SYNOPSIS
Objective
This study measured the relationship between lesions suggestive of subclinical pig illness at harvest to carcass contamination and human foodborne risk.
Methods
Over the course of eight visits (December 2005 to January 2006), we swabbed 280 randomly selected carcasses, during normal slaughter operations, at three points in the slaughter line: skin pre-scald; the bung or pelvic cavity following removal of the distal colon and rectum; and pleural cavity, immediately before the final carcass rinse. Each swab sponge was used on five carcasses in bung and pleural cavity sampling. Swab sponges were cultured quantitatively for Campylobacter spp., Enterococcus spp., and Enterobacteriaceae spp., and qualitatively for Salmonella spp. Data on health indicators were collected for all pigs in the study (2,625 pigs) by experienced plant quality assurance personnel.
Results
Campylobacter spp. were recovered from the pleural cavity in 58.9% (33/56) of pools (five carcasses/pool), and in 44.6% (25/56) of pools from the bung cavity. Enterococcus spp. were recovered from 66.1% (37/56) and 35.7% (20/56) of pleural and bung pools, respectively. The most common lesion identified was the peel-out (pleuritis or adhesions), with a total of 7.1% (186/2,625 total head). Linear regression showed that for every percentage point increase in peel-outs, Enterococcus spp. contamination increased by 4.4% and Campylobacter spp. increased by 5.1% (p<0.05).
Conclusion
This study showed a correlation between animal health and human health risk, as measured by carcass contamination. Therefore, animal management decisions on-farm, such as housing, antibiotic use, environment, and level of veterinary care, may directly impact public health.
Since the passage of the Food and Drugs Act in 1906, the public has agreed that the consumption of healthy food animals is in the best interest of public health.1 Today, public health risks not detected by traditional visual inspection procedures in the packing plant include chemical or antibiotic residues and foodborne pathogens. The “one medicine” concept, or the close association of the medical and veterinary professions for the health of all species,2 is often discussed; however, it is unclear how veterinary care specifically impacts public health risks. Accurate quantification of this risk-risk relationship between animal health and public health is essential for effective policy-making.
The risk-risk approach has been applied to some public health issues in the U.S., such as the Environmental Protection Agency's assessment of cancer risk from chlorinated water relative to bacterial risk from nonchlorinated water.3,4 To conduct these types of analyses in the food safety arena, the link between animal and human health needs to be measured. Antemortem inspection usually removes clinically ill animals from the human food supply. However, subclinically ill animals appearing healthy at slaughter may have internal lesions affecting processing. These lesions may impact the evisceration process leading to cross-contamination and increased pathogen load. Enterococcus is an indicator of fecal contamination on the carcass. Campylobacter spp. and Salmonella spp. are common human foodborne pathogens, and thus indicate the level of public health risk. Additionally, these lesions often indicate chronic or resolved illnesses in the growing or finishing pig.5 The objective of this study was to quantify the relationship between lesions detected at slaughter and bacterial carcass contamination.
METHODS
For bacteriological evaluation, we selected carcasses during normal slaughter operations in a modern, vertically integrated pork production company. We swabbed three locations on the carcasses at three different points in the slaughter line: (1) the skin immediately before scald; (2) the bung (pelvic) cavity immediately after removal of the bung (distal colon and rectum) but before evisceration; (3) and the pleural cavity following complete evisceration and immediately prior to final carcass rinse. Because carcasses were not identified at the point of sample collection, it is likely that we did not obtain samples from all three areas on the same carcass; i.e., we took only one sample from one of the three sites per carcass. We obtained data on eight different visits during December 2005 and January 2006, with an average of 328 pigs per group.
