Abstract
The infant arousal response involves subcortical and cortical responses occurring as a sequence of stereotyped behaviour regardless of the eliciting stimulus. The spontaneous activity of these responses during sleep, however, is uncertain. We examined the spontaneous arousal pattern in normal infants to determine the sequence of responses, and to examine their periodicity and the effects of sleep state. We performed a nap polysomnographic study on 10 normal infants between 2 and 10 weeks of age. Electroencephalographic and electro-oculographic activity, and respiratory airflow and movements were measured, and video recordings were made throughout each study. Different levels of arousal behaviour were examined. We found that spontaneous arousal activity occurred frequently and the majority of responses occurred as a sequence involving an augmented breath followed by a startle and then cortical arousal. Subcortical arousals as reflected by augmented breaths and startles were more common than cortical arousals. Additionally, augmented breaths followed by apnoea were recorded and were not usually associated with other arousal responses. All of the responses occurred periodically either as bursts of activity or as isolated responses. Each of the responses occurred more frequently during rapid eye movement (REM) sleep than during non-rapid eye movement (NREM) sleep. We conclude that there is an endogenous rhythm of spontaneous activity in infants involving excitatory processes from the brainstem, which may or may not be closely followed by cortical excitation. The spontaneous arousal responses occur periodically but with a high level of irregularity and the level of activity is affected by sleep state.
The arousal response to various chemical and mechanical stimuli has been described in infants (Galland et al. 1998; Page & Jeffrey, 1998; Read et al. 1998). Recently, the infant arousal response to a respiratory stimulus was found to be composed of individual subcortical as well as cortical components, which occur in a specific sequence (Lijowska et al. 1997). We found also that arousal elicited using a non-respiratory (tactile) stimulus involved a sequence of subcortical and cortical responses (McNamara et al. 1998). In this study, the investigators reported that spontaneous arousals also occurred, during both NREM and REM sleep, and involved a similar sequence of events as in elicited arousals.
The infant arousal response typically involves an augmented breath, coupled with a startle, followed by a cortical arousal. Each of these responses has been shown previously to occur spontaneously during NREM and REM sleep in infants (Korner, 1969; Fleming et al. 1984; McNamara et al. 1996). Sleep startles have been reported to occur more often during NREM sleep than during REM sleep (Korner, 1969) whereas augmented breaths occur more frequently during REM sleep (Alvarez et al. 1993). The involvement of sleep startles and augmented breaths in the arousal response suggests that subcortical as well as cortical mechanisms are coordinated in the overall arousal process from all stages of sleep.
There are now several reports postulating the importance of arousal mechanisms to protect an infant from a life-threatening stimulus (McNamara et al. 1996, 1998; Lijowska et al. 1997; Galland et al. 1998; Page & Jeffrey, 1998; Wulbrand et al. 1998), and a failure to arouse has been implicated in the sudden infant death syndrome (SIDS). In fact two studies have provided evidence of decreased spontaneous arousals during sleep in SIDS compared with control infants (Kahn et al. 1992; Schechtmann et al. 1992). Information on specific patterns and characteristics of spontaneous arousals in young infants during the developmental period of increased susceptibility to SIDS, however, is minimal. Therefore we felt it of interest to examine the spontaneous arousal behaviour of normal infants. We wanted to determine the spontaneous frequency of these arousal responses in infants and whether there was any difference in arousal behaviour and frequency between NREM and REM sleep. Additionally, as there are conflicting views regarding the physiological basis for the association of apnoea during sleep with augmented breaths, we made a detailed description of respiration and arousal activity in order to better understand mechanisms involved in this type of sleep apnoea.
METHODS
Patients
We studied 10 normal infants (five boys, five girls) with a mean age of 5 ± 0.9 weeks and a range of 2–10 weeks of age. The infants were recruited from advertisements through the hospital and the local media. All infants were normal to a clinical examination. Nine of the infants were full-term, one infant had been born at 36 weeks gestation, but had no complications associated with prematurity. The Washington University Human Study Ethics Committee approved the study protocol, which conformed to the Declaration of Helsinki. Informed consent was obtained from the parent of each infant prior to the study.
