Abstract
Astronauts returning from space have reduced red blood cell masses, hypovolaemia and orthostatic intolerance, marked by greater cardio–acceleration during standing than before spaceflight, and in some, orthostatic hypotension and presyncope. Adaptation of the sympathetic nervous system occurring during spaceflight may be responsible for these postflight alterations. We tested the hypotheses that exposure to microgravity reduces sympathetic neural outflow and impairs sympathetic neural responses to orthostatic stress. We measured heart rate, photoplethysmographic finger arterial pressure, peroneal nerve muscle sympathetic activity and plasma noradrenaline spillover and clearance, in male astronauts before, during (flight day 12 or 13) and after the 16 day Neurolab space shuttle mission. Measurements were made during supine rest and orthostatic stress, as simulated on Earth and in space by 7 min periods of 15 and 30 mmHg lower body suction. Mean (± s.e.m.) heart rates before lower body suction were similar pre–flight and in flight. Heart rate responses to −30 mmHg were greater in flight (from 56 ± 4 to 72 ± 4 beats min−1) than pre–flight (from 56 ± 4 at rest to 62 ± 4 beats min−1, P < 0.05). Noradrenaline spillover and clearance were increased from pre–flight levels during baseline periods and during lower body suction, both in flight (n = 3) and on post–flight days 1 or 2 (n = 5, P < 0.05). In–flight baseline sympathetic nerve activity was increased above pre–flight levels (by 10–33 %) in the same three subjects in whom noradrenaline spillover and clearance were increased. The sympathetic response to 30 mmHg lower body suction was at pre–flight levels or higher in each subject (35 pre–flight vs. 40 bursts min−1 in flight). No astronaut experienced presyncope during lower body suction in space (or during upright tilt following the Neurolab mission). We conclude that in space, baseline sympathetic neural outflow is increased moderately and sympathetic responses to lower body suction are exaggerated. Therefore, notwithstanding hypovolaemia, astronauts respond normally to simulated orthostatic stress and are able to maintain their arterial pressures at normal levels.
Astronauts experience symptoms suggestive of autonomic dysfunction, including nausea, vomiting and dizziness, during the earliest days of space missions (Young et al. 1993), and subsequently develop mild anaemia (Alfrey et al. 1996) and hypovolaemia (Johnson et al. 1977). Despite these changes, astronauts adapt remarkably well to microgravity, and their cardiovascular function remains normal (Rummel et al. 1976). However, upon return to Earth and its gravitational forces, harmful consequences of this adaptation emerge, with orthostatic presyncope occurring in up to 64 % of astronauts (Buckey et al. 1996b).
The orthostatic changes observed in astronauts on return from space travel resemble the clinical syndrome of orthostatic intolerance. The latter is encountered among otherwise healthy young adults, who experience inordinate tachycardia on standing, associated with unusually augmented sympathetic activity. Although genetic (Shannon et al. 2000) and acquired (Jacob et al. 2000) etiologies of orthostatic intolerance have recently been elucidated, most patients' pathophysiology remains poorly understood. A major rationale for the autonomic studies conducted during the Neurolab mission was that a better understanding of the altered physiology that leads to postflight orthostatic symptoms in astronauts might help elucidate pathophysiology in patients with orthostatic intolerance.
Ours is one of three articles (Cox et al. 2002; Levine et al. 2002) reporting autonomic studies performed on astronauts who participated in the Neurolab space shuttle mission. We conducted this research because prior studies of sympathetic nervous system function in space yielded conflicting results. Although postflight plasma noradrenaline levels in astronauts are consistently elevated in supine and upright tilt positions (Whitson et al. 1995; Fritsch–Yelle et al. 1996), in–flight catecholamine levels (compared with pre–flight levels in the supine position) have been shown to be decreased (Leach et al. 1983), unchanged (Leach et al. 1996), or increased (Kvetňansky et al. 1991; Norsk et al. 1995).
We report the first comprehensive measurements of human sympathetic nervous system function in space, comprising plasma noradrenaline concentrations, noradrenaline spillover and clearance and directly recorded muscle sympathetic nerve activity, during supine rest and orthostatic stress, simulated on Earth and in space by lower body negative pressure. We tested the hypotheses that microgravity exposure reduces sympathetic neural outflow and impairs sympathetic neural responses to orthostatic stress. Some of these results have been published in abstract form (Ertl & Neurolab Autonomic Team Investigators, 1998).
METHODS
Subjects
This study was performed as part of the integrated series of the human autonomic investigations of the Neurolab space shuttle mission, as described in detail by Cox et al. (2002). We studied six male astronauts whose mean (± s.e.m.) age was 40 ± 2 years, height 187 ± 2 cm and weight 89.3 ± 3.7 kg. This study conformed with the Declaration of Helsinki, and all subjects signed consent forms approved by the National Aeronautics and Space Administration (NASA) Human Subjects Review Committee and the institutional review boards of the principal investigators' institutions. Four subjects participated in all pre–flight, in–flight and postflight sessions. One additional subject participated in pre–flight sessions and sessions 1 and 5 days after landing, with measurements of noradrenaline kinetics. A sixth subject participated in pre– and postflight sessions, but without measurements of sympathetic nerve activity or noradrenaline kinetics. We indicate the number of subjects for each measurement; because of NASA restrictions and technical difficulties, not all astronauts were studied in all protocols.