In this operation, pigs are marketed from a single barn on two or three separate occasions. The first pigs marketed are those first achieving acceptable weight. We sampled these first pull pigs—the fastest-growing and likely the healthiest—first thing in the morning to minimize cross-contamination among workers, carcasses, and machinery. On Monday, we collected samples from conventional (CON) pigs, and on Tuesday from antibiotic-free (ABF) pigs. CON-raised pigs received antibiotics for disease prevention or treatment, or performance enhancement from late nursery until near slaughter. ABF pigs were raised without performance-enhancing/preventive or treatment antibiotics. Clinically ill animals requiring treatment were moved to separate pens in the same building and were sent to market separate from their ABF cohorts. Management of the two groups, excepting antibiotic treatment, was similar in veterinary care, housing, environment, and feed. Microbiological comparison between ABF and CON pigs was a secondary objective in this study, and is not reported here.
Health indicators
Experienced plant quality assurance (QA) personnel reported cases of the following health indicators per group (a group represented all pigs processed from one barn on that day):
Abscessed heads: condemned heads due to visible abscesses.
Peel-outs (pleuritis or adhesions): the pleural lining is removed after evisceration due to adhesions between the pleura and portions of the lung. Portions of the lung were usually attached.
Fatigued: animals exhibit signs of anaerobic metabolism, including respiratory distress (open-mouth breathing), reluctance to move, or recumbency.
Bacteriology
Specimen collectors wore latex gloves and changed gloves between each sampling. We swabbed the carcasses with Whirl-Pak Speci-Sponges (Nasco, Ft. Atkinson, Wisconsin) swabs hydrated with 10m sterile 0.1% peptone water. For the pre-scald samples, one sponge was used for each carcass. However, for the bung (pelvic) and pleural cavity sampling, one sponge was used for five carcasses—a pooling method commonly used in Denmark to allow for the increased efficiency needed to detect the expected low number of Salmonella spp. positive carcasses.6 The interior surface of the pelvic, abdominal, and thoracic cavity was swabbed, covering as much area as possible in the short time available, as carcasses were still moving down the line. Sponges were maintained on ice during transport to the laboratory. We minimized differences in storage times between the two groups by immediately transporting samples to the laboratory on Monday and Tuesday afternoons.
In the laboratory, we enriched sponges with 15mL sterile 0.1% peptone water, stomached the sponges (Seward Stomacher 3500, Worthing, West Sussex, UK) for one minute, serially diluted the samples in 0.1% peptone water, and then plated on selective agars for Campylobacter spp., Enterobacteriaceae spp., and Enterococcus spp.
We complied with Nannapaneni7 in Campylobacter agar (CA) preparation by combining Campylobacter enrichment broth (Bolton formula, LAB 135; J&K Microbiologics, Inc., North Ridgeville, Ohio) with 1.5% agar (Becton Dickinson Microbiology Systems, Sparks, Maryland), mixing Campylobacter selective antibiotic supplement (X132, J&K Microbiologics, Inc.) and 5% lysed defibrinated horse blood (MT 59074; Quad Five, Ryegate, Montana) thoroughly before pouring CA plates. We aseptically spread 100μL of diluted samples onto CA plates in duplicate. The plates were incubated in a Forma Tri-gas incubator (5% O2, 10% CO2, 85% N2) at 42°C and evaluated at 24 and 48 hours.
To isolate Enterococcus spp., we aseptically spread 100μL of diluted sample onto Enterococcosel® agar (Difco, Becton Dickinson Microbiology Systems) in duplicate and incubated for 24 hours at 35°C. Similarly, we isolated Enterobacteriaceae spp. by aseptically spreading 100μL of diluted sample onto violet red bile agar with glucose (VRBG, Difco, Becton Dickinson Microbiology Systems) in duplicate (24 hours at 37°C). For Salmonella spp., we enriched the remaining buffer and sponge swab in buffered peptone water, and screened these with an antigen-capture enzyme-linked immunosorbent (Reveal Salmonella; Neogen, Lansing, Michigan) assay to determine presumptive Salmonella spp. positives. Presumptive positive samples were enriched in tetrathionate broth (24 hours at 37°C) and streaked for isolation on XLD agar (24 hours at 37°C). Colonies exhibiting typical salmonella morphology and phenotypic reactions were transferred to Triple Sugar Iron agar (TSI) and Lysine Iron agar (LIA) slants. Colonies showing typical salmonella-like reactions on TSI and LIA were confirmed using the BBL Crystal Enteric/Nonfermenter ID kit, following the manufacturer's instructions. Salmonella spp. colonies were serotyped at the U.S. Department of Agriculture (USDA)–Animal and Plant Health Inspection Service (APHIS) National Veterinary Services Laboratory.