Infants were monitored during a natural sleeping period to determine the rates of spontaneous activity and arousal patterns during sleep. Infants were set up for recording by application of electrodes, fed by their usual feeding method, which was either bottle-feeding (four infants) or breast-feeding (six infants), and allowed to fall asleep in a cot in the laboratory. Each study was performed at a time of day that each infant usually slept and the recording was performed for between 1 and 3 h until they spontaneously awoke. In general, the studies were performed between 10 a.m. and 3 p.m. The room temperature was centrally controlled and kept constant between 20 and 21 °C.
Polysomnographic studies
All infants underwent a polysomnographic recording during a daytime nap period. All data were recorded on an 8-channel polygraph recorder (R611, Beckman Instrument Inc., IL, USA). Sleep state was measured with one channel of centrally placed electroencephalogram (EEG) and one channel of electro-oculogram (EOG) to record right eye movements. Electrocardiogram (ECG) was also monitored. All EEG, EOG and ECG electrodes were placed superficially. Airflow was recorded in the infants, by inserting a 1 cm piece of soft rubber tubing 0.5 cm into one nostril and using a thin polyethylene catheter to measure interval pressure at a midpoint in the rubber tube. The cannula was then attached to a differential pressure transducer (Statham, PM15E, USA). The pressure signal was integrated by the polygraph to provide a relative measure of tidal volume (VT). Thoracic and abdominal respiratory movements and the sum of these two recordings were measured using inductance plethysmography (Respitrace, Ambulatory Monitoring Inc., Ardsley, NY, USA). Each infant was set up for the study, fed and allowed to fall asleep in a cot in the laboratory. Polysomnographic recordings were made until the infant woke spontaneously from sleep.
A video recording was made throughout each study. The infant and the respiratory channels of the polygraphic recording were simultaneously videoed by two separate cameras (JVC Professional Products, Elmwood Park, NJ, USA). The images were combined and displayed on a split-screen monitor (Videonix Inc., Campbell, CA, USA) so that the events on the polygraph and the behaviour of the infant could be viewed simultaneously.
Analysis
The polygraphic recordings were reviewed and sleep was staged using EEG and EOG criteria, similar to the established criteria described for neonates and infants (Rechtschaffen & Kales, 1968; Anders et al. 1971). Thirty-second epochs were analysed and assigned as either awake, NREM sleep or REM sleep. The total, NREM and REM sleep times were summed and recorded for each infant.
The video recording of each study was also examined and correlated with the events on the polygraphic recording. Augmented breaths, startles and cortical (EEG) arousals were scored during each sleep state. An augmented breath typically involved the following characteristics: (1) an inspiration with a biphasic pattern with the first phase having an inspiratory time, volume and flow pattern similar to preceding breaths and the second phase consisting of one or more additional bursts of flow, and (2) an increased amplitude indicated by VT and Respitrace, greater than the preceding 10 breaths (Thach & Taeusch, 1976; Lijowska et al. 1997). A startle was characterized by a sudden movement of the body with extension of the arms ending in a bowing of the arms over the thorax (McGraw, 1937). The startles were identified from the video recording that was advanced frame-by-frame to identify the point on the polygraph that the motor activity occurred. A cortical arousal was defined using a criterion similar to that described by the Sleep Disorders Atlas Task Force of the ASDA (1992), that is, a shift in the EEG pattern to frequencies of alpha (8–13 Hz) or above 16 Hz for a minimum of 1 s. In each infant the total number of augmented breaths, startles and cortical arousals during NREM and REM sleep were calculated and each was expressed as an index (number of events per hour) for each sleep state.
Augmented breaths in infants have been associated with apnoea (Fleming et al. 1984). Apnoeas that followed an augmented breath during NREM and REM sleep were noted. An apnoea was defined as a cessation of airflow for a minimum duration of two respiratory cycles, which corresponded to approximately 3 s in length. The apnoeas could be classified as either central, which is associated with an absence of airflow and respiratory movements, or obstructive, which involves a cessation of airflow but continued respiratory efforts on the Respitrace recordings. For each infant the number of augmented breaths that were followed by an apnoea during each sleep state was calculated and expressed as an index, that is, events per hour of sleep and as a percentage of total augmented breaths for each sleep state. Apnoeas that were not associated with a preceding augmented breath were also noted and summed for each sleep state.
