SYNOPSIS
Understanding interactions between animals and humans is critical in preventing outbreaks of zoonotic disease. This is particularly important for avian influenza. Food animal production has been transformed since the 1918 influenza pandemic. Poultry and swine production have changed from small-scale methods to industrial-scale operations. There is substantial evidence of pathogen movement between and among these industrial facilities, release to the external environment, and exposure to farm workers, which challenges the assumption that modern poultry production is more biosecure and biocontained as compared with backyard or small holder operations in preventing introduction and release of pathogens. An analysis of data from the Thai government investigation in 2004 indicates that the odds of H5N1 outbreaks and infections were significantly higher in large-scale commercial poultry operations as compared with backyard flocks. These data suggest that successful strategies to prevent or mitigate the emergence of pandemic avian influenza must consider risk factors specific to modern industrialized food animal production.
The emergence and spread of avian influenza viruses are complex and incompletely understood.1 While preparation for pandemic disease is a critically important public health task, understanding risk factors for disease transmission at the animal-human interface may identify opportunities for disease prevention and outbreak containment. There is great interest in examining decisive events in the 1918 pandemic experience to inform planning.2 These studies have highlighted the significance of domesticated species in the development and emergence of highly pathogenic avian influenza (HPAI) virus and strains that are transmissible to humans. The source of initial human exposure in 1918 remains uncertain, and it is not clear if interspecies transmission was necessarily involved in viral reassortment, which could have occurred in settings where swine, chickens, and humans coexisted in close contact.3,4 Recent studies have presented evidence for and against genetic reassortment that is suggestive of modification in the course of interspecies transmission.5
Since 1918, much has changed in the relationships between human populations and domesticated food animals, including poultry and swine. It is often assumed that modern methods of intensive food animal production provide increased biosecurity and biocontainment and thus reduced risks for transfers of zoonotic disease to humans, but these assumptions need to be critically examined. This article undertakes three objectives: (1) a review of the changes in food animal production that have occurred over the past century worldwide, with a focus on aspects of biosecurity and biocontainment at the animal-human interface; (2) evidence from studies of other pathogen movement from confined poultry and swine operations resulting in environmental releases, interspecies transmission, and opportunities for human infection; and (3) an analysis of data from the Thai government program of H5N1 surveillance and outbreak investigations.
TRANSFORMATION OF FOOD ANIMAL PRODUCTION
Over the past 70 years, food animal production in much of the world, beginning in the U.S., has been transformed from traditional small-scale methods and entrepreneurial organization to industrial-scale operations and vertically integrated management in which most if not all aspects of production (breeding, supply of young animals, feeds, animal husbandry) are controlled by a single entity.6,7 Both of these characteristics are relevant to understanding the current nature of the animal-human interface. Industrial or large-scale food animal production (IFAP) involves high throughput animal husbandry, in which thousands of animals of one breed and for one purpose (i.e., pigs, layer hens, broiler chickens, ducks, turkeys, beef or dairy cattle, finfish, or crustacea) are raised with short-generation intervals at single sites under highly controlled conditions, often in confined housing, with defined feeds replacing access to forage crops. These methods facilitate the uniform and reliable production of consumer products through streamlined organizational and production structure, improvements in breeding and animal husbandry, increased veterinary oversight, and specially formulated diets, including the addition of antibiotics to promote feed conversion efficiency and growth rates.8,9 In the U.S., these facilities are known as animal feeding operations (AFOs). Concentrated AFOs (CAFOs) are a type of AFO that has a regulatory definition as facilities that have animals stabled or confined for at least 45 days out of any 12-month period and holding at least 1,000 animal units (AUs) (1 AU = 1,000 pounds).
In the U.S., this change began in the 1930s and now more than 90% of broiler chickens and turkeys are produced in houses in which between 15,000 and 50,000 birds are confined throughout their lifespan. For swine, this transformation occurred more recently and more rapidly: from 1994 to 2001, the market share of hogs produced in IFAP increased from 10% to 72% of total U.S. production.10 The change to an industrial model of production has also reduced farmer autonomy, as most decisions related to feeding and housing are determined by the large producers (or integrators) with which farmers or growers contract to raise animals.
Industrial-scale poultry production is expanding rapidly in Asia, Africa, Latin America, North Africa, and the Near East (Table 1).11,12 Concerns have been raised over the relatively weak veterinary and public health infrastructure in some of these countries.13 Swine production is also increasing; for example, in China, pork production increased from 42 million tons to 51 million tons from 2001 to 2006.12 This increase is largely related to the expansion of the integrated or industrial model of production14 led by both national and multinational corporations for expanding markets of increasingly urban consumer populations within these countries as well as exports.11
Table 1.
Slingenbergh JI, Gilbert M, de Balogh KI, Wint W. Ecological sources of zoonotic diseases. Rev Sci Tech 2004;23:467-84.
Production figures are in thousands of metric tons per year.
Data represents EU-25.
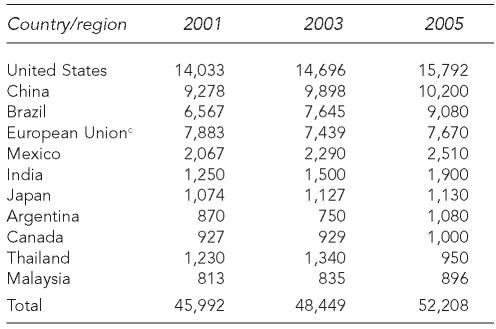
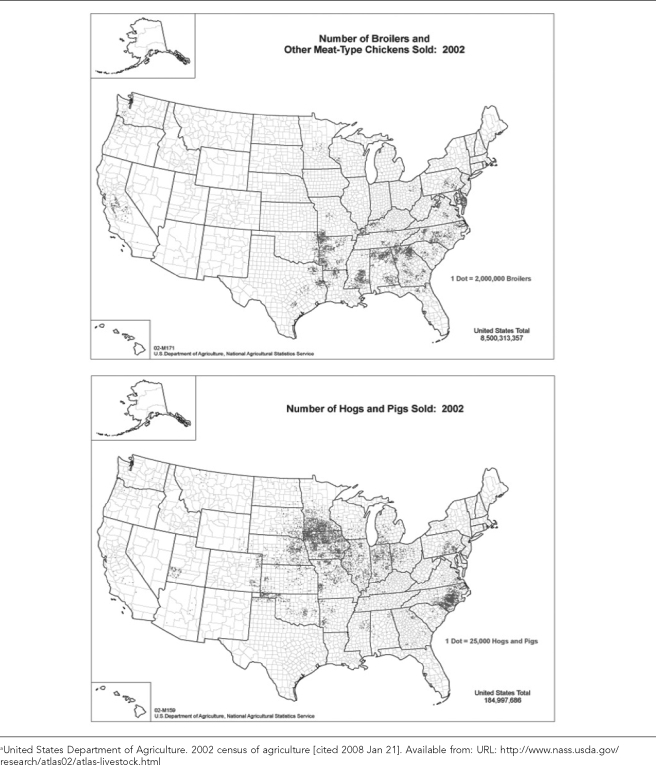
The economic consolidation of poultry and swine production has also affected the geography of food animal populations. Over the past 60 years, the geographic distribution of both swine and poultry production in the U.S. has shrunk, with poultry production now highly concentrated in the southeastern states and hog production concentrated in some of these same states, as well as in the Midwest (Figure 1a). Similar trends have occurred worldwide according to the U.S. Department of Agriculture (USDA).15 For example, pig and poultry densities are highly localized and often coincident in the U.S. (Figure 1a) and Asia (Figure 1b).16 This geographic intensity and coincidence increases the possibility for direct or indirect interactions between large populations of confined poultry and pigs, with potential consequences for the development and transmission of some zoonotic diseases between these species and evolution of pathogens that are transmissible to humans.17,18 As an example, interactions between poultry flocks and swine herds were documented in a case-control study of the 1997–1998 outbreak of classical swine fever in the Netherlands.19
Figure 1a.