Statistical analysis
We compared the means of the variables between the CON and ABF groups with t-tests and the Wilcoxon rank sum test. We did not use the nonparametric Chi-square test as it requires combining all the CON samples into a single group, and there was a substantial variance between the observations by replicate. We evaluated the association between health (percent lesions) and contamination (percent of pools positive) with linear regression using bivariate linear regression between all possible pairs of contamination and health. Contamination was the response variable. For the regression, the health and the contamination variables that were percentages (range=0–100) were treated as unbounded continuous variables. Because the health and the contamination variable values in the CON group differed substantially from those in the ABF group, we used multivariate regression by forcing the antibiotic use group (ABF or CON) indicator variable into the model. We also utilized multivariate linear regression to determine whether the quantity of bacteria (log of the most probable number) was associated with animal health indicators.
RESULTS
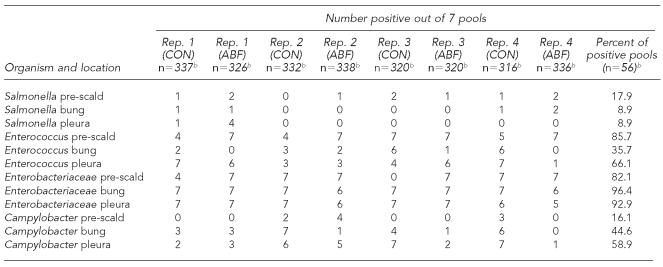
On each visit, 35 carcasses were swabbed at each location (bung [pelvic] cavity, pleural cavity, skin pre-scald). One sponge was used for five carcasses, resulting in seven pools of carcasses swabbed per replicate, except for pre-scald, where only one sponge was used. A total of 280 carcasses over eight replicates were swabbed, resulting in 56 pools for culture. Table 1 shows the results of bacterial contamination by location on the carcass. In Table 1, n represents the number of carcasses available for swabbing in a particular replicate and the number observed for health indicators (Table 2).
Table 1.
Bacterial contamination of swine carcasses at slaughter collected from three different points in the slaughter process: skin pre-scald, pelvic (bung), and pleural cavitiesa
CON animals received antibiotics for growth promotion and disease prevention and treatment; ABF animals never received antibiotics.
n=number of carcasses available for testing in each Rep.
CON = conventionally raised
ABF = antibiotic-free
Rep. = replicate
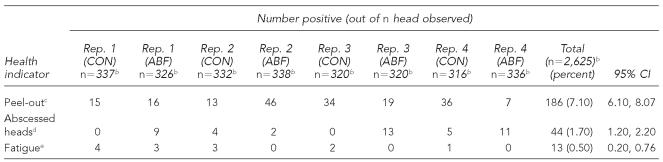
Table 2.
Health indicators for swine at harvesta
CON animals received antibiotics for growth promotion and disease prevention and treatment; ABF animals never received antibiotics.
n=number of carcasses available for testing in each Rep.
Pleuritis or pleural adhesions usually requiring removal of the cavity lining
Heads condemned due to visible abscess(es)
Anaerobic metabolism, respiratory distress, recumbency
CON = conventionally raised
ABF = antibiotic-free
Rep. = replicate
CI = confidence interval
Enterobacteriaceae spp. was recovered in a high percentage of the pools, ranging from a mean of 82.1% pre-scald (46/56 pools) to 96.4% (54/56 pools) of bung swabs. Salmonella spp. was recovered in a relatively low percentage of pools, ranging from a mean of 8.9% in both the bung and pleural cavities (5/56 pools) to 17.9% (10/56 pools) pre-scald. Because the level of bacteria on the skin pre-scald is less a measure of food safety and more an indicator of how dirty the pigs are at slaughter, we excluded this pre-scald measure from further analyses.
