Abstract
Reorganisation of the motor cortex may occur after limb amputation or spinal cord injury. In humans, transcranial magnetic stimulation (TMS) shows expansion of motor cortical representations of muscles proximal to the injury. Similarly, ischaemic block of the hand can increase acutely the representation of the biceps muscle, measured by increased biceps motor potentials evoked by TMS. It is thought that this increase occurs at the expense of the cortical representation of the paralysed and deafferented hand muscles but this has never been investigated. To study what changes occur in the cortical representation of the hand muscles during ischaemic block, a tungsten microelectrode was inserted into the ulnar or median nerve above the elbow and the size of the neural potential elicited by TMS in fascicles supplying the hand was measured in seven subjects. Prior to ischaemia, TMS evoked EMG responses in the intrinsic hand muscles. In the nerve, a brief motor potential preceded the response in the muscle and was followed by a contraction-induced sensory potential. During 40 min of ischaemia produced by a blood pressure cuff inflated around the forearm to 210 mmHg, the EMG response to TMS and the sensory potential from the hand were progressively blocked. However, the motor neural evoked potential showed a significant increase in amplitude during the ischaemic period (30.5 %, P = 0.005). The increase in the neural potential suggests that output to the hand evoked from the cortex by TMS was not decreased by ischaemic block. Thus, we conclude that the increased response of biceps to TMS during distal ischaemia is not accompanied by a corresponding decrease in the motor cortical representation of the hand.
In human subjects transcranial magnetic stimulation (TMS) over the motor cortex can evoke responses in many muscles. Activation of different areas of motor cortex preferentially produces motor-evoked potentials (MEPs) in different muscles, allowing the organisation of the motor cortex to be mapped by measuring the size of MEPs evoked in various muscles by stimulation over different sites on the scalp (Wassermann et al. 1992; Wilson et al. 1993; Classen et al. 1998). Reorganisation of the motor cortex, as demonstrated by changes in these motor maps, has been seen following disturbances of peripheral sensory and motor function. For example, after limb amputation MEPs in muscles proximal to the stump are elicited by lower intensities of stimulation and over wider areas of cortex than in the contralateral muscles (Fuhr et al. 1992; Chen et al. 1998; Röricht et al. 1999; Schwenkreis et al. 2000). Similarly, after spinal cord injury there is expansion of the motor cortical maps of the muscles unaffected by the injury (Levy et al. 1990; Streletz et al. 1995). It is likely that the loss of sensory input alters inhibition within the motor cortex and allows an increase in the size and excitability of the cortical projections to neighbouring muscles (Jacobs & Donoghue, 1991; Chen et al. 1998; Ziemann et al. 1998a,b; Sanes & Donoghue, 2000). This expansion may occur into the cortical areas that previously controlled the deafferented muscles. In experimental animals microstimulation after amputation or nerve lesions can demonstrate such shifts in cortical projections (Donoghue et al. 1990; Sanes et al. 1990). Despite this demonstration of the expansion of the motor cortical areas activating neighbouring muscles, there is little evidence to suggest what happens to the cortical maps of the muscles that have been disconnected from the cortex by amputation or neural injury. One possibility is that these maps may decrease while neighbouring areas expand. In other circumstances, reciprocal changes are seen in the maps of some muscles. In patients with hemifacial spasm the representation of the face becomes larger than normal and that of the hand muscles smaller (Liepert et al. 1999). It is likely that the anatomy of corticomotoneuronal connections is largely unchanged by limb amputation. A retrograde tracer injected into the cervical spinal cord of monkeys with one amputated forelimb showed similar cortical distributions of the forelimb areas both ipsilateral and contralateral to the amputation (Wu & Kaas, 1999). However, the physiological responsiveness of these connections and thus the area of cortical motor map of the amputated muscles remains uncertain.
Ischaemic anaesthetic block of the forearm and hand has been used to induce changes in motor cortical maps acutely in normal human subjects. In these experiments, as in amputees, muscles proximal to the block become more easily activated by TMS. MEPs in biceps brachii increase in size as the hand becomes anaesthetised and paralysed (Brasil-Neto et al. 1992, 1993; Ridding & Rothwell, 1997), and this increase persists for up to 30 min after blood flow resumes. Some of the changes in the responses to cortical stimulation seen in amputees do not occur with acute deafferentation. In amputees, the threshold stimulus intensity that can elicit an MEP is decreased and GABA-dependent intracortical inhibition, as demonstrated by paired pulses, may be diminished (Chen et al. 1998; Schwenkreis et al. 2000; cf. Capaday et al. 2000). With ischaemic block, changes in intracortical inhibition only occur if repetitive TMS is carried out at the same time (Ziemann et al. 1998a). However, expansion of the cortical map of the muscles proximal to the block does occur (Brasil-Neto et al. 1993; Ridding & Rothwell, 1995), although the mechanisms of this expansion are unclear (Ridding & Rothwell, 1997).
