Abstract
The present study examined whether resistance and stretching training programmes altered the viscoelastic properties of human tendon structures in vivo. Eight subjects completed 8 weeks (4 days per week) of resistance training which consisted of unilateral plantar flexion at 70 % of one repetition maximum with 10 repetitions per set (5 sets per day). They performed resistance training (RT) on one side and resistance training and static stretching training (RST; 10 min per day, 7 days per week) on the other side. Before and after training, the elongation of the tendon structures in the medial gastrocnemius muscle was directly measured using ultrasonography, while the subjects performed ramp isometric plantar flexion up to the voluntary maximum, followed by a ramp relaxation. The relationship between estimated muscle force (Fm) and tendon elongation (L) was fitted to a linear regression, the slope of which was defined as stiffness. The hysteresis was calculated as the ratio of the area within the Fm-L loop to the area beneath the load portion of the curve. The stiffness increased significantly by 18.8 ± 10.4 % for RT and 15.3 ± 9.3 % for RST. There was no significant difference in the relative increase of stiffness between RT and RST. The hysteresis, on the other hand, decreased 17 ± 20 % for RST, but was unchanged for RT. These results suggested that the resistance training increased the stiffness of tendon structures as well as muscle strength and size, and the stretching training affected the viscosity of tendon structures but not the elasticity.
Resistance training is generally expected to cause an increase in muscle strength and muscle mass. A number of studies have demonstrated that resistance training increases muscle size and strength (e.g. Narici et al. 1996; Kubo et al. 2001d). These effects would be caused by the ‘overload’ of the resistance training on muscle and tendon. Therefore, the properties and dimension of human tendon structures may be changeable through the execution of regular exercise. However, we have little knowledge of the influences of resistance training on human tendon structures.
Many previous studies have demonstrated that the maximum range of motion, i.e. flexibility, increased after stretching training (Wilson et al. 1992; Magnusson et al. 1996). In addition, Toft et al. (1989) found a 36 % decrease in passive tension of plantar flexors following a 3 week stretching programme. However, the mechanisms for the acute and chronic changes in joint range of motion and/or passive tension remain ambiguous. Further, Wilson et al. (1992), who applied a damped oscillation technique to determine the stiffness of the upper limbs, showed that the rebound bench press performance enhancement observed consequent to flexibility training was caused by a reduction in stiffness of muscle-tendon units, increasing the utilization of elastic strain energy during the rebound bench press lift. Taken together, these previous results would indicate that the stretching training made the viscoelastic properties of tendon structures change. However, no attempt has been made to investigate the influences of stretching on the properties of human tendon structures in vivo.
Resistance training and stretching have been commonly used for increasing performance during various human movements. Although the resistance and stretching training regimens would affect the biomechanical properties of muscle and tendon, many previous studies have shown changes in the dimensions and properties of the muscle component only (e.g. Magnusson et al. 1996; Narici et al. 1996). Recently, some researchers have indicated that the tendon properties affect the various human movements, especially stretch-shortening cycle exercises (Kubo et al. 1999, 2000b). However, we have little knowledge of the influences of these exercise programmes on human tendon structures, compared to the muscle component.
Recent reports have shown that ultrasonography can be used to determine the stiffness and hysteresis of human tendon structures in vivo (Maganaris & Paul, 1999; Kubo et al. 1999, 2000a,b, 2001a-g; Magnusson et al. 2001). Further, we showed that the execution of either muscle contractions or stretching changed transiently the tendon properties (Kubo et al. 2001e,f,g). Therefore, it is likely that the tendon properties will be changeable through regular resistance and/or stretching exercises. The present study examined whether resistance and stretching training programmes altered the viscoelastic properties of human tendon structures in vivo.
METHODS
Subjects
Eight healthy males (age 21 ± 2 years, height 172 ± 4 cm, weight 64 ± 6 kg; means ± s.d.) participated as subjects. At the time of data collection, the subjects participated in recreational sports but had experienced neither any strength training nor any flexibility training programmes. The subjects were fully informed of the procedures to be utilized as well as the purpose of this study and gave written consent. This study confirmed to the Declaration of Helsinki and was approved by the office of the Department of Sports Sciences, University of Tokyo.
