Abstract
We examined the effect of taurine on depolarisation-induced force responses and sarcoplasmic reticulum (SR) function in mechanically skinned skeletal muscle fibres from the extensor digitorum longus (EDL) of the rat. Taurine (20 mm) produced a small but significant (P < 0.01) decrease in the sensitivity of the contractile apparatus to Ca2+ (increase in the [Ca2+] corresponding to 50 % of maximum force of about 7 %; n = 10) and in maximum force (92.0 ± 1.0 % of controls) in the skinned fibres. Taurine had no statistically significant effect on the slope of the force-pCa curve. Depolarisation-induced force responses in the skinned fibres were markedly increased in peak value by 20 mm taurine, to 120.8 ± 5.3 % of control measurements (P = 0.0006, n = 27). Taurine (20 mm) significantly increased the SR Ca2+ accumulation in the skinned fibres by 34.6 ± 9.3 % compared to control conditions (measured by comparing the integral of caffeine contractures in fibres previously loaded with Ca2+ in the absence or presence of taurine; P = 0.0014, n = 10). Taurine (20 mm) also increased both the peak and rate of rise of caffeine-induced force responses in the fibres by 29.2 ± 9.7 % (P = 0.0298, n = 6) and 27.6 ± 8.9 % (P = 0.037), respectively, compared with controls. This study shows that taurine is a modulator of contractile function in mammalian skeletal muscle. Taurine may increase the size of depolarisation-induced force responses by augmenting SR Ca2+ accumulation and release.
The sulfonic amino acid taurine is found in high concentrations in many mammalian excitable cells and is reported to have a variety of functions including osmoregulation, modulation of neuronal excitability, antioxidation and control of Ca2+ homeostasis (Huxtable, 1992).
Taurine is also found in high levels in skeletal muscle (Chesney et al. 1986; Nieminen et al. 1988; Turner et al. 1994). The taurine content in skeletal muscle is reported to vary between muscle type and species. In the rat, the slow-twitch soleus muscle is reported to have twice the taurine content (33 μmol (g wet weight)−1) of the fast-twitch extensor digitorum longus (EDL) muscle (17 μmol (g wet weight)−1; Iwata et al. 1986). In the horse, slow-twitch type I fibres have been reported to have a high taurine content, while in fast-twitch type IIb fibres, taurine was reported to be undetectable (Dunnett et al. 1992). Recent studies suggest that the taurine content within skeletal muscle may also vary markedly between fibres in the same muscle. Wide differences in the taurine immunoreactivity of individual fibres within the same skeletal muscle have been reported for a number of species, including the rat and the cat (Quesada et al. 1993; Lobo et al. 2000). In the cat soleus muscle, the taurine immunoreactivity was reported to be high and relatively homogeneous (Quesada et al. 1993).
The reason for the heterogeneity in the taurine content of mammalian skeletal muscle is unknown. Taurine is actively accumulated by most cells via a Na+-dependent high affinity taurine transporter, and this taurine transporter has been shown to be highly expressed in skeletal muscle (Ramamoorthy et al. 1994). Taurine release has also been demonstrated in many cell types, and one of the main pathways is volume activated (via hyposmotic conditions), and is thought to involve specific anion channels (Perlman & Goldstein, 1999). Hyposmotic conditions and pathological factors such as ischaemia have been shown to lead to taurine release in cardiac myocytes (Kramer et al. 1981) and neurones (Schouboe & Pasantes-Morales, 1992; Saransaari & Oja, 1998) by mechanisms that are poorly understood.
In skeletal muscle, there is evidence that contractile activity may induce changes in myoplasmic taurine content. Chronic sciatic nerve stimulation (100 Hz) has been shown to increase the taurine content of rat EDL muscle, and decrease the taurine content in rat soleus muscle (Kim et al. 1986). After neural stimulation, the proportion of taurine-positive skeletal muscle fibres (determined by immunoreactivity measurements) has been reported to fall in the cat (Quesada et al. 1993).
In cardiac muscle, taurine has been shown to modulate the excitation–contraction (E–C) coupling process by altering sarcoplasmic reticulum (SR) Ca2+ handling (Schaffer et al. 1994), leading to increased cellular Ca2+ and twitch tension (Franconi et al. 1982). These experiments were undertaken using taurine applied externally. However, Steele et al. (1990) found that taurine also potentiated contractile responses induced by caffeine in chemically skinned cardiac trabeculae, and this was shown to be due to a taurine-induced increase in SR Ca2+ accumulation.
The effects of taurine on mammalian skeletal muscle have not been widely studied. Taurine has been shown to increase the mechanical threshold for contraction in skeletal muscle fibres (De Luca et al. 1996). The presence of taurine has also been reported to promote intracellular membrane stabilisation (Huxtable & Bressler, 1973) and sarcolemmal polarisation (Gruener et al. 1975; Conte-Camerino et al. 1987) in skeletal muscle. Taurine has also been shown to increase the Ca2+ uptake and storage capacity of isolated SR vesicles from ‘white’ skeletal muscle of the rat (Huxtable & Bressler, 1973), suggesting that it may have similar effects in skeletal muscle to those described for cardiac muscle. To date, no studies have specifically addressed the issue of whether taurine modulates the activity of the SR in functioning vertebrate skeletal muscle fibres, and what role this plays in the E–C coupling process. In this study, we used the mechanically skinned fibre technique to examine the effects of taurine on depolarisation-induced force responses, SR function and the Ca2+–force relationship of the contractile apparatus in mechanically skinned fast-twitch fibres of the rat.