Heart rate and arterial pressure
Heart rate was derived from a surface electrocardiogram. Finger arterial pressure was measured by finger photoplethysmography (on Earth, Finapres, Ohmeda, Englewood, CO, USA; in space, Finapres, as modified by TNO TPD Biomedical Instrumentation (BMI) Academic Medical Center, Amsterdam, The Netherlands, for the European Space Agency).
Noradrenaline spillover and clearance
Left and right antecubital veins were cannulated for tritiated noradrenaline ([3H]NA) infusion and blood drawing. One–hundred microcuries of [3H]NA (l–[ring 2,5,6–3H] noradrenlaline, Du Pont–NEN, Boston, MA, USA) in 1 ml of ethanol was stored at −20 °C, and then mixed with 55 ml normal saline just prior to performance of the protocols. The infusion syringe contained approximately 13.5 ng ml−1 of tracer noradrenaline. The infusion was initiated with a 13.5 ml (24.0 μCi) loading dose given over 1.5 min. The steady–state maintenance infusion rate was 0.45 ml min−1 (0.81 μCi min−1), delivered with a Baxter INFUSO.R infusion pump (Baxter Healthcare Corp., Deerfield, IL, USA). The infusion was begun at least 30 min prior to the first blood sampling, during baseline recordings prior to lower body suction.
With each blood sampling, two 5 ml samples were drawn into vacuum gel separator tubes containing 1.8 mg ml−1 EGTA and 1.2 mg ml−1 glutathione (Boomsma et al. 1993). Samples were placed on ice on Earth, or in a −4 °C methocel gel pack in space and centrifuged within 30 min, at 1295 g for 15 min. The samples were then placed in a −70 °C freezer on Earth, or in a −20 °C freezer in space until landing, when they were transferred to a −70 °C freezer. Samples were analysed for noradrenaline by high precision liquid chromatography with electrochemical detection (HPLC). The 3H activity in samples was assayed by liquid scintillation counting (LS 6000IC, Beckman Instruments, Fullerton, CA, USA).
Whole–body noradrenaline clearance, expressed in litres per minute, was calculated as [3H]NA infusion rate/plasma [3H]NA specific activity.
Whole–body noradrenaline spillover, expressed in micrograms per minute, was calculated as whole–body noradrenaline clearance × plasma noradrenaline concentration.
Muscle sympathetic nerve activity
Peroneal nerve muscle sympathetic activity was recorded as described previously (Wallin & Eckberg, 1982). Briefly, the nerve was located with cutaneous electrical stimulation (H–Reflex Stimulator, Canadian Space Agency). A tungsten reference electrode (FHC, Bowdoinham, MA, USA) was inserted subcutaneously, ∼2 cm from the nerve, and a tungsten recording electrode with an uninsulated tip diameter of ∼2 μm was inserted through the skin near the nerve. Adjustments of the recording electrode position were made according to auditory signals generated by impaled nerves. Both electrodes were connected in series to a differential preamplifier and an amplifier (NASA, Houston, TX, USA), isolated by two 100 μA current limiters. The nerve signal was amplified (total gain 70 000 to 160 000), rectified, band–pass filtered (700–2000 Hz) and integrated (time constant 0.1 s) to obtain mean voltage neurograms. Satisfactory recordings of muscle sympathetic nerve activity were defined by pulse–synchronous bursts that increased during end–expiratory apnoea or Valsalva straining and did not change during tactile or auditory stimulation.
Sympathetic bursts were identified initially by software written by A. Diedrich, Vanderbilt University, in PV Wave (Visual Numerics Inc., Houston, TX, USA). An automated detection algorithm included artefact elimination, dynamic noise level detection and signal–to–noise estimation. Bursts were selected if the signal–to–noise ratio was greater than 2:1, and bursts occurred about 1.3 s after the previous (one removed) electrocardiographic R wave. The computer selection of sympathetic bursts was overseen and corrected by two experienced microneurographers. Muscle sympathetic nerve activity is expressed in bursts per minute and burst height (each burst recorded during lower body suction was normalized by dividing it by the average burst height prior to the beginning of suction).
Lower body negative pressure
Suction was applied with subjects supine in lower body chambers sealed at the iliac crests. The chamber used on Earth was made of rigid plastic, and the chamber used in space, developed by the Deutsche Agentur Raumfahrtagelegenheiten (DARA), was made of collapsible fabric. Both chambers had windows to allow leg access for microneurography. After 7 min of baseline recording, steady pressure was applied at −15 and −30 mmHg, in fixed order, for 7 min each. Data were analysed during the third to fifth minute of each 7 min period, and blood was drawn at the end of each segment.
Physiological signals were digitized on–line during pre– and postflight sessions with Windaq hardware and software (DA–220, DATAQ Instruments, Akron OH, USA) and during in–flight sessions, with data acquisition programs developed by NASA. In–flight signals were down–linked to Earth to allow investigators to monitor cardiovascular parameters and assist astronauts to evaluate the quality of sympathetic nerve recordings.
Statistical analyses
Statistical comparisons were made with a repeated measures analysis of variance (SigmaStat, SPSS, Chicago, IL, USA). For these analyses, the time factor was the level of lower body pressure (0 (baseline), −15, and −30 mmHg), and the treatment factor was the experimental day. When post hoc analysis was appropriate, multiple comparisons were made with Tukey's honestly significant difference test. If the analysis resulted in unequal variance, Friedman's repeated measures analysis of variance on ranks and Dunnett's method of post hoc multiple comparisons were used. Significance was set at P < 0.05 to accept or reject our null hypotheses.