Magnetic resonance imaging
Measurements of muscle and tendon cross-sectional area (CSA) were carried out by magnetic resonance imaging scans (AIRIS II, Hitachi Medical Corp., Tokyo, Japan). T1-weighted spin-echo, axial-plane imaging was performed with the following parameters: TR 850 ms, TE 25 ms, matrix 256 × 256, field of view 250 mm, slice thickness 10 mm, and interslice gap 0 mm. The subjects were imaged in a spine position with the knee and ankle kept at 180 deg (full extension) and 0 deg (anatomical position), respectively. During the scanning, the subject lay supine with the base of the foot resting on a polystyrene block to maintain an ankle angle of 0 deg. The number of sections obtained for each subject was 42–47. The muscles investigated were m. medial gastrocnemius (MG), m. lateral gastocnemius (LG), and m. soleus (SOL). From the axial image, outlines of each muscle were traced, and the traced images were transferred to a Macintosh computer (Power Macintosh 7200/120, Apple Computer) for calculation of the CSA using the public domain National Institutes of Health (NIH) Image software package (v. 1.61/ppc). The muscle volume was determined by multiplying the anatomical CSA of each image by the thickness (10 mm). In addition, the measurement of tendon CSA was taken at two positions (one above the calcareous and the other at 10 mm proximal from the calcareous), although it was rather difficult to distinguish between tendon structures and other tissues in MRI images. The average of CSA at two positions was calculated as the representative of tendon CSA.
The repeatability for the muscle volume and tendon CSA measurements was investigated on two separate days in a preliminary study with six young males. There were no significant differences between the test and retest values of the muscle volume and tendon CSA. The test-retest correlation coefficient (r) was 0.92 for the muscle volume and 0.97 for the tendon CSA. The coefficients of variance were 2.4 % for the muscle volume and 1.5 % for the tendon CSA.
Measurement of viscoelastic properties of tendon structures
The subject lay prone on a test bench and the waist and shoulders were secured by adjustable lap belts and held in position. The right ankle joint was set at 0 deg (anatomical position) with the knee joint at full extension and the foot was securely strapped to a footplate connected to the lever arm of the dynamometer (Myoret, Asics, Japan). Prior to the test, the subject performed a standardized warm-up and submaximal contractions to become accustomed to the test procedure. The subject was instructed to develop a gradually increasing force from relaxed to maximal voluntary contraction (MVC) within 5 s, followed by a gradual relaxation within 5 s. The task was repeated two times per subject with at least 3 min between trials. Torque signals were A/D converted at a sampling rate of 1 kHz (MacLab/8, type ML780, ADInstruments, Japan) and analysed by a personal computer (Power Macintosh 7200/120). The measured values that are shown below are the means of two trials.
A real-time ultrasonic apparatus (SSD-2000, Aloka, Japan) with an electronic linear-array probe (7.5 MHz wave frequency with 60 mm scanning length; UST 5047–5, Aloka) was used to obtain a longitudinal ultrasonic image of MG at the level of 30 % of the lower leg length, i.e. from the popliteal crease to the centre of the lateral malleolus. The width and depth resolutions of ultrasonography with this probe were 0.67 and 0.4 mm, respectively. The ultrasonic images were recorded on videotape at 30 Hz, synchronized with recordings of a clock timer for subsequent analyses. The tester visually confirmed the echoes from the aponeurosis and MG fascicles. The point at which one fascicle was attached to the aponeurosis (P) was visualized on the ultrasonic image. P moved proximally during isometric torque development up to the maximum (Fig. 1). A marker (X) was placed between the skin and the ultrasonic probe as the landmark to confirm that the probe did not move during measurements. The cross-point between superficial aponeurosis and fascicles did not move. Therefore, the displacement of P (L) is considered to indicate the lengthening of the deep aponeurosis and the distal tendon (Kubo et al. 1999, 2001f).
Figure 1. Ultrasonic images of longitudinal sections of medial gastrocnemius muscle during isometric contraction.
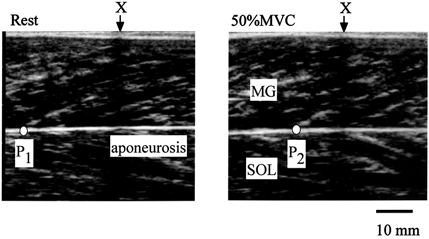
A marker (X) was placed between the skin and the ultrasonic probe as the landmark to confirm that the probe did not move during measurements. The cross-point (P) was determined from the echoes of the deep aponeurosis and fascicles. P moved proximally during isometric torque development from rest (P1) to 50 % MVC (P2). The distance traveled by P (L) was defined as the length change of tendon and aponeurosis during contraction.
