Abstract
Orthostatic intolerance is common when astronauts return to Earth: after brief spaceflight, up to two-thirds are unable to remain standing for 10 min. Previous research suggests that susceptible individuals are unable to increase their systemic vascular resistance and plasma noradrenaline concentrations above pre-flight upright levels. In this study, we tested the hypothesis that adaptation to the microgravity of space impairs sympathetic neural responses to upright posture on Earth. We studied six astronauts ∼72 and 23 days before and on landing day after the 16 day Neurolab space shuttle mission. We measured heart rate, arterial pressure and cardiac output, and calculated stroke volume and total peripheral resistance, during supine rest and 10 min of 60 deg upright tilt. Muscle sympathetic nerve activity was recorded in five subjects, as a direct measure of sympathetic nervous system responses. As in previous studies, mean (± s.e.m.) stroke volume was lower (46 ± 5 vs. 76 ± 3 ml, P = 0.017) and heart rate was higher (93 ± 1 vs. 74 ± 4 beats min−1, P = 0.002) during tilt after spaceflight than before spaceflight. Total peripheral resistance during tilt post flight was higher in some, but not all astronauts (1674 ± 256 vs. 1372 ± 62 dynes s cm−5, P = 0.32). No crew member exhibited orthostatic hypotension or presyncopal symptoms during the 10 min of postflight tilting. Muscle sympathetic nerve activity was higher post flight in all subjects, in supine (27 ± 4 vs. 17 ± 2 bursts min−1, P = 0.04) and tilted (46 ± 4 vs. 38 ± 3 bursts min−1, P = 0.01) positions. A strong (r2 = 0.91–1.00) linear correlation between left ventricular stroke volume and muscle sympathetic nerve activity suggested that sympathetic responses were appropriate for the haemodynamic challenge of upright tilt and were unaffected by spaceflight. We conclude that after 16 days of spaceflight, muscle sympathetic nerve responses to upright tilt are normal.
Adaptation to the microgravity environment of space leads to a series of well-described physiological responses to upright posture after return to Earth, characterized primarily by a large fall in stroke volume and an augmented tachycardia (Blomqvist & Stone, 1983; Bungo et al. 1985; Blomqvist, 1986; Fritsch-Yelle et al. 1994, 1996; Buckey et al. 1996; Fortney et al. 1996). Depending on the type and duration of orthostatic stress, from one-quarter (Fritsch–Yelle et al. 1996) to two–thirds (Buckey et al. 1996) of crew members returning from brief (8–14 days) space missions experience presyncope and are unable to remain standing for 10 min.
Mechanisms responsible for such ‘orthostatic intolerance’ after space travel have been investigated extensively (Blomqvist & Stone, 1983; Blomqvist et al. 1996; Levine, 1996; Watenpaugh & Hargens, 1996). Buckey et al. (1996) showed that those astronauts with the most severe postflight orthostatic intolerance are unable to augment their total peripheral resistances above pre-flight upright levels. Fritsch-Yelle et al. (1996) confirmed this observation in a larger group of astronauts and showed further that the most severely affected crew members have smaller increases of plasma noradrenaline concentrations during or immediately following standing than their more orthostatically tolerant colleagues. These observations suggest that cardiovascular adaptation to spaceflight may fundamentally alter reflex regulation of sympathetic nerve activity, and that this change contributes to postflight orthostatic intolerance (Robertson et al. 1994; Buckey et al. 1996; Fritsch-Yelle et al. 1996).
In this, one of three reports from the Neurolab space shuttle mission (Cox et al. 2002; Ertl et al. 2002), we tested the hypotheses that spaceflight impairs the ability of astronauts to increase their muscle sympathetic nerve activity in the upright position on Earth, and that this impairment translates into inadequate vasoconstriction and hypotension. We report, for the first time, directly measured efferent muscle sympathetic nerve activity in both supine and upright positions, before and after astronauts' adaptation to microgravity. Some of these results have been published in abstract form (Levine, for the Neurolab Autonomic Team Investigators, 1999).
METHODS
Subjects
We studied six male crew members of the 16 day Neurolab mission, described in detail by Cox et al. (2002). Their mean age (± s.e.m.) was 40 ± 2 years, height 187 ± 2 cm and weight 89.3 ± 4 kg. All astronauts were in excellent health, as determined by comprehensive National Aeronautics and Space Administration (NASA) Class III physical examinations. No subject smoked or used any medication regularly. This research conformed with the Declaration of Helsinki, and all subjects signed an informed consent form approved by the NASA Human Subjects Review Committee and the institutional review boards of principal investigators' institutions.
Pre-flight experiments
Experiments were performed 73–70 and 24–21 days pre-flight and on landing day. Subjects refrained from performing high intensity exercise or taking any over-the-counter medication within 24 h of all studies. Subjects were studied at least 2 h after a meal and more than 12 h after the last caffeinated or alcoholic beverage. Experiments were conducted in a quiet, environmentally controlled laboratory with an ambient temperature of 25 °C, at Johnson Space Center, Houston, TX, USA. Subjects were positioned supine in a custom-designed rigid plastic chamber designed to allow both lower body negative pressure and upright tilt. The right leg was supported in the fully extended position by a series of removable footboards, customized for each subject. The left leg was elevated six inches from the bottom of the chamber with the hip flexed. Access to the fibular head and popliteal fossa for microneurography was obtained via a removable window in the chamber.
Landing day experiments
No subject took medication during the 48 h prior to landing. However, just prior to re-entry, while still in space, three astronauts consumed 1.5 l of hyposmolar glucose and electrolyte solution, and three astronauts consumed about 8 g salt and 912 ml water, according to a NASA ‘fluid loading’ protocol (Bungo et al. 1985). Fluid loading induced vomiting in one of the latter three. Immediately after their space shuttle landing at Kennedy Space Center, Cape Canaveral, FL, USA, astronauts were transported to the laboratory in the supine position, where they remained until the experiments were completed. (Two of the six subjects sat upright less than 30 min, to complete another experiment.) Subjects were studied in pairs; the first pair was studied within 1 h, the second within 3 h and the third within 5 h of landing on Earth. All astronauts were allowed free access to water from the time of landing until the beginning of the protocol. Subjects were placed in the supine position on a commercial tilt bed (OT9001, Omni Technologies, Valley City, ND, USA), with the right leg extended. The left leg was elevated for microneurography, which was performed at the fibular head. Wide straps were placed over the right thigh near the knee and at the waist to minimize any body motion, and to prevent knee flexion during tilting.
