SYNOPSIS
Objectives
This study utilized the electronic medical records of six veterinary hospitals (operated by Banfield, The Pet Hospital®) in the vicinity of Fairburn, Georgia, to assess the health of dogs and cats following the unintentional release of propyl mercaptan from a waste-processing facility.
Methods
Standardized electronic medical records were used to define clinical syndromes (eye inflammation, gastrointestinal, respiratory, fever, general weakness/change in mental state) in dogs and cats. The frequency and geographic distribution of each syndrome was evaluated before, during, and after the chemical release, using control charts, density maps, change in average mean distance from a suspected point source of chemical release, space-time statistics, and autoregressive integrated moving averages.
Results
No consistent pattern of change in syndromic events was observed following the suspected release of propyl mercaptan. Some syndromes, including respiratory syndrome in cats, gastrointestinal syndrome in dogs, and eye inflammation syndrome in both cats and dogs, showed a change in time and spatial patterns following the release of propyl mercaptan into the community. These changes were consistent with clinical signs observed in people during a previous propyl mercaptan release in California as well as the release in Fairburn.
Conclusion
A systematic review of electronic medical records of dogs and cats exposed to release of propyl mercaptan showed no conclusive and consistent evidence of adverse health effects. Methods for the use of medical records of pets for detecting environmental hazards require further development and evaluation.
The “canary in the mine” was a sentinel for environmental health hazards.1 Animals outside the laboratory can yield information about each step in risk assessment, including risk characterization, hazard identification, dose-response assessment, and exposure assessment. Under appropriate conditions, the use of domestic and wild animals can help to reveal the presence of unknown chemicals in the environment before they cause harm to humans or to help identify health effects of exposure to known or suspected chemical contaminants. A National Academy of Sciences Committee on Animals as Sentinels of Environmental Health Hazards noted that “domestic animals often share the human environment and are capable of responding to chemical insults manifested by a broad spectrum of pathologic conditions, including behavioral and reproductive dysfunctions, immunologic and biochemical perturbations, and anatomic changes as varied as birth defects and cancers.”1 Despite the obvious advantages of animal sentinels, they are rarely used to investigate the impact of the release of industrial chemicals into a community.
On July 28, 2006, the Georgia Department of Human Resources, Division of Public Health (DPH) was invited by the LaGrange Health District in LaGrange, Georgia (about 60 miles southwest of Atlanta), to assist in an investigation of health effects related to exposure to industrial chemicals. Fairburn, Georgia, is located about 20 miles southwest of Atlanta and has a population of approximately 8,500 with an additional 20,000 people living in the nearby Georgia communities of Fayetteville and Tyrone. During June 2006, a wastewater treatment and nonhazardous waste-processing facility (PSC Recovery Systems) located in Fairburn handled shipments of industrial wastewater containing the pesticide Ethoprop and its breakdown product, propyl mercaptan. On June 29, the facility received and rejected a shipment in four tanker trucks after one of the trucks was tested and found to have an excessive odor caused by propyl mercaptan.
Propyl mercaptan has a strong onion-like odor, and exposure to the chemical has been reported to cause symptoms in people including headache, nausea, and irritation of the skin, eyes, and mucous membranes.2 Community complaints about odors and health consequences were reported to local health officials, and an investigation was initiated. A Web-based community survey of 622 people found that 96% reported one or more symptoms including headache (74%), burning eyes (58%), cough/sore throat (54%), nausea/vomiting (49%), and difficulty breathing (45%). In addition, many people reported a foul odor. A total of 187 people reported seeking medical attention. A case was defined as a person having at least two of the symptoms with onset between May 1 and August 31, 2006; 353 people met the case definition. Among the 233 cases reporting an estimated date of onset, 41% occurred between the weeks of June 25 and July 9 and 82% between the weeks of May 28 and July 9; approximately 60% of these individuals lived within three miles of the PSC Recovery Systems plant. The epidemic curve suggested continued exposure to a toxic chemical.
A telephone survey was conducted of all people whose community survey form included reports of symptoms in a pet animal. Of 36 animals with clinical signs, 29 were dogs, six were cats, and one was a rabbit. The locations of affected animals were similar to the areas where symptomatic residents reported living. The most commonly reported clinical signs in animals were change of behavior, such as lethargy and lack of activity (18), diarrhea (17), loss of appetite (15), runny nose (12), and sneezing (12). The clinical signs ranged in onset from the first week of February through the second week of July. Six of the animals had been seen by a veterinarian, but the findings from these examinations were varied and not specific. Eight affected animals died, but only one of these had a necropsy exam performed by a veterinarian; the findings in this dog were consistent with a diagnosis of gastric torsion (bloat). The pattern of morbidity and mortality in the domestic animals was not considered suggestive of a chemical toxin or other known etiological agent.