The measured torque (TQ) during isometric plantar flexion was converted to muscle force (Fm) by the following equation:
where k (0.18 ± 0.03) is the relative contribution of MG to the plantar flexor muscles in terms of the ratio of the muscle volume, and MA (50.6 ± 1.9 mm) is the moment arm length of triceps surae muscles at 0 deg of ankle joint, which was estimated from the lower leg length of each subject as described by Visser et al. (1990).
As reported in previous studies using animal and human cadavers (e.g. Woo et al. 1981), the Fm-L relation in the tendon structure was curvilinear, consisting of an initial region (toe region) characterized by a large increase in L with increasing force and a linear region immediately after the toe region. In the present study, therefore, the Fm and L values above 50 % of MVC were fitted to a linear regression equation, the slope of which was adopted as stiffness (Kubo et al. 1999, 2001f).
The Fm-L curves during the ascending and descending phases of force development produced a loop. In the present study, the area under each of the curves under both the ascending and descending phases was calculated. Then, the ratio of the area within the Fm-L loop (elastic energy dissipated) to the area beneath the curve during the ascending phase (elastic energy input) was calculated as hysteresis (Kubo et al. 2001f).
The test-retest correlation coefficient (r) was 0.89 for stiffness and 0.85 for hysteresis. The coefficient of variation was 5.6 % for stiffness and 10.2 % for hysteresis.
Measurement of flexibility
To assess the flexibility, the joint angle and the passive torque were measured during a passive stretch of the triceps surae muscles. The posture of the subject and the set-up were similar to those for the measurement of tendon properties mentioned above. The subjects did not warm-up before the stretch manoeuvre. The right ankle joint was set at 0 deg (anatomical position) with the knee joint at full extension and the foot was securely strapped to a footplate connected to the lever arm of the dynamometer. The platform of the dynamometer, which was attached to the sole of the subject's foot, was moved to 25 deg of dorsiflexion with a constant velocity of 5 deg s−1. The passive torque (Nm) during the stretching was detected by the dynamometer. Throughout the stretching, the subjects were requested to relax completely and not offer any voluntary resistance. The passive torque, joint angle and electromyographic activity of triceps surae muscles (see below) were continuously recorded over the entire range of stretch manoeuvres. The slope of the portion of the passive torque-angle curve from 15 to 25 deg was defined as the flexibility index (Kubo et al. 2001b).
The repeatability for the flexibility measurement was investigated on two separate days in a preliminary study with six of the subjects. There were no significant differences between the test and retest values of the flexibility index. The test-retest correlation coefficient (r) and the coefficients of variance were 0.97 and 1.6 %, respectively.
Measurement of electromyogram
The electromyographic activity (EMG) was recorded during the ramp isometric contraction (i.e. measurement of tendon properties) and during the passive stretch (i.e. measurement of flexibility). Bipolar surface electrodes (5 mm in diameter) were placed over the bellies of MG, LG, SOL and tibialis anterior (TA) with a constant interelectrode distance of 25 mm. The positions of the electrodes were marked on the skin by small ink dots. These stained dots ensured the same electrode positioning in each test during the experimental period. The EMG signals were transmitted to a computer (Power Macintosh 7200/120) at a sampling rate of 1 kHz. The EMG was full-wave rectified and integrated for the duration of the contraction and passive stretch to give an integrated EMG (iEMG).
Training
Subjects performed resistance training (RT) on one side and resistance training and static stretching training (RST) on the other side. In each subject, the right and left legs were randomly allocated to the training protocols.
Resistance training
Subjects performed isotonic resistance training of plantar flexor muscles on both sides. Using a horizontal leg press training device (VR-4100, Cybex Corp., USA), they completed 8 weeks (4 days per week) of resistance training which consisted of unilateral plantar flexion at 70 % of one repetition maximum with 10 repetitions per set (5 sets per day). The exercise was done with the range of motion for fully dorsiflexed position to fully plantar flexed position. The measurement of one repetition maximum was made every 2 weeks to adjust the training load.
Stretching training
On the resistance and stretching training side, the subjects performed stretching training in addition to the resistance training. The stretch manoeuvre was performed at 35 deg of dorsiflexion with the ankle joint in the standing position with the stretch leg in a straight position and the hip in neutral rotation. Each session consisted of five stretches for 45 s with a 15 s rest in between. Subjects performed two sessions daily 7 days per week. Two sessions, one in the morning and one in the afternoon, were performed on a daily basis for 8 weeks. Warm-up was not included before stretching training. However, on the days when resistance training was performed, the stretching session was always performed before and after the resistance training session.