RESULTS
Heart rate and arterial pressure
Haemodynamic measurements before and during lower body suction in pre– and in–flight sessions are depicted in Fig. 1. Baseline heart rate was similar pre– and in–flight. As expected, heart rate increased during 15 and 30 mmHg of lower body suction in all sessions (P < 0.05). This cardio–acceleration was greater during in–flight than during pre– or postflight sessions (P < 0.05). Arterial pressure changes are given in Table 1. Systolic and diastolic pressures did not change significantly during lower body suction in any session. Diastolic pressure increased significantly more during lower body suction in flight (Fig. 1, middle panel) and 1 day after landing, compared with pre–flight changes (both P < 0.05).
Figure 1. Haemodymic responses to lower body suction.
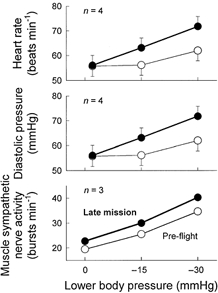
Although average baseline heart rates and diastolic pressures were similar during pre– and in–flight sessions, increments during graded lower body suction were greater in space. All three astronauts had more muscle sympathetic bursts per minute before and during lower body suction in space than on Earth.
Table 1.
Arterial pressure responses to lower body negative pressure (n =4)
| Level | −15 mmHg | −30 mmHg | ||||
|---|---|---|---|---|---|---|
| Session | Systolic | Diastolic | Mean | Systolic | Diastolic | Mean |
| Pre-flight | −1 ± 2 | −1 ± 1 | −1 ± 1 | 0 ± 2 | 1 ± 1 | 1 ± 1 |
| In-flight, day 12 or 13 | 2 ± 3 | 5 ± 2* | 4 ± 2 | 0 ± 2 | 7 ± 3* | 3 ± 2 |
| Day after landing | 10 ± 4* | 6 ± 2* | 7 ± 3* | 11 ± 5* | 9 ± 2* | 10 ± 3* |
| 5 days after landing | 0 ± 3 | 2 ± 2 | 1 ± 2 | 4 ± 4 | 7 ± 2 | 6 ± 3 |
Values are means ± s.e.m.
P< 0.05 for variable on test day relative to pre–flight and 5 days after landing.
Muscle sympathetic nerve activity
Figure 2 depicts muscle sympathetic nerve activity in one astronaut, recorded 73 days pre–flight and 13 days in flight. This subject's muscle sympathetic nerve activity prior to lower body suction increased from 15 bursts min−1 pre–flight, to 20 bursts min−1 in flight. His muscle sympathetic nerve activity increased further during −15 and −30 mmHg lower body pressure, and these increases also were greater during in– than pre–flight sessions (19 vs. 32 and 39 vs. 48 bursts min−1). Average muscle sympathetic nerve activity before and during spaceflight for all three astronauts is depicted in Fig. 1 (bottom panel). Table 2 lists all sympathetic nerve measurements.
Figure 2. Recordings before and during 30 mmHg lower body suction from one astronaut.

All three astronauts had greater sympathetic responses to lower body suction in space than on Earth.
Table 2.
Muscle sympathetic responses to lower body suction
| ∼72 days pre-flight | Mission day 12 or 13 | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | Astronaut | Baseline | 15 mmHg | 30 mmHg | Baseline | 15 mmHg | 30 mmHg |
| Normalized average sympathetic burst area | 2 | 1 | 1.2 | 1.4 | 1 | 0.9 | 1.0 |
| 3 | 1 | 1.0 | 1.0 | 1 | 0.8 | 0.4 | |
| 4 | 1 | 1.6 | 1.7 | 1 | 1.8 | 2.1 | |
| Mean ±s.d. | 1.3 | 1.4 | 1.2 | 1.2 | |||
| Integrated sympathetic burst area (15 s)−1 | 2 | 5.7 | 8.4 | 12.5 | 6.4 | 7.1 | 10.1 |
| 3 | 5.2 | 7.8 | 8.3 | 5.7 | 5.2 | 3.4 | |
| 4 | 3.7 | 7.4 | 16.2 | 5.0 | 14.7 | 25.2 | |
| Mean ±s.d.. | 4.9 | 7.9 | 12.4 | 5.7 | 9.0 | 12.9 | |
| Percentage of heart beats with a sympathetic burst | 2 | 50.3 | 60.1 | 68.0 | 48.2 | 48.7 | 56.1 |
| 3 | 35.4 | 57.0 | 50.5 | 45.4 | 48.2 | 48.9 | |
| 4 | 24.5 | 28.9 | 54.7 | 31.6 | 48.6 | 64.0 | |
| Mean ±s.d.. | 36.7 | 48.6 | 57.8 | 41.7 | 48.5 | 56.3 | |
Plasma noradrenaline and noradrenaline kinetics
Table 3 lists all measurements of muscle sympathetic nerve activity, plasma noradrenaline, and plasma noradrenaline spillover and clearance for all astronauts, for all sessions. In the same three astronauts who underwent sympathetic microneurography, baseline plasma noradrenaline concentrations were higher in flight than pre–flight (range of increases 35–169 %, Fig. 3). In these three astronauts, adrenaline levels at 0 (baseline), 15 and 30 mmHg lower body suction were slightly higher during pre– than in–flight sessions (49, 45 and 58 vs. 38, 43 and 55 pg ml−1). In five astronauts, plasma noradrenaline concentrations and whole body noradrenaline spillover and clearance were significantly higher (P < 0.05) 1 day after landing than ∼72 days pre–flight.
Table 3.
