Abstract
Properties of N-methyl-d-aspartate (NMDA) receptor channels were studied using the patch-clamp technique in fluorescence-labelled control and axotomised motoneurons in thin spinal cord slices. Single-channel currents induced by NMDA in outside-out patches isolated from axotomised motoneurons and voltage clamped at −100 mV, exhibited six amplitude levels with a mean conductance of 14.9 ± 1.9, 22.2 ± 2.7, 35.6 ± 4.4, 49.1 ± 3.5, 59.6 ± 3.5 and 69.0 ± 2.9 pS. In contrast, the conductance of NMDA receptor channels, recorded under identical conditions in control motoneurons was characterised by only four levels corresponding to 20.1 ± 2.5, 38.0 ± 3.0, 58.6 ± 3.4 and 71.5 ± 2.6 pS. The time course of deactivation of NMDA receptor EPSCs in axotomised motoneurons voltage clamped at +40 mV was double exponential. The deactivation had a similar time course in control and axotomised motoneurons from 6–day-old animals; however, the deactivation became faster with increased time after injury. The fast and slow time constants in motoneurons 8 days after axotomy became three times faster than in controls. NMDA receptor-mediated responses were voltage dependent in the presence of extracellular Mg2+. In axotomised motoneurons Boltzmann analysis of the relationship between the peak amplitude of NMDA receptor EPSCs or NMDA-induced responses and membrane potential suggested an apparent Kd for Mg2+ binding (at 0 mV) of 1.2 ± 0.5 and 3.4 ± 3.9 mm, respectively. Single-cell RT-PCR analysis of mRNA revealed that NR2A–D and NR3A subunit transcripts were expressed in axotomised motoneurons. The results of our experiments suggest that in addition to genotypic control of NMDA receptors in motoneurons, axotomy, an experimental model of neurodegeneration, alters functional properties of the receptors in motoneurons destined to die.
Spinal cord motoneurons, like many other neurons within the central nervous system, express ionotropic glutamate receptors, but our knowledge of the regulation of their subunit expression and molecular mechanisms of the receptor assembly are limited. It has been shown previously that the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors are likely to be heteromultimeric proteins, containing a combination of NR1 and NR2A–D and/or NR3A subunits (Dingledine et al. 1999). The functional properties of glutamate receptors are strongly influenced by their subunit composition (Monyer et al. 1992, 1994). Expression of distinct sets of NMDA receptor subunits as well as post-translational modifications and association of NMDA receptors with various regulatory and cytoskeletal proteins precondition differences in the functional and pharmacological properties these receptor channels have in distinct cell populations and during development (Carmignoto & Vicini, 1992; Rosenmund & Westbrook, 1993; Lau & Huganir, 1995; Flint et al. 1997).
We have previously characterised the properties of synaptic and extrasynaptic NMDA receptors in spinal cord motoneurons and shown that their functional and pharmacological properties are substantially different from those described for recombinant and native receptors in other structures of the central nervous system (Paleček et al. 1999). In addition single-cell RT-PCR analysis of mRNA revealed that NR1, NR2A–D and NR3A transcripts were expressed in motoneurons (Abdrachmanova et al. 2000a). Functional properties of both synaptic and extrasynaptic NMDA receptors did not appear to change during the early postnatal period. This is a critical period in rat motoneuron development characterised by loss of sensitivity to target tissue deprivation (Lowrie & Vrbova, 1992). In the rat, deprivation of motoneurons from their target muscle during the first postnatal week induces massive motoneuron death. Furthermore, blockade of NMDA receptors rescues motoneurons otherwise destined to die (Mentis et al. 1993; Greensmith et al. 1994).
Our recent results indicate that the currents induced by activation of synaptic and extrasynaptic (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in motoneurons are mediated by opening of both Ca2+-permeant and Ca2+-impermeant channels. Axotomy performed on the third postnatal day in rats resulted in a gradual and virtually complete motoneuron cell death. In axotomised motoneurons the current mediated by Ca2+-permeant AMPA receptor channels is significantly reduced (Abdrachmanova et al. 2000b).
The aim of the present study was to characterise the effect of axotomy on functional and molecular properties of NMDA receptors in rat spinal cord motoneurons. Our results suggest that functional properties of NMDA receptors are altered in axotomised motoneurons and provide new insight into the regulation of glutamate receptor function.
METHODS
Spinal cord slice preparation
Initially, motoneurons were labelled by fluorescent markers (Fast Blue and Diamidino Yellow; EMS-Polyloy GmbH, Germany) that were injected into the tibialis anterior muscle on the day of birth (PND 0) (for details see Paleček et al. 1999; Abdrachmanova et al. 2000a). The sciatic nerve was transected 1–2 mm proximal to the division of the nerve into the tibial and common peroneal branches on PND 3 (Abdrachmanova et al. 2000b). Subsequently, at PND 6–12, a laminectomy with subsequent spinal cord isolation was performed and the rats were then rapidly decapitated. Slices (200 mm) of the lumbar spinal cord were prepared as described previously (Paleček et al. 1999). Individual fluorochrome-labelled motoneurons were identified in the spinal cord slice using an epifluorescence microscope (Axioskop-FS, Zeiss, Germany). All Wistar rat pups were operated on using ether anaesthesia and aseptic conditions. Efforts were made to minimise animal suffering and to use the minimum number of animals necessary to obtain valid data. All experiments were carried out in accordance with the European Communities Council Directives (86/609/EEC) and with approval of the Institutional Animal Care and Use Committee (1020/491/A/l00).