Statistics
Descriptive data are expressed as means ± s.d. The significance of difference between before and after training was analysed by Student's t test. One-way analysis of variance (ANOVA) was used for the comparison between two conditions. If the F statistic of the analysis of variance was significant, differences between two conditions were assessed by a Scheffé's test. The level of significance was set at P < 0.05.
RESULTS
The muscle volume of the triceps surae muscles increased significantly from 818 ± 29 cm3 to 843 ± 35 cm3 (2.9 ± 1.4 %) for RT and from 824 ± 36 cm3 to 847 ± 31 cm3 (3.1 ± 1.7 %) for RST. No significant difference in the relative increase of muscle volume was found between RT and RST. There were no significant differences in the relative increase in the muscle volume among the constituents of the triceps surae muscles (Fig. 2). Furthermore, no significant change in the Achilles' tendon CSA was found between the two protocols (Table 1).
Figure 2. The relative changes in the muscle volumes of m. medial gastrocnemius (MG), m. lateral gastrocnemius (LG) and m. soleus (SOL) before and after resistance training for 8 weeks.
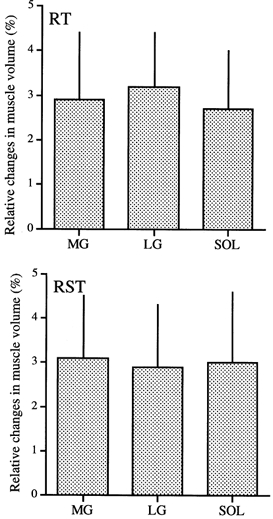
All the muscle volumes increased significantly. However, there seemed to be no differences in the degree of increase in the muscle volumes among the plantar flexor muscles.
Table 1.
Measured variables before and after training
| RT | RST | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| MVC (Nm) | 106 (20) | 128 (17)* | 105 (16) | 124 (15)* |
| Lmax (mm) | 24.2(2.4) | 25.6 (3.2) | 23.3 (3.4) | 23.6 (2.9) |
| Stiffness (N mm−1) | 25.6 (9.0) | 33.6 (9.9)* | 26.5 (5.5) | 32.8 (7.1)* |
| Hysteresis (%) | 17.7 (9.2) | 16.5 (9.4) | 18.7 (10.3) | 11.4 (5.9) |
| Tendon CSA (mm2) | 61 (9) | 59 (8) | 58 (11) | 60 (7) |
| Flexibility index (Nm deg−1) | 1.058 (0.314) | 1.187 (0.333)* | 1.057 (0.193) | 1.112 (0.233) |
Data are means (s.d.).
Significantly different from Before (P <0.05).
Figure 3 shows the relationships between Fm and L before and after training. There were no significant differences in the activation levels (iEMG) of each plantar flexor muscle before and after training (Table 2). In addition, we also confirmed that little co-contraction of TA (< 10 % of MVC) occurred during plantar flexion. The MVC value increased significantly by 19.3 ± 12.8 % (P < 0.01) for RT and 17.0 ± 6.5 % (P < 0.01) for RST. Both protocols produced no significant differences in the L values at all the force production levels between before and after training. For the RST side, on the other hand, the Fm-L loop tended to become smaller after training (Fig. 4). The measured viscoelastic parameters are shown in Table 1. The stiffness increased by 18.8 ± 10.4 % (P = 0.04) for RT and 15.3 ± 9.3 % (P = 0.03) for RST. There was no significant difference in the relative increase of stiffness between RT and RST. The hysteresis, on the other hand, decreased by 17 ± 20 % for RST (P = 0.09), but was unchanged for RT.
Figure 3. The relationships between Fm and L in RT and RST.
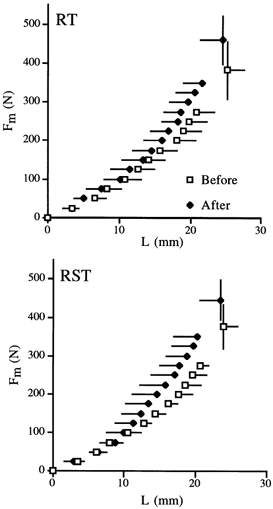
For both sides, there were no significant differences in L values at any force levels.
Table 2.