Dot density maps of swine and broiler production in the U.S. in 2002a
Figure 1b.
Density distribution maps of swine and poultry in Asia estimated for 1998–2000a
ANIMAL-HUMAN INTERFACES IN IFAP: LIMITS OF BIOSECURITY AND BIOCONTAINMENT
These new modes of poultry and swine production have changed the nature of the animal-human interface in both agriculture and the surrounding environment, with important implications for zoonotic diseases and biosecurity more generally. Biosecurity is defined as any practice or system that prevents the spread of infectious agents from infected to susceptible animals, or prevents the introduction of infected animals into a herd, region, or country in which the infection has not yet occurred.20 Another, more strict definition of biosecurity is the outcome of all activities undertaken by an entity to preclude the introduction of disease agents into an area that one is trying to protect, while biocontainment is the effort taken to prevent spread of a disease within a herd or flock when the disease is already present.21 This definition can be extended to include measures for preventing release of pathogens from an infected herd. Although a combination of measures may significantly reduce the risk of pathogen introduction and release, for a variety of pathogens “zero” risk is virtually impossible to achieve in farmed livestock populations, even in highly developed country settings.
Practical implementation of biosecurity and biocontainment requires measures to be tailored to the pathogens that constitute the threat, as well as to the production practices of the farming system at risk. This requires identification of the main pathways of pathogen transmission, quantification of risks and assessment of efficacy, and cost of proposed risk mitigation measures. Given the focus of this article, we illustrate significant pathways of pathogen transmission that are intensified by industrial food animal production and remain largely unaddressed by current prevention measures.
Occupational pathways
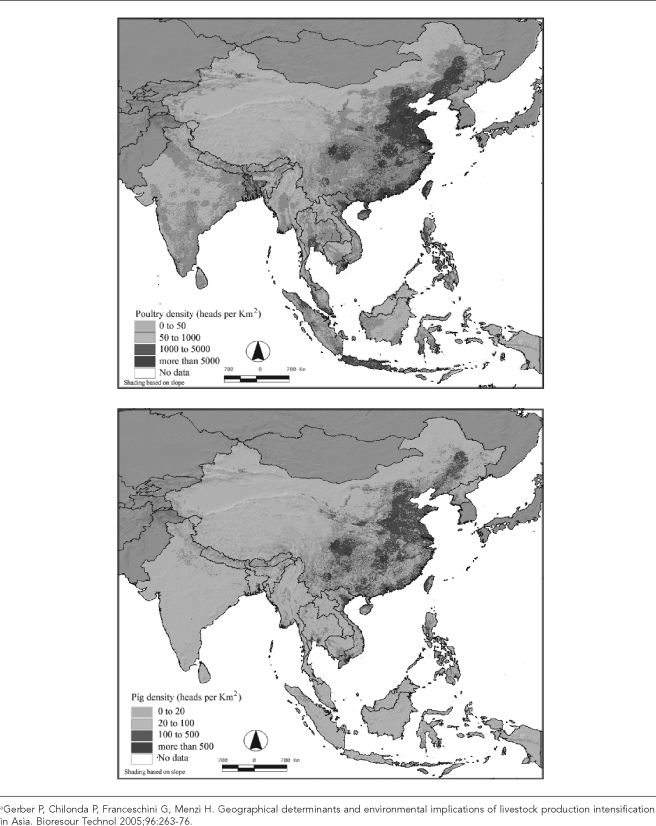
While fewer people are now engaged in animal husbandry at fewer sites (as shown in Figure 2 for hog production in the U.S.), the high throughput and confinement of highly concentrated animal populations increases the intensity of microbial exposures for farmers, their families, farm workers, veterinarians, and others in contact with these operations.
Figure 2.
Temporal trends in the number of hog operations and number of hogs per operation in the U.S., 1965–2003a
In the U.S., as in much of the world, there is little regulation of occupational conditions or worker exposures in most high-density animal houses. The conditions of work, as illustrated in Figure 3, provide many opportunities for both worker infection and transfer to others in the community. With the exception of concerns about disposal of dead chickens during an outbreak,22 there has been minimal attention to animal-human interactions associated with the operation and management of broiler poultry houses. Many workers are provided little or no protective clothing or opportunities for personal hygiene or decontamination on-site. Our studies of poultry house workers in Maryland indicate that workers take their clothes home for washing.23 Thus, it is not surprising that increased risks of pathogen exposure and infections, both bacterial and viral, have been reported among farmers, their families, and farm workers at poultry and swine operations.24–33
Figure 3.
Chicken catchers at work in a poultry concentrated animal feeding operation, U.S., 1999a
Environmental pathways
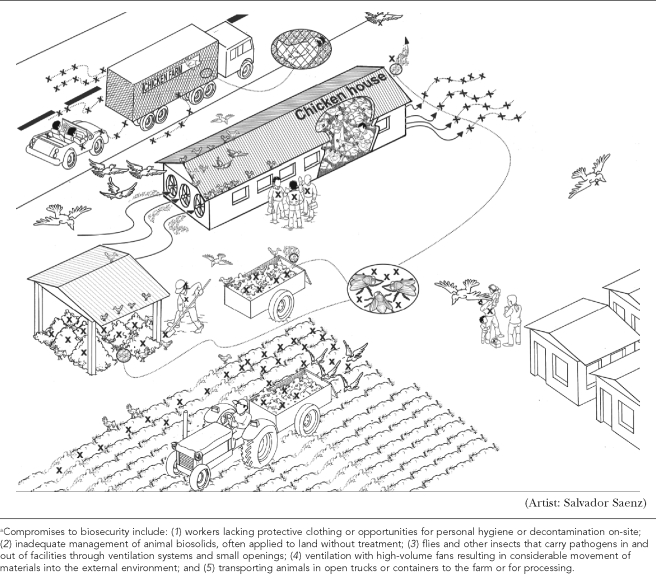
In addition, the design and operational requirements of large-scale swine and broiler poultry houses result in other compromises of biosecurity, as shown schematically in Figure 4. Through these multiple pathways, pathogens move in and out of CAFOs and are then available for exchange among wild avians, domestic avians, swine, and other animals as well as humans. Flock size, number of houses, use of untreated water, and disposal of poultry wastes on the farm all increase risks of Campylobacter spp. colonization of poultry flocks.34 Moreover, because confinement of thousands of animals requires controls to reduce heat and regulate humidity, poultry and swine houses are ventilated with high-volume fans that result in considerable movement of materials into the external environment.35,36
Figure 4.