Instrumentation
Heart rate was monitored continuously by electrocardiography (HP 78801 B, Hewlett-Packard, Andover, MA, USA), and beat-by-beat arterial pressure was estimated with finger photoplethysmography (Finapres, Ohmeda, Englewood, CO, USA). Arm blood pressure was measured intermittently by electrosphygmomanometry (Suntech 4240, Suntech Medical Instruments, Raleigh, NC, USA), with a microphone placed over the brachial artery to detect Korotkoff sounds. An adjustable arm support held the finger at heart level in both supine and upright tilt positions.
Cardiac output was measured with a modification of the acetylene re-breathing technique, with acetylene as the soluble and helium as the insoluble gas (Triebwasser et al. 1977). With this technique, pulmonary blood flow is calculated from the disappearance rate of acetylene in expired air, as measured by a mass spectrometer (MGA 1100, Marquette Instruments, Milwaukee, WI, USA), after adequate mixing in the lung has been confirmed by a stable helium concentration. This method was validated against thermodilution and direct Fick techniques, over a range of cardiac outputs, from 2.8 to 27.0 l min−1 (r2 = 0.91; standard error of the estimate = 1.1 l min−1, Pawelczyk et al. 1995). Stroke volume was calculated by dividing the cardiac output by the heart rate measured during rebreathing, and total peripheral resistance was calculated by dividing mean arm cuff arterial pressure (one-third of the pulse pressure plus diastolic pressure) by cardiac output.
Multiunit, postganglionic muscle sympathetic nerve activity was recorded ∼72 days pre-flight and on landing day, with methods described in detail by Ertl et al. (2002). The nerve signal was amplified (total gain: 70 000–160 000), band-pass filtered (700–2000 Hz) and full-wave rectified and integrated with a resistance-capacitance circuit (time constant 0.1 s) to obtain mean voltage neurograms. Satisfactory muscle sympathetic nerve recordings were defined by pulse-synchronous bursts that increased during end-expiratory apnoea or Valsalva straining and did not change during tactile or auditory stimulation.
Protocol
After at least 20 min of quiet supine rest, plasma and blood volumes were measured with the Evans Blue dye technique (Foldager & Blomqvist, 1991). Cardiac output was measured every 5 min until it reached a stable level, defined as two consecutive measurements within 500 ml min−1 of each other. Upright tilt was performed after a battery of tests of autonomic function, which included controlled-frequency breathing and static handgrip (postflight, as well as pre-flight), and the Valsalva manoeuvre, cold-pressor test and low level (15 and 30 mmHg) lower body negative pressure (pre-flight only). Sufficient time was allowed between each intervention for the baseline haemodynamic state and sympathetic nerve activity to return to baseline levels. A detailed schedule for all Neurolab landing day experiments is given in the Appendix of the article by Cox et al. (2002).
After a minimum 10 min recovery period following the preceding intervention, baseline heart rate, arterial pressure and muscle sympathetic nerve activity were recorded for 5 min. A blood sample for plasma noradrenaline measurement was then collected in EGTA- plus glutathione-containing tubes, placed on ice and centrifuged immediately at −70 °C for later analysis by high precision liquid chromatography. The astronaut was then tilted smoothly to a 60 deg upright position, over 10–15 s. The subject remained in the upright tilt position for 10 min. Data were recorded continuously throughout the duration of upright tilt. Data during tilting were analysed after a 2 min stabilization period, during the second to fourth minute. Then, cardiac output was measured and blood was drawn for plasma noradrenaline determinations. Data were analysed again during the seventh to ninth minute, for comparisons between pre- and postflight conditions. Finally, cardiac output was measured a second time and the astronaut was returned to the supine position.
Data analysis
Sympathetic bursts were identified by a computer program and were then confirmed by an experienced observer who was ‘blind’ to the experimental context. Sympathetic activity was expressed primarily as bursts per minute, a reproducible measure in individual subjects over time (Sundlöf & Wallin, 1977). To obtain an index of the change in total sympathetic nerve activity during tilt, the areas under all bursts were integrated during each recording period. Nerve activity recorded during the final 2 min of the supine baseline recording was assigned a value of 100 %, and subsequent changes in integrated muscle sympathetic nerve activity were expressed as percentages of this baseline value.
Statistical analysis
Data are given as means ± s.e.m. Pre-flight (∼72 days before the mission) and landing day comparisons of baseline data were made with Student's paired t test. Changes in haemodynamic and reflex responses to upright tilt between pre-flight and landing day sessions were examined with a repeated measures, two-way analysis of variance (ANOVA). If a significant (P < 0.05) interaction was observed, a Newman-Keuls post hoc test was used to determine the source of the difference. All statistical analyses were performed with a personal computer-based analysis program (Abstat, Anderson-Bell, Denver, CO, USA). A P value of < 0.05 was considered significant.
RESULTS
Haemodynamics
Plasma volume was not significantly changed (3.3 ± 0.1 vs. 3.2 ± 0.1 l, P = 0.14), but total blood volume was significantly lower (5.1 ± 0.1 vs. 4.8 ± 0.2 l, P = 0.025) after spaceflight than before.
Stroke volumes (Fig. 1) in the supine position and stroke volume reductions during 60 deg upright tilt were remarkably similar during the two pre-flight sessions. Pre-flight stroke volumes decreased by ∼40 % after 5 min and 50 % after 10 min of 60 deg upright tilt. On landing day, stroke volumes during supine rest were lower (P < 0.05) and early and late stroke volume reductions during 60 deg upright tilt were greater than on either pre-flight day. Stroke volumes during postflight tilting were lower than those measured during either pre-flight session in all subjects (P < 0.01).
Figure 1. Left ventricular stroke volume responses to 60 deg upright tilt.