Mechanically skinned fibres have a number of important advantages over intact preparations for a study of this type. One of the main drawbacks for studying the effects of taurine in mammalian cells has been the inability to control accurately the intracellular taurine concentration (Huxtable, 1989). However, in skinned fibres, the sarcolemma of the fibres is mechanically peeled away, allowing the intracellular concentration of taurine to be freely manipulated by altering the taurine concentration in the bath. In addition, the transverse tubular (t-) system seals and repolarises after the sarcolemma has been mechanically removed (Stephenson, 1981; Donaldson, 1985). Therefore, the sealed t-system can be depolarised and force responses elicited by the normal E–C coupling pathway simply by replacing the K+-based bath solution with one in which K+ has been replaced with Na+ (Lamb & Stephenson, 1990). This allows the examination of the effects of taurine on force responses elicited by voltage sensor activation, a clear advantage over using caffeine, which may not activate SR Ca2+ release by the same mechanism as voltage sensor depolarisation. The skinned fibre preparation also allows the quantitative examination of SR Ca2+ uptake and release within the fibres (Bakker et al. 1996, 1998), and examination of the Ca2+ sensitivity of the myofibrils.
METHODS
All experimental procedures undertaken in this study were approved by the University of Western Australia Animal Ethics Committee.
Muscle fibres were isolated from the extensor digitorum longus (EDL) muscle of large (> 350 g) Wistar rats (Rattus norvegicus) killed by an overdose of carbon dioxide (O2 maintained at 20 %). The rat EDL muscle has approximately 94 % fast-twitch fibres (Ariano et al. 1973). The muscle fibres were dissected and skinned in paraffin oil. The fibres were then clamped at one end with a fine pair of fixed forceps and the other end was tied to the arm of a force transducer (SI Heidelberg) with surgical thread (Lamb & Stephenson, 1990). The fibres were maintained in a 2 ml Perspex bath containing a potassium hexamethylene-diamine-tetraacetate (potassium-HDTA) solution composed of (mm): K+, 125; Na+, 36; HDTA2−, 50; ATP (total), 8; Mg2+ (total), 8.6; creatine phosphate, 10; EGTA (total), 0.03; Hepes, 90; NaN3, 1 at pH 7.10 ± 0.01 and pCa (-log[Ca2+]) 7.1 (free Mg2+ concentration, 1 mm) (Lamb & Stephenson, 1990). NaN3 was added to prevent mitochondrial Ca2+ fluxes. In order to maximise the force responses, the fibres were stretched by 20 % from the slack length to bring the sarcomere length to approximately 2.8–3.0 μm (Lamb & Stephenson, 1990). Force responses were recorded (400 Hz) and analysed with a PowerLab data acquisition unit (ADInstruments) attached to a PC.
Taurine (Sigma) and sucrose (Sigma) were added directly to the solutions. The pH of the solutions was not significantly modified by the addition of taurine (change in pH less than 0.01). The effects of both 5 and 20 mm taurine were investigated. Taurine levels of approximately 13 μmol (g wet weight)−1 are commonly reported for rat skeletal muscle (Chesney et al. 1986; Nieminen et al. 1988; Turner et al. 1994). Using a factor of 1/0.58 to convert μmol g−1 muscle into mol kg−1 myoplasmic H2O (Baylor et al. 1983), the intracellular taurine levels in rat skeletal muscle would be approximately 22 mm. Therefore, in this study a maximum taurine concentration of 20 mm was used. Note that this may represent the lower end of the range of taurine concentrations found in rat fast-twitch skeletal muscle, as Iwata et al. (1986) reported taurine levels of 17 μmol (g wet weight)−1 (approximately 29 mm) in rat EDL muscle.
The effect of taurine (20 mm) addition on the free [Ca2+] and free [Mg2+] of the skinned fibre solutions was investigated using the fluorescent Ca2+ indicators Indo-1 and Magfura-2 (Teflabs), respectively. No significant difference in the fluorescence ratio was found in the solutions using either Indo-1 (0.275 ± 0.003 and 0.283 ± 0.003 with and without added taurine, respectively, Student's unpaired t test, P = 0.1, n = 6) or Magfura-2 (0.557 ± 0.003 and 0.564 ± 0.002 with and without added taurine, respectively, Student's unpaired t test, P = 0.1, n = 6), indicating that taurine does not significantly bind Ca2+ or Mg2+ under the conditions of this study.