Baseline sympathetic function and sympathetic responses to lower body negative pressure
| Session | Astronaut | Baseline | 15 mmHg | 30 mmHg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSNA | NA | NAspill | NAclear | MSNA | NA | NAspill | NAclear | MSNA | NA | NAspill | NAclear | ||
| 75 days pre–flight | 1 | — | 110 | 297 | 2.8 | — | 138 | 519 | 3.8 | — | 215 | 602 | 2.8 |
| 2 | 23 | 223 | 435 | 2.0 | 27 | 211 | 506 | 2.4 | 36 | 299 | 705 | 2.4 | |
| 3 | 21 | 282 | 849 | 3.0 | 31 | 267 | 786 | 3.0 | 30 | 431 | 1016 | 2.4 | |
| 4 | 15 | 95 | 244 | 2.6 | 19 | 120 | 304 | 2.5 | 39 | 272 | 524 | 1.9 | |
| 5 | — | 137 | 413 | 3.0 | — | 159 | 598 | 3.8 | — | 203 | 596 | 2.9 | |
| Mean | 20 | 169 | 448 | 2.7 | 26 | 179 | 543 | 3.1 | 35 | 284* | 689* | 2.5 | |
| ±s.e.m. | ± 36 | ± 106 | ± 0.2 | ± 27 | ± 78 | ±0.3 | ± 41 | ± 87 | ± 0.2 | ||||
| In-flight, day 12 or 13 | 1 | — | — | — | — | — | — | — | — | — | — | — | — |
| 2 | 26 | 419 | 901 | 2.4 | 30 | 576 | 1734 | 3.0 | 41 | 457 | 1019 | 2.2 | |
| 3 | 23 | 283 | 770 | 2.8 | 28 | 361 | 1303 | 3.6 | 32 | 439 | 1433 | 3.3 | |
| 4 | 20 | 163 | 1054 | 6.6 | 32 | 162 | 1069 | 6.7 | 48 | 207 | 1219 | 5.9 | |
| 5 | — | — | — | — | — | — | — | — | — | — | — | — | |
| Mean | 23 | 288 | 908 | 3.9 | 30 | 366 | 1369 | 4.4 | 40 | 368 | 1224 | 3.8 | |
| Day after landing | 1 | — | 164 | 645 | 3.9 | — | 185 | 756 | 4.1 | — | 222 | 1035 | 4.7 |
| 2 | — | 419 | 1007 | 2.4 | — | 576 | 1734 | 6.7 | — | 457 | 1019 | 2.2 | |
| 3 | — | 283 | 770 | 2.8 | — | 361 | 1303 | 2.7 | — | 439 | 1433 | 3.3 | |
| 4 | — | 163 | 1054 | 6.6 | — | 162 | 1069 | 3.0 | — | 207 | 1219 | 5.9 | |
| 5 | — | 124 | 473 | 3.8 | — | 183 | 489 | 3.6 | — | 230 | 467 | 2.0 | |
| Mean | — | 231 | 790† | 3.9† | — | 293 | 1070 | 4.0† | — | 311* | 1035† | 3.6† | |
| ±s.e.m. | ± 54 | ± 109 | ± 0.7 | ± 79 | ± 216 | ± 0.7 | ± 56 | ± 160 | ± 0.7 | ||||
| 5 days after landing | 1 | — | 125 | 481 | 3.8 | — | 168 | 704 | 4.4 | — | 215 | 816 | 3.8 |
| 2 | — | 230 | 616 | 2.7 | — | 316 | 554 | 1.8 | — | 344 | 475 | 1.4 | |
| 3 | — | 233 | 484 | 2.1 | — | 257 | 653 | 2.5 | — | 247 | 481 | 2.0 | |
| 4 | — | 203 | 676 | 3.4 | — | 185 | 441 | 2.4 | — | 282 | 535 | 1.9 | |
| 5 | — | 120 | 433 | 3.6 | — | 120 | 428 | 3.6 | — | 180 | 564 | 3.1 | |
| Mean | — | 182 | 538 | 3.1 | — | 209 | 556 | 2.9 | — | 254 | 574 | 2.4* | |
| ±s.e.m. | ± 25 | ± 46 | ± 0.3 | ± 35 | ± 55 | ± 0.5 | ± 28 | ± 63 | ± 0.5 | ||||
Mean ±s.e.m. data from five astronauts. NA,noradrenaline (pg ml−1)MSNA,muscle sympathetic nerveactivity (bursts min−1);NAspill, noradrenaline spillover (μg ml−1). Naclear, noradrenaline clearance (l min−1). No statistical analyses were performed for in–flight measurements (n =3).
P<0.05
P <0.01 forvariables on test day, ∼75 days pre–flight and 1 or 5 days after landing (one-way repeated measures analysisof variance with lower body pressure phase and experimental day as time and treatment factors).
Figure 3. Average noradrenaline data from three astronauts.
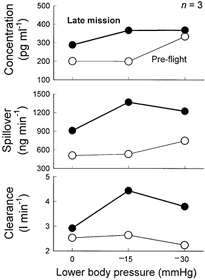
These data are from the same three subjects whose microneurography responses are depicted in Fig. 1, bottom panel.
DISCUSSION
We report the first comprehensive analysis of human sympathetic nervous system function in the microgravity of space. We measured plasma noradrenaline concentrations, whole–body noradrenaline spillover and clearance, and peroneal nerve muscle sympathetic activity in astronauts before, during and after the Neurolab space shuttle mission, during supine rest and graded lower body suction. Our findings are remarkably concordant: in space, baseline sympathetic neural outflow (however measured) is increased moderately and sympathetic responses to lower body suction are exaggerated. Therefore, notwithstanding hypovolaemia, astronauts respond normally to simulated orthostatic stress and are able to maintain their arterial pressures at normal levels.
