Abstract
CONDENSATION
Term or preterm delivery in rats can be effectively predicted using artificial neural network analysis of uterine EMG data.
Objective
To use artificial neural networks (ANN) on uterine electromyography (EMG) data to identify term and preterm labor in rats.
Study Design
Controls (G1:N=4) and preterm labor models (G2:N=4, treated with onapristone) were used. Uterine EMG and intrauterine pressure (IUP) variables were measured by implanted telemetric devices. For each time-point assessed, either a “labor-event” or “non-labor-event” was first assigned using visual and other means. 112 total labor and non-labor events were observed. ANN was then used with EMG and IUP parameters to attempt algorithmic, objective identification for time of labor in each group.
Results
For G1, 8/8 (100%) of labor events and 44/44 (100%) of non-labor events were correctly identified by the ANN. For G2, 22/24 (92%) of labor events and 31/36 (86%) of non-labor events were correctly determined by the ANN.
Conclusion
ANN can effectively predict term and preterm labor during pregnancy using uterine EMG and IUP variables.
Keywords: labor, uterus, electromyography, artificial neural networks, classification
INTRODUCTION
Preterm labor (and subsequent preterm birth) is the most common major complication during pregnancy, with 20% of all pregnant women at high risk. In the United States alone, 12.7% of the four million babies born each year are premature (1). National health care expenditures well over $28 billion have been estimated. In addition, preterm labor accounts for 85% of infant mortality and 50% of infant neurological disorders. One of the keys to treating preterm labor is the early detection of labor. If preterm labor is exposed early, medical specialists can attempt to stop the labor process, or if unsuccessful, are better prepared to handle the premature infant.
Diagnosis of labor either at term or preterm is the most difficult and important task facing medical practitioners in maternity care. Knowing when labor has begun, as well as predicting when it will start, is important for both normal and complicated pregnancies. Prediction of labor in normal pregnancies is important for minimizing unnecessary hospitalizations, interventions and expenses. On the other hand, accurate diagnosis of preterm labor will also allow practitioners to start treatment early in patients with true labor and avert unnecessary treatment and hospitalization in patients who are having preterm contractions, but who are not in true labor.
While several techniques (monitoring contractions by examiner, cervical state, intrauterine pressure and tocodynamometry) have been adopted to monitor labor, they are subjective and do not provide an accurate diagnosis or prediction of when labor will occur. While a few methods can identify some of the signs of oncoming labor, none of the current methods offer objective data that accurately predicts labor over a broad range of patients. It is well documented that in forecasting labor and delivery, the capability of the technologies already generally accepted into clinical practice (multiple preterm labor symptoms, contractions >4/hour, cervical ultrasonography, or fetal fibronectin) are limited, especially by their sensitivity and positive predictive values (2).
Because of this scenario, classification of pregnant patients and correct identification of preterm labor are still critical challenges in obstetrics. One novel technique, uterine electromyography (EMG), has shown great promise for monitoring patients during pregnancy. For example, previous EMG experiments established that the electrical activity of the myometrium is actually responsible for myometrial contractions (3–5). Furthermore, extensive studies have been done in the last 60 years to monitor uterine contractility using the electrical activity measured from electrodes placed on the uterus (6,7), or on the abdominal surface (8–10).
Artificial neural networks (ANN), on the other hand, are a powerful and innovative mathematical method that tracks/categorizes numerical inter-relationships between variables (11). As such, ANNs are ideal for classification of objects (e.g., patients) based upon one or more diagnostic input variables (e.g., uterine EMG). As with people, ANN’s learn by example when configured for specific applications, such as pattern recognition and data classification. They also possess a remarkable ability to derive meaning from complicated or imprecise data. They are often used to extract patterns and detect trends that are too complex to be noticed by humans or other computer techniques. For this study, we wanted to apply a particular ANN algorithm to uterine EMG data in order to identify labor for both term- and preterm-delivering rats.
MATERIALS AND METHODS
Approval for these studies was granted by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. Animals were allowed free access to food and water, and were provided natural day-night cycles. Controls (G1: N=4) and preterm labor models (G2: N=4; treated with onapristone, an antiprogestin agent ,3 mg given subcutaneously once, on day 17 of pregnancy ) were used. Uterine EMG and intrauterine pressure (IUP) were measured by implanted telemetric devices with electrodes and pressure catheter (Data Sciences), and were recorded at multiple time points on gestational days 16–22 and 1 day postpartum for G1 (normal delivery on day 22 of gestation) and similar times for G2 (delivery at gestation days 18 to 20). EMG mean amplitude,(mv), EMG energy (mV2/sec/ohm), EMG duration (sec.), EMG power-spectrum peak frequency (Hz), , IUP mean (mmHg), and IUP integral (mmHg × sec.) were calculated for EMG bursts and contractions.