Contractile apparatus
The effect of taurine on the sensitivity of the contractile apparatus to Ca2+ in the EDL fibres was determined by exposing fibres to solutions of different free [Ca2+] in the presence and absence of 5 and 20 mm taurine. The strongly Ca2+-buffered solutions of different, known, free [Ca2+] were prepared by mixing different proportions of Ca-EGTA- (Sol. A) and EGTA-based (Sol. B) solutions (Stephenson & Williams, 1981). Sol. B was composed of (mm): K+, 117; Na+, 36; ATP (total), 8; free Mg2+, 1; creatine phosphate, 10; EGTA2− (total), 50; Hepes, 60; NaN3, 1 at pH 7.10. Sol. A was similar to Sol. B, with the exception that the [EGTA2−] and [Ca-EGTA] of Sol. A were 0.77 and 49.33 mm, respectively. The free [Ca2+] of the solutions was calculated using a Kapp for EGTA of 4.78 × 106m−1 (Fink et al. 1986). Fibres were exposed to Triton X-100 for 2 min at the start of the experiment, in order to destroy membranous compartments and stop mitochondrial Ca2+ fluxes. During experiments, a brief exposure to Sol. B was used to return the force to baseline before the next force response was measured. Measurements in the presence of taurine were compared to control measurements made before and after taurine exposure in order to compensate for the progressive, small decline in force that occurs during these experiments. Steady-state force elicited after exposure to solutions of increasing free [Ca2+] were expressed as a percentage of maximum Ca2+ -activated force and plotted as a function of pCa. The data were fitted with sigmoidal curves using GraphPad Prizm (GraphPad Software Inc.), and the slope and pCa50 values (pCa value corresponding to 50 % of maximum force) of the curves were determined for control conditions and in the presence of taurine. The effect of taurine on maximum force production was determined using separate solutions consisting of Sol. A alone (with a free [Ca2+] of 3.5× 10−5m), with and without (5 or 20 mm) taurine. The maximal force responses in the taurine solution were compared to control force responses obtained before and after taurine exposure.
Depolarisation-induced force responses
The skinned muscle fibres used in this study have been shown to retain normal E–C coupling due to sealing of the t-system after mechanical skinning. The sealed t-system can be repolarised by a 1 min exposure of the fibres to a potassium-HDTA solution, and normal depolarisation-induced SR Ca2+ release and contraction can be induced by exposure of the fibre to an HDTA solution in which K+ has been replaced by Na+ (Lamb & Stephenson, 1990). The pCa in the potassium-HDTA and sodium-HDTA solutions was weakly buffered to about pCa 7.0 and the solutions were isosmotic (Lamb & Stephenson, 1990). Generally, two responses were elicited under control conditions and then two in the presence of taurine, followed by two more responses under control conditions. Initial depolarisation-induced force responses measured in the presence of 5 and 20 mm taurine were compared to control responses elicited immediately prior to exposure to the drug in the same fibres. Before depolarisation in the presence of taurine, the fibres were first incubated in a potassium-HDTA solution with the appropriate concentration of taurine for 1 min to allow time for the amino acid to diffuse within the fibre. Responses in the presence of taurine were also compared to control experiments where an equivalent number of depolarisation-induced force responses were measured in fibres that were never exposed to taurine. In the skinned fibres, the depolarisation-induced force responses generally increased in size over the first two to four responses and then stabilised. Experiments were always commenced after the force responses had stabilised. In some experiments taurine was replaced with sucrose mole for mole to examine whether the effects of taurine were due to the increased osmolarity of the solutions in the presence of taurine.
Experiments to measure the effect of taurine on SR Ca2+ accumulation
In these experiments the SR of the fibre was initially depleted of all releasable Ca2+ by exposure to a low-Mg2+ potassium-HDTA solution (total Mg2+ of 2.125 mm) with 30 mm caffeine and 0.75 mm EGTA for 2 min (Bakker et al. 1998). The fibre could then be reloaded with Ca2+ by exposure to a highly buffered Ca2+ solution (pCa ≈ 6.55), made by combining Sol. A and Sol. B at a ratio of 1:1, for a time period that produced a consistent, submaximal SR Ca2+ load (approximately 15 s) (Bakker et al. 1996). Loading was rapidly terminated at the end of each loading period by a brief (∼1–2 s) exposure to Sol. B. The fibre was then exposed to the depletion solution again and the time integral of the force response elicited by this depletion was used as an indicator of the amount of Ca2+ taken up during the loading period (Endo, 1977). Depletion measurements made after loading in the presence or absence of 20 mm taurine were compared to control measurements made before and after loading with the drug to minimise errors associated with any deterioration in the size of the control responses. Before exposure to the load solution containing taurine, the fibres were exposed to a potassium-HDTA solution containing taurine (and 1.5 mm EGTA to prevent Ca2+ loading) for 30 s to allow time for taurine to equilibrate within the fibre. Control responses involved exposure to a similar solution without taurine. We also examined the effect of 20 mm sucrose on SR Ca2+ accumulation to ensure that the effects of taurine were not simply due to an increase in the osmolarity of the load solution in the presence of taurine.
Caffeine-induced Ca2+ release experiments
In the experiments carried out to determine the effect of taurine on caffeine-induced SR Ca2+ release, the fibres were initially depleted and then reloaded with Ca2+ using a similar protocol to that described in the previous section. Fibres were then exposed to a potassium-HDTA solution with 15 mm caffeine and 0.35 mm EGTA to induce Ca2+ release from the SR. This solution produced a submaximal force response that was sensitive to changes in SR Ca2+ release channel activity. The peak and mean rate of rise (mean rate of rise during 20–80 % of peak force) of force responses elicited after exposure to the submaximal caffeine solution, in the presence or absence of taurine, were compared. Before exposure to the submaximal caffeine solution, the fibres were exposed to a potassium-HDTA solution (with 0.35 mm EGTA to prevent SR Ca2+ uptake) with taurine to allow the amino acid time to equilibrate within the fibre. Control responses involved exposure to a similar solution without taurine. In separate experiments, taurine was replaced with sucrose mole for mole to ensure that the effects of taurine on caffeine-induced Ca2+ release were not due to the increased osmolarity of the solutions in the presence of taurine.
