Abstract
In many gastrointestinal tissues nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) both play a role as inhibitory non-adrenergic non-cholinergic neurotransmitters. As the mode of interaction between NO and VIP remains controversial, the aim of this study was to investigate the interplay between NO and VIP in the mouse gastric fundus and to evaluate the nitric oxide synthase (NOS) isoform involved in VIP-induced relaxation by using inducible NOS (iNOS), endothelial NOS (eNOS) and neuronal NOS (nNOS) knockout mice. The influence of NOS inhibitors on the relaxant effect of VIP was determined in isolated smooth muscle cells and smooth muscle strips of wild-type and knockout mice. In isolated smooth muscle cells from wild-type, eNOS knockout and nNOS knockout mice, the relaxation induced by VIP (10−9m) was inhibited by approximately 70–95 % by both the non-selective NOS inhibitor NG-nitro-l-arginine (l-NA; 10−4m) and the selective inducible NOS inhibitor N-(3-(aminomethyl)-benzyl)acetamidine (1400W; 10−6m). In cells isolated from iNOS knockout mice, VIP still induced full relaxation but it was not influenced by l-NA or 1400W. In smooth muscle strips from wild-type and knockout mice, the concentration-dependent relaxation by VIP (10−9 to 3 × 10−7m) was not influenced by l-NA or 1400W. These results suggest that the experimental method determines the influence of NOS inhibitors on the relaxant effect of VIP. iNOS, probably induced by the isolation procedure, might be involved in the relaxant effect of VIP in isolated smooth muscle cells but not in classic smooth muscle strips.
It is widely accepted that nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) both play an important role as inhibitory neurotransmitters in non-adrenergic non-cholinergic (NANC) smooth muscle responses throughout the gastrointestinal tract (Sanders & Ward, 1992; Brookes, 1993; Shuttleworth & Keef, 1995; Rand & Li, 1995).
NO is produced from l-arginine by one of three nitric oxide synthase (NOS) enzymes, namely neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) (Förstermann et al. 1995). The source of NO as an inhibitory regulator of gastrointestinal motility is usually thought to be nNOS in so-called nitrergic nerves; by immunochemistry, nNOS has indeed been localized to neurones and nerve endings throughout the gastrointestinal tract (see e.g. Ekblad et al. 1994a). Investigations into whether myenteric nitrergic neuron cell bodies and nerve endings also contain VIP have yielded variable results (Barbiers et al. 1993; Ekblad et al. 1994b). VIP and NO, being released from the same or separate neurones, are in general considered to contribute in parallel to a given inhibitory motor response. At the level of the gastric fundus, NO and VIP initiate and sustain NANC relaxation, respectively, in some species such as the rat and the ferret (Li & Rand, 1990; Boeckxstaens et al. 1992; D'Amato et al. 1992; Grundy et al. 1993), while NO seems to be the major neurotransmitter, to both initiate and sustain NANC relaxation, in the gastric fundus of other species such as the guinea-pig, the cat and the pig (Barbier & Lefebvre, 1993; Desai et al. 1994; Lefebvre & Vandekerkhove, 1998). The stimulation frequency of the myenteric neurones is important, as recently illustrated in the human gastric fundus; low frequency stimulation caused only NO release whereas high frequency stimulation induced both NO and VIP release (Tonini et al. 2000). It has also been proposed that NO is released from gastrointestinal smooth muscle cells in response to VIP. In isolated smooth muscle cells and smooth muscle strips of the guinea-pig gastric fundus and rat colon, the relaxant effect of VIP was indeed inhibited by NOS inhibitors (Grider et al. 1992; Grider, 1993; Jin et al. 1993). Although Western and immunoblot analysis of rabbit gastric smooth muscle did not reveal the presence of NOS, eNOS mRNA was localized in these cells by RT-PCR (Teng et al. 1998). Additionally, nNOS mRNA has been detected in gastrointestinal smooth muscle cells (Chakder et al. 1997). All these results correlate with the possibility that NO is a mediator synthesized in smooth muscle cells by VIP.
We recently demonstrated that, in the guinea-pig and pig gastric fundus, NOS inhibitors exert a completely different effect on VIP-induced relaxation depending on the use of isolated smooth muscle cells or smooth muscle strips (Dick et al. 2000; Dick & Lefebvre, 2000). In the smooth muscle cells the relaxant effect of VIP was inhibited by NOS inhibitors, including the iNOS-selective 1400W, whereas in isolated smooth muscle strips it was not. Although immunocytochemistry did not reveal systematic iNOS expression in isolated cells, these results illustrate the importance of the experimental method used and suggest the involvement of iNOS in VIP-induced relaxation in isolated smooth muscle cells.
RT-PCR for iNOS, eNOS and nNOS in isolated smooth muscle cell preparations might be influenced by contamination with other cellular elements. However, several lines of knockout mice have been developed in which the genes encoding eNOS, nNOS or iNOS have been selectively disrupted (Huang et al. 1993, 1995; Wei et al. 1995). These gene-targeted mice might allow us to further investigate the identity of the NOS isoform involved in VIP-induced relaxation in isolated smooth muscle cells of the gastric fundus. The purpose of the present study was to investigate the interaction between NO and VIP in isolated smooth muscle cells and smooth muscle strips of the mouse gastric fundus by use of iNOS, eNOS and nNOS knockout mice.
METHODS
Animals
Breeding pairs of homozygous mutant mice lacking the gene for inducible NOS (iNOS(−/−)), generated as described previously by Wei et al. (1995), were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Breeding pairs of endothelial NOS-deficient mice (eNOS(−/−)), generated as described previously (Huang et al. 1995), were obtained from Dr Paul Huang (Cardiovascular Research Center, Harvard Medical School, USA). Both types of knockout mice were bred at the Department of Molecular Biology at Ghent University.
The neuronal NOS-deficient mice (nNOS(−/−)), generated as described previously by Huang et al. (1993) and their wild-type (WT) B6.129F2/J controls were obtained from Jackson Laboratories. WT C57BL/6J controls for the iNOS(−/−) mice and WT Sv129 controls for the eNOS(−/−) mice were obtained from Iffa Credo (Saint Germain sur l'Arbresle, France). Animals were housed in a 12 h light-dark cycle in a temperature-controlled, air-conditioned room with food and water ad libitum; they were used at the age of 7–10 weeks.
All experiments were carried out in accordance with the Belgian regulations on the use of experimental animals, as approved by the Ethics Committee on Experimental Animals of the Ghent University Faculty of Medicine and Health Sciences.
The efficacy of the gene deletion was assessed as follows.
