Abstract
Women have a higher prevalence of fibromyalgia and myofascial pain than men, but sex differences in muscle pain are inconsistently detected. We examined sex differences in ratings and effects of recalled and experimentally-induced muscle pain. In Study 1 (N = 188), participants completed a questionnaire about recalled muscle pain. In Study 2 (N = 55), participants’ described muscle pain from an exercise stimulus across three days by telephone. Muscle pain ratings, self-care behaviors for muscle pain, and effects of muscle pain on activities were measured. No significant sex differences were found except that women tended to view exercise as more effective for decreasing muscle pain than men (F1, 187 = 5.43, p = .02, η2 = .03), fewer women performed exercise for induced muscle pain than men, and women’s activity interference was significantly higher than men’s at the third day post-exercise (F2, 42 = 6.54, p= .01, η2 = .14). These findings support the absence of meaningful sex differences in muscle pain ratings. However, additional investigations are needed that consider the daily activities completed by people and the prevalence and incidence of performing a wide range of self-care behaviors for pain.
Perspective: These studies support that sex differences are not present in recalled and experimentally-induced muscle pain ratings. Therefore, we must be cautious about generalizing the musculoskeletal pain literature to muscle pain. Additional research is needed to interpret potential sex differences in self-care behaviors for muscle pain and activity interference from muscle pain.
Keywords: Gender, delayed-onset muscle soreness, self-management
Fibromyalgia40,77 and myofascial pain66 are more prevalent in women than men and the only located epidemiological study of muscle pain within a community sample (N = 780) found that women were more likely to report muscle pain than men.35 In addition, women have higher pain responses to pressure applied onto muscles than men1,5,7,25,32,43,44,67 with an exception located.51 Furthermore, women have greater muscle pain in response to intramuscular injections of algesic substances than men4,27,79 with one exception located.72 Thus, health care providers may assume that women have higher muscle pain responses than men.
In contrast, sex differences in movement-induced muscle pain are inconsistently detected. For example, women reported less muscle pain during cycling than men,9 but women perceived more muscle pain from chewing and biting tasks than men.39,59,72 There is mixed evidence of sex differences in pain from muscle contractions during ischemia.22,26,28,45,52,58 Sex differences have not been detected in most studies of delayed-onset muscle pain/soreness13,16,19,31,41,55,56,60,62 with the exceptions of one study that found women’s pain reports increased more slowly than men’s49 and another study that found less pain in women than men.12 Therefore, additional research is needed regarding sex differences in muscle pain.
Many factors (eg characteristics of the setting, type of pain, etc.) are already known to influence the observation of sex differences in pain.21,63 However, the importance of the type of pain measure has received little consideration with some exceptions.20,23 Previous studies of sex differences in muscle pain have measured the prevalence of pain, pain ratings, and pain thresholds and tolerances, but additional insight about sex differences in muscle pain may be obtained by assessing other types of pain behaviors such as the performance of self-care behaviors (eg taking medication or applying heat). No studies of sex differences in self-care behaviors specifically for muscle pain were located, but women have been found to perform more self-care behaviors in response to musculoskeletal pain than men42,75 and to use women medication and exercise more frequently to relieve musculoskeletal pain than men.30
In addition to the evaluation of self-care behaviors, consideration of the effects of muscle pain on the performance of daily activities (i.e., activity interference) may also advance our understanding of sex differences in muscle pain. No studies of sex differences in activity interference from muscle pain, in particular, were located, but studies about pain, in general, have reported higher activity interference in women than men,64,70 lower activity interference in women than men,17 and no sex difference in activity interference.69,74 Therefore, there is no consensus in the literature regarding sex differences in activity interference and investigations specifically about muscle pain are warranted.
We conducted two studies to advance our understanding of sex differences in recalled muscle pain and movement-induced muscle pain. Muscle pain reports, the performance of self-care behaviors for muscle pain, and the effects of muscle pain on daily activities were examined. We hypothesized that women would report similar levels of recalled and movement-induced muscle pain than men, but women would view self-care behaviors as more effective for muscle pain than men, more women would perform self-care behaviors for muscle pain than men, and women would experience more activity interference from muscle pain than men.
Study 1 Methods
Participants
Participants (N = 188; 61.7% women) were recruited from a university participant pool and their average age was about 19 years (SD = 1.56). There were no restrictions for participation and each participant consented to participate in a manner that was approved by a large southeastern university’s Health Science Center Institutional Review Board.
Procedures
Groups of as many as eight participants completed individual packets of paper and pencil questionnaires in a single-session protocol. These questionnaires asked participants to rate previous muscle pain and to describe their attitudes about and performance of self-care behaviors for recalled muscle pain. In addition, participants were asked to rate the effects of muscle pain on their daily activities.
Muscle pain ratings
Using 0–100 numerical pain scales for pain intensity and pain unpleasantness, participants rated the lowest, usual, and highest levels of muscle pain they had experienced during the past week. Both pain intensity and pain unpleasantness were assessed in order to evaluate the quantity and quality of pain as recommended.61 The anchors for the pain intensity scale were “0 = no pain sensation” and “100 = most intense pain sensation imaginable.” The anchors for the pain unpleasantness scale were “0 = not at all unpleasant” and “100 = most unpleasant imaginable.” Such numerical pain scales have been found to be reliable and valid.36
Concern about and effects of muscle pain
Assessments of concern about muscle pain as a signal of future harm or impaired health, the ability to endure muscle pain, and the amount of activity interference from the muscle pain were based on the Medical College of Virginia questionnaire76 and rated using 0–100 numerical scales. The level of concern for muscle pain as a signal of the future harm or impaired health was rated using the anchors of “0 = not at all concerned” and “100 = most intensely concerned imaginable.” The ability to endure muscle pain was rated using the anchors of “0 = not at all difficult” and “100 = most difficult imaginable.” Activity interference from muscle pain was rated using the anchors of “0 = no interference” and “100 = complete interference.”