Whole-cell and outside-out patch recording
Whole-cell and outside-out currents were recorded by the patch-clamp technique using an Axopatch-1D amplifier (Axon Instruments Inc., Foster City, CA, USA) at room temperature (22–25 °C). Pipettes for whole-cell patch-clamp recording were pulled from borosilicate glass (Kavalier-Otvovice, Czech Republic) and when filled with intracellular solution had a resistance of 3–4 MΩ. Pipettes for outside-out patch recording were Sylgard (Dow Corning Europe S.A., Brussels, Belgium) coated and had a resistance of 5–10 MΩ.
The I–V curves of NMDA-evoked currents recorded in the presence of Mg2+ were constructed by a ramp (0.13 mV ms−1) applied to a neuron voltage clamped at +30 mV during a steady-state response to a focal application of 30 μm NMDA. Responses were low-pass filtered at 0.5 kHz (8-pole Bessel filter, Frequency Devices, Haverhill, MA, USA) and digitally sampled at 1 kHz. Leak currents induced by a voltage ramp in the absence of NMDA were subtracted from the NMDA response and the final I–V relation was fitted by the equation:
| (1) |
in which g0 is the estimated conductance of the NMDA whole-cell response in the absence of extracellular Mg2+ ([Mg2+]o = 0), Vrev is the reversal potential of the NMDA-induced current and a and b are parameters with the following interpretation:
| (2) |
where Kd represents the apparent dissociation constant for Mg2+ binding to the NMDA receptor at a given membrane potential (V) and:
| (3) |
where δ indicates the apparent electrical distance of the Mg2+ binding site from the outside of the membrane and F, R and T have their standard thermodynamic meanings (Kirson & Yaari, 1996).
To elicit NMDA receptor mediated excitatory postsynaptic currents (NMDA receptor EPSCs), a second electrode filled with extracellular solution and positioned near (20–150 μm from) the motoneuron was used. Stimuli of constant amplitude (3–10 μA) and duration (50 μs) were applied at 7 s intervals from an Isolator 11 (Axon Instruments Inc). The apparent Kd for Mg2+ binding to synaptically activated NMDA receptor channels was assessed by fitting eqn (1) to the I–V curves of peak NMDA receptor EPSCs recorded in the presence of Mg2+.
Records of single-channel activity induced by NMDA (3 μm) were low-pass filtered at 2 kHz (8-pole Bessel filter), digitally sampled at 20 kHz and analysed using pCLAMP software version 6.0.2 (Axon Instruments Inc.). Initially, the number and current amplitude of the conductance levels of NMDA receptor channel openings were estimated for each patch individually. These values were used to detect opening and closing transitions using a 50% threshold criterion that was set separately for each conductance level (Colquhoun & Sigworth, 1983). Openings briefer than 425 μs (2.5 × filter rise time) were excluded from the analysis (Colquhoun & Sakmann, 1985). Gaussian functions were fitted to the amplitude distributions using a non-linear least squares routine to define current amplitudes and relative areas of the openings. For statistical analysis the values of current amplitudes and relative areas were used if the initially estimated current amplitudes approached that derived from the fit.
Results are presented as means ±s.d.; one-way ANOVA, Student's t test and the Mann-Whitney rank sum test with P < 0.05 were used to determine the significance of differences in the results.
Solutions
The extracellular solution for the preparation and perfusion of spinal cord slices contained (mm): NaCl, 113; KCl, 3; NaHCO3, 25; NaH2PO4, 1; MgCl2, 1; CaCl2, 2; glucose, 11, bubbled with 95% O2–5% CO2. In some experiments, the extracellular solution also contained bicuculline methiodide (5 μm) to block GABAA receptors, strychnine (5 μm) to block glycine receptors, glycine (5 μm) to activate glycine-binding sites associated with NMDA receptors and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 5 μm) to block AMPA receptors. The extracellular solution for single NMDA receptor channel recording contained (mm): NaCl, 160; KCl, 2.5; CaCl2, 1; glucose, 10; Hepes, 10; glycine, 0.005; strychnine, 0.005 (pH was adjusted to 7.3 with NaOH). The intracellular (pipette) solution for whole-cell and outside-out recordings contained (mm): CsMeSO3, 125; CsCl, 15; CaCl2, 1; EGTA, 10; Hepes, 10 (pH was adjusted to 7.2 with CsOH). Local NMDA application was used to study the sensitivity of NMDA receptor channels to extracellular Mg2+. A pipette similar to the one used for whole-cell recording was filled with 30 μm NMDA dissolved in extracellular solution containing (mm): NaCl, 160; KCl, 2.5; CaCl2, 2; MgCl2, 1; glucose, 10; Hepes, 10; glycine, 0.005; strychnine, 0.005; CNQX, 0.005; tetrodotoxin, 0.0005. The pipette was positioned approximately 20–30 μm from the neuron under study and the whole-cell response was induced by ejecting solution with positive pressure (15–20 mmHg). Ultra pure salts (SigmaUltra grade) were used to prepare the Mg2+-free extracellular solution. All other drugs used in electrophysiological experiments were of analytical grade and purchased from the Sigma Chemical Co. (St Louis, MO, USA) with the exception of NMDA and CNQX, which were purchased from Tocris-Cookson (Bristol, UK).
Single-cell RT-PCR
Methods for single-motoneuron RT-PCR were the same as those described previously (Abdrachmanova et al. 2000a). Large diameter pipettes (0.6–1.2 MΩ) were filled with autoclaved intracellular solution (mm): KCl, 140; MgCl2, 3; EGTA, 5; Hepes, 5 (pH was adjusted to 7.2 with KOH) and used to perform whole-cell patch-clamp recording from fluorescence-labelled motoneurons. While resistance was continually monitored, a steady negative pressure was applied to the pipette to harvest the cytoplasm and nucleus. The harvested cell content was then expelled into a reaction tube and subjected to a reverse transcriptase reaction. The PCR amplification (see Abdrachmanova et al. 2000a) of a fragment of cDNA encoding NR3A was performed according to Das et al. (1998) and for NR2A–C and NR2D the method was as described in Audinat et al. (1994).