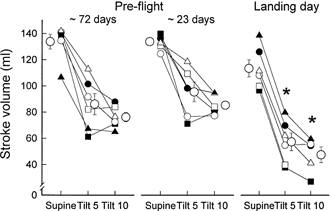
Average (± s.e.m.) and individual stroke volumes in the supine position and after 5 and 10 min 60 deg upright tilt (Tilt 5 and Tilt 10). In this and subsequent figures, s.e.m. bars appear to be missing for some points because of the tight data and small s.e.m. Each astronaut is represented by the same symbols in all figures, in this and the other two Neurolab articles (Cox et al. 2002; Ertl et al. 2002). Astronauts 1–6 are represented by ○, □, ▵, •, ▪ and ▴. *P < 0.05 compared with same time points pre-flight. Although all six subjects completed 10 min of tilting, there was a technical problem with the cardiac output measurement for astronaut 2 (□) at 10 min, and no stroke volume measurement was made.
Heart rate (Fig. 2) during supine rest and heart rate increases during 60 deg upright tilt were similar in the two pre-flight sessions. The greater decreases in stroke volume registered during postflight tilting (Fig. 1) were associated with greater increases in heart rate (P < 0.01) compared with pre-flight levels; as a result, cardiac outputs during tilting were comparable during pre- and postflight sessions. Pre- and postflight cardiac outputs averaged 7.8 ± 0.3 vs. 7.3 ± 0.6 l min (P = 0.54) in the supine position and 5.6 ± 0.3 vs. 4.9 ± 0.6 l min (P = 0.37) at 10 min tilt.
Figure 2. Heart rate responses to 60 deg upright tilt.
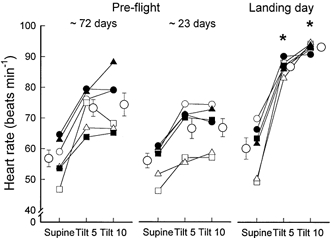
*P < 0.05, compared with same position and time points pre-flight.
Total peripheral resistance during supine rest and total peripheral resistance increases during 60 deg upright tilt (Fig. 3) were similar during the pre-flight sessions. Peripheral resistance in the supine position was insignificantly greater on landing day than during pre-flight sessions. In contrast to the much greater increases in heart rate that occurred during tilting on landing day (Fig. 2), increases in total peripheral resistance with tilting were not greater than those measured pre-flight (P = 0.32). (Thus, on landing day, greater stroke volume reductions were not matched by greater increases of total peripheral resistance.) On landing day, five of the six astronauts had total peripheral resistance increases during tilting similar to those measured pre-flight (in two, peripheral resistance increases were slightly greater and in three, they were slightly smaller). Only one subject, a high-performance jet pilot, experienced a substantially greater increase of total peripheral resistance during tilting on landing day, than during the pre-flight sessions (Fig. 3, right panel, filled squares).
Figure 3. Calculated total peripheral resistance responses to 60 deg upright tilt.
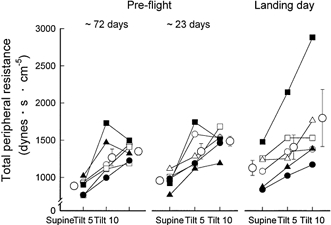
Total peripheral resistance increased significantly (P < 0.05) when subjects were tilted from supine to 60 deg upright positions, in all sessions. These increases were not significantly different among sessions.
The net result of reduced stroke volumes, increased heart rates and unchanged total peripheral resistances was that arterial pressures were preserved (Fig. 4). There were no significant differences between supine and tilting measurements, or among measurements made during pre-flight and landing day sessions. Diastolic pressure increased significantly from supine levels during tilting (P < 0.05) in both pre-flight and landing day trials, with no significant differences among them. Post flight, most individual recordings showed large respiratory oscillations of arterial pressure during tilting, which were not observed pre-flight. (These fluctuations were synchronous with fluctuations in end-tidal carbon dioxide concentration, which we do not report here.) Systolic pressure standard deviations, used as indices of this variability, did not change significantly from supine to tilting pre-flight (7.3 ± 1.2 vs. 8.2 ± 1.6 mmHg, P = 0.11), but increased significantly, by > 50 %, on landing day (6.7 ± 0.8 vs. 10.1 ± 2.1 mmHg, P < 0.01). Increases in systolic pressure standard deviations during tilting on landing day were significantly greater than those measured during pre-flight sessions (P < 0.001).
Figure 4. Arterial pressure responses to 60 deg upright tilt.
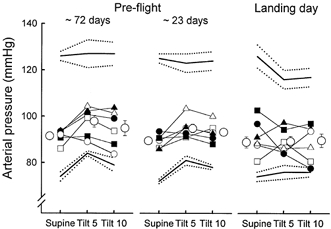
This figure shows mean (± s.e.m.) systolic, mean and diastolic pressure, and individual mean arterial pressure. There were no statistically significant differences among supine and tilt measurements and among pre-flight and landing day measurements.
Sympathetic neural responses
Figure 5 shows muscle sympathetic nerve activity of a representative crew member. Pre-flight recordings (left) document typical pulse-synchronous sympathetic bursting during supine rest and increased burst frequency during tilting. The postflight supine recording (upper right panel) is scaled so that its mean burst height is equivalent to the mean pre-flight burst height. The vertical scale is the same in postflight supine and upright recordings and faithfully indicates the changes of burst amplitude that occurred with tilting. Figure 5 shows increases of sympathetic nerve activity when subjects changed from supine to upright tilt positions, both pre- and postflight; and higher levels of sympathetic nerve activity postflight compared with pre-flight levels, in both supine and upright tilted positions.
Figure 5. Muscle sympathetic nerve responses of one astronaut.
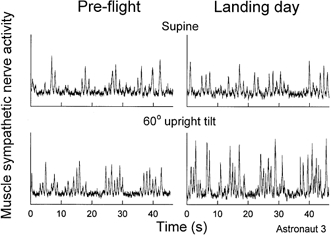
Muscle sympathetic nerve recordings from one astronaut in supine and tilted positions, during pre-flight and landing day sessions. Postflight supine recordings (upper right) is scaled so that its mean burst height is equivalent to the mean pre-flight supine burst height, with no change in scale from supine to 60 deg upright tilt.