Self-care behaviors for muscle pain
The perceived effectiveness of self-care behaviors specifically for muscle pain and the frequency of performing specific self-care behaviors for muscle pain were assessed. The self-care behaviors that were evaluated (i.e., rest, exercise, massage, heat, cold, stretching, over-the-counter medications) are common treatments for muscle injury.6 A 0–100 numerical scale with anchors of “0 = not at all” and “100 = completely” was used to measure the effectiveness of performing each behavior. A 0–100 numerical scale with anchors of “0 = never” and “100 = always” was used to measure the frequency of performing each behavior.
Data Analyses
Sex differences in muscle pain ratings, concerns about and effects of muscle pain, and the perceived effectiveness and performance of self-care behaviors were assessed using one-way analyses of variance (ANOVAs). Due to the number of tests conducted, the alpha level for statistical significance (α = .05) was corrected by the maximum number of family-wise comparisons (i.e., ratings, concerns and effects of muscle pain, and self-care behaviors) such that statistical significance ranged from p < .017 (.05 / 3) to p < .007 (.05 / 7). All analyses were conducted using SPSSx software (SPSS, Inc., Chicago, IL). The meaningfulness of the group differences were determined by calculating eta squared (η2). Eta squared values of .01, .06, and .14 corresponded to small, medium, and large effect sizes, respectively.8
Study 1 Results
No significant sex differences were detected in recalled muscle pain ratings. More specifically, there were no meaningful sex differences in the participants’ ratings of the highest, usual, or lowest muscle pain intensity or muscle pain unpleasantness from the past week. (See Table 1.) However, the levels of muscle pain from the past week were noteworthy; the highest level of pain intensity was 37.76 (SD = 24.62) and pain unpleasantness was 40.98 (SD = 28.19) for the total sample. Self-care behaviors for muscle pain such as stretching, massage, exercise, and rest were commonly performed, but no sex differences were found in the frequency of performing self-care behaviors for muscle pain. In addition, no sex difference in the perceived effectiveness of self-care behaviors for muscle pain were observed except that women tended to view exercise as more effective for decreasing muscle pain than men (F1, 187 = 5.43, p = .02, η2 = .03). (See Table 2 and Table 3.) Furthermore, no sex differences were observed in participants’ concern about muscle pain as a signal of future harm or impaired health, ability to endure muscle pain, and the amount of activity interference from muscle pain (Table 4).
Table 1.
Mean and standard deviations of recalled muscle pain (N = 188)
| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | η2 | |
| Highest Intensity | 37.01 | 22.38 | 38.22 | 26.00 | 0.11 | .75 | <.01 |
| Lowest Intensity | 4.49 | 8.00 | 6.01 | 10.84 | 1.06 | .31 | .01 |
| Usual Intensity | 18.22 | 14.98 | 19.46 | 17.76 | 0.24 | .62 | <.01 |
| Highest Unpleasantness | 39.71 | 25.69 | 41.77 | 29.72 | 0.24 | .63 | <.01 |
| Lowest Unpleasantness | 6.00 | 12.42 | 8.59 | 15.17 | 1.49 | .22 | .01 |
| Usual Unpleasantness | 16.90 | 16.41 | 18.35 | 18.15 | 0.31 | .58 | <.01 |
Note: Rated on a scale from 0–100 with 0 = no pain intensity or unpleasantness and 100 = most intense or unpleasant pain imaginable
Table 2.
Mean and standard deviations of frequencies of performing self-care behaviors for recalled muscle pain (N = 188)
| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | η2 | |
| Stretch | 72.96 | 26.50 | 78.15 | 26.22 | 1.73 | .19 | .01 |
| Massage | 71.22 | 25.50 | 76.96 | 24.22 | 2.39 | .12 | .01 |
| Rest | 59.58 | 30.40 | 61.23 | 30.96 | 0.13 | .72 | <.01 |
| OTC Medication | 22.69 | 27.49 | 25.53 | 30.92 | 0.41 | .52 | <.01 |
| Ice | 25.58 | 23.46 | 26.31 | 30.29 | 0.03 | .86 | <.01 |
| Heat | 33.28 | 29.18 | 30.89 | 31.33 | 0.27 | .60 | <.01 |
| Exercise | 56.07 | 27.28 | 60.76 | 29.35 | 1.20 | .28 | .01 |
Note: Rated on a scale from 0–100 with 0 = never and 100 = always
Table 3.
Mean and standard deviations of perceived effectiveness of self-care behaviors for recalled muscle pain (N = 188)
| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| M | M | M | SD | F | p | η2 | |
| Stretch | 72.24 | 22.05 | 71.20 | 23.63 | 0.09 | .76 | <.01 |
| Massage | 67.71 | 21.69 | 73.63 | 22.53 | 3.16 | .08 | .02 |
| Rest | 69.69 | 25.63 | 71.83 | 27.50 | 0.28 | .60 | <.01 |
| OTC Medication | 56.67 | 26.35 | 59.18 | 27.91 | 0.38 | .54 | <.01 |
| Ice | 51.46 | 25.41 | 55.53 | 27.29 | 1.04 | .31 | <.01 |
| Heat | 51.42 | 26.19 | 54.58 | 25.97 | 0.65 | .42 | <.01 |
| Exercise | 52.00 | 24.24 | 60.67 | 25.14 | 5.43a | .02 | .03 |
p<.05
Note: Rated on a scale from 0–100 with 0 = not at all and 100 = completely
Table 4.