Nitrite assay
Blood from WT C57BL/6J control mice and homozygous mutant iNOS knockout mice challenged i.v. with 20 μg murine tumour necrosis factor alpha (mTNF α) was collected by cardiac puncture under avertin (2,2,2-tribromoethanol) anaesthesia (100 mg kg−1, i.p.), 3, 6, 9 and 12 h after mTNF α challenge. To estimate NO production, the procedure described by Granger et al. (1991), based on the measurement of the nitrite content in serum after reduction of nitrate to nitrite, was followed in a slightly modified form.
Immunoblotting of nNOS
Within 30 min after decapitation, the cerebellum of B6.129F2/J control mice or homozygous mutant nNOS knockout mice was homogenized in ice-cold homogenization buffer (250 mm sucrose, 10 mm Tris, 10 mm MgCl2, 2 mm EDTA, 0.2 mm phenylmethylsulfonylfluoride (PMSF), 1 μm leupeptin and 1 μg ml−1 aprotinin, pH 7.4) in 5–10 volumes (v/w) at 4 °C. The homogenate was centrifuged at 300 g for 10 min at 4 °C. The proteins of the supernatant (post-nuclear supernatant, PNS) were separated by SDS-PAGE under denaturing conditions (Laemmli et al. 1970), blotted onto a nitrocellulose membrane (Towbin et al. 1979) and nNOS was immunologically detected as described below. All incubations were performed at room temperature on a rocking platform. After overnight incubation with 10 % (v/v) Western blocking reagent in phosphate-buffered saline (PBS, 137 mm NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, 8.1 mm Na2HPO4), the blot was incubated for 2 h with polyclonal rabbit anti-nNOS antiserum (diluted 1:5000 in PBS containing 0.05 % (v/v) Tween 20, PBS-Tween). After extensive rinsing with PBS-Tween, the blot was incubated for 2 h with goat anti-rabbit IgG HRP-conjugated antibody (diluted 1:5000 in PBS-Tween). Detection was performed by enhanced chemiluminescence (Amersham Pharmacia Biotech), according to the manufacturer's instructions.
Blood pressure measurement
The blood pressures of WT C57BL/6J control mice and homozygous eNOS(−/−) mice were measured by a computerized non-invasive tail-cuff system as described by Krege et al. (1995). The system measures blood pressure by determining the cuff pressure at which blood flow to the tail is eliminated.
Preparation of isolated smooth muscle cells
Circular smooth muscle cells were isolated from the gastric fundus of mice by collagenase digestion as previously described by Bitar & Makhlouf (1982). Briefly, three to four mice (25–30 g in weight) of either sex were killed by cervical dislocation to obtain cells for one experiment. The gastric fundus was isolated immediately and the circular muscle layer was separated from the rest of the stomach wall by careful dissection under the microscope. Small sheets from the circular muscle layer were incubated for 15 min at 31 °C, in 15 ml of 25 mm Hepes-buffered medium, containing 150 U ml−1 collagenase (Type II) and 0.01 % soybean trypsin inhibitor, and gassed with 95 % O2–5 % CO2. The medium consisted of: NaCl, 98 mm; KCl, 6 mm; NaH2PO4, 2.5 mm; CaCl2, 1.8 mm; d(+)-glucose, 11.5 mm; bovine serum albumin, 0.2 % (w/v). It was supplemented with: sodium pyruvate, 5 mm; sodium fumarate, 5 mm; sodium glutamate, 5 mm; glutamine, 2 mm; amino acid mixture, 1 % (v/v); vitamin mixture, 1 % (v/v); penicillin G, 50 μg ml−1; streptomycin, 50 μg ml−1. The pH of the buffered medium was adjusted to 7.4. At the end of the incubation, the medium was filtered through a 500 μm pore size Nitex filter and the partly digested tissues were washed with 30 ml enzyme-free medium, whereafter they were allowed to disperse spontaneously in enzyme-free medium for 60 min. Finally the spontaneously dissociated muscle cells were harvested by filtration and used for functional measurements.
Viability tests by exclusion of trypan blue showed that 86.6 ± 1.2 % (mean ± s.e.m., n = 6) of the cells in suspension obtained from WT C57BL/6J control mice were viable at the time of the contraction experiments. Cell suspensions were maintained at 31 °C and usually studied within 30 min.
The length of the isolated smooth muscle cells was measured using an image splitting eyepiece connected to a micrometer after fixation with glutaraldehyde. An aliquot of 50 μl treated cell suspension was placed on a Malassez slide. The first 25 or 50 randomly encountered and morphologically intact cells were measured using a Carl Zeiss eyepiece at a magnification of at least ×200. For the vials with control cells and carbachol-treated cells, two different aliquots were taken and 25 or 50 cells were measured from each aliquot. The absolute cell length measurement was performed with a scale mask placed on a video screen, connected to a video camera. Magnification due to the video camera had first been calculated by use of a micrometer.
Measurement of relaxation (inhibition of contraction) in isolated smooth muscle cells
Untreated cells served as controls. Cells were contracted by incubation with 10−8m carbachol for 30 s, followed by fixation of the cells with glutaraldehyde (pH 7.4), added to a final concentration of 2.5 %. In relaxation experiments, VIP (10−13 to 10−8m) was added 60 s before the carbachol. The inhibition of the carbachol-induced contraction was considered to be relaxation as previously described (Grider et al. 1992; Jin et al. 1993; Rekik et al. 1996). The term relaxation will be used throughout this manuscript. To study the possible involvement of NO, the cells were incubated for 5 min before addition of VIP with the NOS inhibitors NG-nitro-l-arginine (l-NA) and N-(3-(aminomethyl)-benzyl)acetamidine (1400W), with or without l- or d-arginine. In parallel control vials, the cells were incubated with the solvent of these agents.
Preparation of smooth muscle strips
After cervical dislocation two smooth muscle strips (10 × 2 mm) were prepared from the mouse gastric fundus by cutting in the direction of the circular muscle layer as described by Öngülener et al. (1995). The strips were suspended between two platinum plate electrodes under a load of 0.5 g in 5 or 20 ml organ baths containing Tyrode solution, maintained at 37 °C and gassed with 95 % O2–5 % CO2. The Tyrode solution had the following composition: NaCl, 137 mm; KCl, 2.7 mm; NaH2PO4, 0.4 mm; MgCl2, 1.0 mm; CaCl2, 1.8 mm; NaHCO3, 12.0 mm; and d(+)-glucose, 5.5 mm. The Tyrode solution always contained 4 × 10−6m guanethidine to inhibit noradrenergic responses. Changes in length were recorded isotonically via type 373 B40 Lever transducers (Hugo Sachs Elektronik-Harvard Apparatus GmbH, March-Hugstetten, Germany) on a Linearcorder 8 WR 3500 (Western Graphtec Inc., Irvine, CA, USA) in the 5 ml baths or via Palmer Bioscience (USA) T3 transducers on a Graphtec Linearcorder WR 3701 F in the 20 ml baths. Electrical field stimulation (EFS) was performed by means of a Hugo Sachs Stimulator I type 215/I in the 5 ml baths or by a Grass S88 Stimulator in the 20 ml baths. The tissues were allowed to equilibrate for 60 min, being rinsed every 15 min.