RESULTS
Motoneuron cell death, both excitotoxic and apoptotic, takes place after axonal injury (Greensmith et al. 1994; de Bilbao & Dubois-Dauphin, 1996). The sensitivity of motoneurons to nerve injury is developmentally regulated and in rats occurs only during the first postnatal week (Greensmith et al. 1994). In accordance with others we have previously shown that sciatic nerve transection performed on PND 3 resulted in a gradual motoneuron loss. On the injured side 50% of the motoneurons survived at PND 10 and only 4% survived at PND 21 (Abdrachmanova et al. 2000b). This study was designed to elucidate the effect of axotomy on the functional and molecular properties of NMDA receptors in motoneurons.
Axotomy affects single-channel conductance of NMDA receptor channels
Figure 1 shows records of channel activity in outside-out patches isolated from axotomised motoneurons, in response to the application of a low concentration of NMDA (3 μm) in the presence of Mg2+-free extracellular solution containing 1 mm Ca2+ and glycine (5 μm). Single-channel openings were sensitive to d-2-amino-5-phosphonovalerate (APV, 50 μm) and Mg2+ (1 mm) at −100 mV and had a reversal potential of ∼0 mV (not illustrated). This strongly suggested that the activity arose from openings of NMDA receptor channels. The distribution of channel openings in Fig. 1A was fitted with the sum of five Gaussian components corresponding to a single-channel conductance of 15, 31, 50, 63 and 72 pS, while the distribution shown in Fig. 1B was fitted with the sum of four Gaussian components corresponding to a conductance of 20, 46, 61 and 73 pS. In 24 patches analysed the distribution of channel openings was fitted with the sum of two components in one patch, three components in four patches, four components in 13 patches and five components in six patches. To determine the number and the mean value of the conductance levels to which NMDA receptor channels open in axotomised motoneurons, single-channel conductances identified in 24 patches (a total of 96 values) were sorted into groups. The criteria used were: (1) the value of the single-channel conductance; (2) the number of conductance levels identified in the patch; (3) the value of the single-channel conductance of two adjacent conductance levels. The mean conductance levels of NMDA receptor openings, their relative distribution, their incidence of occurrence and the simultaneous occurrence of two adjacent conductance levels (coincidence) are shown in Table 1. Neither the single-channel conductance nor the incidence of conductance levels of NMDA receptor channels detected in outside-out patches isolated from motoneurons appeared to be affected by the number of days after axotomy that the recording was made (1–10 days; PND 4–13).
Figure 1. Conductance of NMDA receptor channels in outside-out patches isolated from axotomised motoneurons.
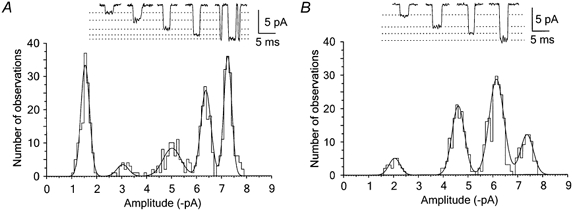
Application of NMDA (3 μm) in the presence of glycine (5 μm) to the outside-out patch isolated from axotomised motoneurons voltage clamped at −100 mV induced single-channel currents of different amplitude levels. A, examples of NMDA-induced single-channel openings to the 5 distinct conductance levels in the motoneuron of an 8-day-old animal that was axotomised on PND 3. Dotted lines show where the current levels identified from the fit of the amplitude distribution would fall. The distribution of channel amplitudes of openings longer than 0.425 ms was fitted with the sum of 5 Gaussian components of amplitude (relative area): −1.5 pA (24%), −3.1 pA (4%), −5.0 pA (12%), −6.4 pA (28%) and −7.2 pA (32%). B, examples of NMDA-induced single-channel openings isolated from a different axotomised motoneuron of a 4-day-old animal, showing openings to each of the 4 distinct conductance levels identified. The distribution of amplitudes of channel openings was fitted with the sum of four Gaussian components of amplitude (relative area): −2.1 pA (6%), −4.6 pA (29%), −6.2 pA (47%) and −7.3 pA (18%).
Table 1.
Summary of NMDA receptor properties in axotomised motoneurons
| Conductance (pS) | Relative distribution (%) | Incidence | Coincidence |
|---|---|---|---|
| 14.9 ± 1.9 | 15 ± 9 | 9 | 4 |
| 22.2 ± 2.7 | 14 ± 11 | 16 | 10 |
| 35.6 ± 4.4 | 22 ± 24 | 16 | 13 |
| 49.1 ± 3.5 | 20 ± 10 | 19 | 16 |
| 60.0 ± 3.5 | 38 ± 22 | 21 | 12 |
| 69.0 ± 2.9 | 32 ± 24 | 15 | — |
The mean chord conductances, their relative distributions and the incidence of occurrence of the conductance levels in NMDA receptor channels in 24 patches.
In control experiments the properties of NMDA receptor channels were elucidated in outside-out patches isolated from uninjured motoneurons (controls) located in the contralateral side of the spinal cord to axotomised motoneurons. The distribution of single-channel openings induced by NMDA (3 μm) (see Fig. 2A) and recorded under the same conditions as activity in patches isolated from axotomised motoneurons was fitted with the sum of four Gaussian components in all five patches analysed. The mean chord conductance of NMDA receptor channels in control motoneurons and their relative distribution are shown in Table 2. These values are similar to those reported for NMDA receptor channels in spinal cord motoneurons from unoperated animals (see Paleček et al. 1999).