Mean and standard deviations of effects of recalled muscle pain (N = 188)
| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p | η2 | |
| Interference in activities | 26.31 | 22.15 | 25.66 | 25.84 | 0.03 | .86 | <.01 |
| Difficulty enduring pain over time | 28.82 | 24.05 | 33.73 | 24.76 | 1.79 | .18 | .01 |
| Concern about future harm or impaired health | 34.35 | 26.97 | 42.61 | 29.77 | 3.68 | .06 | .02 |
Note: Interference rated on a scale from 0–100 with 0 = no interference and 100 = complete interference, difficulty rated on a scale from 0–100 with 0 = not at all difficult and 100 = most difficult imaginable, concern rated on a scale from 0–100 with 0 = not at all concerned and 100 = most intensely concerned imaginable
Study 1 Discussion
In Study 1, no sex differences in ratings of recalled muscle pain were observed despite similarities between the participants’ highest recalled muscle pain ratings and the average muscle pain ratings reported by patients with myopathies.15 Also no sex differences were observed in the amount of activity interference from muscle pain, ability to endure the muscle pain, or concern about the muscle pain as a signal of future harm or impaired health. Furthermore, no sex differences were found in the frequency or perceived effectiveness of self-care behaviors for muscle pain with the exception of a small sex difference in women viewing exercise as more beneficial for muscle pain than men. These findings support our hypothesis that the sexes would be similar in recalled muscle pain ratings, but these findings contradict our hypotheses that women would perform self-care behaviors for muscle pain more frequently than men and women would experience more activity interference than men.
There are several potential explanations for the lack of hypothesized sex differences. Our sample was composed of young adults with an average age of 21 years while the median age of the community sample in the study by Jansen and colleagues was 44 years. Perhaps, young healthy adults simply perform self-care behaviors differently than older adults. Older adults have a higher prevalence of musculoskeletal pain,2,3,50,69 but young adults are more physically active than older adults.73 It is also possible that our questionnaire about recalled muscle pain lacked sufficient sensitivity. Alternatively, sex differences in indicators of musculoskeletal pain may not generalize to recalled muscle pain. For example, musculoskeletal pain measures may be dominated by the joint pain from arthritis, which is very prevalent and different from muscle pain.24
Regardless, the limitations of this questionnaire study of recalled behaviors for recalled muscle pain needed to be addressed by a prospective investigation of induced muscle pain. The administration of controlled stimuli that produce clinically-relevant muscle pain offers a significant advantage over survey methodologies because the amount of stimulation used to induce the muscle pain is quantified and applied in a standardized way. In addition, such a prospective investigation reduces recall bias.
Therefore, in a second study, exercise was completed to induce movement-induced muscle pain; more specifically, delayed-onset muscle pain was induced. Muscle pain ratings and self-care behaviors for and activity interference from delayed-onset muscle pain were assessed. We hypothesized again that women would report similar levels of muscle pain responses than men, but women would perform more self-care behaviors for muscle pain than men and women would experience more activity interference from muscle pain than men.
Study 2 Methods
Participants
Participants (N = 55; 58.2% women) were recruited from a university and their average age was about 21 years (SD = 2.23). The restrictions for participation were the following: (a) had not engaged in upper body strength training on a regular basis (i.e., two times per week) for consecutive weeks within the previous six months, (b) were not currently experiencing arm pain, and (c) had no history of upper arm injury within the previous six months. In addition, participants were screened for potential risk factors to the exercise protocol (e.g., excessive swelling, loss of range or motion, exertional rhabdomyolysis). Participants were restricted from consuming analgesics 12 hours before the eccentric exercise session. Each participant consented to participate in a manner that was approved by a large southeastern university’s Health Science Center Institutional Review Board.
Procedures
The eccentric exercise task was completed in a single session, which was conducted at a physical therapy clinic of Shands Hospital. Then movement-induced muscle pain ratings, effects of the movement-induced muscle pain on daily activities, and the performance of self-care behaviors to decrease movement-induced muscle pain were assessed by a telephone data recording system (VoiceGuide; Katalina Technologies, Sydney, Australia) across three days post-exercise. A female investigator (EAD) conducted the sessions for all the participants and the telephone data recording system used sound files of her voice.
Exercise behavior
The Leisure-Time Exercise Questionnaire (LTEQ) was administered before the eccentric exercise task to provide information about the exercise behavior of the sample.29 The LTEQ is composed of three items that assess the frequency of performing strenuous, moderate, and mild exercise during leisure-time. Examples of each exercise intensity are provided and the respondents are asked to consider a typical week. The frequency of each exercise intensity can be conceptualized as a total weighted score in metabolic equivalents. The correlation between the total weighted score and maximal oxygen intake (VO2max) was r= .56 (p < .05), which was the highest correlation among 10 other self-report measures of exercise behavior.34
Eccentric exercise task
To induce temporary movement-induced muscle pain, lengthening contractions (i.e., eccentric contractions) of the elbow flexors of participants’ dominant arm were completed with a muscle testing apparatus (Biodex System 3; Biodex Medical Systems, Shirley, NY). Participants were initially positioned according to the manufacturer’s recommendations and 6–10 sub-maximal familiarization repetitions were completed. Then an eccentric strength test that consisted of five consecutive maximal lengthening repetitions (5 RM) was performed and the peak torque was defined as eccentric strength. Finally, using a minimum peak torque target of 75% of eccentric strength, participants completed 3 sets of 12 lengthening repetitions with a rest period of 60 s in between each set. All of these repetitions were completed at a velocity of 90°/s through active range of motion and participants were permitted to observe their torque output throughout the range of motion on a display screen. The total work (i.e., torque by distance) during both the eccentric strength test and the 3 sets of repetitions was recorded.
Muscle pain ratings
In order to evaluate the multidimensional nature of pain,61 ratings of muscle pain intensity and muscle pain unpleasantness in the dominant arm were assessed before eccentric exercise by verbal report and daily for three days post-exercise with 0–100 numerical scales via a telephone data collection system. The anchors of the pain intensity scales were “no pain” and “most intense pain sensation imaginable.” The anchors of the pain unpleasantness scales were “no unpleasantness” and “most unpleasant imaginable.” The numerical scales have been found to be reliable and valid.36
Performance of self-care behaviors
The performance of self-care behaviors specifically for the movement-induced muscle pain in the dominant arm over the previous 24 hrs were reported daily for three days post-exercise via the telephone data collection system. (We did not assess pre-exercise self-care behaviors for muscle pain in the arms because participants with any current arm injuries were excluded from participation.) Participants reported whether they had or had not performed any of the following behaviors for muscle pain in their dominant arms over the previous 24 hrs: applied heat or cold, stretched, massaged, rested, exercised, or consumed a medication.