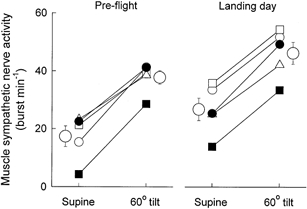
Figure 6 depicts individual and mean measurements of muscle sympathetic nerve burst frequency from all subjects. Increases of muscle sympathetic nerve activity provoked by 60 deg upright tilt were remarkably consistent among astronauts during both pre- and postflight sessions. However, supine muscle sympathetic nerve burst frequency was significantly greater in postflight than pre-flight sessions (P < 0.05). Therefore, increases of muscle sympathetic nerve activity with tilting, which were comparable pre- and postflight, carried muscle sympathetic nerve burst frequency to higher levels postflight. Pre-flight plasma noradrenaline concentrations (not shown) increased from 167 ± 34 supine, to 292 ± 27 ng ml−1 upright (P < 0.01). Postflight, plasma noradrenaline concentrations increased from a significantly higher supine level than pre-flight, 284 ± 48, to 588 ± 71 ng ml−1 upright (both P < 0.05, compared with pre-flight levels).
Figure 6. Muscle sympathetic nerve burst frequencies for all astronauts.
As shown in Fig. 4, steady-state arterial pressures were similar in supine and tilted positions, before and after spaceflight. Therefore, arterial pressure measurements provided no evidence for the changes in baroreceptor activity that must have occurred during pre- and postflight tilting and postflight hypovolaemia. Left ventricular stroke volumes, on the other hand, did change as expected. Figure 7 depicts average and individual pre- and postflight muscle sympathetic nerve activity-left ventricular stroke volume relations. Linear regression coefficients were extremely high, both for individual astronauts (r2 = 0.91−1.00) and mean data (thick line, r2 = 0.99, P < 0.01).
Figure 7. Muscle sympathetic nerve burst frequency plotted as a function of left ventricular stroke volume.
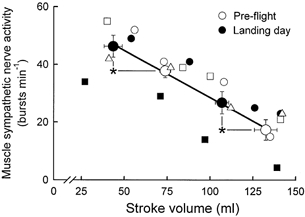
Average measurements (± s.e.m.) made during pre-flight sessions are shown as large open circles and average measurements made on landing day are shown as filled circles. The r2 for linear regression of average data (thick line) was 0.99. Symbols for individual astronauts are the same as in the other figures. r2 values for individual astronauts ranged between 0.91 and 1.00. *P < 0.05 for comparisons between pre- and postflight muscle sympathetic nerve activities and stroke volumes.
Stroke volumes were largest and muscle sympathetic nerve activities were smallest during pre-flight supine rest (Fig. 7, extreme right). Stroke volumes and muscle sympathetic nerve activities during postflight supine rest fell about half -way between pre-flight supine and upright values. Stroke volumes were lowest and muscle sympathetic nerve activity was highest during postflight tilting (Fig. 7, extreme left).
Clinical outcome
All subjects completed the entire protocol and were able to remain upright at 60 deg for 10 min both before and after spaceflight. One subject had a transient ‘vasovagal reaction’ in the supine position prior to postflight tilting, during insertion of the microneurography recording electrode. However, his heart rate, arterial pressure, cardiac output and muscle sympathetic nerve activity returned quickly to conditions present immediately prior to the reaction and he completed the experiment without further incident.
DISCUSSION
This paper, one of three closely related studies from the Neurolab space shuttle mission (Cox et al. 2002; Ertl et al. 2002), directly addressed the issue that has driven most research into the autonomic consequences of spaceflight: the orthostatic intolerance that astronauts may experience when they return to Earth. Although in our study, no astronaut experienced actual presyncope during 10 min of tilting on landing day, all had altered orthostatic responses, including especially, much lower stroke volumes and greater tachycardia. We report two new findings. First, muscle sympathetic nerve activity is significantly greater in the supine position and increases to even higher levels during 60 deg upright tilt, after 16 days of spaceflight than before. Second, postflight increases in muscle sympathetic nerve activity appear to be appropriately calibrated to counter the haemodynamic changes that result from exposure to microgravity. Thus, our results reject the hypothesis that adaptation to microgravity impairs sympathetic responses to upright posture on Earth – sympathetic responses to tilt after spaceflight are well preserved.
Earlier studies
Orthostatic intolerance has been observed after both short- and long-duration space missions, since the inception of the manned space program (Rummel et al. 1976; Blomqvist & Stone, 1983; Watenpaugh & Hargens, 1996). Buckey et al. (1996) reported astronauts' responses to standing, before and after three space shuttle missions. Compared with their pre-flight responses, astronauts had greater reductions in stroke volume during standing, greater tachycardia, and in the case of the nine of 14 of astronauts who were unable to remain standing for 10 min, inability to combat orthostatic hypotension by increasing total peripheral resistance.
Fritsch-Yelle et al. (1996) confirmed these findings in a larger cohort of subjects and showed further that increases in plasma noradrenaline concentrations were significantly smaller in the 25 % of astronauts who were unable to remain standing for 10 min than in those who were. Thus, published evidence suggests that impaired ability to increase sympathetic nerve activity and total peripheral resistance during standing may be a primary mechanism responsible for postflight orthostatic intolerance.
The evidence for this possibility, however, is not definitive. First, there is a technical problem with measurements of postflight noradrenaline concentrations. Fritsch-Yelle et al. (1996) drew blood samples from orthostatically intolerant astronauts after they had been returned to the supine position, but drew samples from orthostatically tolerant astronauts while they were still upright. Second, plasma noradrenaline concentrations provide only indirect estimates of sympathetic nerve activity (Esler et al. 1985; Meredith et al. 1992; Vaz et al. 1995). During standing or tilting, plasma noradrenaline increases are mediated by reductions of noradrenaline clearance, as well as by increases of noradrenaline spillover into plasma (Meredith et al. 1992; Jacob et al. 1998). Therefore, low plasma noradrenaline levels in orthostatically intolerant astronauts may reflect greater clearance and not necessarily reduced spillover of noradrenaline (which correlates directly with muscle sympathetic nerve activity, Esler et al. 1990).