Spaceflight provides a unique opportunity to examine mechanisms involved in cardiovascular responses to changes in gravitational forces. Two earlier observations led us to study the possibility that sympathetic adaptation during spaceflight adversely affects astronauts' orthostatic tolerance on Earth after spaceflight. Astronauts with orthostatic intolerance appear to have less augmentation of plasma noradrenaline levels (Fritsch–Yelle et al. 1996) and smaller increases of systemic vascular resistance (Buckey et al. 1996b) during standing, than astronauts with preserved orthostatic responses.
Prior studies of sympathetic function during simulated or actual microgravity
Although the basic question ‘Does microgravity reduce or increase sympathetic nervous activity?’ had not been answered prior to this study, cogent arguments were made in support of both possibilities (Robertson et al. 1994; Goldstein et al. 1995; Norsk et al. 1995; Convertino et al. 1998). There are several reasons why microgravity might reduce sympathetic neural outflow in space. First, cephalad movement of fluids no longer pulled by gravity (Watenpaugh & Hargens, 1995) might inhibit sympathetic activity by stretching cardiac chambers (Buckey et al. 1996a) and arterial baroreceptive arteries. Second, hypovolaemia might reduce entry of noradrenaline into the circulation, and thereby lead to underestimation of the degree of sympathoinhibition (Goldstein et al. 1995). Finally, absence of the episodic baroreflex–mediated sympathetic stimulation that occurs on Earth with frequent orthostatic fluid shifts might lead to transient baroreflex dysfunction in space, and exaggerated but insufficient sympathetic orthostatic responses when astronauts are re–exposed to Earth's gravitational forces (Fritsch–Yelle et al. 1996; Vernikos et al. 1996).
Actual data on sympathetic nervous system function in space are indirect, scarce and contradictory. Plasma noradrenaline levels in microgravity have been reported as decreased (Leach et al. 1983), unchanged (Leach et al. 1996) or increased (Kvetňanskyý et al. 1991; Norsk et al. 1995). Plasma catecholamine levels are useful indicators of overall sympathetic nervous activity (Robertson et al. 1979) and correlate well with muscle sympathetic nerve activity (Wallin, 1988). However, circulating noradrenaline levels reflect not only noradrenaline release from nerve endings, but also its removal from the circulation (Esler, 1993). Therefore, plasma noradrenaline concentrations may under– or over–represent sympathetic nerve activity, depending upon noradrenaline clearance. One example of such a confound is the reduction of noradrenaline clearance (and consequent augmentation of plasma noradrenaline levels) that occurs when autonomic failure patients and healthy subjects stand (Meredith et al. 1992; Jacob et al. 1998). Noradrenaline spillover measurements provide a more direct indicator of sympathetic nerve activity than plasma noradrenaline levels (Esler, 1993). However, prior to the Neurolab mission, noradrenaline spillover had not been measured in space.
Sympathetic microneurography enables direct on–line monitoring of sympathetic nervous system function, is remarkably stable in individual subjects over time (Fagius & Wallin, 1993) and yields measurements that correlate well with noradrenaline spillover (Wallin et al. 1992). Several groups used sympathetic microneurography to study the effects of microgravity, as simulated on Earth by 6 deg head–down bed rest (Vernikos et al. 1996). Shoemaker et al. (1998) reported reduced baseline muscle sympathetic nerve traffic after 14 days of bed rest. This result was surprising, because the reductions of central blood volume and cardiac filling pressures that occur during prolonged head–down bed rest (Beck et al. 1992; Convertino et al. 1994; Levine et al. 1997) should increase, not decrease sympathetic nerve activity. More recent studies documented increases of sympathetic nerve activity during 6 deg head–down bed rest (Mano et al. 1998; Shoemaker et al. 1999; Kamiya et al. 2000; Pawelczyk et al. 2001).
Neurolab results
Baseline sympathetic outflow
In three astronauts, microgravity moderately increased the number of sympathetic bursts per minute in baseline sympathetic nerve recordings. These limited data are consistent with most studies employing sympathetic microneurography during simulated microgravity on Earth and lead us to reject the first hypothesis we tested, that exposure to microgravity reduces sympathetic neural outflow. The finding of increased levels of muscle sympathetic nerve activity during the Neurolab mission suggests that the significantly increased levels of sympathetic nerve activity recorded in five astronauts after the Neurolab mission (Levine et al. 2002) are not artefacts resulting from uncontrollable stresses on landing day.
Although the number of astronauts who underwent sympathetic microneurography was very small, changes of sympathetic nerve activity were mirrored by catecholamine data. The same three astronauts had elevated plasma noradrenaline levels, compared with values obtained with subjects in the supine position on Earth (Fig. 3 and Table 3), an observation that confirms the earlier findings of Kvetňanskyý (1991) and Norsk et al. (1995) and their coworkers. However, as mentioned, elevations of plasma noradrenaline concentrations may reflect reduced clearance, as well as increased spillover. We found that noradrenaline clearance is increased in space, a change that should reduce, not increase plasma noradrenaline levels. One implication of increased noradrenaline clearance is that plasma noradrenaline levels in space underestimate increases in sympathetic nervous activity. In Neurolab astronauts, plasma noradrenaline spillover was increased more than noradrenaline clearance and therefore plasma noradrenaline levels were higher (Fig. 3 and Table 3), not lower in space.