Effects of the pain
The frequency of feeling movement-induced muscle pain during normal daily activities and the amount of activity interference from the movement-induced muscle pain over the previous 24 hrs were collected daily via the telephone data collection system for three days post-exercise. (We did not assess pre-exercise effects of muscle pain in the arms because participant with any current arm injuries were excluded from participation.) Participants were specifically asked to rate “How frequently you have felt muscle pain from the exercise in your dominant arm while doing your normal daily activities over the last 24 hrs” and “How frequently the muscle pain from the exercise in your dominant arm has interfered with your normal daily activities over the last 24 hrs.” (We did not attempt to define “normal daily activities” for the participants.) The frequency of feeling muscle pain during daily activities and the frequency of muscle pain interfering with daily activities were rated using the anchors of “0 = never” and “100 = constantly.”
Data Analyses
Sex differences in leisure-time exercise behavior were appraised with one-way analyses of variance (ANOVAs). Evaluations of sex differences in responses to the eccentric exercise controlled for the average total work (i.e., torque by displacement) across the three sets of eccentric exercise because muscle strain is believed to be the cause of muscle damage from lengthening contractions48 and men produce more torque than women during eccentric exercise.65 Therefore, sex differences in movement-induced muscle pain ratings were evaluated with 2 (Sex)× 4 (Time) mixed-model analyses of covariance (ANCOVAs) such that muscle pain ratings pre-exercise and across three days post-exercise were included. Also sex differences in the effects of the movement-induced muscle pain on activities across three days post-exercise were assessed with 2 (Sex)× 3 (Time) mixed-model ANCOVAs. Significant interactions were further analyzed by one-way ANCOVAs for each day post-exercise. Finally, sex differences in the performance of self-care behaviors were examined using Fisher’s Exact Tests. All analyses were conducted using SPSSx software (SPSS, Inc., Chicago, IL). Statistical significance was defined as p < .05 and eta squared (η2) was calculated to determine the meaningfulness of the results. Eta squared values of .01, .06, and .14 corresponded to small, medium, and large effect sizes, respectively.8
Study 2 Results
The leisure-time exercise behavior of women (M = 38.73, SD = 23.15) was not significantly different than men (M= 37.30, SD = 29.06; F1, 53 = 0.04, p= .84, η2 < .01). The participants’ compliance with appropriately calling into the telephone data recording system for the three days post-exercise ranged from 85.2% to 100%. The eccentric exercise successfully induced a level of muscle pain that is similar to the average muscle pain ratings reported by patients with myopathies.15 After adjustment by total work completed during the eccentric exercise, both muscle pain intensity (F3, 126 = 4.78, p < .01, η2 = .10) and muscle pain unpleasantness (F3, 126 = 6.54, p < .01, η2 = .14) increased across the three days post-exercise; there were no differences among the three days post-exercise. No sex by time interactions for muscle pain intensity (F3, 126 = 0.93, p = .43, η2 = .02) or muscle pain unpleasantness (F3, 126 = 1.02, p = .39, η2 = .02) were found. Also no main effects of sex on muscle pain intensity (F1, 42 = 0.06, p = .81, η2 < .01) or muscle pain unpleasantness (F1, 42 = 0.16, p = .69, η 2 < .01) were observed. (See Figure 1.)
Figure 1.
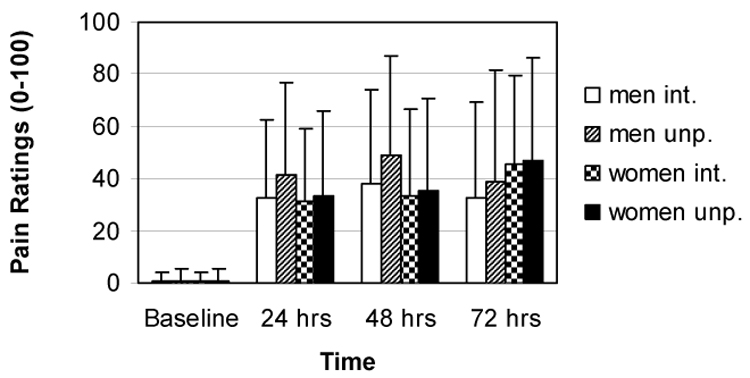
Adjusted means and standard deviations for delayed-onset muscle pain intensity and unpleasantness.
Similarly, the frequency of feeling muscle pain during normal daily activities did not significantly fluctuate across the three days post-exercise (F2, 82 = 0.99, p = .38, η2 = .02) and no sex by time interaction (F2, 82 = 2.03, p = .14, η2 = .05) or main effect of sex (F1, 41 = 0.10, p = .76, η2< .01) were detected after adjustment for total work. (See Figure 2.) Also activity interference from muscle pain did not significantly change across the three days post-exercise (F2, 84 = 1.67, p = .19, η2 = .04) and there was no significant main effect of sex (F1, 42 = 1.14, p= .29, η2 = .03) after adjustment for total work. However, despite similar levels and patterns of muscle pain, the sex by time interaction was significant (F2, 84 = 4.97, p = .01, η2 = .11) such that women’s activity interference from muscle pain was significantly higher than men’s at the third day post-exercise (F2, 42 = 6.54, p = .01, η 2 = .14). (See Figure 2.)
Figure 2.