In the current study, we investigated the hypothesis that the increase in size of MEPs in muscles proximal to an ischaemic block was accompanied by a decrease in cortical activation of the deafferented paralysed hand muscles. To do this, we stimulated the motor cortex to evoke MEPs in hand muscles and monitored the efferent neural potential at a level above the block. Recording through an insulated tungsten microelectrode inserted into either the median or ulnar nerve above the elbow allowed us to follow both the descending evoked motor volley in the nerve (motor nerve evoked potential, NEPm) and the ascending sensory volley while the hand became anaesthetised and paralysed.
METHODS
General procedures
Experiments were performed on seven healthy neurologically normal subjects (4 female, 3 male, age range 18–41 years). All subjects gave written, informed consent and ethical approval was given by the Committee on Experimental Procedures Involving Human Subjects, University of New South Wales. Experiments were conducted in accordance with the Declaration of Helsinki. Subjects were seated in a comfortable chair with the forearm supported in a supinated position on a stable platform. A blood pressure cuff was positioned around the proximal forearm. In each subject, neural volleys were recorded from the ulnar or median nerve in the upper arm while TMS evoked motor responses in the hand muscles (Fig. 1). Recording continued during and after ischaemic block of the hand and forearm.
Figure 1. Experimental set-up.
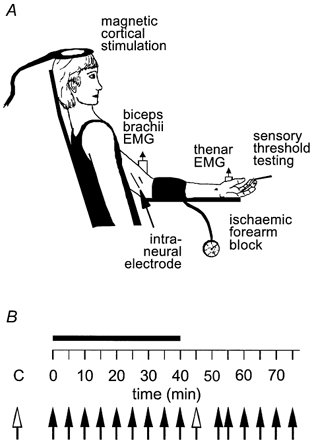
A, typical experimental set-up. B, experiment time sequence: Control trials (C) preceded ischaemia (filled bar), which, after 40 min, was followed by deflation of the cuff and recovery. Each filled arrow represents one series of 10 magnetic shocks; the unfilled arrows represent five series of stimuli. After each series of stimuli both cutaneous sensory thresholds and muscle afferent activity were tested. Note: EMG was also recorded in first dorsal interosseous muscle and abductor digiti minimi.
Intraneural recording
To record neural volleys an insulated tungsten microelectrode (low impedance, 200 μm diameter, 1 μm tip diameter; type TM33B10, World Precision Instruments, USA) was inserted through the skin into either the median or ulnar nerve 5–10 cm proximal to the cubital fossa. A similar electrode with 1 mm of insulation removed from the tip inserted subdermally 2 cm away served as a reference electrode. Intraneural stimulation (0.1 ms pulse, 1 Hz, 0.01–1 mA) was delivered from an optically isolated, constant-current stimulator (ADInstruments, Australia) to guide electrode placement within the nerve in conjunction with verbal feedback from subjects. Optimal recording sites were those that allowed multiunit recording from within a motor area of the nerve that predominantly supplied the intrinsic muscles of the hand. At the level of nerve penetration used in these experiments, the fascicles of both the ulnar and median nerves contain an admixture of sensory and motor axons (Sunderland, 1978). Areas of the nerve innervating either the biceps or the forearm flexor muscles as well as areas of high cutaneous axonal density were deliberately avoided. Motor areas were evident from twitching in appropriate muscles time-locked to the stimulus pulse while cutaneous portions of the nerve elicited paraesthesiae. The neural signal was filtered (bandwidth 300 Hz to 10 kHz, gain 2 × 103), digitised (12.8 kHz) and stored on computer with all other signals for off-line analysis.
Transcranial magnetic stimulation (TMS)
TMS was delivered via a 13 cm round coil positioned over the vertex and oriented to optimise MEPs in the target intrinsic hand muscle (Magstim 200, The Magstim Company, UK). When recording from the ulnar nerve, first dorsal interosseous (FDI), adductor pollicis (AP) or abductor digiti minimi (ADM) was the target muscle. The thenar muscle group was targeted in median nerve recordings. To determine the intensity of magnetic stimulation in each experiment, a stimulus-response curve was recorded for the target muscle. Groups of 10 stimuli were delivered at intensities increasing in steps of 5 % of stimulator output. Stimulation began with an intensity below that needed to evoke any response in the target muscle and continued until obvious twitches were elicited in biceps brachii. Higher intensities of stimulation risked dislodging the intraneural electrode. An intensity of magnetic stimulation which was on the steepest portion of the stimulus-response curve for the target muscle but which minimised activation of more proximal muscles was then chosen for the experiment (Fig. 2A and B). There were corresponding increases in the amplitudes of both the descending peripheral neural volley and the target muscle response with higher magnetic stimulation intensities. Thus, the amplitude of the descending neural volley and the target muscle MEP were significantly correlated (P < 0.001; Fig. 2C). In one case the stimulator intensity chosen for the experiment was the highest point on the stimulus-response curve. However, it is clear from Fig. 5 (data from the same subject) that an increase in the motor nerve evoked potential could still be seen beyond the initial level. The range of intensities used for these experiments was 45–65 % of stimulator output. All magnetic stimuli were given in series of 10 shocks, delivered 4 s apart. Electromyograms (EMGs) from biceps brachii and the target hand muscle were recorded using disposable 6 mm Ag-AgCl surface EMG electrodes placed over the bellies of the muscles (Fig. 1). EMG was amplified (bandwidth 10 Hz to 5 kHz, gain 200), digitised at 3.2 kHz (MacLab BioAmp, ADInstruments) and stored on computer using the SC/ZOOM data acquisition and analysis system (Department of Physiology, University of Umeå, Sweden).