The Georgia Syndromic Surveillance Program that was being developed by the Georgia DPH did not have any reporting hospitals located in the Fairburn area before August 2006. Therefore, data were not available from that system that could be used to further study the health effects in people associated with the release of propyl mercaptan. In September 2006, the director of the National Companion Animal Surveillance Program (NCASP) at Purdue University in West Lafayette, Indiana, was contacted by the Georgia DPH and asked to assist in its ongoing investigation in Fairburn. NCASP was originally established with a $1.2 million grant from the Centers for Disease Control and Prevention (CDC) to conduct syndromic surveillance of companion animals for acts of bioterrorism. However, NCASP may also be utilized to conduct retrospective analyses using medical records of dogs and cats following an unintentional release of industrial chemicals into the environment.
The purpose of this article is to describe how NCASP was used to conduct a preliminary retrospective study of changes in the health status of dogs and cats following the release of propyl mercaptan from the nonhazardous waste-treatment facility in Fairburn.
METHODS AND RESULTS
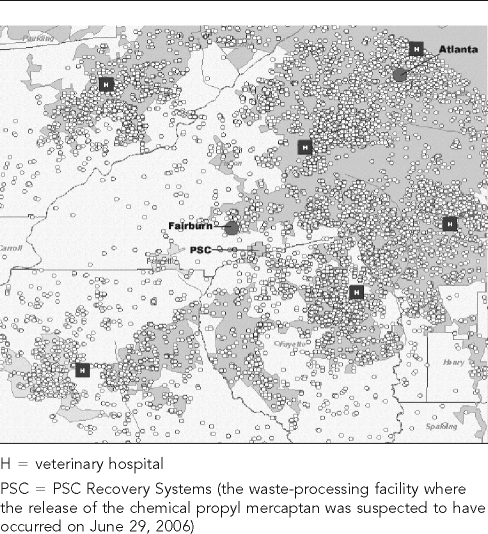
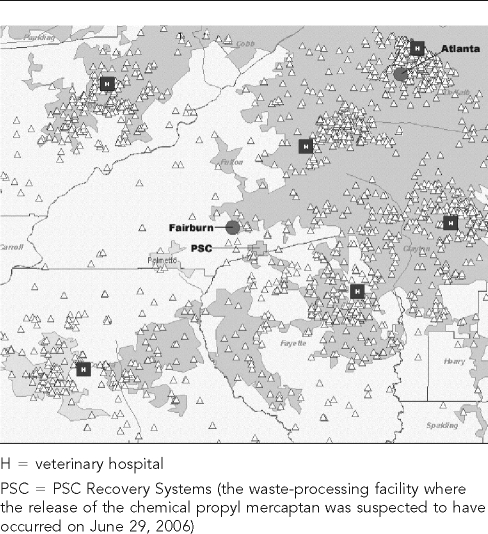
Fairburn is a city in Fulton County, Georgia, in the Atlanta metropolitan area (latitude 33.56°N; longitude 84.58°W). As of June 2006, six primary care Banfield, The Pet Hospital veterinary hospitals were located within a 20-mile radius of Fairburn. From January 1 to December 28, 2006, these six hospitals recorded 36,744 office visits for dogs and 4,608 office visits for cats (Figures 1a and 1b). Banfield, The Pet Hospital is currently the largest provider of health care for companion animals in the U.S., with more than 640 full-service veterinary hospitals located in 44 states that see approximately 80,000 pets weekly.
Figure 1a.
Dog visits to six Banfield, The Pet Hospital® veterinary hospitals within a 20-mile radius of Fairburn, GA, in 2006
Figure 1b.
Cat visits to six Banfield, The Pet Hospital® veterinary hospitals within a 20-mile radius of Fairburn, GA, in 2006
Banfield hospitals use a proprietary software system called PetWare™ to electronically record all demographic and medical information. The medical-records system used by Banfield has been described previously.3 Medical records are uploaded nightly to a central data warehouse. Using the SAS® Pass-Through Facility for Oracle®,4 these records were transferred to Purdue University for use in this study, and all procedures were approved by the Human Subjects Committee of Purdue University. Data transferred to Purdue University were converted into analysis-ready datasets using SAS® 9.1.3 software.5 A data visualization system developed at Purdue University called Linked Animal-Human Health Visual Analytics (LAHVA)6 was used to plot the geographical distribution of dogs and cats with specified clinical syndromes over space and time.