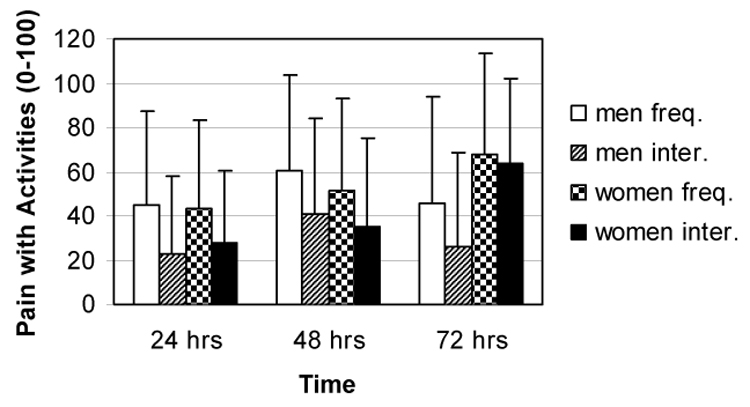
Adjusted means and standard deviations for frequency of feeling delayed-onset muscle pain during normal daily activities and for delayed-onset muscle pain interfering with normal daily activities.
No sex differences in the performance of self-care behaviors for the induced muscle pain were found across three days post-exercise except that significantly fewer women (7.4%) exercised the arm to reduce the muscle pain than men (33.3%) at both one and three days post-exercise. At two days post-exercise 25.9% of women and 38.9% of men exercised the arm to reduce the muscle pain, which were not significantly different. (The results for the third day post-exercise are displayed in Table 5).
Table 5.
Frequencies of performance of self-care behaviors for delayed-onset muscle pain at the third day post-exercise (N = 45)
| Men | Women | |||
|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | |
| Apply heat or cold | 4 | 22.2 | 5 | 18.5 |
| Stretch | 14 | 77.8 | 24 | 88.9 |
| Massage | 15 | 83.3 | 24 | 88.9 |
| Rest | 12 | 66.7 | 22 | 78.3 |
| Exercise | 6 | 26.7 | 2 | 7.4 |
| Consume medication | 3 | 16.7 | 9 | 33.3 |
Study 2 Discussion
In Study 2, despite successful induction of delayed-onset muscle pain at a level that was similar to the average muscle pain ratings reported by patients with myopathies,15 no significant sex differences in ratings of muscle pain intensity or muscle pain unpleasantness were observed. As previously stated, most studies of delayed-onset muscle pain/soreness have not detected significant sex differences13,16,19,31,41,55,56,60,62 with the exceptions of one study that found women’s pain reports increased more slowly than men’s49 and another study that found less pain in women than men.12 Therefore, the results of Study 2 add to the developing consensus that women and men do not differ in delayed-onset muscle pain reports.
Similarly, the women and men in Study 2 did not generally differ in the performance of self-care behaviors with the exception that fewer women than men reported performing exercise to reduce muscle pain at one and two days post-exercise. These findings contradict the previously reviewed literature of women performing more self-care behaviors for musculoskeletal pain than men. Once again, it is possible that the age of sample, the method of assessing self-care behaviors, and/or the type of pain may reasons for the lack of consensus. Also we only induced muscle pain in one location.
The only sex difference in the effects of pain on activities was that women reported significantly higher activity interference from delayed-onset muscle pain at the third day post-exercise than men. This result resembles reports that women with musculoskeletal pain have more activity interference,71 greater disability,30 miss more work,46 and are less likely to return to work and retain work than men.10,54 However, measures of activity interference are alwaysaffected by the actual activities performed and the manner in which the activities were performed. For example, women have more physically repetitive employment in uncomfortable environments, work more frequently and for longer durations with their arms elevated above their shoulders, and spend less time resting than men.11,68 In fact, it has been argued that sex differences in the prevalence of repetitive strain injury (e.g., carpal tunnel syndrome) disappear when the occupational task is controlled.53 However, sex differences in occupational tasks were not likely to be a large factor in this study of young adults and Study 2 did not detect that the women and men differed in leisure time exercise behavior. Future investigations need to assess the actual activities undertaken and avoided by participants with induced muscle pain.
General Discussion
Study 1 administered a retrospective questionnaire to examine recalled muscle pain while Study 2 measured movement-induced muscle pain by telephone data collection. Both studies consistently lacked significant sex differences in ratings of muscle pain, which agree with most studies of delayed-onset muscle pain that have assessed muscle pain/soreness responses.13,16,19,31,41,55,56,60,62 In addition, Studies 1 and 2, for the most part, did not detect significant differences in the performance or perceived effectiveness of self-care behaviors.
However, concluding from these results that there are no sex differences in ratings of recalled or delayed-onset muscle pain may be simplistic. For example, in Study 1, a small sex difference was observed in the perceived effectiveness of exercise for treating muscle pain, but women did not report performing exercise or any other self-care behaviors for muscle pain more frequently than men. In contrast, when muscle pain was actually induced in Study 2, fewer women performed exercise to reduce the induced muscle pain than men at days one and two post-exercise and women reported more activity interference from delayed-onset muscle pain than men at the third day post-exercise. These sex differences are particularly interesting when no sex differences in actual muscle pain ratings were detected. Clearly, it is important to assess a wider range of pain behaviors than just pain ratings.
The distinctions between the two studies’ results support the importance of considering experimentally-induced pain along with recalled pain when interpreting evidence about sex differences in muscle pain. Perhaps, retrospective measures of pain behaviors are inherently inaccurate just like recalled pain ratings.18 Also experimentally-induced muscle pain may be inherently different from recalled muscle pain; certainly concerns about harm (i.e., “torn muscles”) may be different in the two circumstances. In addition, we did not attempt to determine the etiology of recalled muscle pain. Furthermore, different research personnel collected data in the two studies, which may have influenced the results based on data about the influence of investigators’ femininity and/or masculinity, attractiveness, empathy, race, and professional status on pain responses.33,38,47,78 A prospective comparison of recalled muscle pain and experimentally-induced muscle pain within the same participants with the same investigators is needed.