Measurement of relaxation in muscle strips
After the equilibration period, tone was raised by the administration of 5 × 10−7m carbachol. Once a stable contraction was obtained, EFS was performed or VIP was administered. Frequency–response curves to EFS (40 V, 0.2 ms, 0–5–16 Hz) were obtained by stimulating the tissues with 30 s trains at 5 min intervals. VIP (10−9 to 3 × 10−7m) was administered cumulatively. After obtaining the first frequency–response or concentration- response curve, the tissues were regularly rinsed for 30 min. l-NA (10−4m) or 1400W (10−6m) was then added and incubated for 30 min. Tone was then again raised by adding 5 × 10−7m carbachol and a second frequency–response or concentration– response curve was obtained. In parallel control strips, only the solvent of the tested drug was administered. All experiments ended with another cycle of carbachol-induced tone and relaxation by 10−4m papaverine.
Data analysis
The contraction of the isolated smooth muscle cells was expressed as the percentage decrease in cell length compared to untreated controls, using the following formula: ((L0 – Lx)L0−1) × 100, where L0 is the mean length of cells in the control state and Lx is the mean length of carbachol-treated cells. In relaxation experiments, the degree of inhibition of contraction was expressed as the percentage decrease in maximal contractile response, as observed in carbachol-treated cells in the absence of relaxant agent. Relaxations in the smooth muscle strips were expressed as the percentage of the papaverine-induced relaxation at the end of the experiment.
Results are given as means ± s.e.m. and n refers to isolated smooth muscle cell preparations or smooth muscle strips from different animals. Blood pressure values and nitrite levels from eNOS(−/−) and iNOS(−/−) mice were compared with those in controls by Student's unpaired t test. The length of smooth muscle cells from iNOS(−/−), eNOS(−/−) and nNOS(−/−) mice was compared with that from their respective WT mice by Student's unpaired t test. Responses in parallel vials of isolated smooth muscle cells were compared by ANOVA and Student's t test, corrected for multiple comparisons by theBonferroni procedure.
Responses during the first and second curves in the strips were compared by Student's paired t test. When a drug under test induced a significant difference, this was compared with the difference between the first and second curve in the parallel control tissues by Student's unpaired t test.
P values of less than 0.05 were considered statistically significant.
Chemicals
Collagenase was purchased from the Worthington Biochemical Corporation (Freehold, NJ, USA). Vasoactive intestinal polypeptide (VIP) was obtained from Bachem (Bubendorf, Switzerland) and carbamoylcholine chloride (carbachol) from Fluka (Switzerland). N-(3-(aminomethyl)-benzyl)acetamidine (1400W) was obtained from the Alexis Corporation (Nottingham, UK) and essential amino acid mixture from ICN (Costa Mesa, USA). Aprotinin, d-arginine hydrochloride, l-arginine hydrochloride, bovine serum albumin, glutamine, glutaraldehyde, goat anti-rabbit IgG HRP-conjugated antibody, guanethidine sulphate, Griess reagent, leupeptin, NG-nitro-l-arginine (l-NA), penicillin G, phenylmethylsulfonylfluoride (PMSF), polyclonal rabbit anti-nNOS antiserum, sodium fumarate, sodium glutamate, sodium pyruvate, streptomycin, sucrose, trypan blue, Tween 20 and vitamin mixture were bought from Sigma Chemicals (St Louis, MO, USA). EDTA, Hepes, soybean trypsin inhibitor, Tris and Western blocking reagent were obtained from Boehringer Mannheim Biochemicals (Indianapolis, IN, USA) and papaverine from a local pharmacist. Merck (Darmstadt, Germany) provided trichloroacetic acid and tissue culture medium was bought from Gibco BRL (Paisley, UK). Recombinant murine tumour necrosis factor α (mTNF α) was produced and purified at the Department of Molecular Biology. It had a specific activity of 1.9 × 10−8 i.u. mg−1 and contained less than 10 U of endotoxin per milligram of protein, as assessed by a chromogenic substrate test (Chromogenix, Stockholm, Sweden).
All drugs were dissolved in deionized water, except for PMSF (0.2 m), which was dissolved in pure ethanol. Further dilutions were made in physiological salt solution. The solvents, diluted in physiological salt solution to the final concentration applied to the strips or the cells, had no effect per se on the tone of the strips or on control isolated smooth muscle cells. Stock solutions of 1400W (of up to 10−2m) and VIP (of up to 10−4m) were prepared in deionized water and stored at −20 °C. All other solutions were prepared on the day of the experiment.
RESULTS
Control experiments
Preliminary control experiments were performed to verify the accuracy of the iNOS, nNOS and eNOS deletions (Fig. 1). A time-dependent increase in the serum nitrite level was observed in WT C57BL/6J control mice after i.p. challenge with mTNF α, whereas the background nitrite level was maintained in the serum of iNOS(−/−) mice. The blood pressure of eNOS(−/−) mice (138.5 ± 1.8 mmHg) was significantly higher than that of WT C57BL/6J control mice (105.2 ± 0.7 mmHg). A positive 155 kDa nNOS band was found by Western blotting in the postnuclear supernatant of the cerebellum of a WT B6.129F2/J mouse but not in that of a nNOS(−/−) mouse.
Figure 1. Preliminary control experiments performed to verify the accuracy of the iNOS, nNOS and eNOS deletions in the knockout mice.
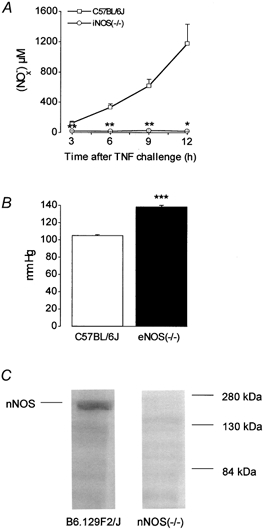
A, concentration of nitrite, after reduction of nitrate to nitrite, detected in serum of WT C57BL/6J control mice and iNOS(−/−) mice 3, 6, 9 and 12 h after mTNF α challenge. Values are means ± s.e.m., n = 2–5. * P < 0.05, ** P < 0.01, significantly different from control. B, blood pressure determined in WT C57BL/6J control mice and eNOS(−/−) mice. Values are means ± s.e.m., n = 8. *** P < 0.001, significantly different from control. C, immunodetection of nNOS in the postnuclear supernatant of WT B6.129F2/J and nNOS(−/−) mouse cerebellum.