A schematic representation (not to scale) of multiple potential pathways for exposure to and transfer of pathogens within the environs of concentrated animal feeding operationsa
In 2004, in British Columbia, Canada, the rapid spread of avian influenza among poultry flocks was partly attributed to air exchanges between confinement facilities located within several hundred meters of one another, highlighting the potential for dissemination of HPAI viruses in air emissions.36 Tunnel ventilation systems, which are increasingly used in the U.S. industry, consist of eight 1-meter-diameter fans positioned at one end of the building. These fans generate large quantities of aerosolized dust such that emissions of small particles (<10 micrometers in size) from broiler houses can range from 25 to 40 g/m3/24 hours, representing a million-fold increase as compared with air sampled in a semirural area.36 This aspect of IFAP has not been previously noted in studies that have assumed benefits of confining domestic avian populations in terms of reducing risks of avian influenza.37 Other points of release and interspecies transfers include methods of handling animal house wastes (as discussed later in this article), the use of poultry house wastes in aquaculture, and open-truck transport of food animals from farms to processing plants. Pathogen contamination of shipping containers and trucks, which are not enclosed, is known to occur, and animal stress during transport increases pathogen shedding.38
The role of food animal wastes in interrupting biosecurity
In addition to the production of animals for human consumption, IFAP also produces large amounts of animal waste, or biosolids. Animal biosolids contain a range of pathogens that may include influenza viruses, which can persist for extended periods of time in the absence of specific treatment. The volume of animal wastes is significant, reflecting the considerable expansion of food animal production globally.39 In the U.S., it is estimated that 238,000 CAFOs produce 314 million metric tons of waste per year, which is 100 times as much biosolids produced by treating human wastewater.40 Global estimates suggest that 140 million metric tons of poultry litter and 460 million metric tons of swine waste were produced in 2003 based on data from the Food and Agriculture Organization.41,42 Furthermore, because of the regional concentration of poultry and swine production, the generation of these biosolids is also highly concentrated geographically. Workers involved in removing the wastes from animal houses, transporting wastes, and spreading wastes on land are especially at risk of exposure to pathogens through inhalation, dermal contact, and hand-to-mouth transfers.43
In contrast to the management of human biosolids,44 there are relatively few regulations for animal waste disposal and no specific requirements for treatment. Apart from some use in animal feeds and aquaculture, poultry and swine wastes are almost entirely managed by land disposal. Pathogens can survive in untreated and land-disposed wastes from food animals for extended periods of time—between two and 12 months for bacteria and between three and six months for viruses.40 Contamination of both surface and ground water can result from these practices.45 Moreover, both holding and land disposal of poultry wastes can attract wild avians because spilled feed is present in these wastes. This is particularly likely in the vicinity of their stopover areas, such as Guangdong Province in China and the Delmarva Peninsula in the U.S., where waterfowl commonly forage in fields near poultry production facilities.
In addition, there is growing use of poultry and duck house wastes for use in land-based aquaculture operations (e.g., tilapia and catfish) in many countries (either in integrated facilities, as in Asia and Africa, or after processing, as in the European Union and the U.S.).46–48 This mode of pathogen transfer is wholly unregulated. Open impoundments for aquaculture are essentially small wetlands, and they are frequently visited by wild avians, setting up another setting for bidirectional pathogen transfers. It should be noted that the fecal-water-oral route is considered a highly probable mode of transmission of avian influenza virus between birds.49
Evidence from studies of animal-human and animal-animal transfers of bacteria in IFAP
There is a paucity of research on transfers of viruses from IFAP; however, there is extensive literature on the exchange of bacteria among confined food animals, wild animals, humans, and the environment. This literature supports concerns about the efficacy of biocontainment at industrial food animal production operations. Research on Campylobacter spp. is particularly relevant in this regard, as Campylobacter is an avian commensal that frequently colonizes commercial broiler flocks. In humans, Campylobacter causes a serious diarrheal disease (Campylobacteriosis) of considerable public health significance.23 As for avian influenzas, wild birds are the natural vertebrate reservoirs of Campylobacter, and they can serve as vectors for transmission to other vertebrates.50–56 Campylobacter moves among avian host species, both domesticated57 and wild,58–60 and the exchange of Campylobacter between broiler flocks and wild avians can occur in both directions.61
There are several pathways for Campylobacter colonization of broiler flocks including in ovo vertical transmission, carryover from previous flocks, horizontal transfer from other animals (wild or domestic), and contaminated feed and water. There is clear evidence that colonization of confined poultry flocks can also result from the entrance of Campylobacter from the immediate external environment (which may originate from nearby wastes or from wild avians), as demonstrated in study of Campylobacter-free broiler flocks, housed in sanitized facilities, using standard biosecurity measures, and fed Campylobacter-free feed and water. Seven out of 10 flocks became colonized with Campylobacter by the time of slaughter and two flocks were colonized by Campylobacter strains genetically indistinguishable from strains isolated from puddles outside of the facility prior to flock placement.62 Although the route of entry was not determined, this study showed the capacity for microbes to enter broiler facilities.
Once a poultry flock is colonized with Campylobacter, the food, water, and air within the house quickly becomes contaminated with the bacterium.62 Contaminated air exiting the house via ventilation systems is then a potential source of Campylobacter to the external environment. Campylobacter strains with identical DNA fingerprints to those colonizing broilers have been measured in air up to 30m downwind from broiler facilities housing colonized flocks.62 Other pathogens originating in confined animal houses have also been identified in the air downwind from facilities and in air and groundwater near IFAP houses.36,63–66 Data from an investigation by the Canadian Food Inspection Agency following an H7N3 outbreak suggest that in the event of an HPAI outbreak within a flock, the virus load is such that biocontainment is nearly impossible and that virus transfers to the external environment via the ventilation system may present exposure risks to nearby poultry houses as well as human communities.36
There are additional mechanisms by which Campylobacter and other microbes enter and leave “biosecure” poultry houses. For example, insects may carry microbes in and out of facilities through ventilation systems and small openings. This was demonstrated in a study in Denmark, which found that Campylobacter carriage was common among flies surrounding the broiler facilities, and that as many as 30,000 flies may enter a broiler facility during a single flock rotation in the summer months.67 Additionally, research conducted during an HPAI outbreak in Kyoto, Japan, in 2004 found that flies caught in proximity to broiler facilities where the outbreak took place carried the same strains of H5N1 influenza virus as found in chickens of an infected poultry farm.68 Further, flies serving as potential mechanical transmitters of HPAI from poultry houses to soils and water may pose a risk in those regions where intensive poultry production takes place within flyways of migratory wild birds. Rats have also been suspected of carrying H5N1 from wild birds to domesticated poultry in Japan during an outbreak in 2007.69 Together, these observations suggest that veterinary and public health officials should consider the potential for non-avian species to carry avian influenza virus from the feces of waterfowl into poultry facilities as well as from infected poultry flocks into the surrounding environment.
Evidence for increased risks of virus exposure for farmers and farm workers
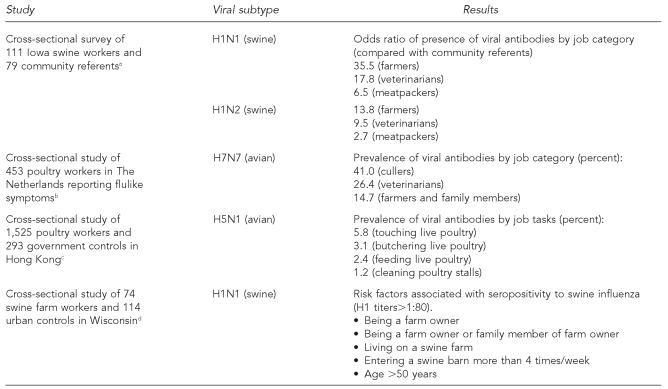
We have outlined several opportunities for pathogen exposures in CAFOs via occupational and environmental pathways. As indicated previously, there are studies demonstrating increased risks of bacterial infections among farmers and farm workers. A number of studies also demonstrate that zoonotic viruses can be transmitted across the animal-human interface in the context of food animal production (Table 2). Studies on seroprevalence of H1 swine influenza virus infections27,70 and hepatitis E22 indicate that farmers and farm workers can be infected by viruses from food animals. Myers et al. reported that swine farmers had higher titers of H1N1 and H1N2 antibodies and greatly elevated risks of seropositivity to these two influenza A viruses (35.3 and 13.8 odds ratios, respectively, as compared with community referents).70
Table 2.
Results of recent studies of zoonotic influenza infection among farm workers
Myers KP, Olsen CW, Setterquist SF, Capuano AW, Donham KJ, Thacker EL, et al. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin Infect Dis 2006;42:14-20.
Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 2004;363:587-93.
Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, Mak KH, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J Infect Dis 2002;185:1005-10.
Olsen CW, Brammer L, Easterday BC, Arden N, Belay E, Baker I, et al. Serologic evidence of H1 swine influenza virus infection in swine farm residents and employees. Emerg Infect Dis 2002;8:814-9.
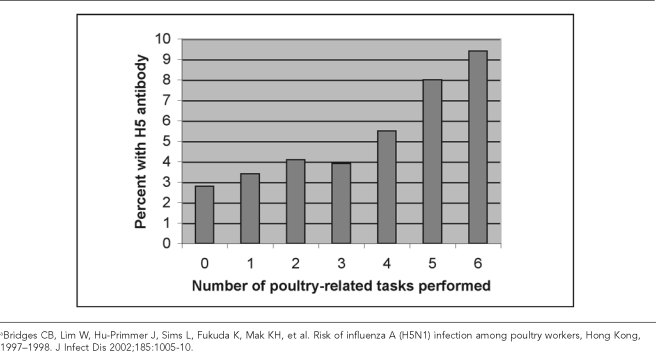
There are also several studies of recent outbreaks of avian influenza in which poultry workers have been studied. Two small studies in Italy and Canada reported on exposure of poultry workers to H7 HPAI viruses.1,71 More comprehensive studies have been conducted in Hong Kong and the Netherlands. In a study of exposures to H5N1 during the Hong Kong outbreak in 1997–1998, 1,525 poultry workers and 293 government workers involved in outbreak investigations were assessed for risk factors for seropositivity.72 Only those occupational tasks involving contact with live poultry were associated with increased risks of seropositivity; as shown in Figure 5, the percentage of people with H5 antibodies rose with increased numbers of such occupational contacts.
Figure 5.
Percentage of poultry workers seropositive for H5 antibody by number of poultry-related tasks performeda
A study of H7N7 infection among people reporting relevant symptoms was conducted during a large outbreak of avian influenza in the Netherlands in 2003.73 At the time of the outbreak in February 2003, all poultry workers, farmers, and their families were asked to report symptoms of conjunctivitis and flulike illness to the health department. Among 453 people calling into the health department from the region of the outbreak, H7 virus was detected from swabs in 86 people, in 26.4% of those with conjunctivitis and in 9.4% of those with conjunctivitis and flulike symptoms. The highest frequencies were observed in cullers (41.2%), veterinarians (26.3%), and farmers and their family members (14.7%). In a second phase, all those in close contact with people confirmed to carry H7 were also asked to enroll in the study. Three of the 83 people contacted in this manner were confirmed to have H7 virus as well. All of them were family members of farmers.
Evidence for increased risks of HPAI in confined poultry flocks
We have tested the hypothesis that confined poultry operations may present increased risks of HPAI through an analysis of data provided by Tiensin et al.74,75 on the 2004 HPAI epidemic in Thailand, which also includes a separate dataset from the nationwide active surveillance program undertaken by the Thai government to detect HPAI infections in poultry. These data are unique and highly relevant to addressing questions on biosecurity and biocontainment risks associated with different modes of poultry production.
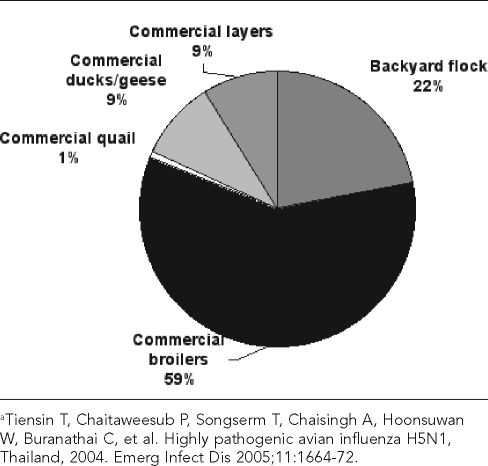
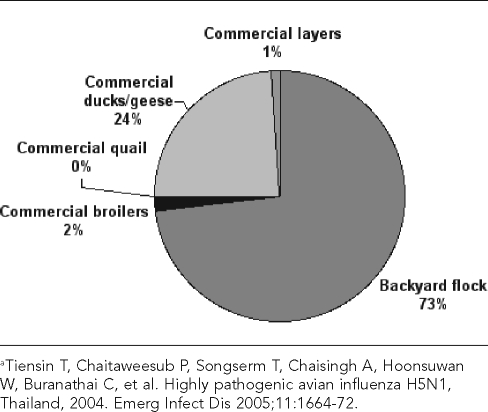
In the original 2005 report, Tiensin et al. classified flocks as “backyard” or “commercial” and classified the poultry by species: duck, geese, quail, layer chickens, or broiler chickens.75 Most of the poultry population in Thailand at the time consisted of broiler chickens kept by commercial enterprises, while backyard operations made up most of the flocks (Figures 6 and 7). Backyard flocks, which consist of 30 birds per flock on average, constituted approximately three-quarters of flocks but accounted for only about one-fifth of the total poultry population. Commercial broiler enterprises, consisting of 3,500 birds per flock on average, constitute only 2% of all “flocks” but account for nearly 60% of the total poultry population.
Figure 6.
Contribution of different flock types to total domestic poultry population (approximately 280 million birds) in Thailand in 2004a
Figure 7.
Contribution of different flock types to total number of flocks (approximately 2.9 million flocks) in Thailand in 2004a
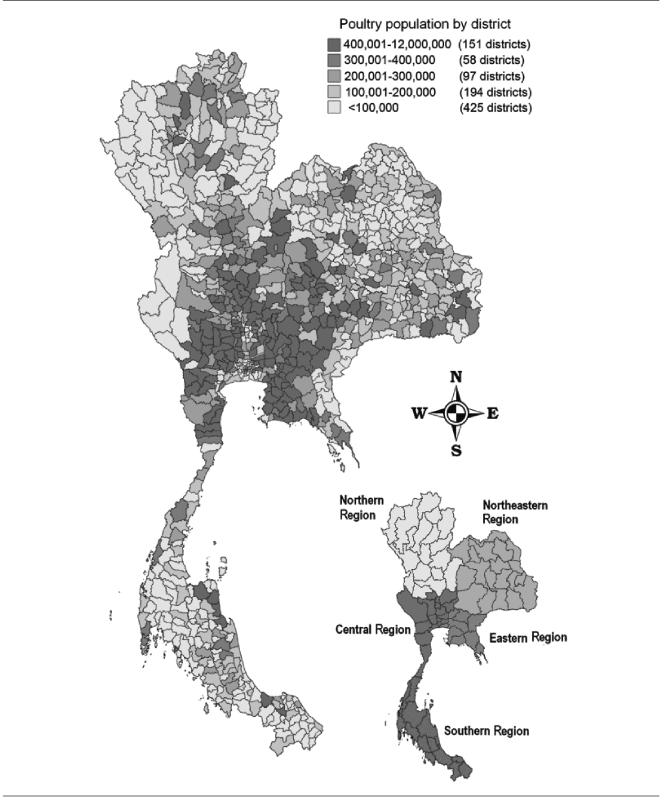
The Thai poultry industry is also regionally concentrated, with commercial production being particularly important in the Central and Eastern regions (Figure 8). In contrast, production of ducks and geese in the North, Northeast, and South are mainly backyard operations.75 The majority of quail also appear to be commercially raised, as the average flock size in the Central region was 9,000 birds.
Figure 8.