Figure 2. Responses to increasing intensities of TMS in one subject.
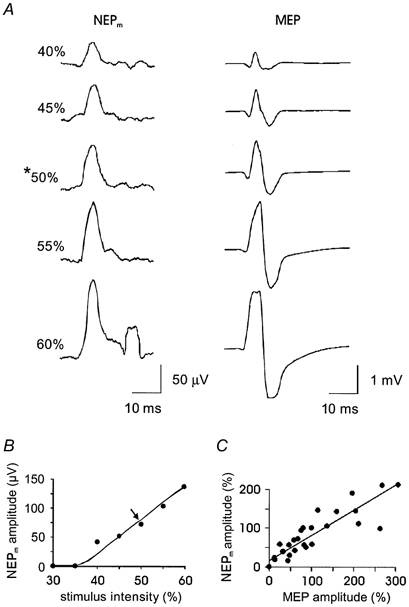
A, traces on the left are averaged RMS processed records of neural activity (NEPm, motor nerve evoked potential). Traces on the right are the responses (MEPs) recorded at the same time from abductor digiti minimi muscle. Both the amplitude of the initial peak in the nerve recording and that of the MEP increase with increasing stimulus intensity. Note that at 60 % stimulus intensity some of the MEPs contributing to the averaged trace were clipped. In the nerve recording also at 60 % stimulus intensity, a movement artefact resulting from a twitch of biceps brachii can be seen as a second peak. The asterisk indicates the intensity chosen for later use. B, amplitude of the peripheral motor neural volley plotted against the intensity of stimulation for the same subject as in A. Amplitudes were measured from averaged traces of 10 stimuli. The relationship is highlighted by a hand-drawn line. An arrow marks the intensity of stimulation chosen for use in the experiment in this subject. C, stimulus-response data for seven subjects with increasing intensities of magnetic stimulation. The amplitude of the NEPm is plotted against the amplitude of the response in the targeted intrinsic hand muscle. Data are normalised so that for each subject the NEPm and the MEP evoked by stimuli of the intensity chosen for the experiment are shown with an amplitude of 100 %. In one subject the amplitude of the NEPm plateaued while that of the MEP continued to increase. The line of best fit has a correlation coefficient of 0.99 (P < 0.001).
Figure 5. Neural recordings from a single subject during and after ischaemic block.
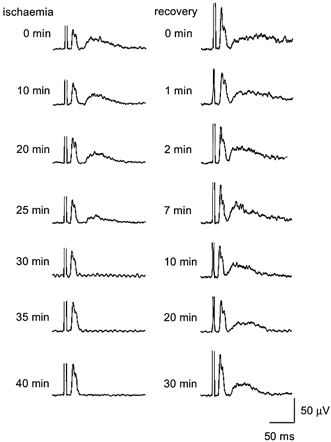
Each trace is an average RMS-processed response to TMS as recorded from the ulnar nerve (n = 10). On the left are responses recorded each 5 min during ischaemia. On the right are responses recorded after blood flow to the hand was allowed to resume. The descending neural volley (which follows the truncated stimulus artefact) does not decrease in amplitude or duration during the period of ischaemia. The broad peak of the ascending sensory volley starts to decrease after 25 min of ischaemia and has disappeared by 30 min indicating deafferentation of the hand. The sensory volley is prolonged immediately after the release of the cuff at a time when subjects report intense paraesthesiae and returns to control values by 10 min of recovery.