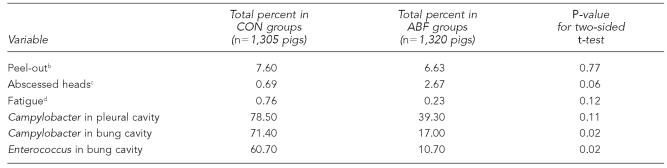
One experienced plant QA person observed all carcasses (2,625 head) for health-related lesions. Results are shown in Table 2. The most common lesion identified was the peel-out, with a total of 7.1% (186/2,625 total head). Abscessed heads were found in 1.7% (44/2,625) of carcasses. Antemortem signs of fatigue were recorded in 0.5% (13/2,625) of observed animals. Similar to contamination rates, variations in health status existed between replicates. Table 3 shows a comparison of health and contamination between antibiotic use groups. There was no statistically significant (p<0.05) difference in health indicators between groups. While ABF pigs had more abscessed heads than CON pigs (2.7% compared with 0.7%, p=0.06), bacterial contamination was consistently higher in the CON pigs. For only this reason, the antibiotic use group was included as a covariate in the regression models measuring the relationship between health and contamination.
Table 3.
Comparison of the key health indicators and contamination rates between ABF and CON pigsa
CON pigs received antibiotics for growth promotion and disease prevention and treatment; ABF pigs never received antibiotics.
Pleuritis and pleural adhesions
Heads condemned due to visible abscess(es)
Anaerobic metabolism, respiratory distress, recumbency
ABF = antibiotic-free
CON = conventionally raised
Rep. = replicate
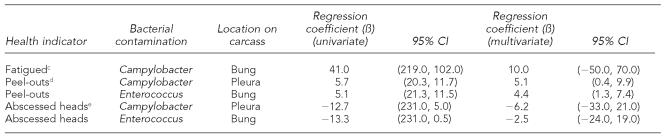
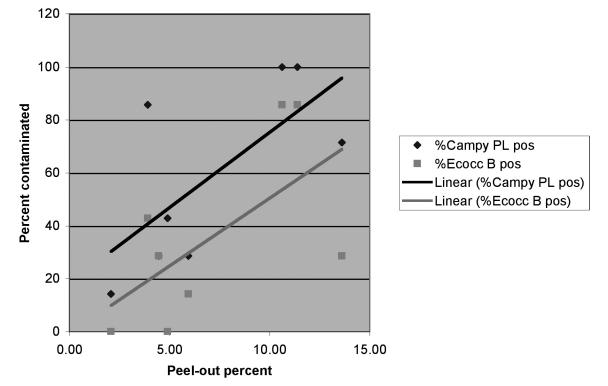
Table 4 shows the results for regression analysis between health indicators and percent of carcasses contaminated. Although some confidence intervals (CIs) are estimated as less than zero, all of the predicted values were within the interval (range=0–100). These results indicate that as the percentage of subclinically ill animals (evidenced by peel-outs [pleuritis or adhesions] and fatigue) increases, so does the percentage of carcasses with bacterial contamination. We found the strongest positive relationships between the percentage of peel-outs and Campylobacter spp. contamination in the pleural cavity (β=5.7) and between peel-out percent and Enterococcus spp. contamination in the bung (pelvic) cavity (β=5.1). In the multivariate model (antibiotic use as a covariate), these relationships were statistically significant (p<0.05), as shown by the 95% CIs that do not include zero. We also identified another positive relationship between the percentage of fatigued pigs and Campylobacter spp. contamination in the bung cavity. The Figure graphically shows the relationship between health and contamination: as the percentage of peel-outs in a group increases by 1 percentage point, the pleural Campylobacter spp. contamination increases 5 percentage points, and Enterococcus spp. contamination in the bung increases 4.4 percentage points.