Isolated smooth muscle cells
Untreated control cells obtained by dissociation of the circular muscle layer of the WT C57BL/6J mouse gastric fundus had a mean cell length of 117.9 ± 1.0 μm (n = 6, Table 1). Incubation for 30 s with 10−8m carbachol produced a 15.3 ± 0.8 % shortening of the cells to 99.9 ± 1.3 μm (n = 6, Table 1).
Table 1.
Effect of l-NA on VIP-induced relaxation in isolated smooth muscle cells obtained from WT C57BL/6J gastric fundus
| Muscle cell length (μm) | Contraction (%) | Relaxation (%) | Inhibition (%) | |
|---|---|---|---|---|
| Controls | 117.9 ± 1.0 | — | — | — |
| Carbachol (10−8m) | 99.9 ± 1.3*** | 15.3 ± 0.8 | — | — |
| Carbachol (10−8m) | ||||
| + VIP (10−9m) | 120.0 ± 2.6 | −1.2 ± 2.1 | 106.3 ± 12.8 | — |
| Carbachol (10−8m) | ||||
| + VIP (10−9m) | ||||
| +l-NA (10−4m) | 100.2 ± 2.1*** | 15.0 ± 1.8 | 11.6 ± 7.3 | 90.8 ± 5.7 |
Effects are described by muscle cell length (μm), contraction (% decrease in cell length), relaxation (% inhibition of carbachol-induced contraction) and inhibition (% inhibition of VIP-induced relaxation). Values are means ± s.e.m.; n = 6
Significantly different from control cells, P < 0.001.
Figure 3. Influence of NOS inhibitors on the relaxant effect of VIP in smooth muscle strips.
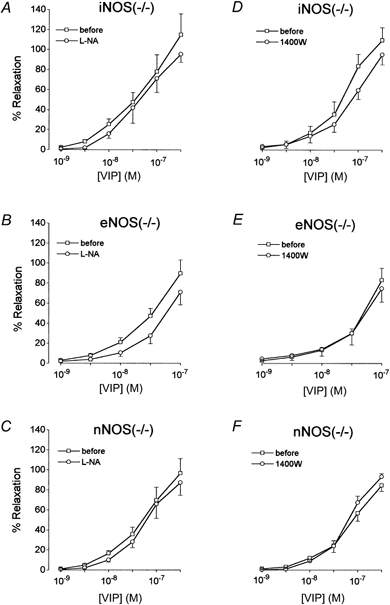
Concentration-response curve for VIP in the absence or presence of 10−4m l-NA (A, B and C) or 10−6m 1400W (D, E and F) in circular gastric smooth muscle strips from iNOS(−/−) (A and D), eNOS(−/−) (B and E) and nNOS(−/−) (C and F) mice. Values are means ± s.e.m., n = 5–6. There were no significant differences in response before and in the presence of a NOS inhibitor, when compared with the change in parallel control tissues.
When cells were preincubated for 60 s with increasing concentrations of VIP (10−13 to 10−8m), the contraction was inhibited in a concentration-dependent manner (data not shown). Full relaxation was obtained with 10−9m VIP and this concentration was selected for further investigation. In the absence of carbachol, VIP did not affect the length of the smooth muscle cells (120.0 ± 2.6 μm, n = 6). The lengths of the smooth muscle cells obtained from WT Sv129 and B6.129F2/J mice (112.9 ± 2.8 and 117.8 ± 2.2 μm, respectively; n = 5–6) were not significantly different from that from C57BL/6J mice. The smooth muscle cell lengths of iNOS(−/−) (106.1 ± 4.5 μm, n = 6) and eNOS(−/−) (100.8 ± 3.7 μm, n = 7) mice were significantly different from those of the corresponding C57BL/6J and Sv129 WT control mice. Smooth muscle cell length in nNOS(−/−) mice (121.3 ± 2.6 μm, n = 5) was not significantly different from that in the corresponding WT B6.129F2/J control mice. The gastric smooth muscle cells of the WT Sv129 and B6.129F2/J and the iNOS(−/−), nNOS(−/−) and eNOS(−/−) mice contracted in response to 10−8m carbachol and relaxed fully in response to 10−9m VIP, as observed for WT C57BL/6J mice. In the absence of carbachol, VIP did not affect the length of the smooth muscle cells. The relaxant effect of 10−9m VIP in gastric smooth muscle cells obtained from WT C57BL/6J, Sv129 and B6.129F2/J mice was inhibited by the non-selective NOS inhibitor l-NA by 90.8 ± 5.7, 72.3 ± 13.6 and 75.0 ± 8.6 % (n = 6–7, Fig. 2), respectively. The inhibitory effect of l-NA was reversed by 10−4m l-arginine, but not by 10−4m d-arginine. In the gastric smooth muscle cells of the three WT mouse strains, the relaxant effect of VIP was also inhibited by the iNOS-selective inhibitor 1400W (10−6m) by 90.5 ± 5.1, 84.7 ± 8.1 and 69.8 ± 6.4 % (n = 5–7, Fig. 2) respectively. The inhibitory effect of 1400W was not reversed by l-arginine in the cells of C57BL/6J mice and B6.129F2/J mice, but it was in the cells of Sv129 mice. In the gastric smooth muscle cells of eNOS(−/−) and nNOS(−/−) mice, the relaxant effect of VIP was reduced by both l-NA and 1400W (Fig. 2); the inhibitory effect of l-NA was reversed by l-arginine, while that of 1400W was reversed by d-arginine in eNOS(−/−) mice. In iNOS(−/−) mice, the relaxant effect of VIP was not significantly influenced by l-NA or 1400W.
Figure 2. Influence of NOS inhibitors on the relaxant effect of VIP in isolated smooth muscle cells from WT, iNOS(−/−), eNOS(−/−) and nNOS(−/−) mice.