Thus, all of our measurements – muscle sympathetic nerve activity, plasma noradrenaline concentrations and noradrenaline spillover – indicate that exposure to microgravity increases baseline sympathetic neural outflow. This finding may explain increases in calf vascular resistance documented in astronauts during brief space missions (Watenpaugh et al. 2001). Following the Neurolab mission, we continued studies of noradrenaline kinetics in five astronauts (Table 3) and found that noradrenaline spillover remains elevated for at least 1–2 days after spaceflight. We were reassured by the agreement between noradrenaline spillover measurements made 5 days post flight and those made during ∼72 days pre–flight.
Lower body suction
Lower body suction in space further augmented muscle sympathetic nerve activity, and these exaggerated increases in muscle sympathetic nerve activity were paralleled by exaggerated increases in noradrenaline spillover. The greater sympathetic responses to lower body suction in space than on Earth (where recordings also were made in the supine position) compensated for the further reduction of the already subnormal effective blood volume, induced by lower body suction, and contributed to maintenance of arterial pressure at pre–flight levels. Thus, we found no support for our second hypothesis, that sympathetic responses to simulated orthostatic stress in space are impaired. The finding of normal sympathetic responses to steady–state lower body suction is consistent with the findings of other Neurolab protocols, which documented normal sympathetic responses to abrupt reductions of baroreceptor input during Valsalva straining in space (Cox et al. 2002), and normal sympathetic and haemodynamic responses to upright tilt after spaceflight (Levine et al. 2002).
Autonomic mechanisms in space
Our haemodynamic measurements confirmed results from earlier studies conducted during simulated or actual microgravity. We found exaggerated cardioacceleration and arterial pressure increases during lower body suction, as did Beck et al. (1992) and Baisch & Petrat (1993). Johnson et al. (1977) reported that such large responses to lower body suction begin after only 2 days of spaceflight, by which time, intravascular volume has declined by 14–17 % (Leach et al. 1996). Greater cardio–acceleration may reflect increased sympathetic stimulation of the sinoatrial node (this study), increased withdrawal of vagal restraint (Cox et al. 2002), or a combination of these factors. Exaggerated responses to lower body suction might also be mediated by cardiac atrophy (Levine et al. 1997). In such a circumstance, the reduction of central blood volume caused by lower body suction would shift the operating position of the already small (because of hypovolaemia) left ventricle to a steeper portion of the Frank–Starling pressure–volume relationship and further diminish stroke volume.
Several mechanisms might explain increased noradrenaline clearance in space. Flow in the pulmonary vascular bed is altered by microgravity (Prisk et al. 1993), and the lung is importantly involved in noradrenaline spillover and clearance (Esler et al. 1990). Cephalad redistribution of fluid might also alter blood flow, capillary exchange and noradrenaline disposition in other vascular beds (Esler et al. 1990). However, if such gravitational shifts alter noradrenaline kinetics in space, it is unclear what mechanism sustains the changes during postflight measurements.
Limitations
The principal limitations of our study are shared by most research conducted in humans in space: the small numbers of subjects, and limited time available for individual research protocols. We were able to record muscle sympathetic nerve activity in only three astronauts. However, we also characterized sympathetic activity with plasma noradrenaline concentrations and noradrenaline spillover in the same astronauts. All indices of sympathetic function changed in parallel. Moreover, we were able to obtain complete sets of noradrenaline kinetic data in five subjects before the Neurolab mission and 1 day after landing. These measurements documented elevations of plasma noradrenaline concentrations and noradrenaline spillover 1 day after landing that were similar to those measured about 4 days earlier in space in three of the five astronauts (Table 3).
There were severe time constraints for all Neurolab protocols, including ours. Nonetheless, we believe that we allowed sufficient time for tritiated noradrenaline to reach a steady–state level for baseline measurements and that the 7 min stages of lower body suction also were adequate for our purposes. In a ground–based study, Baily et al. (1990) obtained comparable noradrenaline kinetic measurements after 5–10 and after 25–30 min of 15 mmHg lower body suction. The diet and fluid intakes of Neurolab astronauts were similar to those of astronauts on earlier NASA missions and were not controlled. We studied short duration spaceflight and therefore do not know if sympathetic mechanisms would have undergone further adaptation, had spaceflight been extended. As detailed in the Appendix of Cox et al. (2002) our studies followed other protocols. We doubt that preceding off–axis rotation influenced our results; although vestibular stimulation increases human muscle sympathetic nerve activity, such influences dissipate within minutes of the end of stimulation (Shortt & Ray, 1997).
In conclusion, we studied the influence of microgravity on sympathetic control mechanisms, with measurements of plasma noradrenaline concentrations, whole–body noradrenaline spillover and clearance and peroneal nerve muscle sympathetic activity on Earth and in space. In space, baseline sympathetic neural outflow (however measured) is increased moderately, and sympathetic responses to lower body suction are exaggerated. The consequence of these changes is that, notwithstanding hypovolaemia, the astronauts we studied responded normally to simulated orthostatic stress (and upright tilt on landing day) and were able to maintain their arterial pressures at normal levels.