Table 4.
Regression coefficients (univariate and multivariate with antibiotic use group as a covariate) between percentage of representative health variables and percentage of carcasses positive for Enterococcus spp. or Campylobacter spp. in the bung or pleural cavitiesa,b
All health and contamination relationships were tested. Only those with p<0.2 in the univariate comparison are shown.
Conventionally raised animals received antibiotics for growth promotion and disease prevention and treatment; antibiotic-free animals never received antibiotics.
Anaerobic metabolism, respiratory distress, recumbency
Pleuritis and pleural adhesions
Heads condemned due to visible abscess(es)
CI = confidence interval
Figure.
Relationship (multivariate linear regression) between percentage of peel-outs and carcass contamination with Campylobacter spp. in the pleura (%Campy PL) and Enterococcus spp. in the bung cavity (%Ecocc B) in pig carcasses cultured during processing
We identified negative relationships between bacterial contamination and the percentage of abscessed heads in the group. In the multivariate regression, as the percentage of abscessed heads increased, Campylobacter spp. contamination in the pleura decreased (β=−6.2), as did Enterococcus spp. in the bung (β=−2.5). Neither of these negative correlations was statistically significant in this dataset.
Additionally, Table 5 shows statistically significant relationships between the percentage of peel-outs in the group and the quantity (log of the mean colony forming units, log10CFU) of Enterococcus spp. present in the bung cavity (β=0.38). A positive correlation was also identified between the quantity of Campylobacter spp. in the pleural cavity and the percentage of peel-outs.
Table 5.
Regression coefficients of the quantity (log10CFU/mL) of Campylobacter spp. and Enterococcus spp. present in the pleura and bung, and the percentage of peel-outs in slaughter pigsa
CON animals received antibiotics for growth promotion and disease prevention and treatment. ABF animals never received antibiotics.
CFU = colony-forming units
CON = conventionally raised animals
ABF = antibiotic-free animals
Campy = Campylobacter spp.
Ecocci = Enterococcus spp.
CI = confidence interval
DISCUSSION
This study shows a correlation between lesions suggestive of subclinical animal illness and human health risk, as measured by carcass contamination (Campylobacter spp., Enterococcus spp.).5 The rationale can be understood from the impact of animal health on the evisceration process: antemortem inspection processes typically remove clinically ill animals. However, if disease is not outwardly evident or has resolved, leaving old lesions, these ill animals may go undetected until evisceration. In previous studies, 50% of abnormalities went undetected by European Commission inspection methods.8 Insufficient or imprecise evisceration was shown to impact the probability of carcass contamination and microbial load more than other processes in slaughter. For example, Enterobacteriaceae spp. contamination increased from 4% to 40% during processing from polishing (beginning) to post-evisceration (end).8 Dressing, splitting, and meat inspection can account for 85% to 90% of carcass contamination.9,10 Therefore, lesions affecting evisceration quality will affect public health risk by increasing carcass handling and cross-contamination. Health conditions that affect evisceration quality include peritonitis, pleuritis, adhesions, volume and fluidity of gut contents, gut friability, and any health condition that leads to additional handling (skin lesions, bruises, abscesses, arthritis).
Health indicators
One experienced plant QA person recorded the health indicators used in this study, which reflect organoleptic (assessment by sight and touch) inspection issues that must be addressed in the slaughter process. The definition of a peel-out, or fatigued pig, is specific from a meat-inspection perspective, but is not a specific veterinary pathological diagnosis. The precise pathological meaning of the indicators needs to be defined. The 13 fatigued pigs in this study exhibited signs of breathlessness and difficulty in moving. After additional rest in the pens, they were ambulatory, and passed USDA Food Safety and Inspection Service (USDA:FSIS) veterinary inspection for normal slaughter. Future research should better define the specific pathological diagnosis of these health indicators. Research has shown that pathology in the thorax, such as pleuritis and adhesions, is likely due to respiratory disease earlier in life.5 Carcasses requiring a peel-out typically have portions of the lung attached to the pleura. Although fatigue and peel-outs are indirect measures of pig health, both of these variables were correlated with an increased amount of bacterial carcass contamination, and thus warrant further exploration.