The differences between NREM and REM sleep in the arousalsequence and rate of spontaneous arousal responses were examined. The differences in the augmented breath, startle and cortical arousal indices between NREM and REM sleep were evaluated using Student's paired t test. The percentage of augmented breaths that either occurred alone, or were followed by a startle, apnoea or arousal, was calculated and the differences between NREM and REM were examined using the paired t test. The differences in the sequence of responses in the arousal pathway were determined using X2 analysis. The arousal responses in infants were also examined according to sleep architecture and the amount of sleep time in each infant, to determine if a shorter total sleep time was associated with more frequent arousals. The infants were separated into two groups, infants with only one cycle of sleep (Group I) and infants who had more than one cycle of NREM and REM sleep (Group II). The time between the arousal responses was recorded for each infant and the average times were calculated. All data are expressed as the mean ± standard error of themean (s.e.m.). A P value of less than 0.05 was consideredsignificant.
RESULTS
Frequency and sequence of arousal responses
Augmented breaths, startles and cortical arousals occurred periodically during NREM and REM sleep in each infant (Table 1). The spontaneous arousal response in most cases during NREM and REM sleep occurred as a specific sequence. The sequence started with an augmented breath, followed by a startle, and ending with a cortical arousal (Fig. 1). The startle would typically occur during the second phase of the augmented breath. Partial sequences of the responses that did not result in cortical arousal were also observed. Augmented breaths alone and augmented breaths coupled with startles frequently occurred (Fig. 1 and Fig. 2). Augmented breaths were the most common response recorded during NREM and REM sleep.
Table 1.
Frequency of arousal responses during NREM and REM sleep
| NREM (464 min total sleep time) | REM (281.5 min total sleep time) | |||
|---|---|---|---|---|
| Total | Events h−1 | Total | Events h−1 | |
| Augmented breaths | 334 | 47.8 ± 9.5 | 254 | 65.1 ± 9.3* |
| Startle | 153 | 24.6 ± 6.4 | 126 | 35.0 ± 5.7 |
| Cortical responses | 112 | 16.8 ± 3.2 | 122 | 31.5 ± 5.2* |
| Apnoea | 98 | 11.1 ± 5.7 | 21 | 5.4 ± 2.6 |
Total sleep time and number of responses in all infants. Means ± s.e.m. of responses, each expressed as an index during NREM and REM.
Denotes significant difference from NREM sleep.
Figure 1. The arousal sequence.
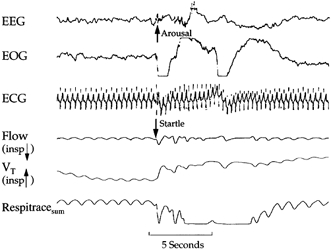
Polygraphic example of a spontaneous arousal response commencing with an augmented breath coupled with a startle and then followed by a cortical arousal response.
Figure 2. Burst of arousal activity.
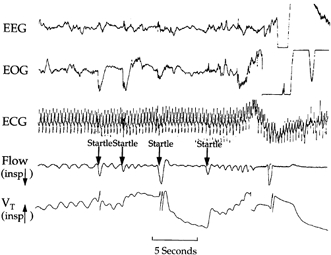
Polygraphic example of a series of responses occurring during NREM sleep in one infant.
Each of the augmented breath, startle and cortical arousal responses occurred more often during REM sleep than during NREM sleep (Table 1). Augmented breaths and cortical arousals occurred significantly more frequently during REM sleep than during NREM sleep (paired t test, P < 0.05 for both comparisons). The frequency of startles was higher during REM sleep than during NREM sleep in eight of the 10 infants, but the overall difference did not reach statistical significance (Table 1; paired t test, P > 0.05).
The proportion of augmented breaths that were followed by a startle was not significantly different between the two sleep states. (Table 2; χ2, P > 0.05). Startles, however, were more closely associated with a cortical arousal during REM sleep than during NREM sleep. A greater proportion of startles ended in a cortical arousal during REM sleep than during NREM sleep (Table 2; χ2, P < 0.05).
Table 2.