Spatial distribution of poultry population counts at district level in Thailand in 2003
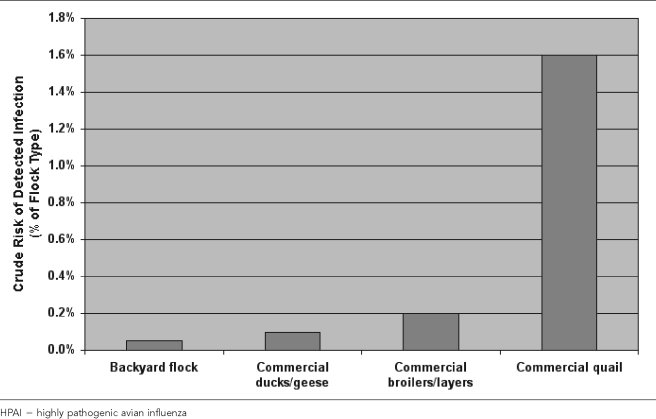
A total of 1,769 flocks with HPAI infection were reported to or detected by the Thai animal health authorities in 2004. The distribution of these infections by flock type indicates that more than 50% of the registered infections involved backyard flocks. However, the proportional contribution of different flock types to registered infections (number of flocks affected by HPAI) was markedly different from their contribution to the total number of flocks. The crude risk of detected infection, expressed as a percentage of the flock type, is shown in Figure 9. Thus, for example, although layer flocks only constituted 1% of all flocks, they accounted for 5% of all registered infected flocks.
Figure 9.
Risk of infection (percent) with HPAI in Thailand in 2004 by flock type
Quail flocks showed the highest risk of infection—roughly 1.6%—followed by layer and broiler flocks, both with infection risks of just above 0.2%. Against expectations, backyard flocks showed the lowest risk of infection with HPAI (0.05%), only one-quarter that of layer and broiler flocks.
These results could reflect differences in outbreak ascertainment. It is likely that HPAI is more readily detectable by the personnel in large commercial operations and more likely to be brought to the attention of animal health authorities. It is not possible to determine the actual impact of underreporting in these data. However, given the focus on backyard operations and the active surveillance programs that were in place in Thailand at this time, this potential ascertainment bias is unlikely to be the sole explanation for the higher risk of identified HPAI outbreaks in commercial layer and broiler flocks compared with backyard operations.
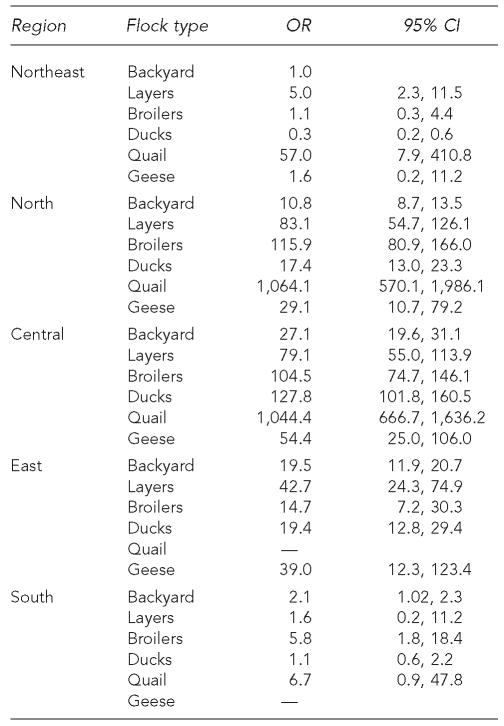
Another explanation for these differences may be due to other risk factors related to HPAI, such as ecological and regional factors, which might modulate transfers from wild avians and among domesticated flocks. From a temporal and a geographical perspective, HPAI outbreaks in Thailand in 2004 were shown to be linked to certain agro-ecological factors, such as the geographical density of wetlands and rice paddies.76 The Central region was particularly affected, followed by the Eastern region, while the Northern, Northeastern, and Southern regions only experienced minor epidemics.75 Given that these different regions also have different mixtures of flock types, the data were subjected to statistical multivariate analysis to control for potential confounding among region, flock type-specific risks, and average flock size within region and species category. Logistic regression analysis was used with variable selection being based on the likelihood ratio statistic. Additionally, the Huber-Sandwich estimator was used to obtain adjusted coefficient estimates due to clustered data. Table 3 displays the adjusted odds ratios (and their 95% confidence intervals) for the selected risk factors, taking backyard operations and the Northeastern region (lowest crude risk) as reference groups (odds ratio = 1).
Table 3.
Adjusted odds ratio (OR) and 95% confidence interval (CI) for selected risk factors for HPAI infection in Thailand in 2004
HPAI = highly pathogenic avian influenza
The multivariate statistical analysis shows that there was an interaction between region and species, such that within the North and Central regions, among the flock types in the region, backyard flocks had the lowest odds ratios of outbreak occurrence. In the East, there was relatively little difference between flock types, but layers and geese had the highest odds ratios. In the South, the odds ratios of infection were not different from backyard flocks in the Northeast for layers, ducks, and quail, but they were higher for backyard and broiler flocks. In the Northeast, layers and quail had higher odds ratios than backyard flocks, whereas they were reduced in ducks, layers, broilers, and geese in the South and Northeast. Across regions, the odds ratios for quail flocks to experience HPAI infection were by far the highest. Overall, these results reflect the geographical pattern of the outbreaks, most of which occurred in the central region of the country, and the lowest number in the South.
In 2004, the Thai government implemented two additional programs designed to improve early detection of avian influenza infections in poultry. One of these, preemptive culling, involved responses based on elevated mortality in a flock, and the other involved an active nationwide surveillance program to detect possible HPAI infections in the absence of increased mortality. In this latter program, swab samples were tested by several methods, including antibody measurement and real-time polymerase chain reaction.74,75 These data indicate higher numbers of detections in birds from backyard flocks as opposed to those from commercial broiler flocks; however, based upon the design, there was a programmatic focus on backyard flocks that resulted in oversampling of backyard flocks relative to commercial flocks. We do not have the data to examine this possibility.
In the active surveillance program, cloacal swabs were randomly collected from five birds per flock and four flocks per village. Over the period of surveillance, swabs were collected from approximately 230,000 flocks in more than 50,000 villages. As indicated previously, for our analysis of outbreak data, on a flock basis, compared with commercial flocks a larger number of backyard flocks may have tested positive, but within these infected flocks much larger numbers of infected animals will have been present in commercial than in backyard flocks. This means that commercial flocks once infected represent a formidable challenge for biocontainment of the infection. This issue includes the problem associated with the disposal of dead birds following culling as part of a control effort.
Taken together, the data do not support the assumption that backyard poultry production in Thailand presents more of a risk, in terms of HPAI outbreaks, than larger commercial poultry operations (either layers, broiler chickens, or quail). There are no data that permit examination of other production factors, apart from flock type and species, which might particularly contribute to increasing or decreasing HPAI infection risks within and between flock types. Similarly, data from Canada, although incomplete, have also indicated that in contrast to backyard flocks, large commercial operations were disproportionately affected by a 2004 HPAI outbreak.36
CONCLUSIONS
Understanding the animal-human interface is a critical element in evaluating and predicting risks of emergent zoonoses, as well as in designing evidence-based programs for prevention and early detection of emerging infectious diseases, such as avian influenza. This has been well documented in terms of zoonoses arising from wild animal species77 and in regard to food safety,78 but it remains underrecognized in terms of workers involved in the production of domesticated animals.
There have been major changes in many aspects of domestic food animal production in the U.S. and other countries during the 20th century, resulting in industrial-scale operations involving high densities of confined animal populations, which is how most of the world's animal protein sources are now derived. These changes in organization, intensity, housing, and waste generation may influence the emergence and transfers of avian influenza virus among wild and domestic species, and from avians to human populations. If this is the case, then inferences based upon the last global and national experience with pandemic avian influenza in 1914–1918 may need reconsideration.