Acknowledgments
We thank the crew of the Neurolab mission and the ground support of Drs Alex Dunlap and Chiaki Mukai, the many scientists, engineers and contractors involved, including Stuart Johnston, Mike Grande, Karen Gunter, Angelene Lee, José Limardo, Ahmat Pahtak, Russel Schultz, Will Gore, Matthew Morrow, Troy Todd, Tia Petersen, Dak Quarles, Boyce Moon, Drs William R. Carpentier, Rhea Seddon, Mel Buderer, Terry Pattinson, Luis Beck, C. Michael Stein, Murray Esler, Niels Christensen, Peter Norsk, Ron White and Jane Park. We also thank Sachin Paranjape, Sylvia Dickinson, Danielle Esler, Robert Carson, Suzanna Lonce, Bonnie Black, Varonica Watts, Dorothea Boemer and the subjects who participated in astronaut microneurography training at Vanderbilt Medical Center and Johnson Space Center. This work was supported in part by NASA grants NAS 9–19483 and NAS 9–19429 and NIH grants M01 RR00095, 5P01 HL56693, 1U01NS33460, 1U01HL53206.
REFERENCES
- Alfrey CP, Udden MM, Leach–Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. Journal of Applied Physiology. 1996;81:98–104. doi: 10.1152/jappl.1996.81.1.98. [DOI] [PubMed] [Google Scholar]
- Baily RG, Prophet SA, Shenberger JS, Zelis R, Sinoway LI. Direct neurohumoral evidence for isolated sympathetic nervous system activation to skeletal muscle in response to cardiopulmonary baroreceptor unloading. Circulation Research. 1990;66:1720–1728. doi: 10.1161/01.res.66.6.1720. [DOI] [PubMed] [Google Scholar]
- Baisch FJ, Petrat G. Body fluid distribution in man in space and effect of lower body negative pressure treatment. The Clinical Investigator. 1993;71:690–699. doi: 10.1007/BF00209722. [DOI] [PubMed] [Google Scholar]
- Beck L, Baisch F, Gaffney FA, Buckey JC, Arbeille Ph, Patat F, Ten Harkel ADJ, Hillebrecht A, Schulz H, Karemaker JM, Meyer M, Blomqvist CG. Cardiovascular responses to lower body negative pressure before, during, and after ten days head–down tilt bedrest. In: Saltin B, Baisch F, Beck L, Blomqvist CG, Karemaker JM, editors. Head–down Tilt Bedrest. HDT' 88 – An International Collaborative Effort in Integrated Systems Physiology, Acta Physiologica Scandinavica. suppl. 604. Vol. 144. 1992. pp. 43–52. [Google Scholar]
- Boomsma F, Alberts G, Van Eijk L, Man In 'T, Veld AJ, Schalekamp MADH. Optimal collection and storage conditions for catecholamine measurements in human plasma and urine. Clinical Chemistry. 1993;39:2503–2508. [PubMed] [Google Scholar]
- Buckey JC, Jr, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancey CW, Jr, Meyer DM, Blomqvist CG. Central venous pressure in space. Journal of Applied Physiology. 1996a;81:19–25. doi: 10.1152/jappl.1996.81.1.19. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. Journal of Applied Physiology. 1996b;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Doerr DF, Ludwig DA, Vernikos J. Effect of simulated microgravity on cardiopulmonary baroreflex control of forearm vascular resistance. American Journal of Physiology. 1994;266:R1962–1969. doi: 10.1152/ajpregu.1994.266.6.R1962. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Ludwig DA, Gray BD, Vernikos J. Effects of exposure to simulated microgravity on neuronal catecholamine release and blood pressure responses to norepinephrine and angiotensin. Clinical Autonomic Research. 1998;8:101–110. doi: 10.1007/BF02267820. [DOI] [PubMed] [Google Scholar]
- Cox JF, Tahvanainen KUO, Kuusela TA, Levine BD, Cooke WH, Mano T, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC, Jr, Baisch FJ, Blomqvist CG, Robertson D, Eckberg DL. Influence of microgravity on astronauts' sympathetic and vagal responses to Valsalva's manoeuvre. Journal of Physiology. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl AC Neurolab Autonomic Team Investigators. Sympathetic response to orthostatic stress is preserved in space. Circulation. 1998;98(suppl. S):I–471. [Google Scholar]
- Esler M. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacology and Toxicology. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological Reviews. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long–term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clinical Autonomic Research. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Fritsch–Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology. 1996;81:2134–2141. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Vernikos J, Holmes C, Convertino VA. Catecholaminergic effects of prolonged head–down bed rest. Journal of Applied Physiology. 1995;78:1023–1029. doi: 10.1152/jappl.1995.78.3.1023. [DOI] [PubMed] [Google Scholar]
- Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. New England Journal of Medicine. 2000;343:1008–1014. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. Journal of Applied Physiology. 1998;84:914–921. doi: 10.1152/jappl.1998.84.3.914. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Driscoll TB, Leblanc AD. Blood volume changes. In: Johnston RS, Dietlein LF, editors. Biomedical Results from Skylab. Washington, DC, USA: National Aeronautics and Space Administration; 1977. pp. 235–241. [Google Scholar]
- Johnson RL, Hoffler GW, Nicogossian AE, Bergman SA, Jr, Jackson MM. Lower body negative pressure: third manned Skylab mission. In: Johnston RS, Dietlein LF, editors. Biomedical Results from Skylab. Washington, DC, USA: National Aeronautics and Space Administration; 1977. pp. 284–312. [Google Scholar]
- Kamiya A, Iwase S, Sugiyama Y, Mano T, Sudoh M. Vasomotor sympathetic nerve activity in men during bed rest and on orthostasis after bed rest. Aviation, Space, and Environmental Medicine. 2000;71:142–149. [PubMed] [Google Scholar]