Figure 2. Conductance of NMDA receptor channels in outside-out patches isolated from control motoneurons located in the contralateral side of the spinal cord to axotomised motoneurons.
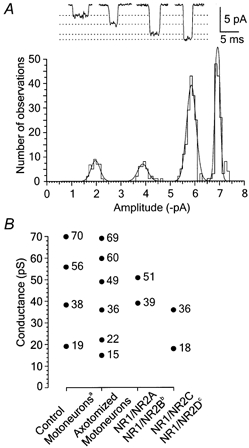
A, application of NMDA (3 μm) in the presence of glycine (5 μm) to the outside-out patch isolated from a PND 10 motoneuron voltage clamped at −100 mV induced single-channel currents to four different amplitude levels. Examples of NMDA-induced single-channel openings to each of the four conductance levels identified. Dotted lines show where the current levels identified from the fit of the amplitude distribution would fall. The distribution of channel amplitudes of openings longer than 0.425 ms was fitted with the sum of 4 Gaussian components of amplitude (relative area): −2.0 pA (9%), −3.9 pA (9%), −5.9 pA (45%), −6.9 pA (37%). B, comparison of conductances of motoneuron and recombinant NMDA receptor channels: aPaleček et al. (1999); bStern et al. (1992, 1994); cStern et al. (1992), Wyllie et al. (1996).
Table 2.
Summary of NMDA receptor properties in control motoneurons
| Conductance (pS) | Relative distribution (%) |
|---|---|
| 20.1 ± 2.5 | 20 ± 12 |
| 38.0 ± 3.0 | 15 ± 11 |
| 58.6 ± 3.4 | 31 ± 15 |
| 71.5 ± 2.6 | 35 ± 18 |
The mean chord conductances and their relative distributions in 5 patches.
Time course of NMDA receptor EPSCs
We have shown recently that the time course of NMDA receptor EPSCs in spinal cord motoneurons is best fitted by a double exponential function with a rapid time course (Abdrachmanova et al. 2000a). Previous studies indicated that differences in the time course of NMDA receptor EPSCs found in diverse anatomical structures of the central nervous system and during development reflect the subunit composition of NMDA receptor channels (Takahashi et al. 1996; Cathala et al. 2000; Tovar et al. 2000). Our next experiments were designed to elucidate whether the appearance of new conductance levels of NMDA receptor channels in axotomised motoneurons would affect the time course of NMDA receptor EPSCs.
Focal electric stimulation was used to elicit NMDA receptor EPSCs in axotomised motoneurons voltage clamped at +40 mV. The slow EPSC component sensitive to 50 μm APV was apparent in all 14 motoneurons, with the deactivation best fitted by two exponentials (Fig. 3A). Figure 3B shows values of time constants and their relative contribution in the deactivation of NMDA receptor EPSCs in axotomised motoneurons plotted versus postnatal age. Analysis indicated a shortening of both constants (Pfast = 0.0015, Pslow = 0.0035; one-way ANOVA); however, the relative contribution of the components was not significantly changed (P = 0.185; one-way ANOVA). The mean amplitude of NMDA receptor EPSCs was 137 ± 58 pA (n = 14) and the amplitude determined for each cell did not change significantly with postnatal age (P = 0.385; one-way ANOVA). We were not able to extend the age of rats used to study the kinetics of NMDA receptor EPSCs beyond PND 11 as it was extremely difficult to prepare viable slices with motoneurons from older animals because of extensive spinal cord myelination and axotomy-induced motoneuron cell death (see Abdrachmanova et al. 2000a).
Figure 3. The decay kinetics of NMDA receptor EPSCs in axotomised motoneurons.
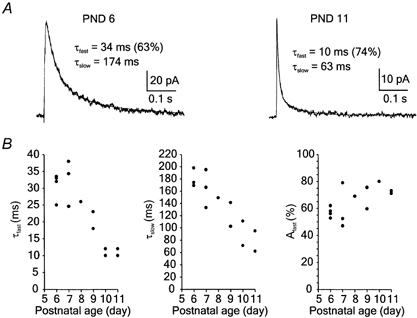
A, examples of EPSCs (6 responses were averaged in both cases) evoked in motoneurons of a 6-day-old (PND 6) and an 11-day-old animal (PND 11). The decay of NMDA receptor EPSCs was fitted by a double exponential function with the time constants and their relative amplitude indicated. Note the differences in the decay kinetics of NMDA receptor EPSCs. EPSCs were recorded from motoneurons at a holding potential of +40 mV in the presence of 1 mm Mg2+ and 5 μm CNQX. B, the graphs indicate the fast component (τfast), the slow component (τslow) and the relative contribution of the fast component (Afast) of the NMDA receptor EPSC in axotomised motoneurons. The values of the fast and slow components of NMDA receptor EPSCs in motoneurons of PND 6–11 significantly decreased as a function of time after axotomy (one-way ANOVA; Pfast = 0.0015; Pslow = 0.0035); however, the relative contribution of these components was not significantly changed (P = 0.185).
Mg2+ block of NMDA receptor channels
NMDA receptor channels on brain neurons have been shown to be sensitive to Mg2+-induced voltage-dependent block (Mayer et al. 1984; Nowak et al. 1984). As for NMDA receptor channel conductance, the affinity of Mg2+ for NMDA receptor channel block is influenced by the receptor subunit composition (Monyer et al. 1992; Stern et al. 1992).