Experiments to measure the effect of taurine on SR Ca2+ leak in skinned fibres
The experiments used to examine the effect of taurine on Ca2+ leak from the SR of the skinned fibres were similar to the SR Ca2+ release experiments described in the previous section. The SR of the fibres was first depleted of Ca2+ and then the SR was reloaded with Ca2+ for a specific time. In these experiments, the fibres were then exposed to a ‘Ca2+ leak’ solution (an HDTA solution with 0.875 mm EGTA to chelate leaked Ca2+ and prevent its re-uptake by the SR) for 90 s before being exposed to a caffeine (15 mm) solution. The caffeine solution had a reduced amount of EGTA (0.25 mm) to ensure force could be measured at low levels of Ca2+ release. The fibres were then fully depleted of Ca2+ by exposure to the low-Mg2+ potassium-HDTA solution with 30 mm caffeine and 0.75 mm EGTA, before again being reloaded with Ca2+. The normal SR Ca2+ leak was demonstrated by comparing the force responses obtained with or without exposure to the leak solution. The effect of taurine on SR Ca2+ leak was examined by comparing the responses elicited after exposure to the Ca2+ leak solution in the presence and absence of 20 mm taurine.
All data are presented as means ± s.e.m. Unless otherwise stated, all responses in the presence of taurine were converted to a percentage of the control response and compared using a two way, one sample t test. All statistical analysis was undertaken using the statistics software package GraphPad InStat. The experiments were all undertaken at room temperature (22–24 °C).
RESULTS
The aim of this study was to examine the effects of taurine on depolarisation-induced force responses and SR function in skinned fast-twitch mammalian skeletal muscle. Two concentrations of taurine (5 and 20 mm) were used in this study.
The effect of taurine on the contractile apparatus
We initially examined the effect of taurine on the function of the contractile apparatus of the skinned fibres, because any change in the relationship between [Ca2+] and force at the level of the contractile proteins elicited by taurine would alter interpretations made with respect to the effects of taurine on other aspects of E–C coupling. The fibres were exposed to two similar sets of solutions exhibiting a range of known free [Ca2+]. One set served as a control, while the other set contained taurine. The steady-state force generated by these solutions was plotted as a percentage of the maximum force against the pCa and fitted with sigmoidal curves (Fig. 1). The mean pCa50 value obtained in the presence of 5 mm taurine (6.12 ± 0.03) was not significantly different from the control value in the same fibres (6.12 ± 0.03; Student's paired t test, P = 0.6218, n = 10). However, in the presence of 20 mm taurine, there was a small but significant decrease in the pCa50 value (6.10 ± 0.03 compared with 6.13 ± 0.03 for controls; Student's paired t test, P = 0.0042, n = 10), indicating that addition of 20 mm taurine decreases the sensitivity of the contractile apparatus to Ca2+. The slopes of the force-pCa curves fitted to the data obtained in the presence of 5 and 20 mm taurine were not significantly different from controls (mean slope under control conditions: 4.9 ± 0.3, n = 20; P = 0.9697 and P = 0.9537, respectively). Both 5 and 20 mm taurine significantly decreased maximum force production in the skinned fibres to 97.9 ± 0.7 % (P = 0.009, n = 10) and 92.0 ± 1.0 % (P = 0.0002, n = 10), respectively, of controls.
Figure 1. The effect of 5 mm (A) and 20 mm taurine (B) on the relationship between Ca2+ and force in skinned EDL fibres of the rat.
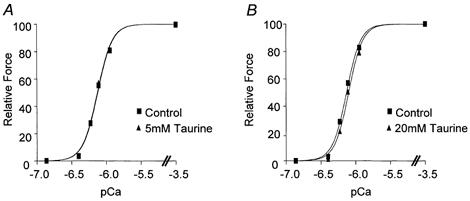
Sigmoidal curves were fitted to force measurements made in a set of highly buffered Ca2+ solutions of different, known free [Ca2+], in the absence and presence of taurine. A small but significant decrease in the pCa50 value was found in the presence of 20 mm taurine but not in 5 mm taurine, indicating that 20 mm taurine decreases the sensitivity of the contractile apparatus to Ca2+. Taurine (5 and 20 mm) had no significant effect on the slope of the Hill plots.
The effect of taurine on depolarisation-induced force responses
In order to investigate the effect of taurine on the E–C coupling process in skeletal muscle, we examined the effect of taurine on depolarisation-induced force responses elicited in skinned fibres by substituting a Na+-based solution for the K+-based bath solution (see Methods; Fig. 2). The depolarisation-induced force measurements made in the presence of 5 and 20 mm taurine were compared to previous control measurements made in the same fibres (Fig. 3A). The presence of 20 mm taurine significantly increased the peak of the depolarisation-induced force responses to 120.8 ± 5.3 % of control measurements made in the same fibres (P = 0.0006, n = 27; Fig. 2 and Fig. 3A). The second depolarisation-induced force response in the presence of 20 mm taurine was also significantly larger than initial controls (110.9 ± 4.2% of controls, P = 0.015, n = 27), but was smaller than the initial response in taurine (P = 0.002). The presence of taurine also increased the mean rate of rise (during 20–80 % of the rising phase of the force responses) of the depolarisation-induced force responses from 0.28 ± 0.06 mN s−1 in the control to 0.31 ± 0.05 mN s−1 after depolarisation in 20 mm taurine (P = 0.009, n = 25). The values for the half-time of relaxation of the depolarisation-induced force responses in the presence and absence of taurine were not significantly different (P = 0.17, n = 25). Taurine (5 mm) had no significant effect on the peak of the depolarisation-induced force responses (mean peak in taurine 113.9 ± 12.6% of controls, P = 0.29, n = 15). These results show that myoplasmic taurine levels of 20 mm significantly increase depolarisation-induced force production in skinned EDL fibres.