Visual observation of time of labor and delivery was the golden standard used in this study, and was used to assess each animal (with labor defined as simultaneous vaginal bleeding and respiratory panting occurring either with or without “pushing” before and during actual delivery). This method has been used for many decades in rat pregnancy studies and has been shown to be quite reliable in labor determination for the rat model (12). In our own studies, for example, this method of diagnosing labor has resulted in prediction of delivery (within approximately 6 hours of observation) for about 95% of the cases. For each time-point assessed with EMG and IUP, either a “labor-event” or “non-labor-event” was assigned for each animal. 112 total labor and non-labor events (52 for G1 and 60 for G2) were observed for all EMG and IUP variables. ANN was then used with these parameters to attempt algorithmic identification for time of labor in each group.
Once all uterine EMG and IUP variables were calculated, the full data set was conditioned according to common practice for ANN data preparation (13), specifically by rescaling all data values to common decimal representation, replacing any missing data values with data set means for that parameter, and by removing outliers (according to Grubb’s test). Training of the ANN was accomplished by running both EMG and IUP data from 10 labor and 10 non-labor events through 10,000 iterations, and using a learning rate of 0.6 (within a range of 0 to 1). Then the ANN (NeuroXL Classifier, www.analyzerXL.com) was used on the calculated EMG input data in an attempt to algorithmically and objectively classify rats into labor and non-labor at each time-point (see Figure 1 for analytic methods used in this study). The particular ANN that was used for classification in this study utilizes the “Kohonen” method (14). The percentage of correctly categorized patients was determined, and finally statistical comparison of the ANN-classified groups was performed using Student’s t-test (P<0.05 used for significance).
Figure 1. Steps Involved in ANN Classification.
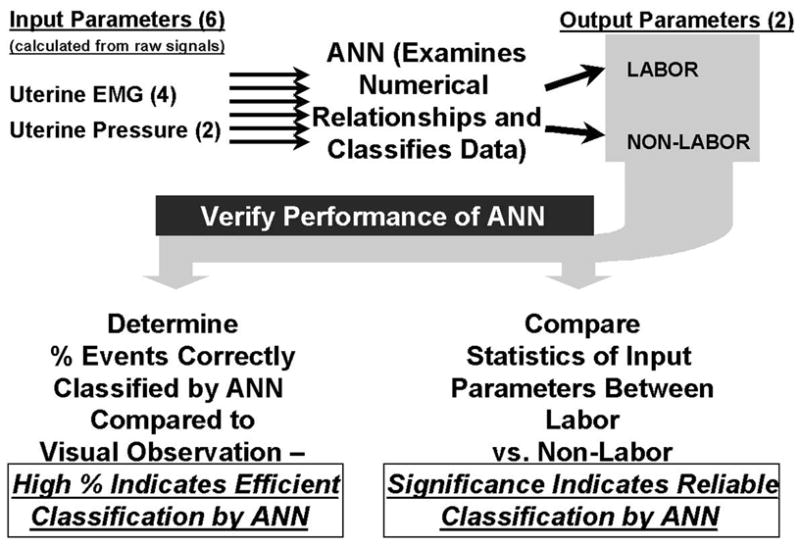
The diagram shows the processing steps in neural network analysis of uterine data, as well as methods to verify the performance of the neural network in classification of subjects. Data are first acquired and then analyzed by an expert, and the calculated parameters (EMG mean amplitude, EMG energy, EMG duration, EMG power density spectrum peak, IUP mean amplitude and integral) are used as inputs for the artificial neural network (ANN). The ANN then categorizes the animals into labor or non-labor based on inter-relations of these input values. The ANN classifications are compared to groupings done by tried and true observational methods (vaginal bleeding, panting, and pushing). The efficacy of the neural network is then quantified by finding the percentage of correctly classified animals (Figure 4) and looking at the post-sorted statistics of the input parameters (Table).