Most importantly, the modern methods of both poultry (particularly broiler chickens) and swine production have changed. These operations result in high numbers of poultry and swine housed under confined conditions at great density and geographic concentration. These operations have also changed the management and autonomy of animal husbandry: individual farmers are increasingly supplanted by large producers that contract with growers to raise animals. This has implications for public health surveillance and interventions, as the farmer no longer makes decisions in relation to feeding, housing, or operations. Access to animal facilities is also increasingly controlled by producers rather than farmers. These changes in some cases may have beneficial impacts in terms of early detection and veterinary oversight.
These new methods of food animal production generate many routes of pathogen transfer among wild and domesticated species and from animals to humans through occupational, peri-occupational, and environmental pathways. At the animal-human interface in these operations, there is inadequate protection of workers and their communities, and, more generally, there is incomplete biocontainment to prevent transfers from the animal house to the general environment. Indeed, the main emphasis of disease prevention with increasing production intensity is typically on enhancing biosecurity, whereas biocontainment is considered less of a priority. Evidence would suggest that once a pathogen has been introduced into such a production facility, it can rapidly multiply; for some pathogens, enormous quantities of infectious organisms can be released and expose other production units. For example, it has not been recognized that the necessity for high ventilation of densely confined animals greatly impairs attempts at biocontainment. Moreover, little attention has been given to the generation and lack of management of the millions of tons of animal wastes generated annually. Food animal wastes are largely disposed on land, and this creates an unrecognized magnet for wild avians because of the presence of undigested feed in the waste. There is some use of poultry wastes as bedding for fish ponds, which creates an additional opportunity for wild avian contact. Studies of bacterial pathogens provide strong evidence for the bidirectional transfers of pathogens among poultry grown in these confined operations, wild birds, and human populations.
Our analysis of the Thai HPAI outbreak and surveillance data suggests that commercial poultry production, an ostensibly more “biosecure” system of production, is not associated with a reduction in risk of HPAI infection at the farm or flock level, as compared with that experienced by subsistence backyard producers. Although the majority of reported HPAI outbreaks in Thailand in 2004 occurred in the latter, this increased cumulative risk of HPAI in the backyard sector is primarily due to their relatively greater numbers rather than more risky production practices.
Some of the measures being considered to make backyard poultry production safer, including the forced housing or confinement of poultry, are not likely to result in a major reduction of HPAI risks. In contrast, the costs will likely be significant and will be imposed upon a marginal group of entrepreneurs and household producers. This may result in an overall reduction of HPAI outbreaks as a consequence of the loss of household production flocks, but not as a result of enhanced biosecurity and biocontainment.
Additionally, the geographic concentration and housing density of commercial poultry production can greatly augment the spread of HPAI in an infected area. A study of an outbreak in The Netherlands found that the transmission rate of the virus was not necessarily affected by improved biosecurity and biocontainment measures but by depletion of susceptible flocks due to complete depopulation of infected areas.79 The authors suggested that reducing flock density in commercial flocks might reduce the probability of a major epidemic in which large numbers of poultry flocks must be culled. Following the aforementioned HPAI outbreak in Canada, restriction permits for the development of new operations were proposed as a method to control the density of commercial operations when deemed too close to existing farms.36
This analysis suggests that renewed attention to the animal-human interface should focus on high-risk populations, especially farm workers. A similar conclusion was reached by Saenz et al.18,80 in an analysis of the risks of influenza outbreaks and transmission to human populations in the context of large-scale commercial poultry and swine production. Monitoring this population may improve detection of early events in emergence of avian influenza. In addition, careful evaluation of operations at all poultry facilities—large and small—should be undertaken to reduce opportunities for the transmission of disease among avian and other species. Moreover, if appropriate protections such as vaccination are identified, the agricultural workforce constitutes a high-risk population for whom protection from zoonotic disease is important not only for their health but for the health of their communities and the population at large. Finally, improved oversight and management of animal wastes—including transport and sale as well as use in aquaculture—should be included in strategies to reduce risks of pandemic HPAI.
Acknowledgments
The authors are grateful for support from the Center for a Livable Future at the Johns Hopkins Bloomberg School of Public Health. The authors also thank Salvador Saenz from the Center for Environmental Resource Management at the University of Texas at El Paso for his wonderful artwork; Dr. Ruth Faden and Ruth Karron of the Johns Hopkins Medical Institutions for providing the opportunity for this collaboration; and Dr. Pierre Gerber from the Food and Agriculture Organization of the United Nations. The authors also acknowledge insights from Jackie Nowell of United Food and Commercial Workers, Dr. Donald Burke, and other colleagues.
REFERENCES
- 1.Webster RG, Peiris M, Chen H, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg Infect Dis. 2006;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–14. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 3.Barry JM. The great influenza: the epic story of the deadliest plague in history. New York: Viking Press; 2004. [Google Scholar]
- 4.Oxford JS, Lambkin R, Sefton A, Daniels R, Elliot A, Brown R, et al. A hypothesis: the conjunction of soldiers, gas, pigs, ducks, geese and horses in northern France during the Great War provided the conditions for the emergence of the “Spanish” influenza pandemic of 1918–1919. Vaccine. 2005;23:940–5. doi: 10.1016/j.vaccine.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86–112. [PMC free article] [PubMed] [Google Scholar]
- 6.Burkart MR. Diffuse pollution from intensive agriculture: sustainability, challenges, and opportunities. Water Sci Technol. 2007;55:17–23. doi: 10.2166/wst.2007.067. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Agriculture. National Agricultural Statistics Service. Trends in U.S. agriculture. 2006.
- 8.Chapman HD, Johnson ZB. Use of antibiotics and roxarsone in broiler chickens in the USA: analysis for the years 1995 to 2000. Poult Sci. 2002;81:356–64. doi: 10.1093/ps/81.3.356. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council. Washington: National Academies Press; 1999. The use of drugs in food animals: benefits and risks. [PubMed] [Google Scholar]
- 10.McBride WD, Key N. Washington: U.S. Department of Agriculture; 2003. Economic and structural relationships in U.S. hog production. [Google Scholar]
- 11.Silverside D, Jones M. Small-scale poultry processing. United Nations Food and Agriculture Organization. [cited 2008 Jan 21]; Available from: URL: http://www.fao.org/docrep/003/t0561e/T0561E01.htm. [Google Scholar]
- 12.Foreign Agricultural Service. U.S. Department of Agriculture. Livestock and poultry: world markets and trade. 2005. Nov, [cited 2008 Jan 21]. Available from: URL: http://www.fas.usda.gov/dlp/circular/2005/05-11LP/production.pdf.
- 13.Schelling E, Wyss K, Bechir M, Moto DD, Zinsstag J. Synergy between public health and veterinary services to deliver human and animal health interventions in rural low income settings. BMJ. 2005;331:1264–7. doi: 10.1136/bmj.331.7527.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nierenberg D. Rethinking the global meat industry. World Watch Institute 2006. [cited 2008 Jan 21]; Available from: URL: http://www.worldwatch.org/node/3993. [Google Scholar]
- 15.Foreign Agricultural Service. U.S. Department of Agriculture. Livestock and poultry world markets and trade. 1997. Oct, [cited 2008 Jan 21]. Available from: URL: http://www.fas.usda.gov/dlp2/circular/1997/97-10LP/poultry.htm.