More recent studies involving ground-based simulation of microgravity have reported direct measurements of muscle sympathetic nerve activity. Although the first of these (Shoemaker et al. 1998), indicated that muscle sympathetic nerve activity in supine subjects is reduced after prolonged head-down bed rest, subsequent studies (Shoemaker et al. 1999; Kamiya et al. 2000a,b; Pawelczyk et al. 2001) documented normal or increased levels of sympathetic nerve activity during supine rest and further increases during upright tilt or lower body negative pressure.
Neurolab findings
Some of our data confirm results published earlier. Although plasma volumes of Neurolab astronauts declined less than those of other astronauts studied earlier (Leach, 1981; Leach et al. 1996), their blood volumes declined similarly (Alfrey et al. 1996; Buckey et al. 1996). Moreover, the haemodynamic measurements we report in both supine and tilted positions, including heart rate, stroke volume and total peripheral resistance, are virtually identical to those published by Buckey (1996) and Fritsch-Yelle et al. (1996) and their coworkers. Although none of our subjects experienced orthostatic hypotension or presyncope during 10 min of tilt, all had large increases in heart rate and respiratory frequency arterial pressure fluctuations, as observed also after head-down bed rest (Ten Harkel et al. 1992; Shoemaker et al. 1999).
Our direct sympathetic nerve recordings provide new insights into the haemodynamic data published earlier. We propose that despite Neurolab astronauts' hypovolaemia, arterial pressures were maintained at pre-flight levels (Fig. 4), in part because their muscle sympathetic nerve activity was higher (Figs 5–7). We suggest further, that astronauts' postflight arterial pressure responses to upright tilt are preserved, because their already increased levels of muscle sympathetic nerve activity rise to even higher levels (aided by much greater cardioacceleration). Finally, we suggest that the autonomic adjustments made by astronauts after microgravity adaptation are appropriate for their altered haemodynamic circumstances.
Left ventricular stroke volume
Although we have no direct evidence regarding what sensors mediated the recorded autonomic response patterns, we suspect that arterial baroreceptors figured prominently. Normal arterial pressure levels in hypovolaemic Neurolab astronauts should not be taken as evidence against arterial baroreflex engagement; if arterial baroreflex responses to hypovolaemia maintain arterial pressure at normal levels, there will be no evidence that arterial baroreceptors are involved. Neurolab data do not include measurements of baroreceptive artery dimensions (which must have been less in supine and tilted positions postflight) and absent changes of arterial pressure, we were challenged to quantify the haemodynamic alterations and steady-state changes of arterial baroreceptor activity that must have been present.
We considered that measurements of left ventricular stroke volume might provide insights that were absent in arterial pressure measurements. Stroke volume was of interest for four reasons. First, stroke volume is the only variable in the heart rate-stroke volume-total peripheral resistance relation (Levine et al. 1991) that is directly affected by gravity. Second, stroke volume is a major determinant of flow in baroreceptive arteries, and flow importantly modulates baroreceptor activity (Hajduczok et al. 1988). Third, stroke volume changes translate into arterial pulse amplitude and pressure changes, and these clearly modulate arterial baroreceptor activity (Angell James, 1971; Chapleau & Abboud, 1989). Fourth, stroke volume is a direct, linear function of central blood volume and left ventricular end-diastolic volume and thereby accurately reflects the stimulus to the myriad receptor populations termed ‘cardiopulmonary’, whose role in orthostatic adjustments is uncertain (Persson et al. 1988). We found near-perfect (r2 = 0.91−1.0) inverse linear relations between stroke volume and muscle sympathetic nerve activity (Fig. 7). We do not imply that such correlations prove direct causality. Rather, we suggest that the strong correlations between muscle sympathetic nerve activity and left ventricular stroke volume indicate that changes in muscle sympathetic nerve activity are appropriate for the haemodynamic alterations imposed by adaptation to microgravity.
This conclusion is supported by other data. Pawelczyk et al. (2001) used pulmonary capillary wedge pressure as a different index of haemodynamic loading and unloading during volume infusion and lower body suction, before and after microgravity simulated by head-down bed rest. That study showed that changes of muscle sympathetic nerve activity during changes of volume loading fall on different portions of the same curvilinear muscle sympathetic nerve activity-pulmonary capillary wedge pressure relation. In that study after bed rest, lower body suction provoked a greater haemodynamic challenge, which was met by correspondingly greater increases in muscle sympathetic nerve activity. Our conclusion is supported also by Valsalva responses of Neurolab astronauts (Cox et al. 2002). Straining provoked greater reductions of diastolic pressure, which were answered by proportionally greater muscle sympathetic nerve responses.
Total peripheral resistance
In this Neurolab study, muscle sympathetic nerve activity increased appropriately during upright tilt, but without a commensurate increase in total peripheral resistance in most astronauts. It is possible that sympathetic nerve traffic to other vascular beds, such as the splanchnic circulation, could be impaired, independent of normal sympathetic responses to skeletal muscle (Ninomiya & Irisawa, 1975). However, Ertl et al. (2002) reported that whole-body noradrenaline spillover, measured with a radioactive tracer technique, was preserved in these subjects, making this possibility unlikely.
Studies of tail-suspended rats have suggested that in this model of microgravity, arterial responses to vasoconstrictors may be impaired because of vascular smooth muscle atrophy (Chew & Segal, 1997; Delp, 1999). However, intravenous (Convertino et al. 1997) and intra-arterial (Pawelczyk & Levine, 1995) infusions of the α-adrenergic agonist, phenylephrine, increase regional (forearm and leg) and systemic vascular resistances normally after microgravity exposure, arguing against a primary loss of vascular responsiveness in humans.
An alternative explanation for increased muscle sympathetic nerve activity, but unchanged total peripheral resistance is that of limited ‘vasoconstrictor reserve’ (Schondorf & Wieling, 2000). Patients predisposed to neurally mediated syncope have less adrenergically mediated vasoconstriction than more tolerant subjects (Brown & Hainsworth, 2000). Such individuals therefore may have insufficient reserve to compensate for the excessive reduction in upright stroke volume that occurs in virtually all individuals after adaptation to microgravity (Levine et al. 1997).