RESULTS
G1 and G2 animals were in labor variously from day 22am through day 22pm, and from day 18am, through day 20am, respectively, with uterine EMG and IUP parameters increasing dramatically during the same time periods (Figures 2, 3). For G2 delivery of any particular animal took anywhere from several hours to nearly a full day, likely because of underdeveloped uteri (producing less-than-ideal contractions), as well as incompletely ripened cervices at the onset of contractions. For G1, 8/8 (100%) of the labor events and 44/44 (100%) of the non-labor events were correctly identified by the ANN (Figure 4), as compared to the golden standard of visual observation of labor, while for G2, 22/24 (92%) of the labor events and 31/36 (86%) of the non-labor events were correctly determined by the ANN (Figure 4).
Figure 2. Changes in, and Correlation of, Area Under Intrauterine Pressure Curve and Uterine EMG Burst Energy for Term-Delivering Animals.
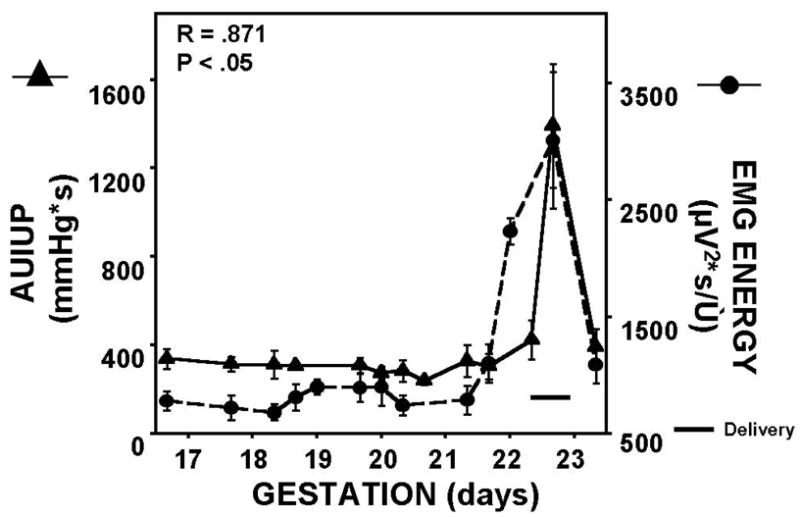
For term-laboring rats, delivery occurred on approximately day 22, with both uterine EMG energy and AUIUP increasing dramatically just prior to and during active labor and subsequent delivery. Delivery for each particular animal took only about 2–3 hours. Correlation coefficient, and corresponding P-value, for the two parameters are shown. Pearson product-moment was used for correlation.
Figure 3. Changes in, and Correlation of, Area Under Intrauterine Pressure Curve and Uterine EMG Burst Energy for Pre-term Delivering Animals.
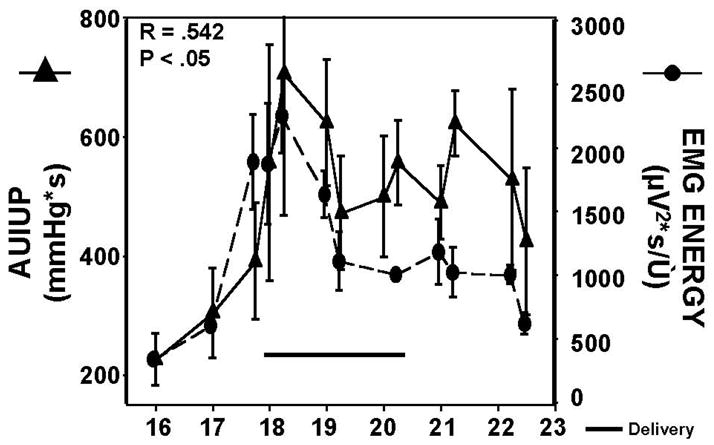
For preterm-laboring rats, delivery occurred variously from day 18 to day 20, with a marked and sustained increase in both uterine EMG and IUP during the entire labor and delivery epoch. Correlation coefficient, and corresponding P-value, for the two parameters are shown. Pearson product-moment was used for correlation.
Figure 4. Percentage of Rats Correctly Classified by ANN as Labor or Nonlabor in Term and Preterm Groups.