Figure 2. An example of the effect of 20 mm taurine on depolarisation-induced force responses elicited in skinned EDL fibres.
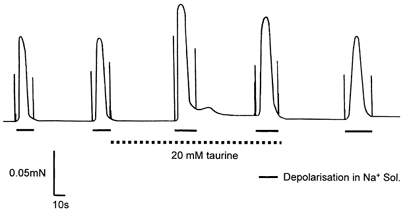
Taurine (third and fourth responses) increased the size of the depolarisation-induced force responses. Force returned to levels similar to initial controls after washout of taurine (fifth response). The fibres were exposed to a potassium-HDTA solution between depolarisations to allow repolarisation of the sealed t-system.
Figure 3.
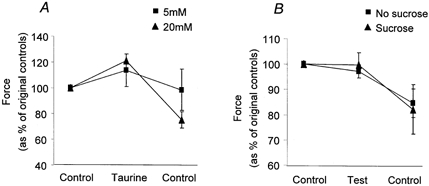
A, the effect of addition and subsequent washout of 5 mm (▪) and 20 mm (▴) taurine on the peak of depolarisation-induced force responses in skinned EDL fibres. Taurine at 20 mm, but not 5 mm, significantly increased the size of the depolarisation-induced force responses in the rat fibres. B, the change in the peak of depolarisation-induced force responses stimulated in rat EDL fibres not exposed to taurine (▪), and after addition and subsequent washout of 20 mm sucrose (▴). Sucrose exposure provided a control for the purely osmotic effects of taurine.
In separate experiments undertaken to examine how large the effects of taurine were in absolute terms, the peaks of the depolarisation-induced force responses in the presence of taurine were compared to maximal force responses (measured by exposure to a solution with a [Ca2+] of ∼3.5 × 10−5m) elicited immediately after taurine exposure, or exposure to control depolarisation solutions alone (not shown). This was undertaken because maximum force production was found to undergo a small but significant decrease in size by the end of experiments such as that shown in Fig. 2. The mean peak of depolarisation-induced force responses elicited in fibres not exposed to taurine was 75.7 ± 5.3% of maximal force (n = 10), while the peak of the depolarisation-induced force responses elicited after exposure to 20 mm taurine was significantly larger, reaching 90.2 ± 12.1% (n = 10) of maximal force (Student's unpaired t test, P = 0.02). This result demonstrates that taurine is able to increase the peak of depolarisation-induced force responses to levels close to maximum force in skinned skeletal muscle fibres.
Control responses elicited after washout of 20 mm taurine were significantly reduced in peak compared to the original controls (P = 0.0008; Fig. 3A). However, this is unlikely to be due to the effects of taurine as in experiments where an equivalent number of responses were elicited under control conditions (no exposure to taurine), the responses also dropped to a similar level (Student's unpaired t test, P = 0.33; Fig. 3B). The peak of the depolarisation-induced force responses elicited in the presence of 20 mm taurine (as a percentage of initial control; Fig. 3A) was also significantly greater than the peak of responses elicited in fibres in which a similar number of responses had been activated without exposure to taurine (Fig. 3B; Student's unpaired t test, P = 0.002).
In order to ensure that the effect of taurine on the depolarisation-induced force responses was not simply due to the increased osmolarity of the solutions caused by the addition of taurine, we examined the effect of 20 mm sucrose on depolarisation-induced force responses (Fig. 3B). Sucrose had no significant effect on the peak of the depolarisation-induced force responses compared with control responses obtained in the same fibres (P = 0.95), indicating that taurine increases the peak of the depolarisation-induced force responses by other means.
The effect of taurine on SR Ca2+ accumulation
In order to test whether taurine increased the size of the depolarisation-induced force responses by facilitating SR Ca2+ accumulation, we examined the effect of 20 mm taurine on SR Ca2+ loading in the skinned fibres. In these experiments, the SR of the fibre was loaded with Ca2+ for a set length of time (that produced submaximal loading under control conditions; Bakker et al. 1998) after having been previously depleted of all releaseable Ca2+ by exposure to a Ca2+-release solution (low-Mg2+, potassium-HDTA solution with 30 mm caffeine and 0.75 mm EGTA). After the loading period, the fibre was exposed to the Ca2+-release solution to again release all releasable Ca2+ from the SR. The level of Ca2+ loading that had occurred during the loading process could then be estimated from the integral of the force response elicited upon re-exposure to the caffeine-EGTA solution (Endo, 1977). The integral of the caffeine-induced force responses elicited after loading in the presence and absence of 5 and 20 mm taurine was compared with control measurements made before and after taurine exposure. Taurine (20 mm) was found to significantly increase SR Ca2+ loading in the skinned fibres to 134.6 ± 9.3% of that under control conditions (P = 0.0014, n = 10; Fig. 4). In the presence of 5 mm taurine, SR Ca2+ loading was 96.3% of control levels (not significantly different, P = 0.539, n = 10). We also examined the effect of 20 mm sucrose, as an osmotic replacement for taurine, on SR Ca2+ loading in the skinned EDL fibres. No significant effect of sucrose on SR Ca2+ loading was found (loading in 20 mm sucrose was 101.1 ± 2.1 % of controls, P = 0.62, n = 10), indicating that the effect of taurine on SR Ca2+ loading was not due to an increase in the osmolarity of the Ca2+ loading solution.