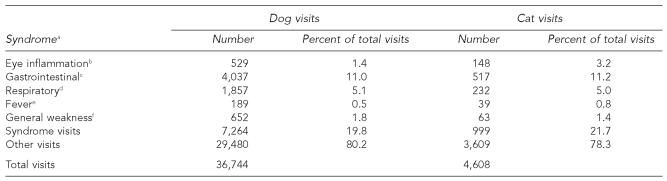
Following notification by the Georgia DPH, NCASP used syndromic definitions based on exam codes rather than specific diagnoses to compare the health status of dogs and cats before, during, and after a possible exposure to propyl mercaptan. Syndromes were studied rather than specific diagnoses, because syndromic surveillance is capable of detecting responses of animals and people to environmental hazards and infectious agents sooner than would otherwise be possible using disease diagnoses based on the results of specific laboratory tests.7 The following syndromes were evaluated in dogs and cats separately because they have previously been associated with exposure to propyl mercaptan in people:2 eye inflammation (allergic inflammation of the conjunctiva); gastrointestinal (GI) (diarrhea, vomiting, or excess gas); respiratory (coughing or trouble breathing); fever (elevated body temperature); and general weakness/change in mental state (fainting, ataxia, depressed mentation, fits, or seizures). During 2006, the most common syndrome observed was GI (11.0% dogs, 11.2% cats) (Table 1).
Table 1.
Number and percent of dog and cat visits by clinical syndrome at Banfield, The Pet Hospital® veterinary hospitals within a 20-mile radius of Fairburn, GA, from January 1 to December 28, 2006
Syndromes were based on exam findings and not diagnoses.
Eye inflammation: allergic inflammation of the conjunctiva
Gastrointestinal: diarrhea, vomiting, or excess gas
Respiratory: coughing or trouble breathing
Fever: elevated body temperature
General weakness: fainting, ataxia, depressed mentation, fits, or seizures
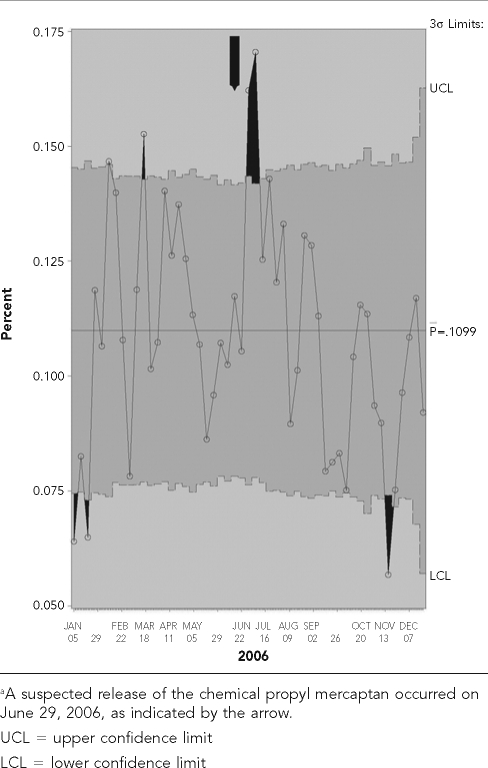
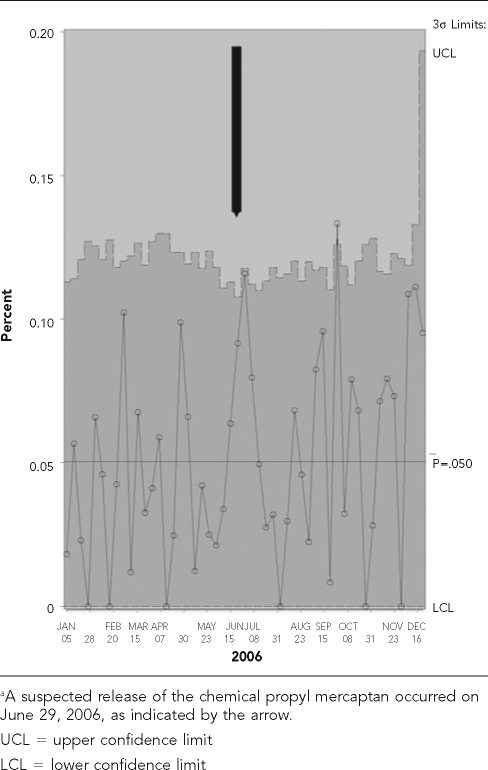
Shewhart control charts8 in SAS were initially created to evaluate the temporal variability in frequency of each syndrome by week in 2006 and to determine at what point the frequency of these events exceeded a prespecified upper limit. The hypothesis tested is that the frequency of events exceeded the upper limit during the four-week period following the chemical release. Only GI syndrome in dogs and respiratory syndrome in cats (Figures 2 and 2b) fulfilled this requirement. However, the upper limit for the frequency of canine GI syndrome was also surpassed earlier in the year (February through March), indicating a lack of specificity of syndromic events associated with a chemical release on or about June 29.