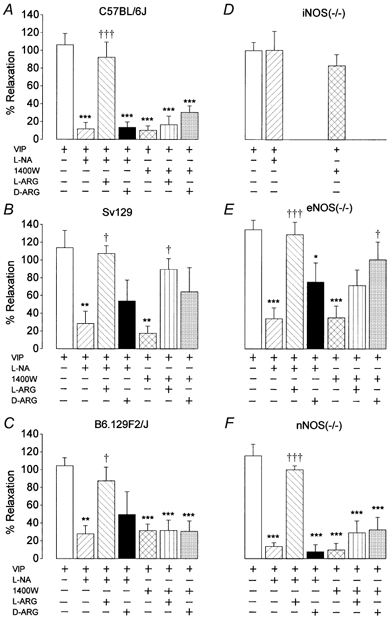
Effect of l-NA (10−4m) and 1400W (10−6m) with or without l-arginine (L-ARG, 10−4m) and d-arginine (D-ARG, 10−4m) on VIP(10−9m)-induced relaxation in isolated smooth muscle cells obtained from WT C57BL/6J (A), Sv129 (B) and B6.129F2/J (C) mice and iNOS(−/−) (D), eNOS(−/−) (E) and nNOS(−/−) (F) mice. Values are means ± s.e.m., n = 5–7. * P < 0.05, ** P < 0.01, *** P < 0.001, significantly different from cells treated with VIP. † P < 0.05, ††† P < 0.001, significantly different from cells treated with VIP and l-NA or 1400W.
Isolated smooth muscle strips
In all strains tested (WT C57BL/6J and B6.129F2/J, not shown; mutant iNOS(−/−), eNOS(−/−) and nNOS(−/−) mice, Fig. 3), VIP (10−9 to 3 × 10−7m) induced concentration-dependent relaxations of gastric circular smooth muscle strips. The response to VIP tended to be lower when obtaining a second concentration- response curve. When comparing responses to VIP before treatment with and in the presence of 10−4m l-NA with those in parallel control strips from the same strain, l-NA had no significant effect except for a decrease in the response to 3 × 10−8m VIP in strips from WT C57BL/6J mice. 1400W (10−6m) had no significant influence on the relaxant effect of VIP in the strains tested (WT C57BL/6J and mutant iNOS(−/−), eNOS(−/−) and nNOS(−/−) mice).
Electrical field stimulation (EFS; 40 V, 0.2 ms, 0.5–16 Hz, 30 s trains at 5 min intervals) in the presence of guanethidine induced frequency-dependent relaxations in gastric fundus circular smooth muscle strips obtained from WT C57BL/6J and B6.129F2/J mice, and from eNOS(−/−) and iNOS(−/−) mice. Representative examples in strips obtained from a WT C57BL/6J mouse and an eNOS(−/−) mouse are shown in Fig. 4A and B. At low frequencies (0.5–2 Hz), EFS induced monophasic short-lasting relaxations; at higher frequencies (4–16 Hz), a quick relaxation occurred but tone did not return completely to baseline and a second, sustained, phase of relaxation was observed. l-NA (10−4m) abolished the responses at 0.5–2 Hz and the first phase of the response at 4–16 Hz; instead a small primary contraction was seen at 4–16 Hz. The second phase of relaxation at the higher frequency range was not influenced by l-NA (Fig. 4A and B). 1400W (10−6m) had no influence on the electrically induced relaxations in the strains tested (C57BL/6J, iNOS(−/−) and eNOS(−/−)). In tissues from nNOS(−/−) mice, EFS only induced sustained relaxations at stimulation frequencies of 4–16 Hz preceded by a small contraction; these responses were not influenced by 10−4 M l-NA. An example is shown in Fig. 4C.
Figure 4. Influence of l-NA on the relaxation induced by electrical field stimulation in smooth muscle strips.
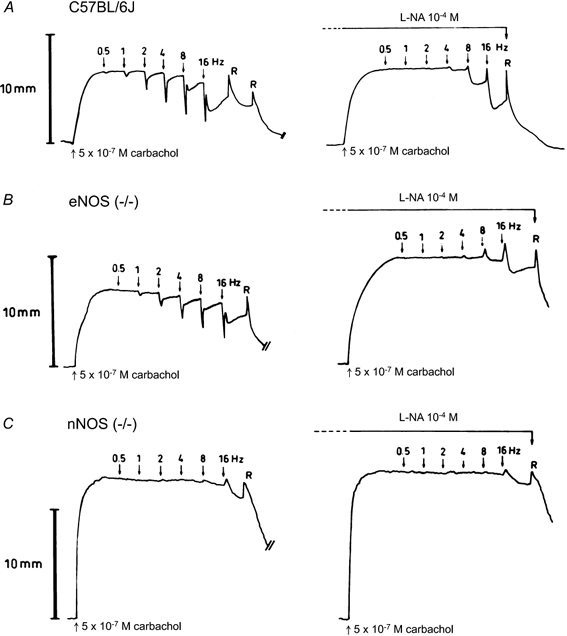
Representative traces from circular smooth muscle strips obtained from a WT C57BL/6J (A), an eNOS(−/−) (B) and an nNOS(−/−) mouse (C) showing the response to electrical field stimulation (40V, 0.2 ms, 0.5–16 Hz) with 30 s trains at 5 min intervals before and after addition of l-NA (10−4m). R, rinsing.
l-NA and 1400W had no influence on the basal tone of the smooth muscle strips or on the carbachol-induced contraction.
DISCUSSION
The aim of the present study was to investigate the interaction between NO and VIP in isolated smooth muscle cells and smooth muscle strips of the mouse gastric fundus in iNOS, eNOS and nNOS knockout mice, to evaluate the NOS isoform involved in VIP-induced relaxation.
Homozygous iNOS, eNOS and nNOS mutant mice, generated by homologous recombination (Huang et al. 1993, 1995; Wei et al. 1995) are viable, fertile and indistinguishable from their respective WT counterparts (C57BL/6J, Sv129 and B6.129F2/J) in appearance and routine behaviour, except for nNOS knockout mice, which have dilated stomachs. The accuracy of the iNOS, nNOS and eNOS deletions was confirmed in preliminary control experiments. iNOS is known to be induced in many cell types by cytokines such as TNF and interleukins (Förstermann et al. 1995). Administration of murine (m)TNF α induced a time-dependent increase in serum nitrite concentrations in WT C57BL/6J mice, whereas only a background level of nitrite was detected in the serum of mutant iNOS mice (see also Cauwels et al. 2000). No nNOS enzyme immunoreactivity was detected in nNOS(−/−) mice, whereas a 155 kDa band was observed in WT B6.129F2/J mice. As Huang et al. (1995) observed, the blood pressure of eNOS(−/−) mice was significantly higher than that of WT C57BL/6J mice, demonstrating that these mutant mice were deficient in eNOS. Because WT Sv129 mice were not available at the time of the preliminary control experiments, and because Huang et al. (1995) reported that the blood pressure was the same in WT C57BL/6J and Sv129 mice, we used WT C57BL/6J mice as controls for eNOS(−/−) mice when measuring blood pressure.