The objective of this study was not to compare ABF and CON pigs. These groups were studied as part of an objective, reported elsewhere, to compare antibiotic resistance patterns.11 Given the data collected, comparison between these two groups is tenuous. Although ABF and CON animals were raised with similar management, feed, and housing, ABF pigs likely represent a different population than CON pigs. The ABF group included only faster-growing animals that were never treated with any antibiotic, representing the “best of the best.” Ill animals in the ABF group were removed from that group and treated, remaining in separate pens until slaughter. CON pigs may have received previous treatment for clinical illness. Consequently, in these data, comparison of health parameters between ABF and CON is to be avoided, especially without more detailed data such as morbidity/mortality rates, rate of gain, and feed efficiency. However, due to the higher contamination rates in the CON group, this variable proved to be an important confounder in our regression models.
Bacterial contamination
The locations tested for contamination (bung [pelvic] and pleural cavities) reflect two temporal extremes of the slaughter process and the carcass itself. The bung cavity was swabbed early in processing, while the pleural cavity was swabbed at the end of the slaughter process, when the carcass represents a near-final product entering the cutting and fabrication process. Higher levels of Enterococcus spp. contamination might be expected in the bung, which lies in close proximity to the anal orifice. However, Campylobacter spp. have been recovered from tonsils, stomach, ileum, cecum, colon, and lymph nodes.12 The association between pathology, such as peel-outs and Campylobacter spp. contamination, does not suggest that Campylobacter causes swine respiratory disease. Passive inhalation of scald water has been implicated in contamination of the lungs. Tearing of the lungs during evisceration may allow that passive contamination onto the pleura.13
Regression relationships
We describe relationships in these data between percent of a group of pooled samples and percent health lesions observed in a truckload of pigs. Sample pooling is an efficient means to test more carcasses, which can be used to estimate individual carcass prevalence of Salmonella spp.5 However, in this study, we compared the percent positive pools rather than estimating carcass prevalence. The statistical results reflect the relatively lower power of only seven observations (pools) per replicate. Therefore, it is noteworthy that statistically significant correlations were detected with such a small study. The health measures, such as peel-outs, are not specific veterinary pathological diagnoses; however, if there is any misclassification, it would have occurred nondifferentially, among the contaminated and noncontaminated carcasses.
The linear regression models suggest that for every percentage point increase in lesions, there is a 4 to 5 percentage point increase in contamination. The Figure and the CIs in Tables 4 and 5 show the uncertainty around these predicted increases. This uncertain information can be used in risk-risk models to estimate the impact of veterinary care on human health risk.
While it is unclear why bacterial contamination rates were so much higher in the CON group (Table 3), multivariate analysis with antibiotic use as a covariate adjusted for this observation. Therefore, the multivariate regression coefficients shown in Table 4 illustrate the relationship between health and contamination, even though contamination in CON pigs was higher.
An insignificant negative association was noted between abscessed heads and bacterial carcass contamination (Table 4). A head was condemned if there were visible abscesses (pockets of inflammatory cells in areas such as muscles or lymph nodes). Carcasses with visible head abscesses were more likely to be removed from the processing line for further inspection or condemnation. Given our study design, these carcasses would not be available for bacteriological examination at either the bung or pleural locations, producing a negative association.
While a few studies have reported minimal correlation between lesions and pathogen contamination within the lesion,14,15 these studies use a different method. They focused on detecting the pathogen in the lesion site, not on apparently healthy parts of the carcass.