Figure 2a.
Shewhart control charts for dogs with gastrointestinal syndrome visiting a Banfield, The Pet Hospital® veterinary hospital within a 20-mile radius of Fairburn, GA, in 2006a
Figure 2b.
Shewhart control charts for cats with respiratory syndrome visiting a Banfield, The Pet Hospital® veterinary hospital within a 20-mile radius of Fairburn, GA, in 2006a
The Fairburn area was divided into one-mile-square grids, and the frequency of syndromic events in dogs and cats was calculated for each grid at two-week intervals from June 6 through July 6. The goal was to determine if any specific syndrome increased in frequency following the suspected chemical release on June 29 and if it did, its geographic relationship to the PSC Recovery Systems waste-processing facility. None of the syndromes evaluated suggested an increase in frequency associated with the chemical exposure on June 29. NCASP thought about constructing a plume analysis to relate the suspected directional dispersion of propyl mercaptan into the community, based on its physical properties and weather conditions at the time, to the occurrence of syndromic events in dogs and cats. However, it was determined that insufficient information was available at the time to create an accurate plume analysis.
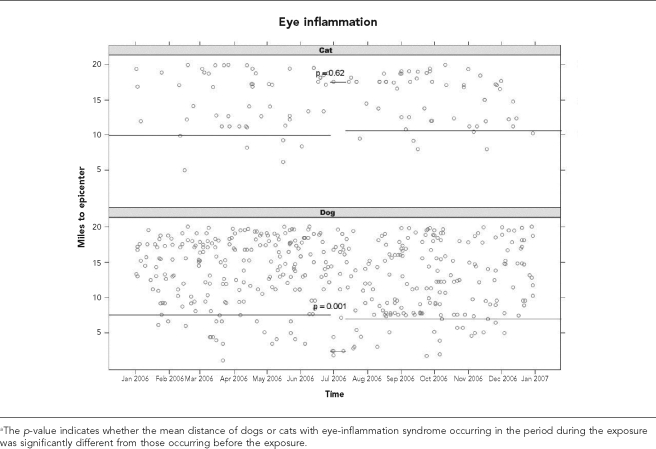
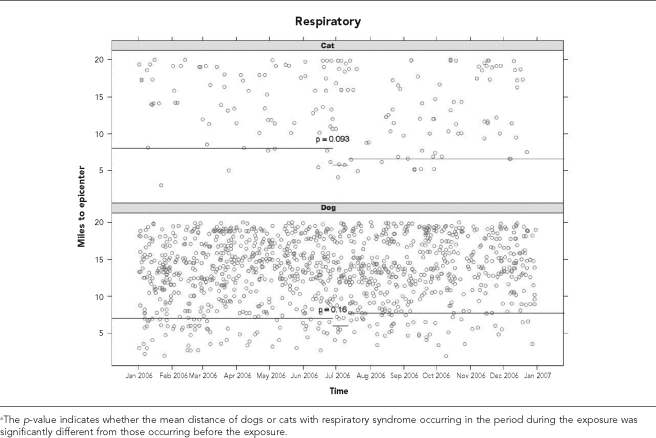
To evaluate a causal relationship between a point-source chemical release and the occurrence of syndromic events, each event was graphed as a function of time of onset (x-axis) and the number of miles a dog or cat with that event was located from the PSC Recovery Systems waste-handling facility (the epicenter) (Figures 3a and 3b). A block bootstrap method9 was used to determine if there was a statistically significant difference in the mean distance between each two-week period and the preceding three-month period for a clinical syndrome in dogs or cats. This was done by evaluating quantiles of distance to the epicenter. That is, the value of q such that during the two-week period of interest, 25% of cases were closer than q miles to the epicenter. Then q was resampled for 999 bootstrap replicates, with replacement from the null distribution of q. For example, if the two-week period before the chemical release was considered indicative of null behavior, then repeated 14-day windows were drawn from that period, with each yielding one value of q. The p-value is the fraction of time that these null q values were more extreme (less than) the observed value of q during the window immediately after the chemical release.