Alternatively, the distinctions between the two studies’ results may have been influenced by measurement issues. Study 1 assessed the recalled frequency of performing self-care behaviors while Study 2 assessed the number of women and men who performed self-care behaviors. Both studies assessed self-care behaviors with questions that the authors constructed due to the absence of a validated measure of self-care behaviors for post-exercise muscle pain. The development and validation of a measure for typical self-care behaviors for muscle pain such as the Pain Coping Inventory37 or Pain Management Inventory 14 is needed.
In summary, these studies support that few sex differences are present in recalled and delayed-onset muscle pain responses. However, the detection of greater activity interference in women than men with movement-induced muscle pain in the absence of sex differences in muscle pain ratings is important. Investigators should assess both muscle pain ratings and the impact of the muscle pain on activities just as is frequently done by health care providers. For example, the severity of overuse injuries is often classified by the timing and duration of pain in relation to activities (e.g., “pain is present during activity, but does not cause alteration of activity”).57 However, both investigators and health care providers should be cautious about generalizing sex differences within the musculoskeletal (e.g., low back pain) and muscle pain (e.g., fibromyalgia) literature to all types of muscle pain.
Additional prospective investigations about sex differences in muscle pain are needed. Investigations should sample a wider age range of adults and measure multiple outcome measures of pain response (e.g., self-care behaviors and activity interference); the frequency, intensity, duration, and type of daily activities undertaken and avoided; and the prevalence and incidence of performing a wide range of self-care behaviors. Also future investigations should compare outcome measures from different types and locations of muscle pain within the same sample.
Acknowledgements
Support was provided from grants (5F32 AR08623-01 and 1KO1 AR050146-01A1) to Dr. Erin A Dannecker from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors would like to thank all the participants who volunteered for this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen JH, Kaergaard A, Frost P, Thomsen JF, Bonde JP, Fallentin N, Borg V, Mikkelsen S. Physical, psychosocial, and individual risk factors for neck/shoulder pain with pressure tenderness in the muscles among workers performing monotonous, repetitive work. Spine. 2002;27:660–667. doi: 10.1097/00007632-200203150-00017. [DOI] [PubMed] [Google Scholar]
- 2.Bergman S, Herrstrom P, Herstrom K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population study. J Rheumatol. 2001;27:1369–1377. [PubMed] [Google Scholar]
- 3.Brattberg G, Parker MG, Thorslund M. A longitudinal study of pain: reported pain from middle age to old age. Clin J Pain. 1997;13:144–149. doi: 10.1097/00002508-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the massester muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- 5.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101:259–266. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 6.Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness: Treatment and performance factors. Sports Med. 2003;33:145–164. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- 7.Christidis N, Kopp S, Ernberg M. The effect of mechanical pain threshold over human muscles by oral administration of granisetron and diclofenac-sodium. Pain. 2005;113:265–270. doi: 10.1016/j.pain.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J. In: Statistical power analysis for the behavioral sciences. Hillsdale NJ, editor. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 9.Cook DB, O'Connor PJ, Oliver SE, Lee Y. Sex differences in naturally occurring leg muscle pain and exertion during maximal cycle ergometry. Int J Neurosci. 1998;95:183–202. doi: 10.3109/00207459809003340. [DOI] [PubMed] [Google Scholar]
- 10.Crook J, Moldofsky H, Shannon H. Determinants of disability after a work related musculoskeletal injury. J Rheumatol. 1998;25:1570–1577. [PubMed] [Google Scholar]
- 11.Dahlberg R, Karlqvist L, Bildt C, Nykvist K. Do work technique and musculoskeletal symptoms differ between men and women performing the same type of work? Appl Ergon. 2004;35:521–529. doi: 10.1016/j.apergo.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Dannecker EA, Koltyn KF, Riley JL, Robinson ME. Sex differences in delayed onset muscle soreness. J Sport Med Phys Fit. 2003;42:458–465. [PubMed] [Google Scholar]
- 13.Dannecker EA, Robinson ME, Hausenblas HA, Kaminski TW. Sex differences in delayed onset muscle pain. Clin J Pain. 2005;21:120–126. doi: 10.1097/00002508-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Davis G. The development of the pain management inventory for patients with arthritis. J Adv Nurs. 1996;24:236–243. doi: 10.1111/j.1365-2648.1996.tb02865.x. [DOI] [PubMed] [Google Scholar]
- 15.Delorme T, Boureau F, Eymard B, Laforet P, Cottrel F. Clinical study of chronic pain in hereditary myopathies. Eur J Pain. 2004;8:55–61. doi: 10.1016/S1090-3801(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 16.Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, Drayson M, Ring C. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. 2007;21:209–217. doi: 10.1016/j.bbi.2006.04.158. [DOI] [PubMed] [Google Scholar]
- 17.Edwards R, Auguston EM, Fillingim R. Sex-specific effects of pain-related anxiety on adjustment to chronic pain. Clin J Pain. 2000;16:46–53. doi: 10.1097/00002508-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Erskine A, Morley S, Pearce S. Memory for pain: a review. Pain. 1990;41:255–265. doi: 10.1016/0304-3959(90)90002-U. [DOI] [PubMed] [Google Scholar]