Figure 4. The effect of 20 mm taurine on SR Ca2+ loading in skinned EDL fibres of the rat.
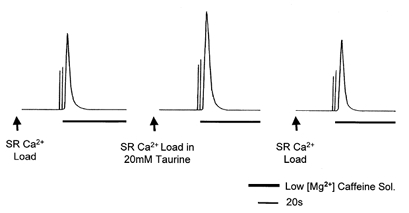
Taurine was present in the Ca2+ loading solution (pCa 6.55) only. Loading time was set so as to give a submaximal force response upon exposure to the low-Mg2+-caffeine Ca2+-release solution. The presence of taurine in the loading solution increased the area of the low-Mg2+-caffeine-induced force responses (middle response) compared to control measurements made before and after with no taurine in the loading solution.
The effect of taurine on caffeine-induced force responses
Taurine has been shown to have a multitude of effects within cells, including the modulation of ion channel activity (Huxtable, 1992). In this study, taurine was found to increase the mean rate of rise of the depolarisation-induced force responses. Therefore, we examined the possibility that, in addition to increasing SR Ca2+ accumulation, taurine may also increase the size of the depolarisation-induced force responses by altering the function of the SR Ca2+ release channels and thereby increasing Ca2+ release. In these experiments, the fibres were initially exposed to an HDTA solution with 30 mm caffeine and 0.75 mm EGTA to completely deplete the SR of Ca2+, and then reloaded with Ca2+ for a set length of time. The fibres were then preincubated in an HDTA solution containing taurine and EGTA (to prevent further loading) for 30 s to allow time for taurine to equilibrate within the fibre (controls were exposed to a similar solution without taurine), and then transferred to a submaximal caffeine–EGTA Ca2+-release solution. The peak and rate of rise of the force responses elicited in the submaximal caffeine solution in the presence or absence of taurine were then compared. Changes in the average rate of rise (during 20–80 % of peak force) and/or peak of the force responses in the presence of taurine would be indicative of taurine-induced changes in the rate of SR Ca2+ release. Taurine (20 mm) was found to significantly increase both the peak and mean rate of rise of the caffeine-induced responses to 129.2 ± 9.7% (P = 0.03, n = 6) and 127.6 ± 8.9% (P = 0.04, n = 6), respectively, of controls (Fig. 5). Addition of 20 mm sucrose to the caffeine release solutions had no significant effect on the peak or rate of rise of caffeine-induced force responses (peak in sucrose: 102.2 ± 6.9% of controls, P = 0.76; mean rate of rise: 102.5 ± 13.7% of controls, P = 0.87, n = 6). This indicates that the increase in the peak and rate of rise of the caffeine-induced force responses observed in the presence of taurine in this study was not due to the osmotic effects of taurine.
Figure 5. The effect of 20 mm taurine on caffeine-induced SR Ca2+ release in skinned EDL fibres of the rat.
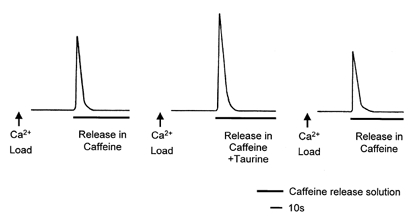
Taurine was present in the Ca2+-release solution (low-Mg2+, potassium-HDTA solution with 30 mm caffeine and 0.75 mm EGTA) and the preincubation solution (which also contained 0.75 mm EGTA to prevent SR Ca2+ loading) only. The SR of the fibre was loaded with Ca2+ under control conditions. The presence of taurine increased the mean rate of rise (during 20–80% of peak force) and the peak of caffeine-induced force responses.
These results indicate that in addition to increasing SR Ca2+ accumulation, taurine can also increase the size of force responses in EDL fibres by altering the activity of the SR Ca2+-release channels. However, it is also possible that the increase in the size of the caffeine-induced force responses in the presence of taurine shown in this study was due to taurine-induced increases in the kinetics of force development.
The effect of taurine on SR Ca2+ leak
Skinned mammalian skeletal muscle has been shown to exhibit a small but significant SR Ca2+ release, or leak, during periods of non-activation (Bakker et al. 1996). In this study, taurine was found to alter caffeine-induced Ca2+ release (see above), suggesting that it may interact with the Ca2+-release channel. Therefore, it was of interest to examine whether 20 mm taurine could alter the SR Ca2+ leak in the skinned fibres, particularly since inhibition of the SR Ca2+ leak by taurine could play a role in the increased SR Ca2+ accumulation shown above. These experiments were similar to the Ca2+-release experiments described above, with the exception that after exposure to the Ca2+ loading solution, the fibres were exposed to a Ca2+ leak solution, with or without 20 mm taurine, for 90 s before exposure to the submaximal caffeine-EGTA Ca2+-release solution. The Ca2+ leak solution had an EGTA concentration of 0.75 mm to chelate leaked Ca2+ and prevent its re-uptake by the SR.
Under control conditions, exposure of the Ca2+-loaded fibres to the leak solution for 90 s reduced the area of the force responses elicited by the ‘submaximal’ Ca2+-release solution to 36.3 ± 12.5% of the area of control force responses (elicited after no exposure to the leak solution), verifying that an SR Ca2+ leak was present in these fibres (Fig. 6). However, force responses elicited after exposure to an identical leak solution containing taurine were not significantly different from control responses elicited after exposure to the control leak solution (taurine response 107.6 ± 7.87% of controls, P = 0.38; Fig. 6), indicating that taurine has no effect on SR Ca2+ leak in skinned EDL fibres.