Figure 3a.
Distance from the epicenter for dogs and cats with eye-inflammation syndrome occurring before, during, or after a suspected release of propyl mercaptan on June 29, 2006a
Figure 3b.
Distance from the epicenter for dogs and cats with respiratory syndrome occurring before, during, or after a suspected release of propyl mercaptan on June 29, 2006a
During the two-week period following the suspected exposure, there was a significant decrease in the location of occurrence of eye inflammation in dogs (p=0.0001), while for cats the mean distance to the epicenter increased (p=0.62). For respiratory syndromic events in dogs and cats, there was a nonsignificant decrease in distance to the epicenter (p=0.16 for dogs and p=0.09 for cats).
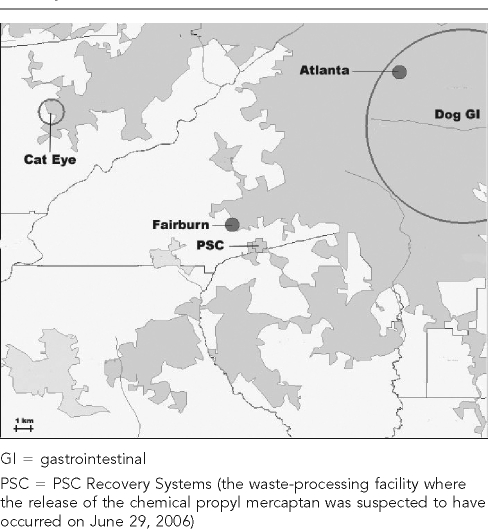
A space-time analysis was performed using the SatScan statistical method proposed by Kulldorff10 to determine if any significant clusters of syndromic events occurred following the suspected chemical release from the PSC Recovery Systems waste-handling facility. This analysis assumed a Bernoulli model and evaluated data one month at a time during 2006, with a one-month sliding window starting on April 1. Three syndromes were considered, including respiratory, eye inflammation, and GI in dogs and cats. One significant cluster of eye-inflammation syndrome was identified in cats, and one significant cluster of GI syndrome was identified in dogs after the propyl mercaptan release on June 29 (Figure 4). The eye-inflammation syndrome cluster in cats occurred from July 21 to August 12 in a population of 16 cats and covered an area of 1.67 km2, with 7.0 cases observed vs. 0.47 expected (p=0.031). The significant cluster of GI syndrome in dogs occurred from July 4 to July 11 in a population of 285 dogs and covered an area of 12.9 km2, with 52 cases observed vs. 23 expected (p=0.038).
Figure 4.
Space-time (SatScan) analysis showing a significant occurrence of a cluster of eye-inflammation syndrome in cats and GI syndrome in dogs that visited a Banfield, The Pet Hospital® veterinary hospital within a 20-mile radius of Fairburn, GA, following a suspected release of the chemical propyl mercaptan on June 29, 2006
Finally, an autoregressive integrated moving average (ARIMA) model was developed11 to evaluate the occurrence of GI syndrome in dogs within 20 miles of Fairburn, the hypothetical release point for propyl mercaptan on June 29, 2006. An exponential weighted moving average (EWMA) was calculated based on the day of the week and the chemical release time as follows:
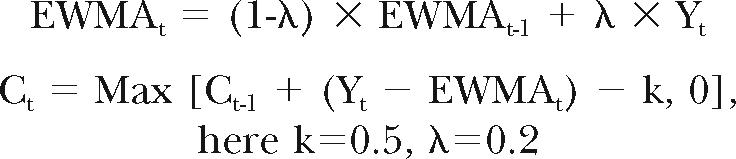 |
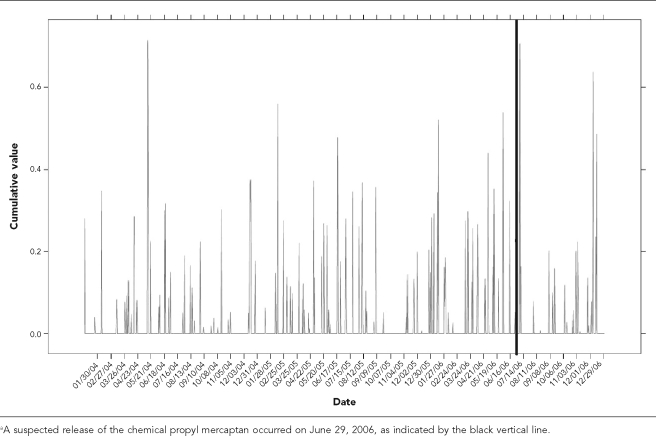
A control chart was developed indicating the relationship between the chemical release date and the cumulative values for EWMA for GI syndrome in dogs (Figure 5). The thick, black vertical line indicates the hypothetical chemical release date that was followed immediately by a spike in GI syndrome (Figure 1a).