Experimental procedures
Once the experimental set-up was completed, baseline sensory thresholds were established at marked sites on both the wrist and distal phalanx of digit III using calibrated von Frey filaments (Simmes-Weinstein Aesthesiometers, Stoelting, IL, USA). All subsequent sensory threshold measurements were normalised to control levels to enable group data to be calculated. Five TMS control series were given with each series beginning 1 min apart. These data were analysed immediately to ensure the intraneural electrode was detecting the peripheral descending (motor) nerve volley. The blood pressure cuff around the proximal forearm was then inflated and maintained at approximately 210 mmHg throughout the ischaemic period. The cessation of blood flow was confirmed by a photo-electric plethysmograph on digit IV (ADInstruments). Ischaemia was sustained for 40 min in each experiment. A series of magnetic stimuli was given immediately following cuff inflation and every 5 min thereafter. Directly after the release of the ischaemia five series of stimuli were given, each beginning 1 min apart, followed by one series of stimuli 7 min after deflation, again at 10 min and then every 5 min for a further 20 min period of recovery (Fig. 1). Following each series of stimuli (or after the block of five series immediately after deflation of the cuff) cutaneous sensory thresholds were tested with von Frey filaments. Audio feedback of the afferent responses to muscle stretch confirmed the electrode's position within a predominantly motor area of the nerve throughout the experiment, with the exception of approximately 10–15 min at the end of the ischaemic period when sensory activity in the hand was abolished.
Pilot experiments confirmed that our set-up replicated earlier studies of forearm ischaemic nerve block. These studies, without intraneural recording, used the same design as seen in Fig. 1. The data from a single subject are illustrated in Fig. 3 where an increase in the biceps MEP can clearly be seen after 30 min of ischaemia of the hand. The size of the MEP had returned to control values by 20 min following the release of ischaemia and showed a slight depression in amplitude at 30 min of recovery. The concurrent FDI MEP was abolished at 30 min of ischaemia, showing an augmentation at 10 min of recovery and a return to control values by 20 min of recovery.
Figure 3. MEPs recorded from biceps brachii (left) and first dorsal interosseous (FDI, right) in a single subject.
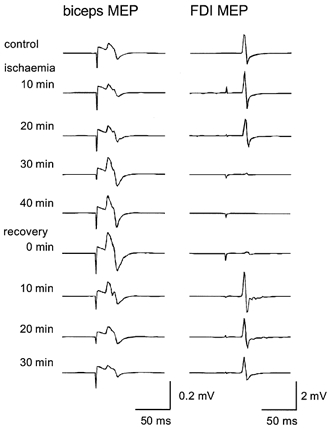
Each trace is the average of 10 stimuli. Traces were recorded during a pilot experiment in which the intensity of TMS was adjusted to evoke responses in biceps brachii and in which no intraneural recording was performed. MEPs in biceps brachii increased during 40 min of ischaemia of the forearm and returned to control values by 20 min of recovery. FDI MEPs decreased during ischaemia until the muscle was completely paralysed 40 min after blood flow was occluded, were slightly above control values by 10 min of recovery and returned to control values 20 min after blood flow was restored.
Data analysis
The responses to each series of 10 magnetic stimuli were averaged with the nerve signal root mean square (RMS) processed (using a sliding 3.2 ms window). The amplitude and duration of the peripheral motor and sensory nerve evoked potentials and the hand MEP were measured using cursor placement at high temporal resolution. EMG responses in FDI, ADM and AP or thenar muscles were monitored in all experiments. However, only the data for the target muscle innervated by the penetrated nerve fascicle were included in the analysis. For statistical analysis the five control series were averaged, as were the first and second halves of the ischaemic period and the first and second halves of the recovery period. The initial series of stimuli in both the first half of the ischaemia and recovery periods were not included in these averages. The first was delivered immediately following the inflation of the cuff when there was essentially no ischaemia and the second directly after the release of the cuff before full blood flow had resumed in the arm. The five periods were analysed using repeated measures ANOVA with Student-Newman-Keuls post hoc analysis, with differences considered significant when P < 0.05.
RESULTS
Seven experiments were conducted, with six intraneural recordings made in the ulnar nerve and one in the median nerve. Despite the proximity of the biceps muscle to the intraneural electrode, the recording site remained secure throughout almost all experiments. In some subjects each stimulus immediately following the release of ischaemia produced high amplitude twitch responses throughout the arm, with the size of responses and the muscles involved varying from subject to subject. In one subject the movement produced was sufficient to dislodge the microelectrode from its recording position and so data from the recovery series were not available, although control and ischaemic data were included in the results. In one other subject the movements produced in the recovery series between 1 and 4 min caused large artefact contamination of other signals, so these were not included in the results given below.
Single subject data
The data in Fig. 4 were taken from the average of a single control series in one subject. In the top intraneural signal two peaks are clear. The first peak corresponds to the descending motor nerve evoked potential or NEPm, as this volley precedes all the EMG responses in the lower traces. The second peak reflects the ascending sensory nerve evoked potential (NEPs) resulting from the stimulus-evoked contraction. These volleys can be more clearly seen in the RMS-processed nerve signal (second trace). Here, the NEPm follows the truncated stimulus artefact, and is followed in turn by a small movement artefact, presumably from the biceps brachii (as this artefact coincides with the MEP in biceps brachii seen in the bottom trace).
Figure 4. Control series of neural and muscle responses to TMS in a single subject.