Absence of presyncope
The postflight Neurolab protocol differed from spaceflight protocols used earlier, in important ways. After Neurolab, we measured the effects of passive tilt to 60 deg, not of active standing, as used by Buckey et al. (1996) and Fritsch-Yelle et al. (1996). Active standing provokes greater initial (during the first 30 s) reductions in arterial pressure than passive tilt (Sprangers et al. 1991) and 90 deg (sin = 1.0) provokes greater autonomic responses than 60 deg (sin = 0.87) of upright tilt (Cooke et al. 1999). Moreover, during tilting, each astronaut supported all his weight on one leg, allowing the other leg to be relaxed for microneurography. Increased muscle tension in the weight-bearing leg (Ten Harkel et al. 1994) may have stimulated group III or group IV muscle afferent nerves and augmented muscle sympathetic nerve activity and arterial pressure via the exercise pressor reflex (Mitchell & Victor, 1996). Such muscle tensing has little effect in healthy subjects, but may exert important effects in individuals, including the astronauts we studied, who have inordinate tachycardia in the upright position (Wieling et al. 1993). However, we took pains to minimize subjects' muscle tension during upright tilt and supported the thigh with a strap to keep the weight-bearing leg extended. Moreover, activation of exercise pressor reflexes would not explain the postflight increases in muscle sympathetic nerve activity that we recorded when subjects were supine.
It is clear that the occurrence of fainting during orthostatic stress is a continuous, rather than a discrete variable, and that presyncope can be induced in all individuals, with haemodynamic stresses of sufficient intensities or durations (Lightfoot & Tsintgiras, 1995; Levine et al. 1997). For example, a subject who does not faint after 10 min of upright tilt, might faint at 11 min. It is unlikely that such fainting would represent a fundamentally different physiological response to orthostasis. More likely, it would represent a quantitative rather than a qualitative difference in the time to (as opposed to the mechanism of) provocable syncope. Thus, more or less tolerant subjects may be designated as ‘non-fainters’ or ‘fainters’ as a function of the specific experimental circumstances, rather than as functions of truly dichotomous populations.
Because we did not observe any instance of presyncope, we cannot determine its specific mechanism following spaceflight. However, published evidence indicates that the final common pathways of neurally mediated syncope are sympathetic withdrawal and vagal cardioinhibition (Morillo et al. 1997; Mosqueda-Garcia et al. 1997). A preliminary report by Kamiya et al. (2000c), shows that after simulated microgravity, muscle sympathetic nerve activity is augmented in the supine position, increases further during upright tilt, and then, at the onset of presyncope, disappears. The data we report from the Neurolab mission are similar, with the exception that the terminal sympathetic withdrawal and presyncope did not occur.
Limitations
Our study suffers from a major limitation shared by most research conducted in astronauts: the number of subjects is small. Conceivably, some of our results might have been different with a larger study population. We believe, but cannot prove, that research protocols conducted prior to our studies before and after the Neurolab mission (see Appendix of Cox et al. 2002), did not influence our results. Autonomic changes provoked by vestibular stimulation (Shortt & Ray, 1997), Valsalva straining (Smith et al. 1996) and controlled breathing should disappear within seconds to minutes after the end of such perturbations. We cannot exclude the possibility that NASA fluid-loading protocols influenced our results. We can say, however, that fluid-loading protocols do not appear to reduce the incidence of postflight presyncope (Buckey et al. 1996).
Integrated interpretation
This is one of three articles in a series (Cox et al. 2002; Ertl et al. 2002) that describes the autonomic responses of astronauts who participated in the Neurolab space shuttle mission. These studies were driven by prior research into the orthostatic intolerance experienced by many astronauts after exposure to microgravity. The three studies tested prospectively defined hypotheses that dealt with very different aspects of autonomic physiology. The study of Cox et al. (2002) evaluated sympathetic and vagal transients provoked by Valsalva straining and tested the hypothesis that exposure to microgravity degrades abrupt sympathetic as well as vagal baroreflex responses. The study of Ertl et al. (2002) correlated muscle sympathetic nerve activity with plasma noradrenaline kinetics before and during steady-state lower body suction and tested the hypotheses that exposure to microgravity reduces baseline muscle sympathetic nerve activity and noradrenaline spillover and the responses of these indexes to simulated orthostatic stress in space. The present study compared measurements of haemodynamic and autonomic responses to 60 deg upright tilt before and after the Neurolab mission and tested the hypothesis that adaptation to the microgravity of space impairs sympathetic neural responses to upright posture on Earth.
Although the three studies employed different methods and addressed different aspects of autonomic physiology, their results are remarkably congruent. First, all three studies rejected the hypotheses tested: with different perturbations and different methods of gauging sympathetic responses, all protocols documented normal sympathetic responses during and after spaceflight. The study of Cox et al. (2002) showed that during very brief reductions of baroreceptor input provoked by Valsalva straining, sympathetic baroreflex gain is normal in space. The study of Ertl et al. (2002) documented elevated baseline levels of muscle sympathetic nerve activity and plasma noradrenaline spillover and greater increases of these measures during graded, steady-state lower body suction in space. Our study showed that muscle sympathetic nerve activity is increased in the supine position and increases further during 60 deg upright tilt immediately following return to Earth. These increases were appropriate for the haemodynamic conditions that followed microgravity exposure – greater haemodynamic stress in the upright position. Together, the three Neurolab studies provide compelling evidence that after 16 days of spaceflight, sympathetic neural responses to orthostatic stresses are intact.
Acknowledgments
In a complex spaceflight experiment such as this one, there are so many individuals who contribute substantively to the success of the project that it is extremely difficult to acknowledge them all. As an absolute minimum, the crew of STS-90 must be acknowledged for an outstanding effort as experimentalists and subjects in flight. Special acknowledgement must also be made of the efforts of Mike Grande and Stuart Johnston from NASA/Lockheed Martin, and Matt Morrow, Troy Todd and Dak Quarles from the Presbyterian Hospital of Dallas who put in effort above and beyond the call of duty to ensure the success of the project.