- 19.Evans GF, Haller RG, Wyrick PS, Parkey RW, Fleckenstein JL. Submaximal delayed-onset muscle soreness: Correlations between MR imaging findings and clinical measures. Radiology. 1998;208:815–820. doi: 10.1148/radiology.208.3.9722865. [DOI] [PubMed] [Google Scholar]
- 20.Fillingim R, Maddux V, Shackelford J. Sex differences in heat pain thresholds as a function of assessment method and rate of rise. Somatosensory Mot Res. 1999;16:57–62. doi: 10.1080/08990229970654. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB. Sex, gender, and pain: women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 22.Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21:387–397. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- 23.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 24.Fontaine KR, Haaz S, Heo M. Projected prevalence of US adults with self-reported doctor-diagnosed arthritis, 2005 to 2050. Clin Rheumatol. 2007;26:772–774. doi: 10.1007/s10067-007-0556-7. [DOI] [PubMed] [Google Scholar]
- 25.Fredriksson L, Alstergren P, Kopp S. Absolute and relative facial pressure-pain thresholds in healthy individuals. J Orofac Pain. 2000;14:98–104. [PubMed] [Google Scholar]
- 26.Frid M, Singer G, Rana C. Interactions between personal expectations and naloxone: Effects on tolerance to ischemic pain. Psychopharmacology. 1979;65:225–231. doi: 10.1007/BF00492208. [DOI] [PubMed] [Google Scholar]
- 27.Ge HY, Madeleine P, Cairns BE, Arendt-Nielsen L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: a potential experimental model of gender-specific differences. Clin J Pain. 2006;22:37–44. doi: 10.1097/01.ajp.0000149799.01123.38. [DOI] [PubMed] [Google Scholar]
- 28.Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 30.Grossi G, Soares JJF, Lundberg U. Gender differences in coping with musculoskeletal pain. Int J Behav Med. 2000;7:305–321. [Google Scholar]
- 31.High DM, Howley ET, Franks BD. The effects of static stretching and warm-up on prevention of delayed-onset muscle soreness. Res Q Exercise Sport. 1989;60:357–361. doi: 10.1080/02701367.1989.10607463. [DOI] [PubMed] [Google Scholar]
- 32.Isselee H, De Laat A, Bogaerts K, Lysens A. Long-term fluctuations of pressure pain thresholds in healthy men, normally menstruating women, and oral contraceptive users. Eur J Pain. 2001;5:27–37. doi: 10.1053/eujp.2000.0213. [DOI] [PubMed] [Google Scholar]
- 33.Jackson T, Iezzi T, Chen H, Ebnet S, Eglitis K. Gender, interpersonal transactions, and the perception of pain: an experimental analysis. J Pain. 2005;6 doi: 10.1016/j.jpain.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs D, Ainsworth B, Hartman T, Leon A. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sport Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Jansen PHP, van Dijck JAAM, Verbeek ALM, Durian FW, Joosten EMG. Estimation of the frequency of the muscular pain-fasciculation syndrome and the muscular cramp-fasciculation syndrome in the adult population. Eur Arch Psychiatry Clin Neurosci. 1991;241:102–104. doi: 10.1007/BF02191150. [DOI] [PubMed] [Google Scholar]
- 36.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford Press; 2001. pp. 135–151. [Google Scholar]
- 37.Jensen MP, Turner JA, Romano JM, Strom SE. The Chronic Pain Coping Inventory: development and preliminary validation. Pain. 1995;60:203–216. doi: 10.1016/0304-3959(94)00118-X. [DOI] [PubMed] [Google Scholar]
- 38.Kállai I, Barke A, Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain. 2004;112:142–147. doi: 10.1016/j.pain.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Karibe H, Goddard G, Gear RW. Sex differences in masticatory muscle pain after chewing. J Dent Res. 2003;82:112–116. doi: 10.1177/154405910308200207. [DOI] [PubMed] [Google Scholar]
- 40.Kassam A, Patten SB. Major depression, fibromyalgia and labour force participation: a population-based cross sectional study. BMC Musculoskelet Disord. 2006;7:1–5. doi: 10.1186/1471-2474-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawczynski A, Nie H, Jaskolaska A, Jaskolaska A, Arendt-Nielsen L, Madeleine P. Mechanomyography and electromyography during and after fatiguing shoulder eccentric contractions in males and females. Scand J Med Sci Spor. 2007;17:172–179. doi: 10.1111/j.1600-0838.2006.00551.x. [DOI] [PubMed] [Google Scholar]
- 42.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 43.Koltyn KF, Trine MR, Stegner AJ, Tobar DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sport Exer. 2001;33:282–290. doi: 10.1097/00005768-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Komiyama O, De Laat A. Tactile and pain thresholds in the intra- and extra-oral regions of symptom-free subjects. Pain. 2005;115:308–315. doi: 10.1016/j.pain.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Lawlis GF, Achterberg J, Kenner L, Kopetz K. Ethnic and sex differences in response to clinical and induced pain in chronic spinal pain patients. Spine. 1984;9:751–754. doi: 10.1097/00007632-198410000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Leijon M, Hensing G, Alexanderson K. Sickness absence due to musculoskeletal diagnoses: association with occupational gender segregation. Scan J Public Health. 2004;32:94–101. doi: 10.1080/14034940310006195. [DOI] [PubMed] [Google Scholar]
- 47.Levine FM, De Simone LL. The effects of experimenter gender on pain report in male and female subjects. Pain. 1991;44:69–72. doi: 10.1016/0304-3959(91)90149-R. [DOI] [PubMed] [Google Scholar]
- 48.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1993;74:520–526. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- 49.MacIntyre DL, Reid WD, Lyster DM, McKenzie DC. Different effects of strenuous eccentric exercise on the accumulation of neutrophils in muscle in women and men. Eur J Appl Physiol. 2000;81:47–53. doi: 10.1007/PL00013796. [DOI] [PubMed] [Google Scholar]
- 50.Magni G, Caldieron C, Rigatti-Luchini S, Merskey H. Chronic musculoskeletal pain and depressive symptoms in the general population. An analysis of the list National Health and Nutrition Exmination Survey data. Pain. 1990;43:299–307. doi: 10.1016/0304-3959(90)90027-B. [DOI] [PubMed] [Google Scholar]