Figure 5.
Occurrence of gastrointestinal syndrome in dogs visiting a Banfield, The Pet Hospital® veterinary hospital within a 20-mile radius of Fairburn, GA, in 2006a
A more suitable time-series model can be specified as follows:
T is the total number of days; εt follows an ARIMA process; WeekDayi represents the day of the week effect for day t (i+1, 2. .. 7); Release + 1 for days between June 29 and July 12 and Release + 0 for any other days.
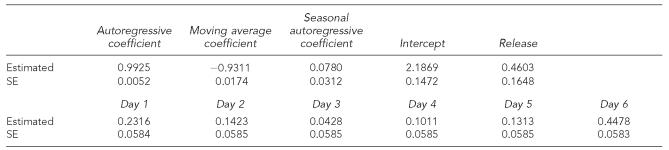
The null hypothesis tested was that the effect of “Release” is 0, which is equivalent to the statement that the number of dogs with GI syndrome did not significantly increase during the time period of June 29 through July 12. The model with the smallest Akaike's information criterion (AIC) is ARIMA(1,0,1)(1,0,0) (autoregressive moving average process with order 1 and 1, seasonal autoregressive order 1 as weeks being the seasons). The parameter estimates are shown in Table 2. Because the parameter estimate for “Release” was 0.4603 with standard error 0.1648 and p<0.05, the null hypothesis was rejected. It was concluded, therefore, that an increase in the frequency of GI syndrome in dogs occurred following the chemical release on June 29.
Table 2.
Results of an ARIMA model for the occurrence of gastrointestinal syndrome in dogs in 2006 within a 20-mile radius of Fairburn, GA
ARIMA = autoregressive integrated moving average
SE = standard error
By comparison, in people who were exposed in a manner similar to the pets, nausea and vomiting were reported to occur in approximately 50% of the people and other GI symptoms in almost 7%. This information was based on the community survey of 622 people conducted by the Georgia DPH.
DISCUSSION
Given the threat of bioterrorism following the events of 9/11 and the increasing availability of electronic databases for surveillance, monitoring systems for the early detection of illnesses and syndromes potentially related to bioterrorism have proliferated.12 While surveillance efforts have largely focused on bioterrorism-related events, the technology being developed is applicable to other areas of public health and clinical practice, such as detection of emerging infections and antibiotic-resistant microbes, drug and vaccine safety, evidence-based medicine, and epidemiologic research. NCASP was initially developed with funding from CDC with the goal of monitoring the medical records of companion animals to detect increases in the frequency of syndromic events related to exposure to biothreat agents or emerging infections that have zoonotic potential.3 However, NCASP can also be used to evaluate whether the release of a chemical into a community or other types of environmental insults such as fires or floods are associated with adverse health consequences for exposed individuals.