The lengths of the smooth muscle cells isolated from iNOS(−/−) and eNOS(−/−) mice were significantly shorter than those from their respective WTs. This might be due to the age of the mice used, as the iNOS(−/−) and eNOS(−/−) mice were younger when used than their respective WTs (e.g. 9.4 ± 0.2 weeks for Sv129, n = 18, and 6.8 ± 0.6 weeks for eNOS(−/−), n = 18). This age-related difference in cell length was also observed in smooth muscle cells of the rat muscular saphenous artery (Todd et al. 1991). The length of the isolated smooth muscle cells from nNOS(−/−) mice was not different from that in the WT B6.129F2/J mice, as their age was similar (8.8 ± 0.7 weeks for B6.129F2/J, n = 6, 8.2 ± 0.7 weeks for nNOS(−/−), n = 5).
As has already been observed in guinea-pig and pig gastric fundus (Dick et al. 2000; Dick & Lefebvre, 2000), both the non-selective NOS inhibitor l-NA and the iNOS-selective inhibitor 1400W (Garvey et al. 1997) reduced the relaxant effect of VIP in isolated gastric fundus smooth muscle cells from WT C57BL/6J, Sv129 and B6.129F2/J mice and from eNOS(−/−) and nNOS(−/−) mice. The inhibitory effect of l-NA was reversed by l-arginine but not by d-arginine, thereby indicating the specificity of the inhibitory effect. The inhibitory effect of 1400W was not reversed by l-arginine in C57BL/6J and B6.129F2/J mice, confirming data obtained in isolated smooth muscle of the guinea-pig gastric fundus (Dick et al. 2000). This might be related to the very tight binding of 1400W to iNOS (Garvey et al. 1997). In WT Sv129 mice, the inhibitory effect of 1400W was partially reversed by l-arginine while it was partially reversed by d-arginine in eNOS(−/−) mice; we have no explanation for this observation. Teng et al. (1998) proposed that eNOS was the isoform involved in the relaxant effect of VIP in gastrointestinal smooth muscle cells but the maintenance of the inhibitory effect of NOS inhibitors on VIP-induced relaxation in the gastric fundus smooth muscle cells of eNOS(−/−) mice argues against this hypothesis. The maintenance of the same effect in cells from nNOS(−/−) mice also excluded any possible role for nNOS in VIP-induced relaxation. In isolated smooth muscle cells from iNOS(−/−) mice, VIP still induced full relaxation, but this relaxant effect was not significantly influenced by either l-NA or 1400W. This demonstrates the fact that the effect of NOS inhibitors in isolated smooth muscle cells of the gastric fundus of mice without the iNOS deletion was due to the inactivation of iNOS. In the latter animals, the iNOS-related mechanism of relaxation by VIP seemed predominant, as only a small relaxation was maintained in the presence of NOS inhibitors. Our previous results in the guinea-pig gastric fundus (Dick et al. 2000) suggested that VIP activated the iNOS-NO pathway via cAMP-protein kinase A. When iNOS cannot be induced, as in the iNOS(−/−) mice, another mechanism of relaxation by VIP can be used. This is probably the classic cAMP-protein kinase A non-NOS related mechanism, which was also observed in strips (see below). How and why this mechanism was suppressed in the cells in which iNOS could be induced was not clear. This might be related to other substrates that are induced in addition to iNOS. It has, for example, been shown that treatment with lipopolysaccharide (LPS) induces the synthesis of tetrahydrobiopterin in rat aorta (Shimizu et al. 1999) and that an excess of tetrahydrobiopterin inhibits phosphorylation of tyrosine hydroxylase (Ribiero & Kaufman, 1994; Alterio et al. 1998). As iNOS is known to be expressed under certain pathophysiological conditions such as stress, bacterial endotoxin and/or cytokine challenge (Schulz et al. 1992; Knowles & Moncada, 1994), a possible explanation for the presence of iNOS in isolated smooth muscle cells could be its induction in response to the stress of the crude enzymatic isolation procedure. Indeed, Imagawa et al. (1999) recently demonstrated the induction of iNOS in response to ischaemia–reperfusion stress in vivo, demonstrating that stress without addition of inflammatory mediators could also induce iNOS. Similarly, the induction of iNOS was shown at mRNA and protein levels in isolated rat hepatocytes obtained by collagenase digestion (Tirmenstein et al. 2000). Our 2 h dissociation procedure seems sufficient to induce iNOS, as Liu et al. (1997) could detect iNOS mRNA 20 min after LPS treatment of vascular preparations. Moreover, iNOS activity has already been measured in cytokine- and LPS-activated vascular endothelial cells after a lag period of 2 h (Radomski et al. 1990).
In smooth muscle strips from WT C57BL/6J and B6.129F2/J mice and from iNOS(−/−) and eNOS(−/−) mice, electrically induced monophasic short relaxations at low frequencies of stimulation, and peak relaxations preceeding the sustained phase at higher frequencies, were inhibited by l-NA but not by 1400W. In nNOS(−/−) mice, l-NA-sensitive relaxations were no longer induced by EFS. These results confirm the role of NO in inhibitory neurotransmission in the mouse gastric fundus (Yano et al. 1995) and demonstrate that nNOS is the enzymatic source of the NO involved. Our results in the gastric fundus of nNOS(−/−) mice correlate with those of Kim et al. (1999) in lower oesophageal sphincter rings, in which the electrically induced relaxation observed in WT mice was absent in nNOS(−/−) but maintained in eNOS(−/−) mice. The electrically induced inhibitory junction potential in gastric fundus smooth muscle was partially reduced in nNOS(−/−) mice, the first purinergic part being maintained (Mashimo et al. 1996). In W/Wv mutant mice, which lack the muscular interstitial cells of Cajal in the gastric fundus, the EFS-induced inhibitory junction potential was reduced and the relaxation was abolished, although the nitrergic innervation was maintained; this still fits with our results as the interstitial cells of Cajal are said to transduce the NO signal into the smooth muscle cells (Burns et al. 1996).