To estimate the sensitivity of NMDA receptor channels to Mg2+ block, the I–V relation of the responses of a motoneuron to local application to the soma of 30 μm NMDA in the presence of 1 mm Mg2+ was constructed. The I–V curves of NMDA responses at holding potentials between −80 and +60 mV had a characteristic ‘J’ shape. For quantitative analysis the I–V curves were fitted by a Boltzmann equation (see Methods for details) and an apparent dissociation constant (Kd) for Mg2+ binding at 0 mV was determined. The mean apparent Kd for Mg2+ binding was 3.4 ± 3.9 mm (range 0.7–11.9 mm; n = 14) (Fig. 4). Analysis of the relationship between the apparent Kd for Mg2+ binding and postnatal age (3–9 days after axotomy; PND 6–12) indicated that there was no statistically significant difference (P = 0.139; one-way ANOVA). To estimate the sensitivity of synaptic NMDA receptors for Mg2+, experiments were performed in which the I–V relation of NMDA receptor EPSCs was constructed. The I–V curve of peak NMDA receptor EPSCs recorded at holding potentials between −80 and +80 mV in the presence of 1 mm Mg2+ is shown in Fig. 5A. The I–V relationship was fitted by a Boltzmann equation and an apparent Kd for Mg2+ binding at 0 mV determined. In contrast to the decay time constants of NMDA receptor EPSCs, which changed as a function of time after axotomy, comparison of the apparent Kd for Mg2+ binding at 0 mV as a function of postnatal age (3–7 days after axotomy; PND 6–10) indicated that there was no statistically significant difference (P = 0.223; one-way ANOVA). The mean apparent Kd for Mg2+ binding was 1.2 ± 0.5 mm (range 0.6–2.0 mm; n = 8) (Fig. 5). The values of the apparent Kd for Mg2+ to synaptic and extrasynaptic NMDA receptors were also not significantly different (Mann-Whitney rank sum test; P = 0.08) (see Fig. 5B).
Figure 4. Voltage dependence of Mg2+ block of NMDA receptor channels activated during agonist application in an axotomised motoneuron.
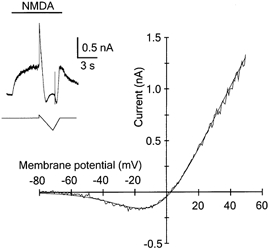
Examples of a current-voltage curve measured by a voltage ramp during a steady-state response to focal application of NMDA in the presence of 1 mm Mg2+. The inset shows the difference in the current induced by NMDA (30 μm) and whole-cell current induced in the absence of NMDA prior to, during and after the holding potential (+30 mV) of a motoneuron (PND 8) was ramped from +60 to −80 mV. Horizontal bar indicates the duration of NMDA application. The continuous line in the I–V relation was obtained by fitting a Boltzmann equation (see Methods for details) to the experimental data. The calculated apparent Kd for Mg2+ binding to NMDA receptor channels at 0 mV was 1.4 mm.
Figure 5. Voltage dependence of Mg2+ block of NMDA receptor channels activated during synaptic transmission.
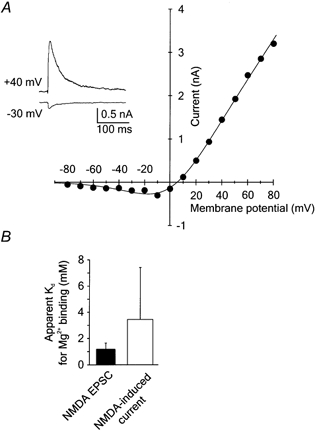
A, examples of NMDA receptor EPSCs recorded in the presence of 1 mm Mg2+ and 5 μm CNQX at holding potentials of −30 and +40 mV. Four records were averaged in each case. Peak NMDA receptor EPSCs were plotted versus the membrane potential. The continuous line in the I–V relation was obtained by fitting eqn (1) (see Methods) to the experimental data. The calculated apparent Kd for Mg2+ binding to synaptically activated NMDA receptor channels at 0 mV was 1.1 mm. B, bar graph shows the means ±s.d. of the apparent Kd for Mg2+ binding to synaptically activated NMDA receptors (NMDA EPSC) and receptors activated by focal NMDA application (NMDA-induced current). The difference between the apparent Kd for Mg2+ binding to NMDA receptor channels activated during synaptic transmission (n = 8) and by focal NMDA application (n = 14) was not statistically significant (Mann-Whitney rank sum test; P = 0.08).
Single-cell RT-PCR in motoneurons
To determine the type of mRNA encoding NMDA receptor subunits expressed in motoneurons, we performed single-cell RT-PCR (Lambolez et al. 1992). The cell content of fluorescence-labelled axotomised motoneurons was isolated and analysed for mRNA encoding NR2A–D and NR3A in a total of 18 cells of PND 8–12. PCR products of NR2A-C were detected in 7/8 motoneurons, NR2D in 5/8 motoneurons and NR3A in 6/8 motoneurons (see Fig. 6). To determine the expression of individual NR2A-C subunits, the PCR products were re-amplified and cut by Bpm I, Bfa I and Sca I, restriction enzymes specific for NR2A, NR2B and NR2C, respectively (Lambolez et al. 1992). Fragments of amplified PCR products of NR2A, NR2B and NR2C were detected in all NR2A-C positive motoneurons (Fig. 6).
Figure 6. Single-cell RT-PCR performed on axotomised motoneurons in rat spinal cord slices.