- Kvetňanskyý R, Noskov VB, Blazicek P, Gharib C, Popova IA, Gauquelin G, Macho L, Guell A, Grigoriev AI. Activity of the sympathoadrenal system in cosmonauts during 25–day space flight on Station Mir. Acta Astronautica. 1991;23:109–116. doi: 10.1016/0094-5765(91)90106-f. [DOI] [PubMed] [Google Scholar]
- Leach CS, Alfrey CP, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW, Krauhs JM. Regulation of body fluid compartments during short–term spaceflight. Journal of Applied Physiology. 1996;81:105–116. doi: 10.1152/jappl.1996.81.1.105. [DOI] [PubMed] [Google Scholar]
- Leach CS, Altchuler SI, Cintron–Trevino NM. The endocrine and metabolic responses to space flight. Medicine and Science in Sports and Exercise. 1983;15:432–440. [PubMed] [Google Scholar]
- Leach CS, Rambaut PC. Biochemical responses of the Skylab crewmen: an overview. In: Johnston RS, Dietlein LS, editors. Biomedical Results from Skylab. Washington, DC, USA: National Aeronautics and Space Administration; 1977. pp. 204–215. [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Robertson D, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamnic responses to tilt following spaceflight. Journal of Physiology. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed–rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Mano T, Iwase S, Kamiya A. Sympathetic nerve responses in humans to short and long term simulation of microgravity. Journal of Gravitational Physiology. 1998;5:93–96. [PubMed] [Google Scholar]
- Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head–up tilt are misleading in autonomic failure. Hypertension. 1992;19:628–633. doi: 10.1161/01.hyp.19.6.628. [DOI] [PubMed] [Google Scholar]
- Norsk P, Drummer C, Röcker L, Strollo F, Christensen NJ, Warberg J, Bie P, Stadeager C, Johansen LB, Heer M, Gunga H-C, Gerzer R. Renal and endocrine responses in humans to isotonic saline infusion during microgravity. Journal of Applied Physiology. 1995;78:2253–2259. doi: 10.1152/jappl.1995.78.6.2253. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Zuckerman JH, Blomqvist CG, Levine BD. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. American Journal of Physiology. 2001;280:H2230–2239. doi: 10.1152/ajpheart.2001.280.5.H2230. [DOI] [PubMed] [Google Scholar]
- Prisk GK, Guy HJB, Elliott AR, Deutschman R A III, Wes t JB. Pulmonary diffusing capacity, capillary blood volume, and cardiac output during sustained microgravity. Journal of Applied Physiology. 1993;75:15–26. doi: 10.1152/jappl.1993.75.1.15. [DOI] [PubMed] [Google Scholar]
- Robertson D, Convertino VA, Vernikos J. The sympathetic nervous system and the physiological consequences of spaceflight: a hypothesis. American Journal of the Medical Sciences. 1994;308:126–132. doi: 10.1097/00000441-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Robertson D, Johnson GA, Robertson RM, Nies AS, Shand DG, Oates JA. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979;59:637–642. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Rummel JA, Michel EL, Sawin CF, Buderer MC. Medical experiment M–171: results from the second manned Skylab mission. Aviation, Space, and Environmental Medicine. 1976;47:1056–1060. [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine–transporter deficiency. New England Journal of Medicine. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Leuenberger UA, Herr MD, Gray K, Silber DH, Sinoway LI. Sympathetic discharge and vascular resistance after bed rest. Journal of Applied Physiology. 1998;84:612–617. doi: 10.1152/jappl.1998.84.2.612. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Sinoway LI. Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology. 1999;277:R1084–1090. doi: 10.1152/ajpregu.1999.277.4.r1084. [DOI] [PubMed] [Google Scholar]
- Shortt TL, Ray CA. Sympathetic and vascular responses to head–down neck flexion in humans. American Journal of Physiology. 1997;272:H1780–1784. doi: 10.1152/ajpheart.1997.272.4.H1780. [DOI] [PubMed] [Google Scholar]
- Vernikos J, Ludwig DA, Ertl AC, Wade CE, Keil L, O'Hara D. Effect of standing or walking on physiological changes induced by head down bed rest: implications for spaceflight. Aviation, Space, and Environmental Medicine. 1996;67:1069–1079. [PubMed] [Google Scholar]
- Wallin BG. Relationship between sympathetic nerve traffic and plasma concentrations of noradrenaline in man. Pharmacology & Toxicology. 1988;63(suppl. 1):9–11. doi: 10.1111/j.1600-0773.1988.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. American Journal of Physiology. 1982;242:H185–190. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. Journal of Physiology. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watenpaugh DE, Buckey JC, Lane LD, Gaffney FA, Levine BD, Moore WE, Wright SJ, Blomqvist CG. Effects of spaceflight on human calf hemodynamics. Journal of Applied Physiology. 2001;90:1552–1558. doi: 10.1152/jappl.2001.90.4.1552. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Hargens AR. The cardiovascular system in microgravity. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. Vol. 1. New York, NY, USA: Oxford University Press; 1995. pp. 631–674. [Google Scholar]
- Whitson PA, Charles JB, Williams WJ, Cintrón NM. Changes in sympathoadrenal response to standing in humans after spaceflight. Journal of Applied Physiology. 1995;79:428–433. doi: 10.1152/jappl.1995.79.2.428. [DOI] [PubMed] [Google Scholar]
- Young LR, Oman CM, Merfeld D, Watt D, Roy S, Deluca C, Balkwill D, Christie J, Groleau N, Jackson DK, Law G, Modestino S, Mayer W. Spatial orientation and posture during and following weightlessness: human experiments on Spacelab Life Sciences 1. Journal of Vestibular Research. 1993;3:231–239. [PubMed] [Google Scholar]