Sequence of arousal responses
| NREM | REM | |
|---|---|---|
| Augmented breaths followed by startle | 49.9 ± 8.7 | 49.1 ± 6.7 |
| Augmented breaths followed by startle and cortical | 34.3 ± 4.2 | 42.1 ± 6.1 |
| Startles followed by cortical | 76.9 ± 5.9 | 85.3 ± 4.1* |
| Augmented breaths followed by apnoea | 21.8 ± 7.3 | 7.6 ± 3.5* |
Means ±s.e.m. of proportion of responses (expressed as a percentage) in the sequence averaged for all infants.
Denotes significant difference from NREM sleep.
Arousal responses from five of the infants who had only one sleep cycle during the recording period (Group I) were compared with the other five infants who had more than one sleep cycle (Group II). Group I infants had relatively more startles during NREM and REM sleep than Group II infants. During NREM sleep, 36.2 ± 8.6 and 13.1 ± 2.4 startles h−1 were recorded for Group I and Group II infants respectively (t test, P < 0.05). During REM sleep, 46.5 ± 8.6 and 23.5 ± 2.3 startles h−1 occurred for Group I and Group II infants respectively (t test, P < 0.05).
Apnoea and associated arousal responses
Augmented breaths were frequently associated with apnoea, and the apnoea typically occurred following the augmented breaths. All the apnoeas recorded in the infants were central in nature; obstructive apnoeas were not observed in the recordings of the infants studied. Augmented breaths followed by apnoeas were more frequent during NREM than during REM sleep (paired t test, P < 0.05) (Table 2). In addition, a significantly greater proportion of the augmented breaths were followed by apnoea during NREM sleep than during REM sleep (paired t test, P < 0.05) (Table 2). The majority of the augmented breaths that were followed by an apnoea were not associated with other arousal responses. Startle responses were coupled with the augmented breaths prior to the apnoea in seven of the 98 cases during NREM sleep; however, during REM sleep, startles did not occur concurrently with apnoea. Cortical arousals were not observed with any of the augmented breaths that were followed by an apnoea in either NREM or REM sleep.
Additionally, there were differences in the augmented breaths followed by apnoea between Group I and II infants. Group II infants had a greater number of augmented breaths that were associated with an apnoea during NREM sleep. Group II infants had 21.8 ± 9.4 apnoeas h−1 and Group I had 0.5 ± 0.5 apnoeas h−1 following augmented breaths (t test, P < 0.05).
Periodicity of arousal responses
Augmented breaths, startles and cortical arousals occurred at varying intervals during NREM and REM sleep. During both NREM and REM sleep there were periods of uninterrupted continuous sleep and periods of high levels of arousal-related activity. The complete and partial arousal sequences commonly occurred as isolated events during NREM and REM sleep, but also sometimes occurred as bursts of activity (Fig. 2). The intervals between consecutive augmented breaths, startles and cortical arousals were varied. Consecutive responses would occur as short as 1 s apart and as long as minutes during both NREM and REM sleep. The mean interval length between each consecutive response was significantly shorter during REM sleep than during NREM sleep (t test, P < 0.05 for each comparison) (Table 3).
Table 3.
Periodicity of arousal responses during NREM and REM sleep
| NREM | REM | |
|---|---|---|
| Augmented breaths | 102.9 ± 26.1 | 56.9 ± 9.9 |
| Startles | 243.6 ± 69.1 | 106.5 ± 11.9 |
| Cortical arousals | 244.4 ± 61.5 | 113.1 ± 10.7 |
Means ±s.e.m. of average time intervals (expressed in seconds) between consecutive responses.
Atypical arousal responses
During both sleep states cortical arousals that were not in the sequence of augmented breath followed by a startle and then cortical arousal were occasionally observed in five of the 10 infants. Cortical arousals occurred at times with a startle but without an augmented breath, or occurred following an augmented breath and without a startle. Startle responses were absent in five of 112 cortical arousals during NREM sleep and in eight of 122 cortical arousals during REM sleep. There was no significant difference in these responses during the two sleep states. Augmented breaths were absent in six of the 112 cortical arousal responses during NREM sleep and in 22 of 122 cortical responses during REM sleep. Cortical arousal responses without augmented breaths occurred significantly more often during REM sleep than during NREM sleep (χ2, P < 0.05). The atypical responses were not associated with age; the age range of the infants with atypical responses was from 2 to 10 weeks of age.