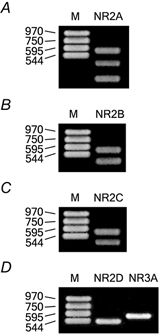
A, ethidium bromide-stained agarose gel electrophoresis of PCR product amplified with primers specific for NR2A. The product was digested with the restriction enzyme Bmp I. B, PCR product amplified with primers specific for NR2B and digested with Bfa I. C, PCR product amplified with primers specific for NR2C and digested with Sca I. D, PCR product amplified with primers specific for NR2D and NR3A. M: marker DNA (bp).
DISCUSSION
The cytotoxic effect of excessive activation of NMDA receptor channels has been suggested to take part in the events leading to the death of motoneurons after neonatal axotomy. In this study we characterised the properties of NMDA receptors in motoneurons in neonatal rat spinal cord slices and found that axotomy resulted in the expression of receptors with different single-channel properties, although all the transcripts encoding different subunits of the NMDA receptor were expressed as they are in uninjured motoneurons. These findings provide a basis for the molecular composition of NMDA receptors and indicate that assembly of the receptor from different subunits is phenotypically controlled.
Molecular biology of NMDA receptors
Our data an showed increase in the conductance levels and a shortening of NMDA receptor EPSCs in motoneurons after axotomy and suggested that peripheral nerve injury resulted in phenotypically induced changes in the functional properties of the NMDA receptors. The results of our experiments extend our knowledge of the functional properties of NMDA receptors in axotomised motoneurons and, together with our previous results from uninjured motoneurons, provide evidence of a functional diversity of native NMDA receptors in a single cell type (Paleček et al. 1999; Abdrachmanova et al. 2000a).
By the use of in situ hybridisation and immunohistochemistry it has been shown that motoneuron expression of mRNA encoding the NMDA receptor subunits and of subunit proteins is altered in models leading to motoneuron death (Hay et al. 1995; Piehl et al. 1995; Virgo et al. 2000). RT-PCR amplification was used to detect transcripts of subunits present in axotomised motoneurons. As in our previous results on uninjured motoneurons (see Abdrachmanova et al. 2000a), axotomised motoneurons express all the NMDA receptor subunits (NR2A–D, NR3A) known to form NMDA receptors and to affect their functional properties. Single-cell RT-PCR analysis does not provide us with quantitative data to allow an estimation of the ratio of the individual NMDA receptor subunits expressed in a single motoneuron; however, rough estimates of ethidium bromide fluorescence of DNA fragments suggest no obvious difference in the expression of individual subunits.
Conductance of NMDA receptor channels
The results of our experiments in which the outside-out configuration of the patch-clamp technique was used to characterise glutamate receptors indicate that NMDA receptors in control and axotomised motoneurons can open to four and six conductance levels, respectively (see Tables 1 and 2). The number and mean values of the conductance levels of NMDA receptor channels in control motoneurons, located in the contralateral side of the spinal cord to axotomised motoneurons, was similar to those reported for NMDA receptor channels in spinal cord motoneurons in unoperated animals (see Paleček et al. 1999; Abdrachmanova et al. 2000a). This indicates that the axotomy-induced changes in the properties of NMDA receptors are confined only to injured motoneurons and makes it unlikely that it is due to a non-specific effect.
To determine the number of conductance levels to which NMDA receptor channels open in spinal cord motoneurons the data obtained from axotomised motoneurons were compared with those reported previously, gained from a large number of patches on motoneurons from unoperated animals (n = 41) (19.2 ± 2.4, 38.4 ± 3.5, 56.3 ± 2.4, 69.6 ± 3.7 pS; see Paleček et al. 1999). Statistical evaluation of the data showed that there was no statistically significant difference (P = 0.7; Student's t test) between the mean values of the principal NMDA receptor channel conductances, 70 and 69 pS, found in control and axotomised motoneurons, respectively, and indicates expression of the same NMDA receptor type. However, statistical comparisons of the other conductance levels showed statistically significant differences (P ≤ 0.002; Student's t test) between the mean values determined in control (56, 38 and 19 pS) and axotomised (60, 49, 36, 22 and 15 pS) motoneurons. This indicates that NMDA receptor channels can open to at least six and possibly up to nine or more conductance levels.
The conductance of NMDA receptor channels is determined by the presence of a specific NR2 subunit in the receptor (Stern et al. 1992, 1994; Wyllie et al. 1996). The conductance levels 36 and 49 pS were similar to those found for recombinant receptors containing NR1–NR2C/D and NR1–NR2A/B subunits, respectively. This may suggest that receptors of this subunit composition are expressed in motoneurons. The subunit composition of NMDA receptor channels that have conductances different from those described for the recombinant receptors has yet to be clarified. It has been suggested that native NMDA receptors are heteromultimeric proteins containing, in addition to the NR1 subunit, a combination of two different NR2A–D and/or NR3A subunits (for review see Dingledine et al. 1999). It is therefore possible that heteromultimeric receptors composed of three distinct subunits form channels that open to conductance levels distinct from those described for recombinant heterodimeric receptors, e.g. 70 pS.
Using the single-cell RT-PCR technique we have shown that uninjured motoneurons express mRNA encoding NMDA receptor subunits (NR2A–D, NR3A) and proposed the existence of a cellular mechanism that permits selective assembly of NMDA receptor subunits that allow the formation of channels with a conductance of 70 pS and excludes those that have a conductance of 50 pS (Abdrachmanova et al. 2000a). Axotomised motoneurons express the same NMDA receptor subunits as uninjured motoneurons; however, the values of single-channel conductances are quite different. It may be that in injured motoneurons the mechanism of assembly of NMDA receptor subunits into functional channels is altered and therefore ion channels with conductance levels different from those in controls are formed.