The sustained, l-NA-insensitive relaxations at higher frequencies of stimulation, which were also present in the gastric fundus of nNOS(−/−) mice, suggest that a second neurotransmitter was involved in electrically induced relaxant responses of the mouse gastric fundus. This is similar to the situation in the rat, where the more delayed relaxations are thought to be due to VIP (Li & Rand, 1990; D'Amato et al. 1992). In gastric fundus smooth muscle strips from the strains tested, relaxation by exogenous VIP was not influenced by either l-NA or 1400W, arguing against the involvement of NO in the relaxant effect of VIP. This contrast between strips and cells of the mouse gastric fundus confirms our data on the guinea-pig and pig gastric fundus (Dick et al. 2000; Dick & Lefebvre, 2000). When measuring smooth muscle membrane potential in gastric fundus circular muscle strips, Mashimo et al. (1996) reported that VIP induced hyperpolarization which was abolished by l-NA and absent in nNOS(−/−) mice; they suggested that VIP induced NO release from nitrergic nerve endings. No evidence for this serial interaction between VIP and NO was obtained from measuring smooth muscle contractile activity in our study, as VIP-induced relaxation was not influenced by l-NA and was still present in nNOS(−/−) mice. Also, Burns et al. (1996) found that VIP-induced hyperpolarization in mouse gastric fundus was not influenced by NG-nitro-l-arginine methyl ester and relaxation by exogenous VIP was not influenced by NOS inhibition in smooth muscle strips from the rat (Boeckxstaens et al. 1992), cat (Barbier & Lefebvre, 1993), dog (Bayguinov et al. 1999) and human gastric fundus (Tonini et al. 2000). This suggests that NO and VIP induce relaxation of gastric fundus smooth muscle strips via parallel pathways. This is corroborated by the observation in gastric fundus circular smooth muscle strips from mice lacking cyclic GMP-dependent protein kinase I, which is the subtype highly expressed in smooth muscle, that VIP-induced relaxations did not differ qualitatively from those in wild-type animals (Ny et al. 2000)
In conclusion, in isolated circular smooth muscle cells of WT and eNOS(−/−) and nNOS(−/−) mouse gastric fundus, the relaxant effect of VIP was inhibited by NOS inhibitors, including the iNOS-selective NOS inhibitor 1400W, whereas no inhibitory influence of NOS inhibitors could be observed in isolated smooth muscle cells of iNOS(−/−) mice. In contrast, in gastric fundus circular smooth muscle strips of WT, iNOS(−/−), eNOS(−/−) and nNOS(−/−) mice, no influence of the NOS inhibitors on the relaxation by VIP was observed, demonstrating that VIP induced relaxation via an NO-independent pathway. These results suggest that the experimental method determines the influence of NOS inhibitors on the relaxant effect of VIP. iNOS, probably induced by the isolation procedure of the smooth muscle cells, seems to be involved in the relaxant effect of VIP in isolated circular smooth muscle cells but not in classic smooth muscle strips from the mouse gastric fundus.
Acknowledgments
This work was supported by grant no. 3G0031.96 from the Fund for Scientific Research Flanders, grant O11A1696 from the Special Investigation Fund of the Ghent University and by Interuniversity Pole of Attraction Programme P4/16 (Services to the Prime Minister – Federal Services for Scientific, Technical and Cultural Affairs). The authors thank Lieve Van Geldre for her help with the Western blots.
REFERENCES
- Alterio J, Ravassard P, Haavik J, Le Caer JP, Biguet NF, Waksman G, Mallet J. Human tyrosine hydroxylase isoforms. Inhibition by excess tetrahydrobiopterin and unusual behavior of isoform 3 after cAMP-dependent protein kinase phosphorylation. Journal of Biological Chemistry. 1998;273:10196–10201. doi: 10.1074/jbc.273.17.10196. [DOI] [PubMed] [Google Scholar]
- Barbier AJ, Lefebvre RA. Involvement of the l-arginine: nitric oxide pathway in non-adrenergic non-cholinergic relaxation of the cat gastric fundus. Journal of Pharmacology and Experimental Therapeutics. 1993;266:172–178. [PubMed] [Google Scholar]
- Barbiers M, Timmermans J-P, Scheuermann DW, Andriaensen D, Mayer B, De Groodt-Lasseel MHA. Distribution and morphological features of nitrergic neurons in the porcine large intestine. Histochemistry. 1993;100:27–34. doi: 10.1007/BF00268875. [DOI] [PubMed] [Google Scholar]
- Bayguinov O, Keef KD, Hagen B, Sanders KM. Parallel pathways mediate inhibitory effects of vasoactive intestinal polypeptide and nitric oxide in canine fundus. British Journal of Pharmacology. 1999;126:1543–1552. doi: 10.1038/sj.bjp.0702450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar KN, Makhlouf GM. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. American Journal of Physiology. 1982;242:G400–407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens GE, Pelckmans PA, De Man JG, Bult H, Herman AG, Van Maercke YM. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by non-adrenergic non-cholinergic nerves in the rat gastric fundus. Archives Internationales de Pharmacodynamie et de Therapie. 1992;318:107–115. [PubMed] [Google Scholar]
- Brookes SJH. Neuronal nitric oxide in the gut. Journal of Gastroenterology and Hepatology. 1993;8:590–603. doi: 10.1111/j.1440-1746.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AEJ, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proceedings of the National Academy of Sciences of the USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauwels A, Van Molle W, Janssen B, Everaert B, Huang P, Fiers W, Brouckaert P. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase. Immunity. 2000;13:223–231. doi: 10.1016/s1074-7613(00)00022-4. [DOI] [PubMed] [Google Scholar]
- Chakder S, Bandyopadhyay A, Rattan S. Neuronal NOS gene expression in gastrointestinal myenteric neurons and smooth muscle cells. American Journal of Physiology. 1997;273:C1868–1875. doi: 10.1152/ajpcell.1997.273.6.C1868. [DOI] [PubMed] [Google Scholar]
- D'Amato M, Curro D, Montuschi P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. Journal of the Autonomic Nervous System. 1992;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- Desai KM, Warner TD, Bishop AE, Polak JM, Vane JR. Nitric oxide, and not vasoactive intestinal polypeptide, as the main neurotransmitter of vagally induced relaxation of the guinea-pig stomach. British Journal of Pharmacology. 1994;113:1197–1202. doi: 10.1111/j.1476-5381.1994.tb17124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JMC, Lefebvre RA. Interplay between NO and VIP in the pig gastric fundus. European Journal of Pharmacology. 2000;297:389–397. doi: 10.1016/s0014-2999(00)00299-5. [DOI] [PubMed] [Google Scholar]