DISCUSSION
Arousal from sleep is considered an important protective response in an infant. Arousal has been described previously in infants in response to a variety of respiratory and non-respiratory stimuli during NREM and REM sleep (McNamara et al. 1996, 1998; Lijowska et al. 1997; Galland et al. 1998; Page & Jeffrey, 1998; Wulbrand et al. 1998). Spontaneously occurring awakenings and associated responses without an apparent preceding stimulus also, have been recorded during sleep in infants (McGraw, 1937; Wolff, 1959; Korner, 1969; Alvarez et al. 1993; Cohen & Henderson-Smart, 1996; McNamara et al. 1996; McNamara & Sullivan, 1999). We have found previously that the arousal response to an asphyxiating stimulus and a non-respiratory stimulus occurs as a sequence of stereotyped behaviours involving cortical and subcortical responses (Lijowska et al. 1997; McNamara et al. 1998). The new findings of this study are that the majority of spontaneous arousals similarly occur as a specific sequence of subcortical then cortical responses, which are affected by sleep state.
The spontaneous arousal responses in our infants nearly always occurred as a sequence and could be a complete sequence ending in cortical arousal or part of the sequence. The complete sequence of responses usually commenced with an augmented breath, followed by a startle and then cortical arousal. The sequence of spontaneous arousal behaviours in infants was similar to our previously demonstrated findings of arousal responses elicited by either an increased level of inspired CO2 (Lijowska et al. 1997) or a tactile stimulus (McNamara et al. 1998). Other investigators have similarly reported that augmented breaths and/or motor activity are frequently observed in association with elicited arousal responses (Glogowska et al. 1972; McGinty et al. 1979; Orem & Trotter, 1993; Carley et al. 1997; Galland et al. 1998). The similarity between arousals elicited by exogenous stimuli and those elicited by endogenous mechanisms (i.e. spontaneous arousal) suggests that both forms of arousal may have a common neural pathway.
The partial sequences, that is, augmented breaths alone or augmented breaths with an accompanied startle, without any evidence of a cortical response, occurred frequently. In the absence of a cortical arousal, the majority of startles were still coupled with a preceding augmented breath. The partial responses occur in the same sequence and are identical to those leading to cortical arousal, suggesting that the same excitatory neural pathways are involved in both cases. Previous studies have shown that arousal-eliciting stimuli can also elicit respiratory and motor responses without resulting in cortical arousal (Glogowska et al. 1972; McGinty et al. 1979; Carley et al. 1997; Lijowska et al. 1997; Galland et al. 1998; McNamara et al. 1998). Augmented breaths are commonly recorded during sleep in infants and are believed to be important in maintenance of lung volume (Mead & Collier, 1959; Thach & Taeusch, 1976). Sleep startles are also common during sleep in infants but their functional importance until recently was conjectural (McGraw, 1937; Korner, 1969). Lijowska et al. (1997) have shown that startles have an important airway protective function in sleeping infants.
The sequence of events involved in the spontaneous infant arousal response was similar during NREM and REM sleep. The same sequence during both sleep states suggests that similar brainstem and cortical efferent pathways are involved. There were differences, however, demonstrated in the frequency of each arousal response between NREM and REM sleep. In addition, the startle response was more commonly associated with cortical responses during REM sleep than during NREM sleep. Previous studies that have examined arousal have reported differences in the arousability between sleep stages (Issa & Sullivan, 1983; Page & Jeffrey, 1998; Read et al. 1998). Using a model suggested by the work of McGinty et al. (1979) and that of Wulbrand et al. (1998), these findings are explainable were we to assume that an inhibitory influence which acts to prevent the spread of arousal activity along the pathways from the brainstem to the cortex is more prominent in NREM than REM sleep.
Each of the responses occurred more frequently during REM sleep than during NREM sleep. Similar to our findings, it has been demonstrated that spontaneous augmented breaths occur more frequently in infants during REM sleep than NREM sleep (Alvarez et al. 1993; Cohen & Henderson-Smart, 1996). Startles during sleep in infants, however, are reported to occur more often during NREM sleep than during REM sleep (Korner, 1969). In contrast to these previous findings, we did not find a difference in the startle response between NREM and REM sleep. A limitation of our study, however, is the small number of infants studied. In the current study, eight of the 10 infants studied startled more frequently during REM sleep than during NREM sleep; a larger number of infants may have demonstrated a difference. Spontaneous cortical arousals have been demonstrated previously to occur more frequently during REM sleep in infants (McNamara et al. 1996; McNamara & Sullivan, 1999). Cortical activity is believed to increase more during REM sleep than during NREM sleep, to a level similar to that recorded during wakefulness (Hess et al. 1987). The excitatory processes that elicit the brainstem and cortical responses during sleep are possibly enhanced during REM sleep.