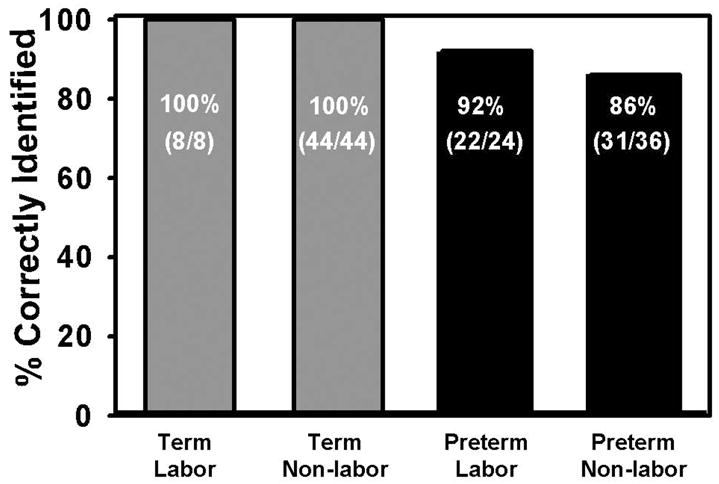
The ANN classification of term-delivering animals into labor and non-labor occurred successfully for all the animals investigated. The results were also very good for pre-term delivering animals, with 92% and 86% correctly classified labor and non-labor animals, respectively.
After classification by the ANN, the labor vs. non-labor classified groups were compared to one another with respect to each EMG and IUP parameter investigated (Table). The EMG variables showed significant differences for both the term and preterm labor groups, while IUP only showed a significant difference in the term labor group.
TABLE 1.
COMPARISON OF UTILITY VALUES
| TERM GROUP | PRETERM GROUP | |||||
|---|---|---|---|---|---|---|
| LABOR | NON-LABOR | P | LABOR | NON-LABOR | P | |
| EMG Mean (mV) | 3.35 ±1.06 | 1.42 ±0.37 | <.001 | 2.00 ± 0.56 | 0.92 ± 0.30 | <.001 |
| EMG Energy (mV2 x sec/Ω) | 3.88 ±1.31 | 1.26 ±0.36 | <.001 | 2.55 ± 0.79 | 1.15 ±0.43 | <.001 |
| EMG Duration (sec) | 18.61 ± 4.09 | 13.71 ±1.76 | <.001 | 18.47 ±3.69 | 15.69 ±2.79 | <.001 |
| EMG PDS Peak Frequency (Hz) | 3.15 ±0.35 | 0.89 ±0.16 | <.001 | 2.57 ± 0.75 | 1.18 ±0.36 | <.001 |
| IUP Mean (mmHg) | 54.75 ± 27.48 | 25.13 ± 10.67 | <.001 | 37.40 ±13.50 | 34.25 ± 12.12 | N/S |
| IUP Integral (mmHg x sec) | 907.28 ± 645.39 | 307.84 ± 95.11 | <.001 | 538.49 ± 268.53 | 436.32 ± 170.23 | N/S |
COMMENT
This study examines the use of EMG and ANN, as complimentary technologies, in an effort to demonstrate their capability in identifying labor, most importantly in the case of preterm labor. After ANN classification of the animals, a clear difference in EMG parameter values is seen between the groups as categorized. EMG seems to be superior to IUP in the determination of preterm labor, as reflected in the table presented above, since highly significant differences are noted in EMG variables between the labor vs nonlabor groups and no significant values are found between IUP. The lack of significance in the IUP variables after ANN classification means that there may be an even stronger relation between uterine EMG and labor than there is between IUP and labor, at least for preterm-delivering animals.
EMG parameters are thought to indicate how well prepared the uterus is for labor (8–10). The significant difference in uterine EMG parameters after ANN classification is an indirect measure of the ability of the ANN classification method to detect the important changes and numerical relationships of the uterine parameters which are indicative of labor. On the other hand, the high percentage of correctly classified labor/non-labor events indicates explicitly just how efficient the ANN method is at achieving such classification.
The reliability of the pregnant rat model, and the dependability of the visual determination of labor in those animals, implies that the golden standard used for the comparison to the ANN-EMG technique of identifying labor is an optimal one. And since the ANN-EMG procedure was highly effective at determining labor based on this golden standard, we can infer that the ANN-EMG method itself is a powerful tool for prediction of labor or nonlabor states in general. That being the case, it stands to reason that the ANN-EMG predictor could prove to have critical functionality when applied to species whose currently available predictors are inadequate, and this is precisely the present condition in the clinical forecasting of labor and delivery in humans. ANN with EMG data has been used in humans and it effectively classifies term/preterm labor/nonlabor patients (15 ). The ANN-EMG method offers higher sensitivity and postivie predictive values over other common clinical predictors (such as fetal fibronectin or cervical ultrasound). This makes this method useful not only in “ruling out” preterm labor, as fetal fibronectin and cervical ultrasound do, but also for ‘ruling in” preterm labor.