- Dick JMC, Van Geldre LA, Timmermans JP, Lefebvre RA. Investigation of the interaction between nitric oxide and vasoactive intestinal polypeptide in the guinea-pig gastric fundus. British Journal of Pharmacology. 2000;129:751–763. doi: 10.1038/sj.bjp.0703089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Alm P, Sundler F. Distribution and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience. 1994a;63:233–248. doi: 10.1016/0306-4522(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Mulder H, Uddman R, Sundler F. NOS-containing neurons in the rat gut and coeliac ganglia. Neuropharmacology. 1994b;33:1323–1331. doi: 10.1016/0028-3908(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Gath I, Schwarz P, Closs EI, Kleinert H. Isoforms of nitric synthase; properties, cellular distribution and expressional control. Biochemical Pharmacology. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJR, Knowles RG. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric oxide synthase in vitro and in vivo. Journal of Biological Chemistry. 1997;172:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Granger DL, Hibbs JB, Broadnax LM. Urinary nitrate excretion in relation to murine macrophage activation: influence of dietary l-arginine and oral NG-monomethyl-l-arginine. Journal of Immunology. 1991;146:1294–1302. [PubMed] [Google Scholar]
- Grider JR. Interplay of VIP and nitric oxide in regulation of the descending relaxation phase of peristalsis. American Journal of Physiology. 1993;264:G334–340. doi: 10.1152/ajpgi.1993.264.2.G334. [DOI] [PubMed] [Google Scholar]
- Grider JR, Murthy KS, Jin J-G, Makhlouf GM. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. American Journal of Physiology. 1992;262:G774–778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Grundy D, Gharib-Naseri MK, Hutson D. Role of nitric oxide and vasoactive intestinal polypeptide in vagally mediated relaxation of the gastric corpus in the anaesthetized ferret. Journal of Autonomic Nervous System. 1993;43:241–246. doi: 10.1016/0165-1838(93)90330-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Imagawa J, Yellon DM, Baxter GF. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. British Journal of Pharmacology. 1999;126:701–708. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J-G, Murthy KS, Grider JR, Makhlouf GM. Activation of distinct cAMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. American Journal of Physiology. 1993;264:G470–477. doi: 10.1152/ajpgi.1993.264.3.G470. [DOI] [PubMed] [Google Scholar]
- Kim CD, Goyal RK, Mashimo H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. American Journal of Physiology. 1999;277:G280–284. doi: 10.1152/ajpgi.1999.277.2.G280. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric oxide synthases in mammals. Journal of Biochemistry. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Hagaman JR, Smithies O. A non-invasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre RA, Vandekerkhove K. Effect of nitroglycerin and long-term electrical stimulation on nitrergic relaxation in the pig gastric fundus. British Journal of Pharmacology. 1998;123:143–149. doi: 10.1038/sj.bjp.0701582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Rand MJ. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. European Journal of Pharmacology. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- Liu SF, Barnes PJ, Evans TW. Time course and cellular localization of lipopolysaccharide-induced nitric oxide synthase messenger RNA expression in the rat in vivo. Critical Care Medicine. 1997;25:512–518. doi: 10.1097/00003246-199703000-00022. [DOI] [PubMed] [Google Scholar]
- Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. Journal of Clinical Investigation. 1996;98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Pfeifer A, Aszodi A, Ahmad M, Alm P, Hedlund P, Fassler R, Andersson K-E. Impaired relaxation of stomach smooth muscle in mice lacking cyclic GMP-dependent protein kinase I. British Journal of Pharmacology. 2000;129:395–401. doi: 10.1038/sj.bjp.0703061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongülener N, Karabal E, Baysal F, Dikmen A. Possible roles of nitric oxide and vasoactive intestinal polypeptide on relaxation induced by isoprenaline in isolated muscle strips of the mouse gastric fundus. Acta Medica Okayama. 1995;49:231–236. doi: 10.18926/AMO/30401. [DOI] [PubMed] [Google Scholar]
- Radomski MW, Palmer RMJ, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MJ, Li CG. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annual Review of Physiology. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Rekik M, Delvaux M, Tack I, Frexinos J, Bueno L. VIP-induced relaxation of guinea-pig intestinal smooth muscle cells: sequential involvement of cAMP and nitric oxide. British Journal of Pharmacology. 1996;118:477–484. doi: 10.1111/j.1476-5381.1996.tb15428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro P, Kaufman S. The effect of tetrahydrobiopterin on the in situ phosphorylation of tyrosine hydroxylase in rat striatal synaptosomes. Neurochemical Research. 1994;19:541–548. doi: 10.1007/BF00971328. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Nitric oxide as a mediator of non-adrenergic non cholinergic neurotransmission. American Journal of Physiology. 1992;262:G379–392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Schulz R, Nava E, Moncada S. Induction and potential biological relevance of a Ca2+-independent nitric oxide synthase in the myocardium. British Journal of Pharmacology. 1992;105:575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Ishii M, Kawakami Y, Kiuchi Y, Momose K, Yamamoto T. Presence of excess tetrahydrobiopterin during nitric oxide production from inducible nitric oxide synthase in LPS-treated rat aorta. Life Sciences. 1999;65:2769–2779. doi: 10.1016/s0024-3205(99)00545-7. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CWR, Keef KD. Roles of peptides in enteric neuromuscular transmission. Regulatory Peptides. 1995;56:101–120. doi: 10.1016/0167-0115(95)00013-2. [DOI] [PubMed] [Google Scholar]
- Teng B-Q, Murthy KS, Keummerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. American Journal of Physiology. 1998;275:G342–351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nicholls-Grzemski FA, Schmittgen TD, Zakrujsek BA, Fariss W. Characterization of nitric oxide production following isolation of rat hepatocytes. Toxicological Sciences. 2000;53:56–62. doi: 10.1093/toxsci/53.1.56. [DOI] [PubMed] [Google Scholar]
- Todd ME, Brent G. Arterial wall and smooth muscle cell development in young Wistar rats and the effects of surgical denervation. Circulation Research. 1991;69:438–446. doi: 10.1161/01.res.69.2.438. [DOI] [PubMed] [Google Scholar]
- Tonini M, De Giorgio R, De Ponti F, Stermini C, Spelta V, Dionigi P, Barbara G, Stanghellini V, Corinaldesi R. Role of nitric oxide- and vasoactive intestinal polypeptide-containing neurones in human gastric fundus strip relaxations. British Journal of Pharmacology. 2000;129:12–20. doi: 10.1038/sj.bjp.0702977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X-Q, Charles IG, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Yano S, Kiyota Y, Yamamoto M, Watanabe K. Pharmacological features of non-adrenergic non-cholinergic (NANC) relaxation induced by electrical vagal stimulation in isolated mouse stomach. Japanese Journal of Pharmacology. 1995;69:9–15. doi: 10.1254/jjp.69.9. [DOI] [PubMed] [Google Scholar]