- 51.Manning EL, Fillingim RB. The influence of athletic status and gender on experimental pain responses. J Pain. 2002;3:421–428. doi: 10.1054/jpai.2002.128068. [DOI] [PubMed] [Google Scholar]
- 52.Maurset A, Skoglund LA, Hustveit O, Klepstad P, Oye I. A new version of the ischemic tourniquet pain test. Methods Find Exp Clin Pharmacol. 1991;13:643–647. [PubMed] [Google Scholar]
- 53.McDiarmid M, Oliver M, Ruser J, Gucer P. Male and female rate differences in carpal tunnel syndrome injuries: personal attributes of job tasks? Environ Res. 2000;83:23–32. doi: 10.1006/enrs.2000.4042. [DOI] [PubMed] [Google Scholar]
- 54.McGeary D, Mayer TG, GR J, Anagnostis C, Proctor TJ. Gender-related differences in treatment outcomes for patients with musculoskeletal disorders. Spine. 2003;3:197–203. doi: 10.1016/s1529-9430(02)00599-5. [DOI] [PubMed] [Google Scholar]
- 55.Nie H, Arendt-Nielsen L, Madeleine P, Graven-Nielsen T. Enhanced temporal summation of pressure pain in the trapezius muscle after delayed onset muscle soreness. Exp Brain Res. 2006;170:182–190. doi: 10.1007/s00221-005-0196-6. [DOI] [PubMed] [Google Scholar]
- 56.Nie H, Kawczynski A, Madeleine P, Arendt-Nielsen L. Delayed onset muscle soreness in neck/shoulder muscles. Eur J Pain. 2005;9:653–660. doi: 10.1016/j.ejpain.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor FG, Nirschl RP. Five-step treatment for overuse injuries. Phys Sportsmed. 1992;20:128. doi: 10.1080/00913847.1992.11947508. [DOI] [PubMed] [Google Scholar]
- 58.Pinerua-Shuhaibar L, Prieto-Rincon D, Ferrer A, Bonilla E, Maixner W, Suarez-Roca H. Reduced tolerance and cardiovascular response to ischemic pain in minor depression. J Affect Disorders. 1999;56:119–126. doi: 10.1016/s0165-0327(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 59.Plesh O, Curtis DA, Hall LJ, Miller A. Gender difference in jaw pain induced by clenching. J Oral Rehabil. 1998;25:258–263. doi: 10.1111/j.1365-2842.1998.00270.x. [DOI] [PubMed] [Google Scholar]
- 60.Poudevigne M, O'Connor P, Pasley J. Does sex or blood pressure affect delayed-onset muscle pain intensity? Clin J Pain. 2002;18:386–393. doi: 10.1097/00002508-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1987;28:297–307. doi: 10.1016/0304-3959(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 62.Rinard J, Clarkson PM, Smith LL, Grossman M. Responses of males and females to high-force eccentric exercise. J Sport Sci. 2000;18:229–236. doi: 10.1080/026404100364965. [DOI] [PubMed] [Google Scholar]
- 63.Robinson ME, Riley JL, Myers CD. Psychosocial contributions to sex-related differences in pain responses. In: Fillingim RB, editor. Sex, Gender, and Pain. Seattle, WA: International Association for the Study of Pain; 2000. pp. 41–68. [Google Scholar]
- 64.Scudds RJ, Ostbye T. Pain and pain-related interference with function in older Canadians: the Canadian study of health and aging. Disabil Rehabil. 2001;23:654–664. doi: 10.1080/09638280110043942. [DOI] [PubMed] [Google Scholar]
- 65.Seger JY, Thorstensson A. Muscle strength and myoelectric activity in prepubertal and adult males and females. Eur J Appl Physiol. 1994;69:81–87. doi: 10.1007/BF00867932. [DOI] [PubMed] [Google Scholar]
- 66.Skootsky SA, Bernadette J, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;151:157–160. [PMC free article] [PubMed] [Google Scholar]
- 67.Soetanto AL, Chung JW, Wong TK. Are there gender differences in pain perception? J Neurosci Nurs. 2006;38:172–176. doi: 10.1097/01376517-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Strazdins L, Bammer G. Women, work and musculoskeletal health. Soc Sci Med. 2004;58:997–1005. doi: 10.1016/s0277-9536(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 69.Suka M, Yoshida K. Musculoskeletal pain in Japan: prevalence and interference with daily activities. Mod Rheumatol. 2005;15:41–47. doi: 10.1007/s10165-004-0362-x. [DOI] [PubMed] [Google Scholar]
- 70.Thomas E, Mottram S, Peat G, Wilkie R, Croft P. The effect of age on the onset of pain interference in a general population of older adults: Prospective findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2007;129:21–27. doi: 10.1016/j.pain.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 71.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 72.Torisu T, Wang K, Svensson P, De Laat A, Fujii H, Arendt-Nielsen L. Effects of muscle fatigue induced by low-level clenching on experimental muscle pain and resting jaw muscle activity: gender differences. Exp Brain Res. 2006;174:566–574. doi: 10.1007/s00221-006-0497-4. [DOI] [PubMed] [Google Scholar]
- 73.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sport Exer. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 74.Unruh AM, Ritchie JA, Mersky H. Does gender affect appraisal of pain and pain coping strategies? Clin J Pain. 1999;15:31–40. doi: 10.1097/00002508-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Verbrugge LM, Ascione FJ. Exploring the iceberg. Med Care. 1987;25:539–569. [PubMed] [Google Scholar]
- 76.Wade J, Dougherty L, Archer C, Price D. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68:157–167. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- 77.Weir PT, Harlan GA, Nkoy FL, Jones SS, Hegmann KT, Gren LH, Lyon JL. The incidence of fibromyalgia and its associated comorbities: a population-based retrospective cohort study based on International Classification of Diseases, 9th revision codes. J Clin Rheumatol. 2006;12:124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
- 78.Weisse CS, Foster KK, Fisher EA. The influence of experimenter gender and race on pain reporting: does racial or gender concordance matter? Pain Medicine. 2005;6:80–87. doi: 10.1111/j.1526-4637.2005.05004.x. [DOI] [PubMed] [Google Scholar]
- 79.Zubieta J, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opiod receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