- 16.Gerber P, Chilonda P, Franceschini G, Menzi H. Geographical determinants and environmental implications of livestock production intensification in Asia. Bioresour Technol. 2005;96:263–76. doi: 10.1016/j.biortech.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Slingenbergh JI, Gilbert M, de Balogh KI, Wint W. Ecological sources of zoonotic diseases. Rev Sci Tech. 2004;23:467–84. doi: 10.20506/rst.23.2.1492. [DOI] [PubMed] [Google Scholar]
- 18.Saenz RA, Hethcote HW, Gray GC. Confined animal feeding operations as amplifiers of influenza. Vector Borne Zoonotic Dis. 2006;6:338–46. doi: 10.1089/vbz.2006.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbers AR, Stegeman JA, de Jong MC. Factors associated with the introduction of classical swine fever virus into pig herds in the central area of the 1997/98 epidemic in The Netherlands. Vet Rec. 2001;149:377–82. doi: 10.1136/vr.149.13.377. [DOI] [PubMed] [Google Scholar]
- 20.Radostits OM. Herd health: food animal production medicine. 3rd ed. Philadelphia: W.B. Saunders Company; 2001. Control of infectious diseases of food-producing animals. [Google Scholar]
- 21.Dargatz DA, Garry FB, Traub-Dargatz JL. An introduction to biosecurity of cattle operations. Vet Clin North Am Food Anim Pract. 2002;18:1–5. doi: 10.1016/s0749-0720(02)00002-6. [DOI] [PubMed] [Google Scholar]
- 22.McQuiston JH, Garber LP, Porter-Spalding BA, Hahn JW, Pierson FW, Wainwright SH, et al. Evaluation of risk factors for the spread of low pathogenicity H7N2 avian influenza virus among commercial poultry farms. J Am Vet Med Assoc. 2005;226:767–72. doi: 10.2460/javma.2005.226.767. [DOI] [PubMed] [Google Scholar]
- 23.Price LB, Roess A, Graham JP, Baqar S, Vailes R, Sheikh KA, et al. Neurologic symptoms and neuropathologic antibodies in poultry workers exposed to Campylobacter jejuni. J Occup Environ Med. 2007;49:748–55. doi: 10.1097/JOM.0b013e3180d09ec5. [DOI] [PubMed] [Google Scholar]
- 24.Akwar TH, Poppe C, Wilson J, Reid-Smith RJ, Dyck M, Waddington J, et al. Risk factors for antimicrobial resistance among fecal Escherichia coli from residents on forty-three swine farms. Microb Drug Resist. 2007;13:69–76. doi: 10.1089/mdr.2006.9999. [DOI] [PubMed] [Google Scholar]
- 25.Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, et al. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg. 2002;66:384–8. doi: 10.4269/ajtmh.2002.66.384. [DOI] [PubMed] [Google Scholar]
- 26.Olsen B, Axelsson-Olsson D, Thelin A, Weiland O. Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis. 2006;38:55–8. doi: 10.1080/00365540500321470. [DOI] [PubMed] [Google Scholar]
- 27.Olsen CW, Brammer L, Easterday BC, Arden N, Belay E, Baker I, et al. Serologic evidence of H1 swine influenza virus infection in swine farm residents and employees. Emerg Infect Dis. 2002;8:814–9. doi: 10.3201/eid0808.010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fey PD, Safranek TJ, Rupp ME, Dunne EF, Ribot E, Iwen PC, et al. Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N Engl J Med. 2000;342:1242–9. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- 29.Levy SB, FitzGerald GB, Macone AB. Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature. 1976;260:40–2. doi: 10.1038/260040a0. [DOI] [PubMed] [Google Scholar]
- 30.Kariuki S, Gilks C, Kimari J, Obanda A, Muyodi J, Waiyaki P, et al. Genotype analysis of Escherichia coli strains isolated from children and chickens living in close contact. Appl Environ Microbiol. 1999;65:472–6. doi: 10.1128/aem.65.2.472-476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bogaard AE, London N, Criessen C, Stobberingh EE. Antibiotic resistance of faecal Escherichia coli in poultry poultry farmers and poultry slaughterers. J Antimicrob Chemother. 2001;47:763–71. doi: 10.1093/jac/47.6.763. [DOI] [PubMed] [Google Scholar]
- 32.van den Bogaard AE, Willems R, London N, Top J, Stobberingh EE. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. J Antimicrob Chemother. 2002;49:497–505. doi: 10.1093/jac/49.3.497. [DOI] [PubMed] [Google Scholar]
- 33.Al-Ghamdi MS, El-Morsy F, Al-Musafa ZH, Al-Ramadhan M, Hanif M. Antibiotic resistance of Escherichia coli isolated from poultry workers, patients and chickens in the eastern province of Saudi Arabia. Trop Med Int Health. 1999;4:278–83. doi: 10.1046/j.1365-3156.1999.00392.x. [DOI] [PubMed] [Google Scholar]
- 34.Guerin MT, Martin W, Reiersen J, Berke O, McEwen SA, Bisaillon JR, et al. A farm-level study of risk factors associated with the colonization of broiler flocks with Campylobacter spp. in Iceland, 2001–2004. Acta Vet Scand. 2007;49:18. doi: 10.1186/1751-0147-49-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones TA, Donnelly CA, Stamp Dawkins M. Environmental and management factors affecting the welfare of chickens on commercial farms in the United Kingdom and Denmark stocked at five densities. Poult Sci. 2005;84:1155–65. doi: 10.1093/ps/84.8.1155. [DOI] [PubMed] [Google Scholar]
- 36.Power C Canadian Food Inspection Agency, Animal Disease Surveillance Unit. The source and means of spread of the avian influenza virus in the lower Fraser Valley of British Columbia during an outbreak in the winter of 2004: an interim report. 2005 [Google Scholar]
- 37.Songserm T, Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, et al. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg Infect Dis. 2006;12:575–81. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramesh N, Joseph SW, Carr LE, Douglass LW, Wheaton FW. Serial disinfection with heat and chlorine to reduce microorganism populations on poultry transport containers. J Food Prot. 2003;66:793–7. doi: 10.4315/0362-028x-66.5.793. [DOI] [PubMed] [Google Scholar]
- 39.Spencer JL, Guan J. Public health implications related to spread of pathogens in manure from livestock and poultry operations. Methods Mol Biol. 2004;268:503–15. doi: 10.1385/1-59259-766-1:503. [DOI] [PubMed] [Google Scholar]
- 40.Gerba CP, Smith JE., Jr Sources of pathogenic microorganisms and their fate during land application of wastes. J Environ Qual. 2005;34:42–8. [PubMed] [Google Scholar]
- 41.Miner JR, Humenik FJ, Overcash MR. Managing livestock wastes to preserve environmental quality. Ames (IA): Blackwell Publishing Professional; 2000. [Google Scholar]
- 42.Food and Agriculture Organization of the United Nations. FAOSTAT. [cited 2008 Jan 21]. Available from: URL: http://faostat.fao.org.