Figure 6. The effect of 20 mm taurine on passive Ca2+ leak from the SR.
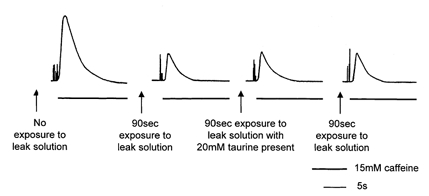
The first response was elicited after Ca2+ loading and subsequent exposure to the Ca2+-release solution. The second response was recorded after Ca2+ loading and exposure to a Ca2+ leak solution followed by exposure to the Ca2+-release solution. The difference between the areas of the first and second response gives a measure of the SR Ca2+ leak under normal conditions. The third response, elicited after exposure to a leak solution with 20 mm added taurine, was similar to the control response, indicating that taurine did not alter the normal passive SR Ca2+ leak in the skinned fibres. The final control response after exposure to the leak solution was also similar to the initial control response (second response overall).
DISCUSSION
This study shows, for the first time, that taurine significantly increases the size of force responses elicited via the normal depolarisation-activated pathway in mammalian skeletal muscle. These results suggest that endogenous concentrations of taurine play an important role in the maintenance of the appropriate force output during mammalian skeletal muscle contraction.
In skeletal muscle, recent reports using immunoreactivity techniques suggest that a proportion of skeletal muscle fibres have little or no detectable taurine (Quesada et al. 1993; Lobo et al. 2000). Furthermore, in cat fibres, neural stimulation is reported to reduce the number of taurine-positive (immunoreactive) fibres in a particular muscle, suggesting that stimulation leads to taurine loss in certain skeletal muscle fibres (Quesada et al. 1993). The results of this study indicate that fibres with a low taurine content would produce significantly less force in response to stimulation than those with a high taurine content. Therefore, skeletal muscle fibres may have the ability to modulate the contractile response to neural stimulation by increasing or decreasing the intracellular taurine levels.
Taurine has been shown to increase the Cl− conductance, leading to membrane hyperpolarisation in rat skeletal muscle fibres, in both cells preloaded with taurine and cells exposed to increases in extracellular taurine (Gruener et al. 1975; Conte-Camerino et al. 1987). Coonan & Lamb (1998) showed that the t-tubular membrane of skinned rat EDL fibres is highly permeable to Cl− ions. This raises the possibility that taurine may have increased the size of the depolarisation-induced force responses in this study by increasing the polarisation of the t-tubule membrane through increased Cl− efflux from the sealed t-system. If the sealed t-system in the skinned fibres is depolarised compared to intact fibres, an increased level of polarisation could decrease voltage sensor inactivation. However, Coonan & Lamb (1998) showed, using fibres that were skinned after incubation in Cl−-free Ringer solution, that the effects of t-tubule Cl− efflux were transient, affecting only the first few responses. The t-tubule Cl− efflux was hypothesised to be responsible for the initial ‘work up’ in the size of the force responses (Coonan & Lamb, 1998). In the present study, the experiments were commenced when control depolarisation-induced force responses had already reached a stable level (after 2–4 responses), making it unlikely that taurine could have any effect on the t-tubular membrane potential via an action on t-tubule Cl− channels under these conditions.
The results of this study show that taurine (20 mm) can increase SR Ca2+ accumulation by 35% in skeletal muscle fibres. While this figure is dependent on the specific conditions of this study, it is consistent with the sizeable (25%) increase in the rate of SR Ca2+ uptake observed in the presence of 15 mm taurine in SR vesicles prepared from ‘white’ muscles of the rat (Huxtable & Bressler, 1973). Our results show that taurine has no significant effect on the passive Ca2+ leak from the SR in skinned fibres. Therefore, the increase in Ca2+ accumulation elicited by taurine in the skinned fibres in this study is likely to be due to a taurine-induced increase in the activity of the SR Ca2+ pump.
The findings of this study indicate that the potentiation observed in the depolarisation-induced force responses in the presence of 20 mm taurine is due to increased SR Ca2+ loading, and the resulting increase in the amount of Ca2+ available for release upon activation. The taurine-induced increase in Ca2+ accumulation reported here is consistent with the hypothesis put forward to explain the effect of taurine deficiency on lowering the mechanical threshold for contraction in rat skeletal muscle fibres (De Luca et al. 1996; Pierno et al. 1998). That is, decreased intracellular taurine levels will lead to less Ca2+ uptake, resulting in an elevation in the resting [Ca2+] within the fibres. This would bring the intracellular [Ca2+] closer to the threshold for contraction, and as a result less Ca2+ release would be required for the onset of contraction, leading to contraction at more negative potentials.
When 20 mm taurine was added to the caffeine release solution in this study, both the rise time and the peak of the caffeine-induced force responses were increased by approximately 28%. These results suggest that taurine is also able to increase the activity of the SR Ca2+-release channels in fast-twitch skeletal muscle. Note that the increase in the peak value of the caffeine-induced force responses in the presence of taurine is likely to represent an underestimate of the effects of taurine on SR Ca2+ release, as taurine addition also produces a small decrease in the sensitivity of the contractile apparatus to Ca2+ and in maximal force production. These results also suggest that increased ryanodine receptor activity in the presence of taurine was, at least partially, responsible for the increase in the mean rise time and peak of the depolarisation-induced force responses elicited in the presence of taurine. This study provides the first evidence that taurine may alter the function of the ryanodine receptor in skeletal muscle cells. However, as mentioned previously, it is also possible that taurine increased the size of the caffeine-induced force responses in this study by increasing the kinetics of force development.