Public health implications
Implications to human health found in this study build on the assumption that swine carcass contamination is a reasonable proxy for human health risk. USDA:FSIS inspection standards accept this assumption. The prevalence of Salmonella positive swine carcasses is the standard for meeting Hazard Analysis and Critical Control Point requirements.16 In Denmark, goals for swine Salmonella control are set relative to carcass contamination rates, and the same standards are used in the European Union community.17 Careful modeling of the swine slaughter process corroborated this assumption. Both Dutch and Danish scientists report that reducing carcass contamination is the most effective way to reduce public health risk.18–21 Notably, some of these studies reported that control methods after carcass rinse (e.g., fabrication and cutting) would not be effective due to additional cross-contamination.18
CONCLUSION
This study gives a preliminary indication of a risk-risk relationship between animal and human health, which has been explored by others. Russell showed that decreased poultry health at processing increases the probability of carcass contamination with Campylobacter spp. and Salmonella spp.22 Others have shown that small changes in animal health can have large impacts on the number of foodborne illness days, even when adjusting for the hypothetical increase in illness days from resistant infections.23,24 If the findings from this study can be supported by further research and repetition, then human foodborne illness risk is impacted by swine health. Therefore, animal management decisions on-farm—such as housing, antibiotic use, environment, and veterinary care—directly impact public health.
Acknowledgments
The authors thank Sasidhar Malladi for assistance in data analysis; Brenda Patton, Department of Animal Science, for data collection and bacteriology; and Elanco Animal Health, Greenfield, Indiana, for partial funding.
Footnotes
At the time of publication, Dr. Hurd was serving as Deputy Undersecretary for Food Safety at the U.S. Department of Agriculture (USDA) Food Safety and Inspection Service. The opinions expressed in this article do not necessarily reflect those of the USDA.
REFERENCES
- 1.U.S. Food and Drug Administration. Federal Food and Drugs Act of 1906 (The “Wiley Act”) [cited 2007 May 31]. Available from: URL: http://www.fda.gov/opacom/laws/wileyact.htm.
- 2.Easton G, Alder M. Editor's choice: one medicine? BMJ. 2005;331:7527. [Google Scholar]
- 3.Environmental Protection Agency (US) Drinking water criteria document for chloramines. 1994. [cited 2006 Dec 9]. Available from: URL: http://www.epa.gov/ncea/pdfs/water/chloramine/dwchloramine.pdf.
- 4.Kennedy D. Risks and risks. Science. 2005;309:2137. doi: 10.1126/science.1119787. [DOI] [PubMed] [Google Scholar]
- 5.Radostits OM. Herd health: food animal production medicine. 3rd ed. Philadelphia: W.B. Saunders Company; 2001. pp. p. 738–9. [Google Scholar]
- 6.Sørensen LL, Alban L, Nielsen B, Dahl J. The correlation between Salmonella serology and isolation of Salmonella in Danish pigs at slaughter. Vet Microbiol. 2004;100:131–41. doi: 10.1016/j.vetmic.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Nannapaneni R, Story R, Wiggins KC, Johnson MG. Concurrent quantitation of total campylobacter and total ciprofloxacin-resistant campylobacter loads in rinses from retail raw chicken carcasses from 2001 to 2003 by direct plating at 42 degrees C. Appl Environ Microbiol. 2005;71:4510–5. doi: 10.1128/AEM.71.8.4510-4515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berends BR, Snijders JM, van Logtestijn JG. Efficacy of current EC meat inspection procedures and some proposed revisions with respect to microbiological safety: a critical review. Vet Rec. 1993;133:411–5. doi: 10.1136/vr.133.17.411. [DOI] [PubMed] [Google Scholar]