- 43.Cole D, Todd L, Wing S. Concentrated swine feeding operations and public health: a review of occupational and community health effects. Environ Health Perspect. 2000;108:685–99. doi: 10.1289/ehp.00108685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Research Council. Washington: National Academies Press; 2002. Biosolids applied to land: advancing standards and practices. [Google Scholar]
- 45.Sapkota AR, Curriero FC, Gibson KE, Schwab KJ. Antibiotic-resistant enterococci and fecal indicators in surface water and groundwater impacted by a concentrated swine feeding operation. Environ Health Perspect. 2007;115:1040–5. doi: 10.1289/ehp.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little D, Satapornvanit K. Poultry and fish production—a framework for their integration in Asia. Paper presented at the Second Food and Agriculture Organization of the United Nations Electronic Conference on Tropical Feeds; 1996 Sep 9–1997 Feb 28. [Google Scholar]
- 47.Hertrampf JW, Piedad-Pascual F. Handbook on ingredients for aquaculture feeds. 1st ed. Boston: Kluwer Academic Publishers; 2000. [Google Scholar]
- 48.Fowler LG. Poultry by-product meal as a dietary protein source in fall chinook salmon diets. Aquaculture. 1991;99:309–21. [Google Scholar]
- 49.Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA, Humberd J, Seiler P, Puthavathana P, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–79. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabrita J, Rodrigues J, Braganca F, Morgado C, Pires I, Goncalves AP. Prevalence, biotypes, plasmid profile and antimicrobial resistance of Campylobacter isolated from wild and domestic animals from northeast Portugal. J Appl Bacteriol. 1992;73:279–85. doi: 10.1111/j.1365-2672.1992.tb04978.x. [DOI] [PubMed] [Google Scholar]
- 51.Yogasundram K, Shane SM, Harrington KS. Prevalence of Campylobacter jejuni in selected domestic and wild birds in Louisiana. Avian Dis. 1989;33:664–7. [PubMed] [Google Scholar]
- 52.Fernandez H, Gesche W, Montefusco A, Schlatter R. Wild birds as reservoir of thermophilic enteropathogenic Campylobacter species in southern Chile. Mem Inst Oswaldo Cruz. 1996;91:699–700. doi: 10.1590/s0074-02761996000600007. [DOI] [PubMed] [Google Scholar]
- 53.Stern NJ, Myszewski MA, Barnhart HM, Dreesen DW. Flagellin A gene restriction fragment length polymorphism patterns of Campylobacter spp. isolates from broiler production sources. Avian Dis. 1997;41:899–905. [PubMed] [Google Scholar]
- 54.Southern JP, Smith RM, Palmer SR. Bird attack on milk bottles: possible mode of transmission of Campylobacter jejuni to man. Lancet. 1990;336:1425–7. doi: 10.1016/0140-6736(90)93114-5. [DOI] [PubMed] [Google Scholar]
- 55.Altekruse SF, Swerdlow DL, Stern NJ. Microbial food borne pathogens. Campylobacter jejuni. Vet Clin North Am Food Anim Pract. 1998;14:31–40. [PubMed] [Google Scholar]
- 56.Hanninen ML, Perko-Makela P, Pitkala A, Rautelin H. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J Clin Microbiol. 2000;38:1998–2000. doi: 10.1128/jcm.38.5.1998-2000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents. 2000;14:327–35. doi: 10.1016/s0924-8579(00)00145-x. [DOI] [PubMed] [Google Scholar]
- 58.Broman T, Waldenstrom J, Dahlgren D, Carlsson I, Eliasson I, Olsen B. Diversities and similarities in PFGE profiles of Campylobacter jejuni isolated from migrating birds and humans. J Appl Microbiol. 2004;96:834–43. doi: 10.1111/j.1365-2672.2004.02232.x. [DOI] [PubMed] [Google Scholar]
- 59.Petersen L, Nielsen EM, Engberg J, On SL, Dietz HH. Comparison of genotypes and serotypes of Campylobacter jejuni isolated from Danish wild mammals and birds and from broiler flocks and humans. Appl Environ Microbiol. 2001;67:3115–21. doi: 10.1128/AEM.67.7.3115-3121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanley KN, Jones K. High frequency of metronidazole resistance among strains of Campylobacter jejuni isolated from birds. Lett Appl Microbiol. 1998;27:247–50. doi: 10.1046/j.1472-765x.1998.t01-12-00449.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee MD, Sanchez S, Zimmer M, Idris U, Berrang ME, McDermott PF. Class 1 integron-associated tobramycin-gentamicin resistance in Campylobacter jejuni isolated from the broiler chicken house environment. Antimicrob Agents Chemother. 2002;46:3660–4. doi: 10.1128/AAC.46.11.3660-3664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bull SA, Allen VM, Domingue G, Jorgensen F, Frost JA, Ure R, et al. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl Environ Microbiol. 2006;72:645–52. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campagnolo ER, Johnson KR, Karpati A, Rubin CS, Kolpin DW, Meyer MT, et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci Total Environ. 2002;299:89–95. doi: 10.1016/s0048-9697(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 64.Esiobu N, Armenta L, Ike J. Antibiotic resistance in soil and water environments. Int J Environ Health Res. 2002;12:133–44. doi: 10.1080/09603120220129292. [DOI] [PubMed] [Google Scholar]
- 65.Hanninen ML, Niskanen M, Korhonen L. Water as a reservoir for Campylobacter jejuni infection in cows studied by serotyping and pulsed-field gel electrophoresis (PFGE) Zentralbl Veterinarmed B. 1998;45:37–42. doi: 10.1111/j.1439-0450.1998.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 66.Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol. 2001;67:1494–502. doi: 10.1128/AEM.67.4.1494-1502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hald B, Skovgard H, Bang DD, Pedersen K, Dybdahl J, Jespersen JB, et al. Flies and Campylobacter infection of broiler flocks. Emerg Infect Dis. 2004;10:1490–2. doi: 10.3201/eid1008.040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawabe K, Hoshino K, Isawa H, Sasaki T, Hayashi T, Tsuda Y, et al. Detection and isolation of highly pathogenic H5N1 avian influenza A viruses from blow flies collected in the vicinity of an infected poultry farm in Kyoto, Japan, 2004. Am J Trop Med Hyg. 2006;75:327–32. [PubMed] [Google Scholar]
- 69.Rodents suspected in spread of avian flu. The Asahi Shimbun. 2007. Feb 16,
- 70.Myers KP, Olsen CW, Setterquist SF, Capuano AW, Donham KJ, Thacker EL, et al. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clin Infect Dis. 2006;42:14–20. doi: 10.1086/498977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puzelli S, Di Trani L, Fabiani C, Campitelli L, De Marco MA, Capua I, et al. Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J Infect Dis. 2005;192:1318–22. doi: 10.1086/444390. [DOI] [PubMed] [Google Scholar]
- 72.Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, Mak KH, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J Infect Dis. 2002;185:1005–10. doi: 10.1086/340044. [DOI] [PubMed] [Google Scholar]
- 73.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–93. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 74.Tiensin T, Chaitaweesub P, Songserm T, Chaisingh A, Hoonsuwan W, Buranathai C, et al. Highly pathogenic avian influenza H5N1, Thailand, 2004. Emerg Infect Dis. 2005;11:1664–72. doi: 10.3201/eid1111.050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tiensin T, Nielen M, Songserm T, Kalpravidh W, Chaitaweesub P, Amonsin A, et al. Geographic and temporal distribution of highly pathogenic avian influenza A virus (H5N1) in Thailand, 2004–2005: an overview. Avian Dis. 2007;51(Suppl 1):182–8. doi: 10.1637/7635-042806R.1. [DOI] [PubMed] [Google Scholar]
- 76.Gilbert M, Chaitaweesub P, Parakamawongsa T, Premashthira S, Tiensin T, Kalpravidh W, et al. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg Infect Dis. 2006;12:227–34. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karesh WB, Cook RA, Bennett EL, Newcomb J. Wildlife trade and global disease emergence. Emerg Infect Dis. 2005;11:1000–2. doi: 10.3201/eid1107.050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schlundt J, Toyofuku H, Jansen J, Jerbst SA. Emerging food-borne zoonoses. Rev Sci Tech. 2004;23:513–33. doi: 10.20506/rst.23.2.1506. [DOI] [PubMed] [Google Scholar]
- 79.Stegeman A, Bouma A, Elbers AR, de Jong MC, Nodelijk G, de Klerk F, et al. Avian influenza A virus (H7N7) epidemic in The Netherlands in 2003: course of the epidemic and effectiveness of control measures. J Infect Dis. 2004;190:2088–95. doi: 10.1086/425583. [DOI] [PubMed] [Google Scholar]
- 80.Gray GC, Trampel DW, Roth JA. Pandemic influenza planning: shouldn't swine and poultry workers be included? Vaccine. 2007;25:4376–81. doi: 10.1016/j.vaccine.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]