Artificial neural networks objectively, automatically, and accurately identify term and preterm labor using uterine EMG variables. This paradigm applies to both term and preterm labor but seems to be more efficient when applied to term labor, yet the results are still highly significant for preterm labor. The ANN and uterine EMG methods used together as in this study gives us the ability to identify true labor, at term or preterm, in pregnant women (11,16,17). Other methods such as multivariable predictive models and CART analysis may also be useful to identify labor. Since true labor most often leads imminently to delivery, our present study suggests that by identifying true labor using uterine EMG signals in humans, ANN should give obstetricians an instrument for actually predicting time of delivery in the clinic.
Acknowledgments
Source of Financial Support: NIH R01 HD037480
Footnotes
Presented orally at the Annual Meeting of the Society for Gynecologic Investigation in Toronto, Ontario, Canada (held from March 22-25, 2006).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Center for Disease Control and Prevention and National Center for Health Statistics data for 2005.
- 2.Iams JD. Prediction and Early Detection of Preterm Labor. Obstet Gynecol. 2003;101(2):402–412. doi: 10.1016/s0029-7844(02)02505-x. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JM. Regulation of the activity in uterine muscle. Physiol Rev. 1962;42:213–227. [PubMed] [Google Scholar]
- 4.Kuriyama H, Csapo A. A study of the parturient uterus with the microelectrode technique. Endocrinology. 1967;80:748–753. doi: 10.1210/endo-68-6-1010. [DOI] [PubMed] [Google Scholar]
- 5.Wolfs GMJA, Van Leeuwen Electromyographic observations on the human uterus during labor. Acta Obstet Gynecol Scand Suppl. 1979;90:1–61. doi: 10.3109/00016347909156375. [DOI] [PubMed] [Google Scholar]
- 6.Devedeux D, Marque C, Mansour S, Germain G, Duchene J. Uterine electromyography: A critical review. Am J Obstet Gynecol. 1993;169:1636–1653. doi: 10.1016/0002-9378(93)90456-s. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeff A, Ramondt J, Garfield RE, Wallenburg HCS. Modulation of spontaneous myometrial activity in chronically instrumented ovariectomized sheep. Eur J Obstet Gynecol Reproduc Biol. 1985;19:113–124. doi: 10.1016/0028-2243(85)90028-0. [DOI] [PubMed] [Google Scholar]
- 8.Garfield RE, Saade G, Buhimschi C, Buhimschi I, Shi L, Shi SQ, Chwalisz K. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4:673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- 9.Buhimschi C, Boyle MB, Saade GR, Garfield RE. Uterine activity during pregnancy and labor assessed by simultaneous recordings from the myometrium and abdominal surface in the rat. Am J Obstet Gynecol. 1998;178:811–822. doi: 10.1016/s0002-9378(98)70498-3. [DOI] [PubMed] [Google Scholar]
- 10.Maner WL, Garfield RE, Maul H, Olson G, Saade G. Predicting term and preterm delivery with trans-abdominal uterine electromyography. Obstet Gynecol. 2003 Jun;101(6):1254–60. doi: 10.1016/s0029-7844(03)00341-7. [DOI] [PubMed] [Google Scholar]
- 11.Haykin S. Neural Networks. 2. Prentice Hall; Upper Saddle River NJ: 1999. [Google Scholar]
- 12.Ducommun D. Rats: Complete Care Guide. BowTie Press; Irvine, CA: 2004. [Google Scholar]
- 13.Lawrence J. Data preparation for a neural network. AI Expert. 1991;11:34–41. [Google Scholar]
- 14.Wyns B, Sette S, Boullart L, Baeten D, Hoffman IE, De Keyser F. Prediction of diagnosis in patients with early arthritis using a combined Kohonen mapping and instance-based evaluation criterion. Artif Intell Med. 2004 May;31(1):45–55. doi: 10.1016/j.artmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Maner WL, Garfield RE. Identification of human term and preterm labor using artificial neural networks on uterine electromyography data. Ann Biomed Engineering. 2007;35:465–473. doi: 10.1007/s10439-006-9248-8. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001 Jul;15(Suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 17.Maner WL, MacKay LB, Saade GR, Garfield RE. Characterization of abdominally acquired uterine electrical signals in humans, using a non-linear analytic method. Med Biol Eng Comput. 2006 Mar;44(1–2):117–123. doi: 10.1007/s11517-005-0011-3. [DOI] [PubMed] [Google Scholar]


