears to be dependent on which cell is being infected. In Vero cell only late transcription is affected by NFAT activity, while in HeLa and 293A cells both early and late are dependent on NFAT. This difference could be due to a number of reasons. First it could merely be the strength of the Sp1 responsive 21bp repeats in Vero does not require additional activation from transcription factor sites in the enhancer region. Second, species specific factors might play a role, as Vero cells are green monkey kidney cells and both HeLa and 293A cells are of human origin. Finally, the difference could be due to the version of NFAT that is expressed in the cells. Vero cells express NFAT4 while HeLa and 293A cells express NFAT3.
Both JCV and SV40 are capable of activating NFAT mediated transcription by expression of their early viral proteins. This appears to be a common feature of the polyomavirus family. Both hamster and mouse polyomavirus have previously been shown to activate NFAT via phophorylation of phospholipase C-gamma (PLC-γ) by the viral protein, middle T-Ag (Brizuela et al., 1995; Kennedy et al., 1998). JCV and SV40 do not have a middle T-Ag protein so the mechanism by which they activate NFAT is likely to be different, but the theme of NFAT activation to aid viral transcription appears to be common.
The potential emergence of SV40 as a human pathogen highlights the need to identify cellular targets for potential drug treatment. Any target for drug treatment must be a factor that is required for SV40 infection of a variety of cell types. As SV40 T-Ag is oncogenic, it must also result in a block to early gene expression. Provided that SV40 requires NFAT/NFκB activity for both early and late transcription in human cells, blocking a combination of NFAT and NFκB activity may be one avenue of drug treatment.
Materials and Methods
Cells, antibodies, and virus
Vero cells were cultured in media supplemented with 10% fetal calf serum (Mediatech, Inc.). 293A and HeLa cells were cultured in media supplemented with 10% fetal calf serum (Mediatech, Inc.). Cells were maintained in a humidified 37°C CO2 incubator. The SV40 strain used in these experiments and its production has been previously described (Liu and Atwood, 2001). The antibodies used for both western blot experiments and chromatin immunoprecipitation analysis (NFATc2 (4G6-G5), NFATc1 (7A6), NFATc4 (H-74), NFATc3 (F-1), α-tubulin, and the normal mouse IgG) were obtained from Santa Cruz Biotechnology.
Infection and indirect immunoflouresence
Cells were grown to ∼70% confluency infected for 1 hr with 1.92×106 pfu/ml of virus in the presence of EMEM plus 2% serum in a total volume of 250μl. This correlates to an MOI of approximately 10. At 3 days post-infection (p.i.), cells were fixed in 2% paraformaldehyde for 30 mins and permeabilized for 20mins in 0.2% Triton-X. Infected cells were detected either using a monoclonal antibody (PAB597) against the major capsid protein VP1, or a monoclonal antibody (PAB416) against SV40 T-Antigen (Oncogene). Primary antibody was detected with an Alexaflour 488-labeled goat anti-mouse secondary antibody (Molecular Probes). Cells were counter-stained with 0.02% Evans blue. Positive cells were visualized on a Nikon epifluorescence microscope (Eclipse E800; Nikon Inc.).
Western blots
Whole cell extracts were separated on Tris-HCl Ready gels (Bio-Rad). Proteins were transferred to nitrocellulose membranes and blocked with 1x casein blocking buffer. Blots were probed with the respective antibodies all diluted in 1x blocking buffer, and then incubated with goat anti-mouse Alexa-Fluor 680 antibodies (Molecular Probes) diluted 1:1000 in blocking buffer. Blots were viewed using an infrared scanner from LICOR and analyzed using Odyssey software.