A period of apnoea immediately following an augmented breath during sleep is a well known phenomenon in human infants and adults as well as in animal models (Hoppenbrouwers, et al. 1978; McGinty et al. 1979; Waggener et al. 1982; Perez-Padilla et al. 1983; Orem & Fleming et al. 1984; Trotter, 1993; Hoch et al. 1998). There are two different explanations for this association. One is that the immediate consequences of the large lung inflation such as increased Pa,O2, decreased Pa,CO2 and increased activity of slowly adapting stretch receptors, transiently dampen respiratory drive thereby causing the apnoea (Fleming et al. 1984; Perez-Padilla et al. 1983; Waggener et al. 1982). The other explanation is that arousal activity in the brainstem causes both the augmented breath and independently the apnoea, without a causal connection between the two (McGinty et al. 1979; Orem & Trotter, 1993). It has also been suggested that these theories are not necessarily mutually exclusive (Hoppenbrouwers et al. 1978; McGinty et al. 1979). Prior work suggests that the arousal process is initiated in the brainstem as a ‘micro-arousal’ which then has the potential to grow in intensity and spread rostrally to other centres including the thalamus and finally the cortex to produce a general or complete arousal (Steriade & McCarley, 1990; McNamara et al. 1998; Wulbrand et al. 1998). Our present findings suggest that occurrence of the apnoea following an augmented breath is favoured by ‘micro-arousals’ in the brainstem as suggested by an isolated augmented breath in the absence of other arousal responses. We found that when there was evidence of increased intensity and spreading of the arousal as suggested by an associated startle as well as by cortical arousal, apnoea was much less likely to occur. Therefore, the present findings do not directly support either of the proposed explanations for the link between apnoeas and augmented breaths. Our findings, however, are consistent with an explanation in which the chemoreceptor and mechanoreceptor stimuli, associated with an augmented breath and inhibitory to respiration, are rapidly counteracted when and if arousals spread to the cortex and other areas associated with positive respiratory stimuli.
Each of the arousal responses in each infant occurred periodically. Arousal activity in each infant could involve bursts of activity with several responses in rapid succession or as isolated responses. There was also marked variability between infants grouped according to their sleeping pattern. In the infants we studied, those who had longer continuous sleeping periods had less arousal responses, whilst infants who had a short total sleep time had more frequent arousal responses. Overall, in our study there was marked variability in arousal responses between infants and within each sleeping period. Although the number of infants we studied is small this may suggest an interaction between high frequency arousal activity during sleep and the lower frequency cycles of neural activity that determine daily cycles of sleep and wakefulness. McGinty et al. (1979) suggested that the excitatory process that elicits the arousal responses occurs periodically. The arousal threshold in individual patients has been shown to have within-night variability (Montserrat et al. 1996) and a cyclic variation in arousability within a given stage of sleep in an individual has been demonstrated recently (Berry et al. 1998). Additionally, Korner (1969) found that the frequency of spontaneous behaviours, including startles, varied considerably within a sleeping period in individual infants and between infants. Startle and cortical response are most probably functionally important in infants, but there is a large range of variability. Although the nature and cause of variation in arousal behaviour has not been determined, there is possibly a cyclic variation in the endogenous rhythm of spontaneous arousal responses in infants during NREM and REM sleep. Further studies in a larger group of infants may provide insights into spontaneous sleep arousals and could offer additional ideas as to why infants with increased sleep arousals appear to be less susceptible to sudden infant death syndrome (Schechtmann et al. 1992; Kahn et al. 1992; Harper et al. 2000).