It is possible that the conductance of NMDA receptor channels in motoneurons is controlled not only by the receptor subunit composition but also by association of the receptor with other membrane components. This has been proposed previously to explain differences in the conductance of NMDA receptor channels recorded in cell-attached (64 and 53 pS) and outside-out patches (51 and 40 pS) isolated from cerebellar granule cells (Clark et al. 1997). If we also assume the same mechanism for NMDA receptors in motoneurons, it would be possible to explain the existence of 50 and 60 pS conductance openings in axotomised motoneurons as those induced by opening of the receptor with the same subunit composition but with attached and detached factors modulating the single-channel conductance. However, for channels with a conductance of 70 pS we would have to assume a different mechanism, as this conductance was observed in both control and axotomised motoneurons. In addition it has to be clarified whether low-conductance NMDA receptor channels are subject to the same single-channel conductance modulating mechanism as those with a high conductance.
We have shown previously that AMPA receptor channels in spinal cord motoneurons exhibited inward rectification in the presence of intracellular spermine, suggesting the presence of Ca2+-permeable AMPA receptor channels. Axotomy resulted in a decrease in spermine-induced inward rectification of AMPA receptor mediated responses and suggested a phenotypically induced alteration of AMPA receptor properties, perhaps through changes in their subunit composition (Abdrachmanova et al. 2000b). This indicates that axotomy results in profound molecular and functional changes in both the AMPA and NMDA subtype of glutamate receptor channels.
Synaptic transmission
The deactivation of NMDA receptor EPSCs in axotomised motoneurons was best described by a double exponential function similar to that found in uninjured motoneurons (Abdrachmanova et al. 2000a). The fast and slow components of the deactivation shortened gradually, reaching at PND 11 (9 days after axotomy) one-third of the value at PND 6 (3 days after axotomy). It appears that shortening of the deactivation of NMDA receptor EPSCs in injured motoneurons is induced by cellular mechanisms triggered by axotomy because in control motoneurons no correlation was found between the values of decay time constants and the motoneuron postnatal age (Abdrachmanova et al. 2000a).
The molecular mechanism responsible for shortening of the NMDA receptor EPSCs in axotomised motoneurons is not clear. The time course of the NMDA receptor EPSCs recorded in some central synapses shortens as a consequence of postnatal development (Carmignoto & Vicini, 1992; Hestrin, 1992). It has been proposed that the decrease in duration of NMDA receptor EPSCs in cortical neurons occurs as a result of a molecular alteration of structure by the regulation of NMDA receptor subunit gene expression (Flint et al. 1997). It is therefore possible that shortening of the NMDA receptor EPSCs reflects the appearance of new NMDA receptors with a different subunit composition and their targeting into synapses in axotomised motoneurons. This mechanism is also supported by the appearance of new conductance levels of NMDA receptors, not previously detected in outside-out patches isolated from control motoneurons. It is, however, possible that factors other than the NMDA receptor subunit composition determine the deactivation of synaptic currents, as the time course of deactivation of NMDA receptor EPSCs in motoneurons 8 days after axotomy is much faster than that in other neurons in the central nervous system.
The mean amplitude of NMDA receptor EPSCs recorded from axotomised motoneurons (137 ± 58 pA) was significantly smaller than that recorded under similar conditions in motoneurons from unoperated animals (328 ± 251 pA). This agrees with our recent finding of the significantly smaller amplitude of AMPA receptor EPSCs in axotomised motoneurons, which was not accompanied by a change in the ratio of the amplitude of AMPA and NMDA receptor EPSCs (see Abdrachmanova et al. 2000b). These results indicate that the number of excitatory synaptic inputs and/or the density of NMDA receptors in motoneurons is reduced following axotomy. The latter hypothesis is further supported by the consistent finding of the appreciably lower activity of NMDA receptor channels in outside-out patches isolated from axotomised motoneurons than in those from control motoneurons, when recorded under identical conditions, with pipettes of similar resistance.
Mg2+ affinity for NMDA receptor EPSC
Our data provide quantitative estimates of Mg2+ blocking efficacy at NMDA receptor channels in axotomised motoneurons and indicate that the apparent Kd for Mg2+ binding to both synaptic and extrasynaptic NMDA receptors was not significantly changed when compared with control motoneurons (see Paleček et al. 1999; Abdrachmanova et al. 2000a). Values of apparent Kd for Mg2+ binding to NMDA receptors similar to those in axotomised motoneurons were also reported for native NMDA receptors in isolated trigeminal, dentate gyrus and cultured hippocampal neurons (Jahr & Stevens, 1990; Chen & Huang, 1992; Kohr et al. 1993).
It is likely that the value of apparent Kd for Mg2+ binding reflects a weighted mean of the different Kd values of the pharmacologically and biophysically different types of NMDA receptor channels present in motoneurons. The values of apparent Kd for Mg2+ binding to synaptic and extrasynaptic NMDA receptors in both control and axotomised motoneurons (0.9–3.4 mm) suggest that, on average, these receptors have a higher affinity for Mg2+ than recombinant heterodimeric NMDA receptors: 8.2 mm NR1–NR2A, 8.2 mm NR1–NR2B, 18.9 mm NR1–NR2C and 36.3 mm NR1–NR2D (Monyer et al. 1994; Kirson & Yaari, 1996). Even though this does not exclude the possibility that heterodimeric receptors are also expressed in motoneurons, it is likely that the majority of the NMDA receptors are heteromultimeric proteins or that factors other than the receptor subunit composition determine their high affinity for Mg2+.