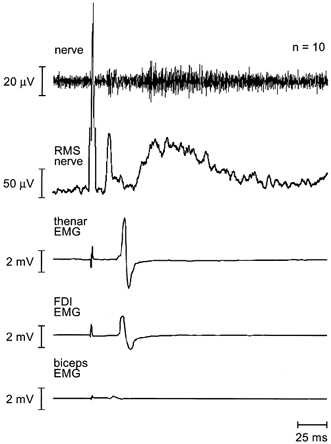
Responses (n = 10) were recorded simultaneously from the ulnar nerve, thenar muscles, first dorsal interosseous (FDI) and biceps brachii. The top trace shows the averaged filtered nerve recording whereas the second trace shows the same nerve signal RMS-processed and then averaged. The stimulus artefact in both traces has been truncated for illustration. The next two traces show MEPs elicited in the hand muscles by TMS and the lowest trace shows a small MEP in biceps brachii. The sequence of descending neural motor volley followed by muscle response and subsequent ascending neural sensory volley can be clearly seen. The peak of the descending volley occurs before the onset of the MEP in biceps brachii.
A sequence of averaged intraneural recordings from a single subject is illustrated in Fig. 5. During the ischaemic and recovery periods there was a marked increase in the amplitude of the NEPm to the hand muscles. However, the duration of this potential remained unchanged. The changes in the NEPs reflect the ischaemic block. NEPs started to decrease after 25 min of ischaemia and was abolished by 30 min. Following the release of ischaemia, an intense afferent barrage occurred. An increased duration of the post-ischaemic NEPs can be seen from 0–7 min, with the sensory volley returning to control values by 10 min.
Pooled results
The data in Fig. 6 represent the pooled mean responses for seven subjects to each series of magnetic stimuli. When the five different periods of the experiment (as described in Methods) were compared, the amplitude of the NEPm (upper left panel) was significantly increased in the second half of the ischaemic period and the first half of recovery compared to the control period (P = 0.005). The second half of ischaemia was 30.5 % larger than the control trials. There was no significant change in the duration of the motor volley. The amplitude of the NEPs was significantly decreased in the second half of ischaemia (P < 0.001) while the duration of this volley not only showed a decrease in the second half of ischaemia (P < 0.001) but also increased significantly in the first half of recovery (P < 0.001). The MEP recorded from the intrinsic hand muscles was decreased in both amplitude and duration in the second half of ischaemia (P < 0.001). The amplitude of the MEP also showed a significant increase in the first half of recovery (P = 0.04). Thus, as the size of the MEP decreases with ischaemia the amplitude of the NEPm, recorded proximal to the site of the ischaemic block, increases.
Figure 6. Amplitude (left panel) and duration (right panel) of the peripheral neural and muscle responses to TMS.
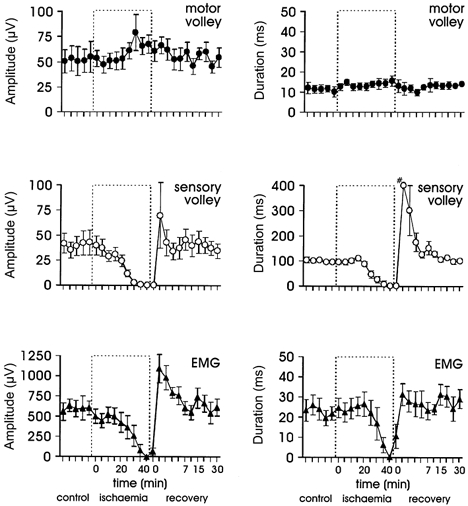
Pooled data for seven subjects. The amplitude of the sensory nerve volley (middle panel) and muscle response (bottom panel) both decreased during ischaemia whereas that of the peripheral motor nerve volley (top panel) increased. These changes during the second half of ischaemia were significantly different to the control period. The duration of the volley in the second half of ischaemia was significantly decreased for both the muscle response (bottom panel) and the sensory potential (middle panel), with the latter also significantly increased in the first half of the recovery. Note: # this data point does not reflect the true duration of the sensory volley but is the limit of the averaging window. An intense continuous sensory barrage following the release of the cuff obscured the end of the sensory volley over this period.
Changes in the cutaneous sensory thresholds of the finger and wrist reflect the expected distal-proximal gradient of ischaemic block. Pooled data for the finger tip site showed complete anaesthesia by 25 min of ischaemia. However, sensation at the wrist was not completely abolished. The threshold for cutaneous sensation increased to 165 % of control levels at 40 min of ischaemia.