The iEMG values (mV s)during ramp isometric contraction
| RT | RST | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| MG | 1.34 (0.41) | 1.45 (0.46) | 1.37 (0.46) | 1.44 (0.49) |
| LG | 1.54 (0.33) | 1.59 (0.27) | 1.54 (0.31) | 1.60 (0.27) |
| SOL | 1.47 (0.39) | 1.53 (0.40) | 1.48 (0.42) | 1.54 (0.35) |
| TA | 0.11 (0.02) | 0.10 (0.02) | 0.10 (0.04) | 0.12 (0.02) |
Data are means (s.d.). There are no significant differences between Before and After. MG, medial gastrocnemius; LG, lateral gastrocnemius; SOL, soleus; TA, tibialis anterior.
Figure 4. The relationship between % MVC and L before and after training.
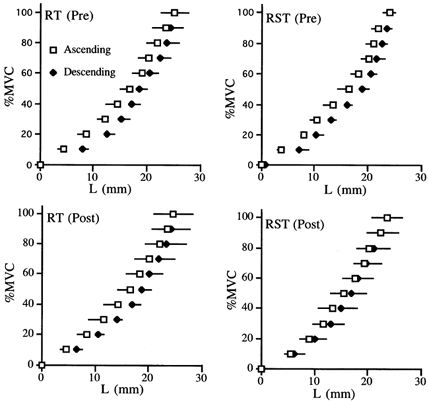
For the RST side, the % MVC-L loop tended to became smaller after training.
The relationship between passive torque and ankle joint angle during the passive stretch is shown in Fig. 5. During the stretching, EMG activities of all the muscles tested were very small (< 1 % of MVC), which confirmed the lack of contribution from the contractile component to the measured resistance to stretch. On the RST side, there were no significant differences in the passive torque values before and after training at any ankle angle. For the RT side, the passive torque values at all ankle angles increased significantly after training. The flexibility index value increased significantly by 13.7 ± 8.9 % (P = 0.02) for RT, but was unchanged for RST (P = 0.26) (Table 1).
Figure 5. The relationship between passive torque and ankle joint angle during the passive stretch of the triceps surae muscle in RT and RST.
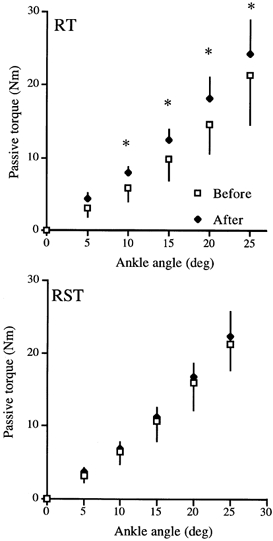
For the RT side, the passive torque values at all ankle angles increased significantly after training. On the RST side, there were no significant differences in the passive torque values at any ankle angles. * Significantly greater than Before at P < 0.05.
DISCUSSION
The intent of the present study was to examine whether the viscoelastic properties of tendon structures were altered with resistance training alone or with resistance training coupled with flexibility training. Both training protocols resulted in an increment of stiffness of tendon structures, although the addition of flexibility training to the isotonic training regimen did not significantly change this result. Furthermore, the added stretching training decreased the hysteresis of tendon structures and prevented the increase in the flexibility index, i.e. the slope of the linear portion in the passive torque-angle curve, by resistance training.
The Fm-L relation should be converted to a stress-strain relation to determine accurately the effect of training on the tendon structures. In this study, no significant change in the tendon CSA was found after training (Table 1). Most of the previous studies using animals have documented that no change in the size of tendons is induced by immobilization (Amiel et al. 1982; Almeida-Silveira et al. 2000) or training (Rollhauser, 1954; Viidik, 1972; Woo et al. 1981). With regard to human tendon, Kubo et al. (2001d) observed that the quadriceps femoris tendon CSA did not change after 12 weeks of isometric knee extension training. Therefore, it is likely that the Achilles' tendon did not change its mass and/or collagen concentration in the 8 weeks of training. The mechanisms which resulted in the increase in the stiffness remain in question, although the training did not induce a significant hypertrophic change in the Achilles' tendon. To explain the increased stiffness, it might be hypothesized that the training induced changes in the internal structures of the tendon and/or aponeurosis. Rollhauser (1954) pointed out that, as a result of 42 days of training in pigs, the only way in which mature tendons could make positive responses to the chronic exercise was to improve the internal structures of the tendons. Further, the variability of the mechanical quality of collagen originates from differences in either the cross-link pattern of the collagen or the structure and packing of the collagen fibres (Danielsen & Andereassen, 1988). Considering these findings, this justifies further discussion of the changes in the viscoelasticity of the tendon structures after training using the Fm-L relationship instead of the stress-strain relationship.