The results of our experiments do not allow us to directly infer the Mg2+ affinity of NMDA receptor channels in axotomised motoneurons with conductances different from those in uninjured motoneurons. It may be that they have a similar Kd for Mg2+ binding to those in controls. However, there was a small increase in the values of apparent Kd for Mg2+ binding to NMDA receptors following axotomy. It is more likely that the receptor channels that open to conductance levels different from those in controls have a lower Mg2+ affinity, which is masked by the prevalence of NMDA receptor channels with a high Mg2+ affinity that open to the same conductance levels as those in uninjured motoneurons.
In conclusion, the results of our experiments suggest that axotomy-induced changes in NMDA receptor channel properties are not caused by the expression of a different subset of receptor subunits, but rather by phenotypically induced changes in the receptor assembly from different subunits and/or alteration of the receptor channel properties by receptor-associated cell components. These results, together with those reported in our previous study (Paleček et al. 1999), suggest that axotomy results in profound changes in the properties of both NMDA and AMPA receptors. Further experiments must be carried out to show whether these changes play a role in axotomy-induced motoneuron death.
Acknowledgments
This work was supported by Grant Agency of the Czech Republic 309/00/1654 and Ministry of Education, Youth and Sports of the Czech Republic (LN00B122).
REFERENCES
- Abdrachmanova G, Teisinger J, Vlachova V, Vyklicky L. Molecular and functional properties of synaptically activated NMDA receptors in neonatal motoneurons in rat spinal cord slices. European Journal of Neuroscience. 2000;12:955–963. doi: 10.1046/j.1460-9568.2000.00989.x. [DOI] [PubMed] [Google Scholar]
- Abdrachmanova G, Vlachova V, Vyklicky L. Axotomy-induced change in the properties of (S)-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor channels in rat motoneurons. Neuroscience. 2000;99:119–131. doi: 10.1016/s0306-4522(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Audinat E, Lambolez B, Rossier J, Crepel F. Activity-dependent regulation of N-methyl-D-aspartate receptor subunit expression in rat cerebellar granule cells. European Journal of Neuroscience. 1994;6:1792–1800. doi: 10.1111/j.1460-9568.1994.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. Journal of Neuroscience. 2000;20:5899–5905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Clark BA, Farrant M, Cull-Candy SG. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. Journal of Neuroscience. 1997;17:107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. Journal of Physiology. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann BN, editor. Single Channel Recording. New York: Plenum Press; 1983. pp. 191–263. [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- De Bilbao F, Dubois-Dauphin M. Time course of axotomy-induced apoptotic cell death in facial motoneurons of neonatal wild type and bcl-2 transgenic mice. Neuroscience. 1996;71:1111–1119. doi: 10.1016/0306-4522(95)00505-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. Journal of Neuroscience. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greensmith L, Mentis GZ, Vrbova G. Blockade of N-methyl-D-aspartate receptors by MK-801 (dizocilpine maleate) rescues motoneurones in developing rats. Brain Research. Developmental Brain Research. 1994;81:162–170. doi: 10.1016/0165-3806(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Hay C, Virgo L, Mentis G, Navarrete R, de Belleroche J. Changes in expression of NR-1 and c-jun mRNA in rat lumbar spinal cord after neonatal common peroneal nerve crush. Brain Research. 1995;704:145–150. doi: 10.1016/0006-8993(95)01286-9. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. Journal of Neuroscience. 1990;10:1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson ED, Yaari Y. Synaptic NMDA receptors in developing mouse hippocampal neurones: functional properties and sensitivity to ifenprodil. Journal of Physiology. 1996;497:437–455. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, De Koninck Y, Mody I. Properties of NMDA receptor channels in neurons acutely isolated from epileptic (kindled) rats. Journal of Neuroscience. 1993;13:3612–3627. doi: 10.1523/JNEUROSCI.13-08-03612.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. Journal of Biological Chemistry. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- Lowrie MB, Vrbova G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends in Neurosciences. 1992;15:80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Greensmith L, Vrbova G. Motoneurons destined to die are rescued by blocking N-methyl-D-aspartate receptors by MK-801. Neuroscience. 1993;54:283–285. doi: 10.1016/0306-4522(93)90253-c. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Paleček J, Abdrachmanova G, Vlachova V, Vyklicky L. Properties of NMDA receptors in rat spinal cord motoneurons. European Journal of Neuroscience. 1999;11:101–111. doi: 10.1046/j.1460-9568.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- Piehl F, Tabar G, Cullheim S. Expression of NMDA receptor mRNAs in rat motoneurons is down-regulated after axotomy. European Journal of Neuroscience. 1995;7:2101–2110. doi: 10.1111/j.1460-9568.1995.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Stern P, Behe P, Schoepfer R, Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proceedings of the Royal Society B. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Stern P, Cik M, Colquhoun D, Stephenson FA. Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. Journal of Physiology. 1994;476:391–397. doi: 10.1113/jphysiol.1994.sp020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K, Mishina M. Functional correlation of NMDA receptor epsilon subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. Journal of Neuroscience. 1996;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Sprouffske K, Westbrook GL. Fast NMDA receptor-mediated synaptic currents in neurons from mice lacking the epsilon2(NR2B) subunit. Journal of Neurophysiology. 2000;83:616–620. doi: 10.1152/jn.2000.83.1.616. [DOI] [PubMed] [Google Scholar]
- Virgo L, Dekkers J, Mentis GZ, Navarrete R, de Belleroche J. Changes in expression of NMDA receptor subunits in the rat lumbar spinal cord following neonatal nerve injury. Neuropathology and Applied Neurobiology. 2000;26:258–272. doi: 10.1046/j.1365-2990.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed inXenopus oocytes. Proceedings of the Royal Society B. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]