DISCUSSION
In this study we recorded for the first time the peripheral nerve volley evoked by transcranial magnetic stimulation (TMS) during ischaemic block of the forearm and hand. In addition to the descending peripheral motor volley (NEPm), we also recorded an ascending sensory volley (NEPs) subsequent to the evoked muscle twitch. During ischaemia, we found that the peripheral neural volley to the hand was increased during anaesthesia and paralysis of the hand. A number of studies have previously reported increases in the size of the motor-evoked potentials (MEPs) of muscles proximal to an ischaemic block and have inferred expansion of the area of motor cortex activating these muscles. Our findings suggest that any change in the motor cortical representation of proximal muscles such as biceps brachii does not occur through a decrease in the motor representation of the hand; rather there may be a more general increase in cortical excitability. Muscles proximal to an ischaemic block may have increased cortical representations for up to 30 min after the resumption of blood flow. Here, the motor neural volley did not return to control size until the second half of recovery, more than 7 min after blood flow resumed. However, intense peripheral paraesthesiae accompany the release of the ischaemic block. This afferent barrage is likely to alter the excitability of spinal motoneurones, as well as acting on the motor cortex, and thus obscure the time course of recovery from any cortical changes associated with anaesthesia and paralysis of the hand.
Some questions of methodology arise from our experiments. One question involves the destination of the descending neural volley. The ulnar and median nerves in the upper arm carry axons innervating skin and muscle in both the forearm and the hand. If the recorded descending volleys largely activated forearm muscles rather than hand muscles the effects of ischaemia might have been diminished. To counter this problem, we were careful to locate the intraneural electrode in a site in which intraneural stimulation at the lowest intensities elicited motor responses in the hand but with minimal cutaneous sensations. The disappearance of the sensory volley as the hand became anaesthetised in the later stages of the ischaemic block further confirmed appropriate placement of the electrode in nerve fascicles innervating the hand. A second possible problem in the neural recording is movement or EMG artefact from twitches in nearby muscle. To minimise EMG responses in the nearby biceps brachii we used the lowest intensities of magnetic cortical stimulation that still evoked appropriate responses in the hand muscles. Although occasional small biceps twitches still occurred in most subjects, in one subject TMS did not evoke any responses in biceps brachii. When twitches did occur, filtering of the neural signal was usually successful in eliminating the low-frequency movement artefacts. The possibility of contamination of the descending motor volley by EMG responses cannot be completely ruled out. However, the behaviour of the nerve volley during ischaemia was the same in the subject in whom there was no twitch evoked in biceps brachii as it was in those in whom there was a twitch. Furthermore, the longer latency of the MEP in biceps brachii compared to the nerve volley suggests that the onset and peak in the neural recording are clear of artefact. A final question is the stability of the recording site, which needed to be maintained for more than 40 min. Although we had anticipated that this might be a problem, these multi-unit recordings were remarkably stable, with similar muscle spindle afferent activity evoked by stretches at the beginning and end of almost all experiments. Motor and sensory volleys recorded in response to TMS were also very similar before ischaemia and after 15–20 min of recovery. In only one subject was the intraneural site lost by very large proximal twitches evoked by TMS in the period immediately after the resumption of blood flow. Thus, we are confident that the initial response recorded through the intraneural electrode was the descending motor volley to the hand and that a decrease in the volley would have been detected.
As expected, while blood flow to the hand was blocked by the cuff inflated around the forearm, subjects gradually lost cutaneous sensation in the hand in a distal to proximal gradient. At the same time, muscle afferent responses to stretch of the intrinsic muscles of the hand were abolished. The sensory volley that followed the twitch evoked by TMS tended to decrease sooner than the MEP in the hand muscles. This is consistent with the greater susceptibility to ischaemia of afferent than efferent axons (Mogyoros et al. 1997). MEPs in the hand muscles were abolished in all subjects after 35 min of ischaemia. In contrast, the evoked motor potential recorded from the peripheral nerve above the block did not decrease but was increased during the latter part of ischaemia. This increase indicates an increased response within the central nervous system to the cortical stimulus. Either the stimulus evoked additional cortical output to the hand or the responsiveness of the motoneurone pool was increased.
Previous studies that have examined MEPs in biceps brachii and deltoid during forearm ischaemic block have demonstrated increases in size after 20–35 min of ischaemia and also increases in the number of sites on the scalp that can evoke the responses. These increased responses in muscles proximal to the block are believed to be due to an increased responsiveness of neurones in the cortex to the magnetic stimulus rather than to a change in α-motoneurone excitability at the spinal level. In the lower limb, H-reflexes and EMG responses in vastus medialis to transcranial electrical stimulation and to electrical spinal stimulation do not grow during below-knee ischaemia (Brasi-Neto et al. 1993). It has been proposed that the changes in cortical excitability come about through a decrease in afferent input from the anaesthetised limb leading to decreased inhibition of neighbouring areas within the cortex (Jacobs & Donoghue, 1991; Ziemann et al. 1998a,b; Sanes & Donoghue, 2000). Existing excitatory connections between cortical areas may be unmasked by the decreased inhibition (Ziemann et al. 1998a). In primates, collaterals of pyramidal cells in the motor cortex form both excitatory and inhibitory connections with other pyramidal cells at distances of up to 3 mm (Ghosh & Porter, 1988).