In the present study, the isotonic training stimulus was sufficient to produce a training effect on the tendon stiffness. However, the relative increase of stiffness (16–19 %) tended to be lower than the previously published result using isometric knee extension training (58 %; Kubo et al. 2001d). Similarly, the increases of muscle strength (17–19 %) and muscle volume (2.9- 3.1 %) were lower than that in knee extensors (e.g. Narici et al. 1996; Kubo et al. 2001d). Weiss et al. (1996) showed that 8 weeks of heavy resistance training involving the triceps surae muscles increased isotonic muscle strength by about 13 % without a concurrent increase in muscularity. McCarthy et al. (1997) also reported that the muscle volume of the plantar flexors increased by 3.8 % after 12 weeks of resistance training. Although the reasons for these discrepancies are unknown, there are two possible explanations. Firstly, these discrepancies may be due to the differing muscle groups. Recently, we reported that the elasticity of tendon structures in knee extensors was more compliant in sprinters than in untrained men, but those in plantar flexors was not (Kubo et al. 2000b). In addition, the decrease in stiffness after bed rest for 3 weeks tended to be lower in plantar flexors (14 %) than in knee extensors (33 %) (Kubo et al. 2000a; authors' unpublished data). Therefore, these discrepancies may be due to the differing daily activity levels between knee extensors and plantar flexors. Secondly, these discrepancies may be due to the differing training modes. In the present study, subjects performed the isotonic training at slow velocity contractions (about 30 deg s−1). Our recent study, on the other hand, showed that the stiffness of tendon structures in knee extensors increased after 12 weeks of the isometric training (Kubo et al. 2001d). Furthermore, another type of isometric training (short duration of muscle contraction) characterized as a training of a ‘ballistic’ or ‘explosive’ type did not increase the stiffness of tendon structures (Kubo et al. 2001c). This type of isometric training has a similar effect on the velocity-torque relation to that produced by dynamic resistance training at high velocity contractions, i.e. a predominant increase in torque at the higher contraction velocities (Behm & Sale, 1993). Hence, it might be assumed that the dynamic contraction mode did not change the tendon stiffness. In any case, further investigations are needed to clarify this point.
Muscle force and stiffness in this study tended to be lower than the previously reported values for human tendon in vivo (Magnusson et al. 2001). With regard to the calculation of stiffness, we adopted the slope of the Fm-L relationship during 50–100 % MVC, although Magnusson et al. (2001) obtained the slope of the Fm-L relationship during 90–100 % MVC. Furthermore, Magnusson et al. (2001) calculated ‘Achilles’ tendon force’, although we calculated ‘muscle force of MG’. In the present study, tendon stress at MVC (tendon force/tendon CSA: before, 28 MPa; after, 34 MPa) was in agreement with the findings of Maganaris & Paul (1999) and Magnusson et al. (2001). On the other hand, our recent observations showed that Young's modulus values of human tendon structures in vivo were 250–430 MPa (Kubo et al. 1999, 2001d,f), but substantially lower than those previously reported for animal tendons (Woo et al. 1981; Almeida-Silveira et al. 2000). This discrepancy can be attributed to the fact that Young's modulus values determined in our studies represent the elasticity of both outer tendon and aponeurosis, while the above quoted research on Young's modulus investigated the outer tendon only (Woo et al. 1981; Almeida-Silveira et al. 2000).
In previous human isometric tests, the joint angle has been assumed to be constant without directly monitoring the joint angle. Thus, since isometric contraction of muscle about a joint will produce more or less angular joint rotation in the direction of the intended movement, the resulting tendon and aponeurosis displacement is the result of displacement attributed to both joint angular rotation and contractile tensile loading. Magnusson et al. (2001) demonstrated the importance of accounting for even small amounts of joint motion: despite a rigid frame that was adjusted separately for each subject, the average plantar flexion motion was 3.6 deg, which resulted in an overestimation of the displacement. However, it seems reasonable to suppose that there was no difference in this kind of error between before and after training. In the present study, therefore, we may say that a slight overestimation of the displacement in ankle joint would not affect the present result.
