It is naturally very gratifying that the Editors of The Journal of Physiology have decided to make available online a paper that Alan Hodgkin and I published 50 years ago (Hodgkin & Huxley, 1952). This was the final paper in the series on the voltage clamp experiments by us and Bernard Katz. In this note, I will set out the background against which this work was done.
Alan Hodgkin began research after graduating in 1935 at Trinity College, Cambridge and, in the same year, I came to the same college as an undergraduate. At that time, it was accepted in Cambridge that the propagated impulse in a nerve or muscle fibre is an all-or-none event in which the membrane potential change generated at each point on the fibre spreads forward along the cable structure of the fibre and acts as a stimulus exciting the next section. The theory of the actual generation of the action potential that we were taught was that of Bernstein (1902), according to which the resting negativity of the interior is due to the membrane being somewhat permeable to potassium but not to sodium ions; since the former are more concentrated inside the fibre than in the body fluids outside, they diffuse outwards carrying their positive charge. The action potential was attributed to a great increase in permeability of the membrane to all ions, occurring when the internal potential was raised by a threshold amount; the internal potential would therefore rise to the same level as outside.
There was, however, no experimental evidence that the electrical change spreading ahead of the action potential was sufficient to stimulate the next segment of a nerve fibre, and such evidence was provided by Hodgkin in his first year of research (Hodgkin, 1937). This experiment was originally planned with the hope of demonstrating the decrease of membrane resistance postulated by Bernstein; in fact, this was achieved by Cole & Curtis (1939) in a beautiful experiment using a high-frequency AC bridge to measure the impedance of the membrane of the giant nerve fibre of the squid. They found that the membrane resistance dropped to a low value during the action potential but the capacitance in parallel with it did not change.
Fig. 15.
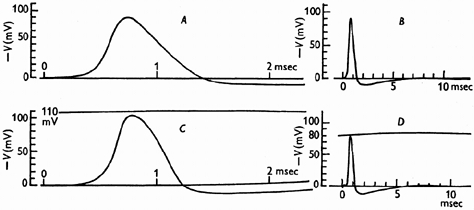
A, solution of eqn. (31) calculated for K of 10.47 msec−1 and temperature of 18.5° C. B, same solution plotted on slower time scale. C, tracing of propagated action potential on same vertical and horizontal scales as A. Temperatire 18.5° C. D, tracing of progagated action potential from another exon on approximately the same vertical and horizontal scales as B. Temperature 19.2° C. This axon had been used for several hours; its spike was initially 100 mV. Reprinted from Hodgkin & Huxley (1952).
From experiments on single fibres from motor nerves of crabs, Hodgkin came to believe that this increase of permeability was not a sudden event but was graded with the change of membrane potential.
In the summer vacation of 1939, when I had just finished undergraduate work, Hodgkin went to the laboratory of the Marine Biological Association at Plymouth to do experiments on the squid giant fibre, and he invited me to join him. We pushed an electrode down inside the fibre and recorded potential directly across the membrane, finding that the interior became substantially positive during the action potential. This was contrary to Bernstein's theory, but it was not altogether a surprise to Hodgkin as he already had hints (unpublished) of this discrepancy from external measurements on fibres of crabs and lobsters. We could not pursue the problem further because war was obviously imminent, and we left Plymouth two days before Hitler invaded Poland. We published the result in a letter to Nature (1939) with no discussion or explanation. In a full paper (1945), we gave four possible explanations, all wrong. It was also in 1945 that we began discussing the explanation that turned out to be correct, namely that the increase in permeability was highly specific for sodium ions, which were thereby enabled to diffuse inwards carrying their positive charge. This was confirmed experimentally by Hodgkin & Katz (1949). If we had known the paper of Overton (1902), I am sure we would have reached that conclusion immediately in 1939.
Both Cole and Hodgkin realised that the explosive character of the action potential mechanism made it difficult to investigate the current-voltage characteristic of the membrane, but that this could be overcome by using feedback to control the internal potential. The laboratory at Plymouth was badly bombed during the war, so Hodgkin, Katz and I could not start such experiments until the summer of 1948. Cole (1949), however, had equipment of this type running in 1947 and showed that the current-voltage relation is indeed continuous, with a region of negative slope that makes it unstable, causing the explosive all-or-none response. However, they did not take the analysis further. In our experiments we altered the external sodium concentration and thereby analysed the membrane current into components carried by sodium and by potassium ions. We fitted equations to the dependence of the permeabilities of the membranes to these two ions as functions of membrane potential and time. Finally, we solved the resulting set of non-linear differential equations for the time course of potential change if there were no feedback, i.e. the expected action potential (upper curves in the figure reproduced here from Hodgkin & Huxley, 1952). This agreed satisfactorily with the time course of the action potential recorded experimentally (lower curves). It is this work that is described in the paper made available online with this issue of The Journal of Physiology.
Supplementary Material
References
- Bernstein J. Pflügers Archiv. 1902;92:521–562. [Google Scholar]
- Cole KS. Archives des Sciences Physiologiques. 1949;3:253–258. [Google Scholar]
- Cole KS, Curtis HJ. Journal of General Physiology. 1939;22:649–670. doi: 10.1085/jgp.22.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL. Journal of Physiology. 1937;90:183–210. 211–232. doi: 10.1113/jphysiol.1937.sp003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Nature. 1939;144:710–711. [Google Scholar]
- Hodgkin AL, Huxley AF. Journal of Physiology. 1945;104:176–195. doi: 10.1113/jphysiol.1945.sp004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Journal of Physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. Journal of Physiology. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton E. Pflügers Archiv. 1902;92:346–386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