Our finding of an increase in the cortically evoked motor nerve volley to the deafferented hand at the same time as the increase in MEPs to muscles proximal to the block might suggest that instead of a specific expansion of the motor cortical areas close to the hand area, there may be a more general increase in cortical excitability. However, previous studies suggest a largely targeted effect. With ischaemic block of the forearm, increases in MEPs were more pronounced in biceps brachii than in deltoid and a muscle in the contralateral hand was not affected (Brasil-Neto et al. 1992). When changes did occur in the number of sites on the scalp from which MEPs could be evoked in deltoid, the expansion was not symmetrical, as might be expected with a general increase in excitability, but was towards the hand area (Brasil-Neto et al. 1993). Furthermore, the threshold stimulus intensity at the optimal site of stimulation was unchanged. With ischaemic block of the lower leg, increased MEPs did occur in muscles in the contralateral leg but the effect was small compared to the increase in MEPs in a muscle proximal to the block (Brasil-Neto et al. 1993).
When the cortical representation of one muscle grows the neighbouring representation does not necessarily shrink. When a GABA antagonist was applied to decrease inhibition in the forelimb area of motor cortex in the rat, microstimulation within the neighbouring vibrissa (face) area soon elicited responses in the forelimb (Jacobs & Donoghue, 1991). That is, there was an expansion of the area of motor cortex from which a response in the forelimb could be elicited. This was thought to occur because excitatory connections from the vibrissa area to the forelimb area were now disinhibited. However, stimulation within the vibrissa area continued to also evoke responses in the vibrissae (Jacobs & Donoghue, 1991). The finding in the current study that the peripheral neural volley to the hand evoked by magnetic stimulation increased at the time when the MEP in more proximal muscles also increased, suggests that similar events may occur in the human cortex during acute ischaemic block. Stimulation of an area of motor cortex that previously elicited responses in only one part of the arm now elicits responses in multiple muscles.
After blood flow was allowed to resume, MEPs in the hand recovered over the first minute. After 1–2 min, subjects reported strong paraesthesiae and continuous afferent activity was recorded through the intraneural electrode. The ectopic firing of sensory axons following ischaemia is well known (Bostock et al. 1994); ectopic motor activity was also apparent in some subjects. During this period of intense afferent activity, MEPs in the hand muscles were significantly increased in size and twitches were often evoked in muscles which had previously not been activated. Each twitch evoked a sensory volley that was greatly increased in duration over control values. During the initial part of recovery, the evoked motor nerve volley was also increased in size compared to control values. While this might in part represent a continuation of the increase in size seen during the ischaemic period, the high levels of afferent activity must also affect both the motoneurone pool and the cortex and may contribute to the increased efferent volley.
The changes in responses to TMS following acute deafferentation mimic some of the changes that follow chronic deafferentation, but some alterations after amputation or spinal cord injury are not seen acutely. For example, the threshold intensity of stimulation needed to elicit a response in a muscle proximal to the lesion becomes lower and a change in intracortical inhibition, which is thought to be mediated through GABAA receptors, can sometimes be identified with paired-pulse testing (Chen et al. 1998; Capaday et al. 2000; Schwenkreis et al. 2000). These changes presumably represent the ongoing process of reorganisation, which is likely to involve alterations in synaptic weighting and the growth of new synapses. Positron emission tomography (PET) has shown that proximal movements are associated with abnormal increases in blood flow in both sensory and motor areas of cortex in amputees (Kew et al. 1994) and that there are changes in the distribution of GABAA receptors (Capaday et al. 2000). Clearly, our experiments using temporary deafferentation in human subjects can only reveal acute changes in cortical responsiveness and do not allow prediction of any long-term changes to motor cortical neurones supplying amputated or deafferented and paralysed muscles. However, two recent studies suggest that even in the longer term deafferented areas of cortex may retain function in human subjects. First, in patients with peripheral nerve injuries, Moore & Schady (2000) performed intraneural microstimulation in the injured nerve proximal to the lesion. Subjects' perceptions of these stimuli were normal despite anaesthesia in the territory of the nerve. That is, subjects reported sensations that they referred to the anaesthetised area. This suggests that the area of sensory cortex receiving these inputs continued to function normally after 3–14 weeks of deafferentation. Second, Röricht & Meyer (2000) have shown that after more than 20 years following hand or arm amputation, magnetic stimulation of the hand area of motor cortex can inhibit the ipsilateral hand muscles in a manner not different to that in control subjects. This demonstration of appropriate interhemispheric responses suggests that the motor cortical representation of the amputated hand is not entirely obliterated but has residual function.
Under some conditions there can be a trade-off in neighbouring representations in the motor cortex. In patients with hemifacial spasm the representation of the face is large, whereas that of hand muscles is abnormally small. When the facial spasm is treated with botulinum toxin both representations return to normal size (Liepert et al. 1999). Furthermore, chronic decreases in afferent input may decrease the area of cortical motor maps. Immobilisation lasting for weeks decreased the area of motor cortex from which responses in tibialis anterior could be evoked (Zanette et al. 1997). However, the current study shows that acute anaesthesia and paralysis of the hand causes no immediate decrease in the ability of the motor cortex to produce output to the hand despite the increase in the area of cortex from which the more proximal muscles can be activated. The responsiveness of the hand area itself may be increased by the altered afferent input.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Program Grant 963206).