Both training protocols produced a significant increase in the stiffness of tendon structures, 19 % for RT and 15 % for RST. However, the additional flexibility training prevented an increase in the flexibility index by the isotonic strength training. Recently, we reported an acute change in stiffness after the static stretching, but this change was relatively small (−8.9 %; Kubo et al. 2001f). Furthermore, the stretching training for 3 weeks did not decrease the stiffness but did decrease the flexibility index (Kubo et al. 2001a). Furthermore, we observed that the flexibility index was unrelated to the stiffness of tendon structures (Kubo et al. 2001b). Considering these findings, it seems reasonable to suppose that the stretching training increases the flexibility but not the extensibility of tendon structures.
An interesting finding in the present study was that the isotonic training with stretching (RST) tended to decrease the hysteresis (P = 0.09). Our recent observations showed that acute and chronic static stretching made the hysteresis of tendon structures decrease (Kubo et al. 2001a,f). The hysteresis is considered to be an indication of the viscosity of the tissue (Butler, 1978). And so, the observed lower hysteresis after stretching training can be interpreted as a decline of viscosity within tendon structures. The in vivo data of the present study are consistent with previous findings on the influences of stretching on the viscoelasticity of tendons, obtained through observations in vitro. Frisen et al. (1969) and Viidik (1972) showed that repeated cyclic stretches of rat tendons decreased hysteresis, suggesting a reduction of energy dissipation in the tissues after stretching. The mechanisms that resulted in the decrease of hysteresis after acute and long-term stretching are unknown. At least for the lowered hysteresis observed in the present study, however, a change in the structure of the tendons might be involved.
The present result implied that the addition of flexibility training to isotonic training might favour performance during various movements. In most exercise and rehabilitation scenarios, the stretching exercises are applied after various resistance training programmes in order to prevent muscle contractures supposed to be a consequence of resistance training. In general, the following effects are attributed to the stretching: lowering of muscular resting tension (Magnusson et al. 1996); prevention of muscle tightness (Toft et al. 1989); increase of the joint's range of motion (e.g. Magnusson et al. 1996); prophylaxis against injuries (Wilson et al. 1991); and, due to these stretching effects, a general increase of muscular performance (Dintiman, 1964; Wilson et al. 1992). For example, Wilson et al. (1992) reported that flexibility training for the upper limbs induced a significant increase in work during the initial concentric portion of the rebound bench press lift. Also, Dintiman (1964) found that the sprint performance was improved when a stretching regimen was included with regular sprint training. These led us to speculate that stretching may be an effective way to increase the re-used elastic energy during exercise involving a stretch-shortening cycle, by reducing the hysteresis.
These results suggest that the resistance training increased the stiffness of tendon structures as well as muscle strength and size, and the stretching training affected the viscosity of tendon structures but not the elasticity.
REFERENCES
- Almeida-Silveira MI, Lambertz D, Perot C, Goubel F. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. European Journal of Applied Physiology. 2000;81:252–257. doi: 10.1007/s004210050039. [DOI] [PubMed] [Google Scholar]
- Amiel D, Woo SLY, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthopaedica Scandinavica. 1982;53:325–332. doi: 10.3109/17453678208992224. [DOI] [PubMed] [Google Scholar]
- Behm DG, Sale DG. Intended rather than actual movement velocity determines velocity-specific training response. Journal of Applied Physiology. 1993;74:359–368. doi: 10.1152/jappl.1993.74.1.359. [DOI] [PubMed] [Google Scholar]
- Butler DL, Grood ES, Noyes FK, Zernicke RF. Biomechanics of ligaments and tendons. Exercise and Sports Science Reviews. 1978;6:125–181. [PubMed] [Google Scholar]
- Danielsen CC, Andereassen TT. Mechanical properties of rat tail tendon in relation to proximal-distal sampling position and age. Journal of Biomechanics. 1988;21:207–212. doi: 10.1016/0021-9290(88)90171-6. [DOI] [PubMed] [Google Scholar]
- Dintiman GB. Effects of various training programs on running speed. Research Quarterly. 1964;35:456–463. [PubMed] [Google Scholar]