Constructs and mutagenesis
The pGFP-VIVIT construct expresses the NFAT peptide inhibitor fused with GFP under the control of the CMV promoter (Aramburu et al., 1999). pSG5-T is the pSG5 plasmid (Stratagene) with the full-length SV40 large T-Antigen inserted into the Bam HI site, and constitutively expresses the SV40 T-Ag protein (Zalvide and DeCaprio, 1995). The pGL4.13 (pluc2/SV40E), pRL-TK and phMGFP constructs were purchased from Promega. The pluc2/SV40L was generated by the PCR amplification of the SV40 promoter region of the pluc2/SV40E construct, with primers that inserted XhoI and BglII sites at either end. The PCR product was then directionally cloned into the pGL4.10 construct (Promega). Therefore, both pluc2/SV40E and pluc2/SV40L constructs carry the entire SV40 promoter region, are identical in sequence, and differ only in the orientation of the promoter. The pRL-TK, which expresses Renilla luciferase from the thymidine kinase promoter of HSV, and phMGFP, which only expresses GFP from the CMV promoter, were both purchased from Promega. The pNFAT-luc reporter plasmid expresses firefly luciferase under the control of a promoter that contains four repeats of the IL-2 NFAT binding site, and the pTA-luc, which is the pNFAT-luc construct minus any NFAT sites, were purchased from Panomics. Reporter constructs were mutated using the Stratgene Quikchange mutagenesis kit according to the manufacturers’ instructions.
Transfection and Luciferase Assays
Transient transfections were carried out using lipofectamine (Invitrogen) as described by the manufacturers’ protocol. Vectors were co-transfected with the Renilla luciferase control vector (pRL-TK) (Promega). Renilla and Firefly luciferase activity were measured 48 h post-transfection using the Promega Dual-Luciferase Reporter Assay System. Relative luciferase units were measured using a Berthoid Lumat LB9501 luminometer. Firefly luciferase measurements for each experiment were normalized using the Renilla luciferase measurements. The results of each experiment were confirmed by three independent transfections.
Chromatin Immunoprecipitation Assays
75cm3 flasks of cells at ∼70% confluency were infected with 105 pfu/ml of SV40 in the presence of EMEM plus 2% serum in a total volume of 1ml for 1hr. The cells were then maintained in EMEM plus 10% serum for 2 days. The ChIP was performed according to a protocol obtained from Santa Cruz Biotechnology. Briefly, cells were harvested by scraping and washed twice in 1XPBS. Cells were then treated for 8mins with 1% formaldehyde. Cells were washed three times in ChIP Lysis buffer (Santa Cruz Biotech. Inc.), and resuspended in 1.0ml of ChIP lysis buffer with high salt (Santa Cruz Biotech. Inc.). Samples were sonicated using a Branson Sonifier 150 at power setting 2 four times for 1min. Chromatin was pre-cleared with Protein A/G PLUSAgarose (Santa Cruz Biotech. Inc.) at 4°C for 30mins. Primary antibody was incubated with chromatin overnight at 4°C. Immune complexes were harvested by Protein A/G PLUSAgarose for 2hrs at 4°C. Beads washed twice with lysis buffer and three times with wash buffer (Santa Cruz Biotech. Inc.). Samples were resuspended in elution buffer (Santa Cruz Biotech. Inc.) and incubated at 67°C overnight. DNA samples were extracted using the Stratagene PCR purification kit according to the manufacturers’ protocol. Sybr green quantitiative PCR (Applied Biosystems) was performed using 5μl of the immunoprecipitated DNA and primers that encompass the entire SV40 promoter region (5′-TGGAATAGCTCAGAGGCCGAG-3′ and 5′-CATGGCCTGAAATAACCTCTG-3′).
Acknowledgements
We thank all members of the Atwood lab for critical discussion during the course of this work. We thank Dr. A. Rao, Dr. J. DeCaprio, and Dr. E. Harlow for providing constructs and antibodies. We also thank Wendy Virgadamo, Heather Forand, and Tammy Glass for administrative assistance. Work in our laboratory was supported by a grant from the National Cancer Institute, R01 CA71878, and by a grant from the National Institute of Neurological Disorders and Stroke, R01 NS43097 to W.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285(5436):2129–33. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Benoist C, Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981;290(5804):304–10. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Brizuela L, Ulug ET, Jones MA, Courtneidge SA. Induction of interleukin-2 transcription by the hamster polyomavirus middle T antigen: a role for Fyn in T cell signal transduction. Eur J Immunol. 1995;25(2):385–93. doi: 10.1002/eji.1830250212. [DOI] [PubMed] [Google Scholar]