REFERENCES
- Bostock H, Burke D, Hales JP. Differences in behaviour of sensory and motor axons following release of ischaemia. Brain. 1994;117:225–234. doi: 10.1093/brain/117.2.225. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Capaday C, Richardson MP, Rothwell JC, Brooks DJ. Long-term changes of GABAergic function in the sensorimotor cortex of amputees. A combined magnetic stimulation and 11C-flumazenil PET study. Experimental Brain Research. 2000;133:552–556. doi: 10.1007/s002210000477. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. Journal of Neuroscience. 1998;18:3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Knorr U, Werhahn KJ, Schlaug G, Kunesch E, Cohen LG, Seitz RJ, Benecke R. Multimodal output mapping of human central motor representation on different spatial scales. Journal of Physiology. 1998;512:163–179. doi: 10.1111/j.1469-7793.1998.163bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Experimental Brain Research. 1990;79:492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Cohen LG, Dang N, Findley TW, Haghighi S, Oro J, Hallett M. Physiological analysis of motor reorganization following lower limb amputation. Electroencephalography and Clinical Neurophysiology. 1992;85:53–60. doi: 10.1016/0168-5597(92)90102-h. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Porter R. Morphology of pyramidal neurones in monkey motor cortex and the synaptic actions of their intracortical axon collaterals. Journal of Physiology. 1988;400:593–615. doi: 10.1113/jphysiol.1988.sp017138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Ridding MC, Rothwell JC, Passingham RE, Leigh PN, Sooriakumaran S, Frackowiak RS, Brooks DJ. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. Journal of Neurophysiology. 1994;72:2517–2524. doi: 10.1152/jn.1994.72.5.2517. [DOI] [PubMed] [Google Scholar]
- Levy WJJ, Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Research. 1990;510:130–134. doi: 10.1016/0006-8993(90)90738-w. [DOI] [PubMed] [Google Scholar]
- Liepert J, Oreja-Guevara C, Cohen LG, Tegenthoff M, Hallett M, Malin JP. Plasticity of cortical hand muscle representation in patients with hemifacial spasm. Neuroscience Letters. 1999;272:33–36. doi: 10.1016/s0304-3940(99)00574-1. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D, Bostock H. Excitability changes in human sensory and motor axons during hyperventilation and ischaemia. Brain. 1997;120:317–325. doi: 10.1093/brain/120.2.317. [DOI] [PubMed] [Google Scholar]
- Moore CE, Schady W. Investigation of the functional correlates of reorganization within the human somatosensory cortex. Brain. 2000;123:1883–1895. doi: 10.1093/brain/123.9.1883. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Reorganisation in human motor cortex. Canadian Journal of Physiology and Pharmacology. 1995;73:218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalography and Clinical Neurophysiology. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Röricht S, Meyer BU. Residual function in motor cortex contralateral to amputated hand. Neurology. 2000;54:984–987. doi: 10.1212/wnl.54.4.984. [DOI] [PubMed] [Google Scholar]
- Röricht S, Meyer BU, Niehaus L, Brandt SA. Long-term reorganization of motor cortex outputs after arm amputation. Neurology. 1999;53:106–111. doi: 10.1212/wnl.53.1.106. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annual Review of Neuroscience. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Experimental Brain Research. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Dertwinkel R, Zenz M, Malin J, Tegenthoff M. Changes of cortical excitability in patients with upper limb amputation. Neuroscience Letters. 2000;293:143–146. doi: 10.1016/s0304-3940(00)01517-2. [DOI] [PubMed] [Google Scholar]
- Streletz LJ, Belevich JK, Jones SM, Bhushan A, Shah SH, Herbison GJ. Transcranial magnetic stimulation: cortical motor maps in acute spinal cord injury. Brain Topography. 1995;7:245–250. doi: 10.1007/BF01202383. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Nerves and Nerve Injuries. Edinburgh: Churchill Livingstone; 1978. [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalography and Clinical Neurophysiology. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Thickbroom GW, Mastaglia FL. Topography of excitatory and inhibitory muscle responses evoked by transcranial magnetic stimulation in the human motor cortex. Neuroscience Letters. 1993;154:52–56. doi: 10.1016/0304-3940(93)90169-l. [DOI] [PubMed] [Google Scholar]
- Wu CWH, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. Journal of Neuroscience. 1999;19:7679–7697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalography and Clinical Neurophysiology. 1997;105:269–279. doi: 10.1016/s0924-980x(97)00024-6. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. Journal of Neuroscience. 1998a;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. Journal of Neuroscience. 1998b;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