- 9.Berends BR, Snijders JMA. Risk factors and control measures during slaughter and processing. Proceedings of the Second International Symposium on Epidemiology and Control of Salmonella in Pork; 1997 Aug 20–22; Cophenhagen, Denmark. [Google Scholar]
- 10.Botteldoorn N, Heyndrickx M, Rijpens N, Grijspeerdt K, Herman L. Salmonella on pig carcasses: positive pigs and cross contamination in the slaughterhouse. J Appl Microbiol. 2003;95:891–903. doi: 10.1046/j.1365-2672.2003.02042.x. [DOI] [PubMed] [Google Scholar]
- 11.Patton BS, Case WR, Kocher ME, Matthews JO, Griffith RW, Hurd HS, et al. The effect of swine production system on bacterial prevalence and antibiotic resistance. International Association for Food Protection Annual Meeting; 2006 Aug 13–16; Calgary, Alberta. [Google Scholar]
- 12.Nesbakken T, Eckner K, Høidal HK, Røtterud OJ. Occurrence of Yersinia enterocolitica and Campylobacter spp. in slaughter pigs and consequences for meat inspection, slaughtering, and dressing procedures. Int J Food Microbiol. 2003;80:231–40. doi: 10.1016/s0168-1605(02)00165-4. [DOI] [PubMed] [Google Scholar]
- 13.Borch E, Nesbakken T, Christensen H. Hazard identification in swine slaughter with respect to foodborne bacteria. Int J Food Microbiol. 1996;30:9–25. doi: 10.1016/0168-1605(96)00988-9. [DOI] [PubMed] [Google Scholar]
- 14.Pointon AM, Hamilton D, Kolega V, Hathaway S. Risk assessment of organoleptic postmortem inspection procedures for pigs. Vet Rec. 2000;146:124–31. doi: 10.1136/vr.146.5.124. [DOI] [PubMed] [Google Scholar]
- 15.Mousing J, Kyrval J, Jensen TK, Aalbæk B, Buttenschøn J, Svensmark B, et al. Meat safety consequences of implementing visual postemortem meat inspection procedures in Danish slaughter pigs. Vet Rec. 1997;140:472–7. doi: 10.1136/vr.140.18.472. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Agriculture, Food Safety and Inspection Service. Progress report on Salmonella testing of raw meat and poultry products, 1998-2001. [cited 2007 Feb 5]. Available from: URL: http://www.fsis.usda.gov/ophs/haccp/salm4year.htm.
- 17.Borck B, Hald T. Annual report on zoonoses in Denmark 2003. Ministry of Food, Agriculture and Fisheries; [Google Scholar]
- 18.Berends BR, van Knapen F, Mossel DAA, Burt SA, Snijders JMA. Salmonella spp. on pork at cutting plants and at the retail level and the influence of particular risk factors. Int J Food Microbiol. 1998;44:207–17. doi: 10.1016/s0168-1605(98)00122-6. [DOI] [PubMed] [Google Scholar]
- 19.Alban L, Stäark KD. Where should the effort be put to reduce the Salmonella prevalence in the slaughtered swine carcass effectively? Prev Vet Med. 2005;68:63–79. doi: 10.1016/j.prevetmed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen B, Dahl J, Goldbach S, Christensen H. Cost-effectiveness analysis of the Danish Salmonella control strategy. Proceedings of the 6th International Symposium on the Epidemiology and Control of Foodborne Pathogens in Pork; 2005 Sep 6–9; Rohnert Park, California. [Google Scholar]
- 21.Goldbach SG, Alban L. A cost-benefit analysis of Salmonella-control strategies in Danish pork production. Prev Vet Med. 2006;77:1–14. doi: 10.1016/j.prevetmed.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Russell SM. The effect of airsacculitis on bird weights, uniformity, fecal contamination, processing errors, and populations of Campylobacter spp. and Escherichia coli. Poult Sci. 2003;82:1326–31. doi: 10.1093/ps/82.8.1326. [DOI] [PubMed] [Google Scholar]
- 23.Cox LA, Jr., Popken DA. Quantifying potential human health impacts of animal antibiotic use: enrofloxacin and macrolides in chickens. Risk Anal. 2006;26:135–46. doi: 10.1111/j.1539-6924.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 24.Singer RS, Cox LA, Jr., Dickson JS, Hurd HS, Phillips I, Miller GY. Modeling the relationship between food animal health and human foodborne illness. Prev Vet Med. 2007;79:186–203. doi: 10.1016/j.prevetmed.2006.12.003. [DOI] [PubMed] [Google Scholar]