Arousal from sleep is considered important in an infant in response to a life-threatening stimulus to initiate defensive behaviours. At least some of these behaviours also occur in spontaneously occurring arousals. We conclude that spontaneous arousal during NREM and REM sleep in infants involves excitatory processes in both the brainstem and cortex. The similarity to evoked arousal responses suggests that there is a common neural pathway to arousal. The spontaneous arousal responses occur periodically, and the level of activity is markedly affected by sleep state, with possibly increased excitability during REM sleep. There appears to be an inhibitory interaction between the apnoea associated with early arousal and the spread of arousal to higher brain centres such that one rarely occurs in the presence of the other. The spontaneous arousal pattern in infants suggests that there is a possible endogenous rhythm of spontaneous activity given these several considerations.
Acknowledgments
The National Institute of Child Health and Diseases (NICHD) (HD 10993) funded this work. The authors gratefully acknowledge the technical assistance of Ms Davida Wilkins. Dr McNamara performed this research whilst on sabbatical leave from the University of Sydney, Australia.
REFERENCES
- Alvarez JE, Bodani J, Fajardo CA, Kwiatkowski K, Cates DB, Rigatto H. Sighs and their relationship to apnea in the newborn infant. Biology of the Neonate. 1993;63:139–146. doi: 10.1159/000243923. [DOI] [PubMed] [Google Scholar]
- Anders T, Emde R, Parmalee AH, editors. A Manual of Standardised Terminology, Techniques and Criteria for Scoring States of Sleep and Wakefulness in Newborn Infants. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1971. [Google Scholar]
- Berry RB, Asyall MA, McNellis MI, Khoo MCK. Within-night variation in respiratory effort preceding apnea termination and EEG delta power in sleep apnea. Journal of Applied Physiology. 1998;85:1434–1441. doi: 10.1152/jappl.1998.85.4.1434. [DOI] [PubMed] [Google Scholar]
- Carley DW, Applebaum R, Basner RC, Onal E, Lopata M. Respiratory and arousal responses to acoustic stimulation. Chest. 1997;112:1567–1571. doi: 10.1378/chest.112.6.1567. [DOI] [PubMed] [Google Scholar]
- Cohen G, Henderson-Smart DJ. The characteristics and frequency of augmented breaths during CO2-induced hyperpnoea of newborn infants. Journal of Physiology. 1996;490:551–557. doi: 10.1113/jphysiol.1996.sp021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming PJ, Goncalves AL, Levine MR, Woollard S. The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. Journal of Physiology. 1984;347:1–16. doi: 10.1113/jphysiol.1984.sp015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland BC, Reeves G, Taylor BJ, Bolton DPG. Sleep position, autonomic function and arousal. Archives of Diseases of Children. Fetal Neonatal Edition. 1998;78:F189–194. doi: 10.1136/fn.78.3.f189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogowska M, Richardson PS, Widdicombe JG, Winning AJ. The role of the vagus nerves, peripheral chemoreceptors and other afferent pathways in the genesis of augmented breaths in cats and rabbits. Respiration Physiology. 1972;16:179–196. doi: 10.1016/0034-5687(72)90050-3. [DOI] [PubMed] [Google Scholar]
- Harper RM, Kinney HC, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Respiration Physiology. 2000;119:123–132. doi: 10.1016/s0034-5687(99)00107-3. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NMF, Schriefer TN. Excitability of the human motor cortex is enhanced during REM sleep. Neuroscience Letters. 1987;82:47–52. doi: 10.1016/0304-3940(87)90169-8. [DOI] [PubMed] [Google Scholar]
- Hoch B, Bernhard M, Hinsch A. Different patterns of sighs in neonates and young infants. Biology of the Neonate. 1998;74:16–21. doi: 10.1159/000014006. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers T, Hodgman JE, Arakawa K, McGinty DJ, Mason J, Harper RM, Sterman MB. Sleep apnea as part of a sequence of events: a comparison of three months old infants at low and increased risk for sudden infant death syndrome (SIDS) Neuropädiatrie. 1978;9:320–337. doi: 10.1055/s-0028-1091492. [DOI] [PubMed] [Google Scholar]