The release of propyl mercaptan in Fairburn prompted an investigation by the Georgia DPH into concerns about community exposure to industrial chemicals. Systematic surveys were conducted in response to community concerns about an onion-like odor and its possible relation to chemical exposures. The results of these surveys indicated that several hundred people in Fulton and Fayette counties in Georgia had noted a foul odor and/or symptoms consistent with irritation of eyes, mucous membranes, and the upper respiratory tract. These symptoms were nonspecific and did not implicate a specific source within the community or a specific exposure. In general, the findings were similar to those that occurred in 1989 in California in people exposed to propyl mercaptan following pesticide treatment of a nearby potato field.2 In that incident, a significant increase in health effects was found among individuals who reported smelling a strong onion-like odor characteristic of propyl mercaptan. The most prominent signs reported were headache, diarrhea, sore throat, burning/itching eyes, fever, and asthma attacks. Both the reported health events in Fairburn and in California following exposure of people to propyl mercaptan were consistent with statements on the Material Safety Data Sheet for n-propyl mercaptan that warned of eye irritation and skin irritation following exposure, as well as a repulsive odor.13
When surveyed, a relatively small number of people living in the vicinity of the chemical release in Fairburn indicated observing dogs and cats with clinical signs possibly related to exposure to propyl mercaptan. A newspaper article reported the occurrence of “illness and death in pet dogs and cats between late June and mid-August that lived within a two-mile radius of the PSC plant.”14 These dogs and cats were observed to have diarrhea, vomiting, scratching, hair loss, weight loss, gagging, and coughing, and they refused to go outside. In no case, however, did a veterinarian make a definitive diagnosis as to the cause of the illness. A concern was raised at the time, however, about whether the noticeable odor produced by propyl mercaptan would prevent an objective assessment of the clinical signs observed in people or animals. That is, were people who were aware of the presence of a potentially toxic substance more likely to report clinical signs in either themselves or their pets than they would have had they been unaware of an unusual odor? It was considered that the presence of routinely collected medical data in electronic format for pets might overcome some of the potential bias caused by the presence of a detectable odor in the community and could be used, therefore, to assess whether a significant increase in the frequency of clinical signs had occurred in dogs and cats following the chemical exposure when compared with the pre-exposure period.
A CDC working group has established a framework for evaluating public health surveillance systems for detection of disease outbreaks.15 One public health function of surveillance is outbreak detection—that is, identifying an increase in frequency of disease above background occurrence of disease. Many new surveillance systems focus on syndrome events. That is, they use data that are not diagnostic of a disease, but might indicate the early stages of an outbreak. Chief patient complaints from human hospital emergency departments are often used for this purpose. However, no such human hospital system was in place in Fairburn at the time of the suspected propyl mercaptan release in June 2006. In contrast, medical data from dogs and cats were available, but NCASP was not being used at the time to conduct prospective surveillance for outbreaks of disease. A decision was made to try to use NCASP data retrospectively to look for a change in syndrome frequency that might be associated with the release of propyl mercaptan.
No systematic methods had been evaluated previously for using NCASP to detect temporal or spatial changes in syndromic occurrence in dogs or cats. Therefore, a variety of methods were applied, including control charts, density maps, change in average mean distance from a suspected point source of chemical release, space-time statistics, and ARIMAs. As expected, different methods pointed to different clinical syndromes being associated with the time of propyl mercaptan release in Fairburn. In general, NCASP found an indication of change over time or in space of respiratory syndrome in cats, GI syndrome in dogs, and eye inflammation syndrome in both cats and dogs. These changes were consistent with the clinical signs observed in people during a previous propyl mercaptan release in California and with the 2006 propyl mercaptan release in Fairburn. It is clear from the 2006 study using NCASP that additional studies are needed to determine what methods are reproducible and might reliably provide the earliest warning of a chemical release in a community.
This study showed no conclusive or consistent evidence of adverse health effects in dogs and cats living in Fairburn following release of the chemical propyl mercaptan. These findings were similar to those reported by the Georgia DPH for humans living in Fairburn, based on the previously described convenience sample and Web-based survey.
Limitations
While the human survey was not population based, our study in pets also had several limitations. First, we did not know the physical activity and home range for each of the pets. Therefore, we could not determine actual exposures for individual pets. We used where each pet resided as a surrogate for exposure. Second, regarding exposure, we did not have sufficient data to develop a plume model to determine probable dispersion patterns for propyl mercaptan in the study area. Third, we were unable to link a pet's clinical signs with that of its owners living in the same home. This would be important in evaluation of whether pets are good sentinels for environmental hazards.
CONCLUSIONS
Use of animal data to evaluate environmental hazards should not be considered as an alternative to hospital- or emergency department-based syndromic surveillance of humans. When human syndromic surveillance data are not available, as was the case in Fairburn, animal data can be particularly useful. Given that Banfield, The Pet Hospital currently has more than 640 veterinary hospitals located in 44 states, sees about 80,000 dogs and cats weekly, is growing at a rate of about two new hospitals each week, and maintains a centralized and standardized medical-records database, NCASP could be used either prospectively to detect a chemical hazard before it manifests itself in people or retrospectively to evaluate the impact of a suspected chemical release. Not only could clinical data be utilized for syndrome monitoring, but routinely collected laboratory data—such as blood urea nitrogen, creatinine, hematological data, and liver enzymes—could also be used to assess specific organ function and toxicity. Longer-term impacts of chemicals could also be evaluated by monitoring rates of chronic diseases such as cancer and autoimmune conditions.