REFERENCES
- Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. Journal of Applied Physiology. 1996;81:98–104. doi: 10.1152/jappl.1996.81.1.98. [DOI] [PubMed] [Google Scholar]
- Angell James JE. The effects of altering mean pressure, pulse pressure and pulse frequency on the impulse activity in baroreceptor fibres from the aortic arch and right subclavian artery in the rabbit. Journal of Physiology. 1971;214:65–88. doi: 10.1113/jphysiol.1971.sp009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist CG. Orthostatic hypotension. Hypertension. 1986;8:722–731. doi: 10.1161/01.hyp.8.8.722. [DOI] [PubMed] [Google Scholar]
- Blomqvist CG, Levine BD, Lane LD. Evaluating cardiac patients at special risk: noncardiac surgery and occupational, environmental, and recreational considerations. In: Brunwald E, Crawford MH, editors. Atlas of Heart Diseases, Heart Disease in the Presence of Disorders of other Organ Systems. Vol. 6. Philadelphia, PA, USA: Mosby; 1996. pp. 10.1–10.29. [Google Scholar]
- Blomqvist CG, Stone HL. Cardiovascular adjustments to gravitational stress. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System. Vol. 3. Bethesda, MD, USA: American Physiological Society; 1983. pp. 1025–1063. part iii. [Google Scholar]
- Brown CM, Hainsworth R. Forearm vascular responses during orthostatic stress in control subjects and patients with posturally related syncope. Clinical Autonomic Research. 2000;10:57–61. doi: 10.1007/BF02279892. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. Journal of Applied Physiology. 1996;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- Bungo MW, Charles JB, Johnson PC., Jr Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviation, Space, and Environmental Medicine. 1985;56:985–990. [PubMed] [Google Scholar]
- Chapleau MW, Abboud FM. Determinants of sensitization of carotid baroreceptors by pulsatile pressure in dogs. Circulation Research. 1989;65:566–577. doi: 10.1161/01.res.65.3.566. [DOI] [PubMed] [Google Scholar]
- Chew HG, Jr, Segal SS. Arterial morphology and blood volumes of rats following 10–14 weeks of tail suspension. Medicine and Science in Sports and Exercise. 1997;29:1304–1310. doi: 10.1097/00005768-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Polet JL, Engelke KA, Hoffler GW, Lane LD, Blomqvist CG. Evidence for increased β-adrenoreceptor responsiveness induced by 14 days of simulated microgravity in humans. American Journal of Physiology. 1997;273:R93–99. doi: 10.1152/ajpregu.1997.273.1.R93. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JF, Tahvanainen KUO, Kuusela TA, Levine BD, Cooke WH, Mano T, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC, Jr, Baisch FJ, Blomqvist CG, Robertson D, Eckberg DL. Influence of microgravity on astronauts' sympathetic and vagal responses to Valsava's manoeuvre. Journal of Physiology. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD. Myogenic and vasoconstrictor responsiveness of skeletal muscle arterioles is diminished by hindlimb unloading. Journal of Applied Physiology. 1999;86:1178–1184. doi: 10.1152/jappl.1999.86.4.1178. [DOI] [PubMed] [Google Scholar]
- Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC, Jr, Lane LD, Shiavi R, Gaffney FA, Costa F, Holt C, Blomqvist CG, Eckberg DL, Baisch FJ, Robertson D. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. Journal of Physiology. 2002;538:321–329. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler MD, Hasking GJ, Willett IR, Leonard PW, Jennings GL. Noradrenaline release and sympathetic nervous system activity. Journal of Hypertension. 1985;3:117–129. doi: 10.1097/00004872-198504000-00003. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiological Reviews. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Foldager N, Blomqvist CG. Repeated plasma volume determination with the Evans blue dye dilution technique: the method and a computer program. Computers in Biology and Medicine. 1991;21:35–41. doi: 10.1016/0010-4825(91)90033-6. [DOI] [PubMed] [Google Scholar]
- Fortney SM, Schneider VS, Greenleaf JE. The physiology of bed rest. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. Vol. 2. Oxford, UK: Oxford University Press; 1996. pp. 889–939. [Google Scholar]
- Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. Journal of Applied Physiology. 1994;77:1776–1783. doi: 10.1152/jappl.1994.77.4.1776. [DOI] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. Journal of Applied Physiology. 1996;81:2134–2141. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiological Reviews. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Hajduczok G, Chapleau MW, Abboud FM. Rheoreceptors in the carotid sinus of dog. Proceedings of the National Academy of Sciences USA. 1988;85:7399–7403. doi: 10.1073/pnas.85.19.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. Journal of Applied Physiology. 1998;84:914–921. doi: 10.1152/jappl.1998.84.3.914. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Iwase S, Kitazawa H, Mano T, Vinogradova OL, Kharchenko IB. Baroreflex control of muscle sympathetic nerve activity after 120 days of 6 ° head-down bed rest. American Journal of Physiology. 2000a;278:R445–452. doi: 10.1152/ajpregu.2000.278.2.R445. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Iwase S, Sugiyama Y, Mano T, Sudoh M. Vasomotor sympathetic nerve activity in men during bed rest and on orthostasis after bed rest. Aviation, Space, and Environmental Medicine. 2000b;71:142–149. [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Fu Q, Atsuta S, Iwase S, Mano T. Arterial baroreflex control of sympathetic vasocontrictor traffic and orthostatic intolerance after head-down bed rest. Journal of Gravitational Physiology. 2000c;7:P109. [PubMed] [Google Scholar]
- Leach CS. An overview of the endocrine and metabolic changes in manned space flight. Acta Astronautica. 1981;8:977–986. doi: 10.1016/0094-5765(81)90068-0. [DOI] [PubMed] [Google Scholar]
- Leach CS, Alfrey CP, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW, Krauhs JM. Regulation of body fluid compartments during short-term spaceflight. Journal of Applied Physiology. 1996;81:105–116. doi: 10.1152/jappl.1996.81.1.105. [DOI] [PubMed] [Google Scholar]
- Levine BD. Critical discussion of research issues in mechanisms of cardiovascular adaptation to actual and simulated μG. Medicine and Science in Sports and Exercise. 1996;28:S90–S93. doi: 10.1097/00005768-199610000-00040. [DOI] [PubMed] [Google Scholar]