- Frisen M, Magi M, Sonnerup L, Viidik A. Rhelogical analysis of soft collagenous tissue. Part I. Theoretical consideration. Journal of Biomechanics. 1969;2:13–20. doi: 10.1016/0021-9290(69)90037-2. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T. Changes in the elastic properties of tendon structures following 20 days bed rest. European Journal of Applied Physiology. 2000a;83:463–468. doi: 10.1007/s004210000309. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Effect of stretching training on the viscoelastic properties of human tendon structures in vivo. Journal of Applied Physiology. 2001a doi: 10.1152/japplphysiol.00658.2001. (in the Press) [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Is passive stiffness related to the elasticity of tendon structures? European Journal of Applied Physiology. 2001b;85:226–232. doi: 10.1007/s004210100463. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. Journal of Applied Physiology. 2001d;91:26–32. doi: 10.1152/jappl.2001.91.1.26. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Elasticity of tendon structures of lower limbs in sprinters. Acta Physiologica Scandinavica. 2000b;168:327–335. doi: 10.1046/j.1365-201x.2000.00653.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Effects of repeated muscle contractions on the tendon structures in humans. European Journal of Applied Physiology. 2001e;84:162–166. doi: 10.1007/s004210000337. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influence of static stretching on viscoelastic properties of human tendon structures in vivo. Journal of Applied Physiology. 2001f;90:511–519. doi: 10.1152/jappl.2001.90.2.520. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influences of repetitive muscle contractions with different modes on the elasticity of tendon structures in vivo. Journal of Applied Physiology. 2001g;91:277–282. doi: 10.1152/jappl.2001.91.1.277. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kawakami Y, Fukunaga T. The influence of elastic properties of tendon structures on jump performance in humans. Journal of Applied Physiology. 1999;87:2090–2096. doi: 10.1152/jappl.1999.87.6.2090. [DOI] [PubMed] [Google Scholar]
- McCarthy JP, Bamman MM, Yelle JM, LeBlanc AD, Rowe RM, Greenisen MC, Lee SMC, Spector ER, Fortney SM. Resistance exercise training and the orthostatic response. European Journal of Applied Physiology. 1997;76:32–40. doi: 10.1007/s004210050209. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendon mechanical properties. Journal of Physiology. 1999;521:307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Rosager S, Poulsen PD, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. Journal of Physiology. 2001;531:277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Simonsen EB, Aagaard P, Sorensen H, Kjaer M. A mechanism for altered flexibility in human skeletal muscle. Journal of Physiology. 1996;497:291–298. doi: 10.1113/jphysiol.1996.sp021768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli F. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiologica Scandinavica. 1996;157:175–186. doi: 10.1046/j.1365-201X.1996.483230000.x. [DOI] [PubMed] [Google Scholar]
- Rollhauser H. Funktionelle Anpassung der Sehnenfaser im submikroskopischen Bereich. Anatomical Anzeiger. 1954;51:318–322. [Google Scholar]
- Toft E, Espersn GT, Kalund S, Sinkjaer T, Hornemann BC. Passive tension of the ankle before and after stretching. American Journal of Sports Medicine. 1989;17:489–494. doi: 10.1177/036354658901700407. [DOI] [PubMed] [Google Scholar]
- Viidik A. Simultaneous mechanical and light microscopic studies of collagen fibers. Zeitschrift fur Anatomie und Entwicklungsgeschichte. 1972;136:204–212. doi: 10.1007/BF00519178. [DOI] [PubMed] [Google Scholar]
- Visser JJ, Hoogkamer JE, Bobbert MF, Huijing PA. Length and moment arm of human leg muscles as a function of knee and hip-joint angles. European Journal of Applied Physiology. 1990;61:453–460. doi: 10.1007/BF00236067. [DOI] [PubMed] [Google Scholar]
- Weiss LW, Clark FC, Howard DG. Effects of heavy-resistance triceps surae muscle training on strength and muscularity of men and women. Physical Therapy. 1996;68:208–213. doi: 10.1093/ptj/68.2.208. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Elliott BC, Wood GA. Stretch shorten cycle performance enhancement through flexibility training. Medicine and Science in Sports and Exercise. 1992;24:116–123. [PubMed] [Google Scholar]
- Wilson GJ, Wood GA, Elliot BC. The relationship between stiffness of the musculature and static flexibility: an alternative explanation for the occurrence of muscular injury. International Journal of Sports Medicine. 1991;12:403–407. doi: 10.1055/s-2007-1024702. [DOI] [PubMed] [Google Scholar]
- Woo SL, Gomez MA, Amiel D, Ritter MA, Gelberman RH, Akeson WH. The effects of exercise on the biomechanical and biochemical properties of swine digital flexor tendons. Journal of Biomechanics Engineering. 1981;103:51–56. doi: 10.1115/1.3138246. [DOI] [PubMed] [Google Scholar]