- Butel JS, Lednicky JA. Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst. 1999;91(2):119–34. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- Diamandopoulos GT. Leukemia, lymphoma, and osteosarcoma induced in the Syrian golden hamster by simian virus 40. Science. 1972;176(31):173–5. doi: 10.1126/science.176.4031.173. [DOI] [PubMed] [Google Scholar]
- Espel E, Fromental C, Reichenbach P, Nabholz M. Activity and interleukin 1 responsiveness of SV40 enhancer motifs in a rodent immature T cell line. Embo J. 1990;9(3):929–37. doi: 10.1002/j.1460-2075.1990.tb08191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett RD, Baty D, Chambon P. The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter. Nucleic Acids Res. 1983;11(8):2447–64. doi: 10.1093/nar/11.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1(5):457–81. [PubMed] [Google Scholar]
- Kennedy AP, Sekulic A, Irvin BJ, Nilson AE, Dilworth SM, Abraham RT. Polyomavirus middle T antigen as a probe for T cell antigen receptor-coupled signaling pathways. J Biol Chem. 1998;273(19):11505–13. doi: 10.1074/jbc.273.19.11505. [DOI] [PubMed] [Google Scholar]
- Lattion AL, Espel E, Reichenbach P, Fromental C, Bucher P, Israel A, Baeuerle P, Rice NR, Nabholz M. Characterization of a new tissue-specific transcription factor binding to the simian virus 40 enhancer TC-II (NF-kappa B) element. Mol Cell Biol. 1992;12(11):5217–27. doi: 10.1128/mcb.12.11.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CK, Atwood WJ. Propagation and assay of the JC virus. Methods Mol Biol. 2001;165:9–17. doi: 10.1385/1-59259-117-5:9. [DOI] [PubMed] [Google Scholar]
- Macchi M, Bornert JM, Davidson I, Kanno M, Rosales R, Vigneron M, Xiao JH, Fromental C, Chambon P. The SV40 TC-II(kappa B) enhanson binds ubiquitous and cell type specifically inducible nuclear proteins from lymphoid and non-lymphoid cell lines. Embo J. 1989;8(13):4215–27. doi: 10.1002/j.1460-2075.1989.tb08607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley K, O’Hara B A, Gee GV, Simkevich CP, Sedivy JM, Atwood WJ. NFAT4 is required for JC virus infection of glial cells. J Virol. 2006;80(24):12079–85. doi: 10.1128/JVI.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermod N, Williams TJ, Tjian R. Enhancer binding factors AP-4 and AP-1 act in concert to activate SV40 late transcription in vitro. Nature. 1988;332(6164):557–61. doi: 10.1038/332557a0. [DOI] [PubMed] [Google Scholar]
- Pass HI, Donington JS, Wu P, Rizzo P, Nishimura M, Kennedy R, Carbone M. Human mesotheliomas contain the simian virus-40 regulatory region and large tumor antigen DNA sequences. J Thorac Cardiovasc Surg. 1998;116(5):854–9. doi: 10.1016/S0022-5223(98)00438-3. [DOI] [PubMed] [Google Scholar]
- Rollison DE, Shah K. The epidemiology of SV40 infection due to contaminated polio vaccines: relation of the virus to human cancer. In: Khalili K, Stoner GL, editors. Human polyomaviruses: molecular and clinical perspectives. Wiley-Liss Inc.; 2001. pp. 561–584. [Google Scholar]
- Shah K, Nathanson N. Human exposure to SV40: review and comment. Am J Epidemiol. 1976;103(1):1–12. doi: 10.1093/oxfordjournals.aje.a112197. [DOI] [PubMed] [Google Scholar]
- Stratton K, Almario DA, McCormick MC. SV40 contamination of polio vaccine and cancer. Immunization safety review committee, Board of Health Promotion and Disease Prevention, Institute of Medicine of the National Academies. The National Academies Press. 2002 [PubMed] [Google Scholar]
- Tjian R. The biochemistry of transcription and gene regulation. Harvey Lect. 1994;90:19–39. [PubMed] [Google Scholar]
- Vilchez RA, Kozinetz CA, Arrington AS, Madden CR, Butel JS. Simian virus 40 in human cancers. Am J Med. 2003;114(8):675–84. doi: 10.1016/s0002-9343(03)00087-1. [DOI] [PubMed] [Google Scholar]
- Vilchez RA, Kozinetz CA, Butel JS. Conventional epidemiology and the link between SV40 and human cancers. Lancet Oncol. 2003;4(3):188–91. doi: 10.1016/s1470-2045(03)01024-6. [DOI] [PubMed] [Google Scholar]
- Zalvide J, DeCaprio JA. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol. 1995;15(10):5800–10. doi: 10.1128/mcb.15.10.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]