- Issa FG, Sullivan CE. Arousal and breathing responses to airway occlusion in healthy sleeping adults. Journal of Applied Physiology. 1983;55:1113–1119. doi: 10.1152/jappl.1983.55.4.1113. [DOI] [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, Richard P, Verghote M, Le Polain D, Wayenberg JL. Sleep and cardiorespiratory characteristics of infant victims of sudden infant death: a prospective case-control study. Sleep. 1992;15:287–292. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- Korner AF. Neonatal startles, smiles, erections and reflex sucks as related to state, sex and individuality. Child Development. 1969;40:1039–1053. [PubMed] [Google Scholar]
- Lijowska AS, Reed NW, Mertins Chiodini BA, Thach BT. Sequential arousal and airway defensive behavior of infants in asphyxial sleep environments. Journal of Applied Physiology. 1997;83:219–228. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, London MS, Baker TL, Stevenson M, Hoppenbrouwers T, Harper RM, Sterman MB, Hodgman J. Sleep apnea in normal kittens. Sleep. 1979;1:393–421. [PubMed] [Google Scholar]
- McGraw MB. The moro reflex. American Journal of Diseases of Childhood. 1937;51:240–251. [Google Scholar]
- McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalities in infants and children. Journal of Applied Physiology. 1996;81:2651–2657. doi: 10.1152/jappl.1996.81.6.2651. [DOI] [PubMed] [Google Scholar]
- McNamara F, Sullivan CE. Effects of nasal CPAP therapy on respiratory and spontaneous arousals in infants with OSA. Journal of Applied Physiology. 1999;87:889–896. doi: 10.1152/jappl.1999.87.3.889. [DOI] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. Journal of Applied Physiology. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- Mead J, Collier C. Relation of volume history of lungs to respiratory mechanics in anesthetised dogs. Journal of Applied Physiology. 1959;14:669–678. [Google Scholar]
- Montserrat JM, Kosmas EN, Cosio MG, Kimoff RJ. Mechanisms of apnea lengthening across the night in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 1996;154:988–993. doi: 10.1164/ajrccm.154.4.8887596. [DOI] [PubMed] [Google Scholar]
- Orem J, Trotter RH. Medullary respiratory neuronal activity during augmented breaths in intact unanesthetised cats. Journal of Applied Physiology. 1993;74:761–769. doi: 10.1152/jappl.1993.74.2.761. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE. Airway protection in sleeping infants in response to pharyngeal fluid stimulation in the supine position. Pediatric Research. 1998;44:691–698. doi: 10.1203/00006450-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, West P, Kryger MH. Sighs during sleep in adult humans. Sleep. 1983;6:234–243. doi: 10.1093/sleep/6.3.234. [DOI] [PubMed] [Google Scholar]
- Read PA, Horne RSC, Cranage SM, Walker AM, Walker DW, Adamson TM. Dynamic changes in arousal threshold during sleep in the human infant. Pediatric Research. 1998;43:697–703. doi: 10.1203/00006450-199805000-00020. [DOI] [PubMed] [Google Scholar]
- Rechschtaffen A, Kales A, editors. A Manual of Standardised Terminology, Techniques and Scoring System of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Schechtmann VL, Harper RM, Wilson AJ, Southall DP. Sleep state organisation in normal infants and victims of the sudden infant death syndrome. Pediatrics. 1992;89:865–870. [PubMed] [Google Scholar]
- Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. New York: Plenum Press; 1990. p. 223. [Google Scholar]
- Thach BT, Taeusch HW., Jr Sighing in newborn human infants: Role of inflation-augmenting reflex. Journal of Applied Physiology. 1976;41:502–507. doi: 10.1152/jappl.1976.41.4.502. [DOI] [PubMed] [Google Scholar]
- Waggener TB, Frantz III ID, Stark AR, Kronauer RE. Oscillatory breathing patterns leading to apneic spells in infants. Journal of Applied Physiology: Respiration, Environment and Exercise Physiology. 1982;52:1288–1295. doi: 10.1152/jappl.1982.52.5.1288. [DOI] [PubMed] [Google Scholar]
- Wolff PH. Observations on newborn infants. Psychosomatic Medicine. 1959;21:110–118. doi: 10.1097/00006842-195903000-00004. [DOI] [PubMed] [Google Scholar]
- Wulbrand H, McNamara F, Thach BT. Suppression of sigma spindle EEG activity as a measure of transient arousal following spontaneous and occlusion evoked sighs and startles. Pediatric Research. 1998;44:767–773. doi: 10.1203/00006450-199811000-00021. [DOI] [PubMed] [Google Scholar]