In 1991, an expert committee on Animals as Monitors of Environmental Health Hazards recommended that “when reporting systems are established in a defined geographic area, appropriate efforts should be made to compare the frequency and pattern of these diseases with those of corresponding diseases in humans, and it should be determined whether animals can provide early warning of health hazards to humans.”1 This recommendation still seems appropriate today.
REFERENCES
- 1.National Research Council, Commission on Life Sciences, Board on Environmental Studies and Toxicology, Committee on Animals as Monitors of Environmental Health Hazards. Animals as sentinels of environmental health hazards. Washington: National Academy Press; 1991. [Google Scholar]
- 2.Ames RG, Stratton JW. Acute health effects from community exposure to n-propyl mercaptan from an ethoprop (Mocap)-treated potato field in Siskiyou County, California. Arch Environ Health. 1991;46:213–7. doi: 10.1080/00039896.1991.9937451. [DOI] [PubMed] [Google Scholar]
- 3.Glickman LT, Moore GE, Glickman NW, Caldanaro RJ, Aucoin D, Lewis HB. Purdue University-Banfield national companion animal surveillance program for emerging and zoonotic diseases. Vector Borne Zoonotic Dis. 2006;6:14–23. doi: 10.1089/vbz.2006.6.14. [DOI] [PubMed] [Google Scholar]
- 4.SAS Institute Inc. SAS/ACCESS¯ interface to Oracle. Cary (NC): SAS Institute Inc.; 2007. [Google Scholar]
- 5.SAS Institute Inc. SAS¯ 9.1.3. Cary (NC): SAS Institute Inc.; 2007. [Google Scholar]
- 6.Maciejewski R, Tyner B, Jang Y, Zheng C, Nehme R, Ebert DS, et al. LAHVA: linked animal-human health visual analytics. the IEEE Symposium on Visual Analytics Science and Technology; 2007 Oct 30; Sacramento, California. Paper presented at. [Google Scholar]
- 7.Mandl KD, Overhage JM, Wagner MM, Lober WB, Sebastiani P, Mostashari F, et al. Implementing syndromic surveillance: a practical guide informed by the early experience. J Am Med Inform Assn. 2004;11:141–50. doi: 10.1197/jamia.M1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SAS Institute Inc. SAS/QC® 9.1 user's guide, the SHEWHART procedure. Cary (NC): SAS Institute Inc.; 2004. [cited 2007 May 22]. Also available from: URL: http://support.sas.com/onlinedoc/913/getDoc/en/qcug.hlp/shw_overview_index.htm. [Google Scholar]
- 9.Buhlmann P. Bootstraps for time series. Statistical Science. 2002;17:52–72. [Google Scholar]
- 10.Kulldorff M. A spatial scan statistic. Commun Stat A-Theor. 1997;26:1481–96. [Google Scholar]
- 11.SAS Institute Inc. SAS/ETS¯ 9.1 user's guide, the ARIMA procedure. Cary (NC): SAS Institute Inc.; pp. 2004pp. p. 363–486. [Google Scholar]
- 12.Bravata DM, McDonald KM, Smith WM, Rydzak C, Szeto H, Buckeridge DL, et al. Systematic review: surveillance systems for early detection of bioterrorism-related diseases. Ann Intern Med. 2004;140:910–22. doi: 10.7326/0003-4819-140-11-200406010-00013. [DOI] [PubMed] [Google Scholar]
- 13.Chevron Phillips Chemical Company LP. Material Safety Data Sheet: n-propyl mercaptan MSDS: 74260. 2006. Dec 28, [cited 2007 May 22]. pp. 1–8. p. Available from: URL: http://www.cpchem.com/enu/msds_unsecured/Import_74260_MSDS_O_ENGLISH_A _ENGLISH_A_N.pdf.
- 14.Nelms B. North Fayette's own “silent summer”? The Citizen 2006 Aug 29. [cited 2007 May 22]. Available from: URL: http://www.thecitizen.com/node/9795.
- 15.Buehler JW, Hopkins RS, Overhage JM, Sosin DM, Tong V. Framework for evaluating public health surveillance systems for early detection of outbreaks: recommendations from the CDC working group. MMWR Recomm Rep. 2004;53(RR-5):1–11. [PubMed] [Google Scholar]