- Levine BD, Buckey JC, Fritsch JM, Yancy CW, Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. Journal of Applied Physiology. 1991;70:112–122. doi: 10.1152/jappl.1991.70.1.112. [DOI] [PubMed] [Google Scholar]
- Levine BD. Sympathetic neural response to upright tilt is preserved after space flight. Medicine and Science in Sports and Exercise. 1999;31:S337. [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Tsintgiras KM. Quantification of tolerance to lower body negative pressure in a healthy population. Medicine and Science in Sports and Exercise. 1995;27:697–706. [PubMed] [Google Scholar]
- Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension. 1992;19:628–633. doi: 10.1161/01.hyp.19.6.628. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Victor RG. Neural control of the cardiovascular system: insights from muscle sympathetic nerve recordings in humans. Medicine and Science in Sports and Exercise. 1996;28(suppl. 10):S60–69. doi: 10.1097/00005768-199610000-00036. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Eckberg DL, Ellenbogen KA, Beightol LA, Hoag JB, Tahvanainen KUO, Kuusela TA, Diedrich AM. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509–2513. doi: 10.1161/01.cir.96.8.2509. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, Desai T, Snell M, Jarai Z, Ananthram V, Robertson RM, Robertson D. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. Journal of Clinical Investigation. 1997;99:2736–2744. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya I, Irisawa H. Non-uniformity of the sympathetic nerve activity in response to baroceptor inputs. Brain Research. 1975;87:313–322. doi: 10.1016/0006-8993(75)90428-x. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Limb vascular responsiveness to adrenergic agonists following physical deconditioning. Medicine and Science in Sports and Exercise. 1995;27:S31. [Google Scholar]
- Pawelczyk JA, Levine BD, Prisk GK, Shykoff BE, Elliot AR, Rosow E. Nasa/Aiaa Life Sciences and Space Medicine Conference. Houston, TX, USA: National Aeronautics and Space Administration; 1995. Accuracy and precision of flight systems for determination of cardiac output by soluble gas rebreathing; pp. Ls95–Ls130. [Google Scholar]
- Pawelczyk JA, Zuckerman JH, Blomqvist CG, Levine BD. Regulation of muscle sympathetic nerve activity after bed rest deconditioning. American Journal of Physiology. 2001;280:H2230–2239. doi: 10.1152/ajpheart.2001.280.5.H2230. [DOI] [PubMed] [Google Scholar]
- Persson P, Ehmke H, Kirchheim H, Seller H. Effect of sino-aortic denervation in comparison to cardiopulmonary deafferentiation on long-term blood pressure in conscious dogs. Pflügers Archiv. 1988;411:160–166. doi: 10.1007/BF00582309. [DOI] [PubMed] [Google Scholar]
- Robertson D, Convertino VA, Vernikos J. The sympathetic nervous system and the physiologic consequences of spaceflight: a hypothesis. American Journal of the Medical Sciences. 1994;308:126–132. doi: 10.1097/00000441-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Rummel JA, Michel EL, Sawin CF, Buderer MC. Medical experiment M-171: results from the second manned Skylab mission. Aviation, Space, and Environmental Medicine. 1976;47:1056–1060. [PubMed] [Google Scholar]
- Schondorf R, Wieling W. Vasoconstrictor reserve in neurally mediated syncope. Clinical Autonomic Research. 2000;10:53–55. doi: 10.1007/BF02279891. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Leuenberger UA, Herr MD, Gray K, Silber DH, Sinoway LI. Sympathetic discharge and vascular resistance after bed rest. Journal of Applied Physiology. 1998;84:612–617. doi: 10.1152/jappl.1998.84.2.612. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Sinoway LI. Contributions of msna and stroke volume to orthostatic intolerance following bed rest. American Journal of Physiology. 1999;277:R1084–1090. doi: 10.1152/ajpregu.1999.277.4.r1084. [DOI] [PubMed] [Google Scholar]
- Shortt TL, Ray CA. Sympathetic and vascular responses to head-down neck flexion in humans. American Journal of Physiology. 1997;272:H1780–1784. doi: 10.1152/ajpheart.1997.272.4.H1780. [DOI] [PubMed] [Google Scholar]
- Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva's maneuver revisited: a quantitative method yielding insights into human autonomic control. American Journal of Physiology. 1996;271:H1240–1249. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- Sprangers RLH, Veerman DP, Karemaker JM, Wieling W. Initial circulatory responses to changes in posture: influence of the angle and speed of tilt. Clinical Physiology. 1991;11:211–220. doi: 10.1111/j.1475-097x.1991.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. Journal of Physiology. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Harkel ADJ, Baisch F, Karemaker JM. Increased orthostatic blood pressure variability after prolonged head-down tilt. In: Saltin B, Baisch F, Beck L, Blomqvist CG, Karemaker JM, editors. Head-Down Tilt Bedrest. HDT' 88 – An International Collaborative Effort In Integrated Systems Physiology, Acta Physiologica Scandinavica. suppl.604. Vol. 144. 1992. pp. 88–99. [PubMed] [Google Scholar]
- Ten Harkel ADJ, Van Lieshout JJ, Wieling W. Effects of leg muscle pumping and tensing on orthostatic arterial pressure: a study in normal subjects and patients with autonomic failure. Clinical Science. 1994;87:553–558. doi: 10.1042/cs0870553. [DOI] [PubMed] [Google Scholar]
- Triebwasser JH, Johnson RL, Jr, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviation, Space and Environmental Medicine. 1977;48:203–209. [PubMed] [Google Scholar]
- Vaz M, Cox HS, Kaye DM, Turner AG, Jennings GL, Esler MD. Fallibility of plasma noradrenaline measurements in studying postprandial sympathetic nervous responses. Journal of the Autonomic Nervous System. 1995;56:97–104. doi: 10.1016/0165-1838(95)00063-4. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Hargens AR. The cardiovascular system in microgravity. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology, The Gravitational Environment. i. New York, NY, USA: Oxford University Press; 1996. pp. 631–674. chap. iii. [Google Scholar]
- Wieling W, Van Leishout JJ, Van Leeuwen AM. Physical manoeuvres that reduce postural hypotension in autonomic failure. Clinical Autonomic Research. 1993;3:57–65. doi: 10.1007/BF01819146. [DOI] [PubMed] [Google Scholar]