Kuo & Miki (1980) also found evidence that taurine increases both Ca2+ uptake and release in disc membranes of photoreceptor cells. The ability of taurine to alter ion channel function has been widely documented (Huxtable, 1989). Taurine reduces the time course of the action potential in skeletal muscle (Gruener et al. 1975), and activates sarcolemmal Cl− channels leading to hyperpolarisation (Conte-Camerino et al. 1987). How taurine could alter ryanodine receptor activity is unknown. It has been reported that taurine indirectly alters Cl− channel function in skeletal muscle by interacting with the phospholipids surrounding the channel, rather than through an action on the channel itself. The presence of taurine has also been shown to be important for the maintenance of SR membrane integrity in the isolation of SR vesicles (Huxtable & Bressler, 1973), suggesting that the interaction of taurine with the SR membrane is important for normal SR function.
Steele et al. (1990) reported no effect of taurine on caffeine responses elicited in skinned cardiac trabeculae (taurine added to the caffeine solution alone). The dissimilarity between this result and the results of our study may be due to differences in the sensitivity to taurine of the different ryanodine receptor isoforms found in skeletal and cardiac muscle, or to the different Ca2+-release mechanisms present in skeletal and cardiac muscle.
In this study, 20 mm taurine was found to produce a small but significant decrease in the sensitivity of the contractile apparatus to Ca2+ in the EDL fibres. The effect of taurine on the Ca2+ sensitivity of the contractile apparatus and maximal force production in the EDL fibres in this study is likely to be due to the direct osmotic effects of taurine addition as the contractile apparatus of rat EDL skeletal muscle has been shown to be sensitive to small changes in osmolarity (Lamb et al. 1993). In rat trabeculae (1–40 mm taurine) (Steele et al. 1990), pig atrial preparations (5 mm taurine) and slow crayfish striated fibres (5 mm taurine) (Galler & Hutzler, 1988, 1990), taurine was reported to produce a small increase in the Ca2+ sensitivity of the contractile apparatus. The difference between these findings and the results reported in this study for fast skeletal muscle may, in part, reflect the different protein isoforms involved in the regulation of contractile protein function in fast-twitch fibres and slower muscle types.
In conclusion, the results of this study indicate that physiologically relevant intracellular concentrations of taurine significantly increase the size of depolarisation-induced force responses in mammalian skeletal muscle, by increasing SR Ca2+ accumulation and Ca2+ release. The findings of this study indicate that taurine is an important modulator of contractile function in mammalian skeletal muscle, and alterations of myoplasmic taurine levels may provide a means for skeletal muscle fibres to alter contractile output in response to neural stimulation.
Acknowledgments
This work was supported by grants from the Raine Medical Research Foundation and the Australian Research Council. Thanks to Professor Don Robertson for helpful suggestions regarding the manuscript.
REFERENCES
- Ariano MA, Armstrong RB, Ederton VR. Hindlimb muscle fiber populations of five mammals. Journal of Histochemistry and Cytochemistry. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Head SI, Wareham AC, Stephenson DG. Effect of clenbuterol on sarcoplasmic reticulum function in single skinned mammalian skeletal muscle fibers. American Journal of Physiology. 1998;274:C1718–1726. doi: 10.1152/ajpcell.1998.274.6.C1718. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Baylor SM, Chandler WK, Marshall MW. Sarcoplasmic reticulum Ca2+ release in frog skeletal muscle fibre estimated from arsenazo III calcium transients. Journal of Physiology. 1983;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney RW, Lippincott S, Gusowski N, Padilla M, Zelikovic I. Studies on renal adaptation to altered dietary amino acid intake: tissue taurine responses in nursing and adult rats. Journal of Nutrition. 1986;116:1965–1976. doi: 10.1093/jn/116.10.1965. [DOI] [PubMed] [Google Scholar]
- Conte-Camerino D, Franconi F, Mambrini M, Bennardini F, Failli P, Bryant SH, Giotti A. The action of taurine on chloride conductance and excitability characteristics of rat striated muscle fibres. Pharmacological Research Communications. 1987;19:685–701. doi: 10.1016/0031-6989(87)90099-3. [DOI] [PubMed] [Google Scholar]
- Coonan JR, Lamb GD. Effect of transverse-tubular chloride conductance on excitability in skinned skeletal muscle fibres of the rat. Journal of Physiology. 1998;509:551–564. doi: 10.1111/j.1469-7793.1998.551bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Pierno S, Conte-Camerino DC. Effect of taurine depletion on excitation-contraction coupling and Cl− conductance of rat skeletal muscle. European Journal of Pharmacology. 1996;296:215–222. doi: 10.1016/0014-2999(95)00702-4. [DOI] [PubMed] [Google Scholar]
- Donaldson SKB. Peeled mammalian skeletal muscle fibres. Possible stimulation of Ca2+ release via a transverse tubule-sarcoplasmic reticulum mechanism. Journal of General Physiology. 1985;86:501–525. doi: 10.1085/jgp.86.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett M, Harris RC, Sewell DA. Taurine content and distribution in equine skeletal muscle. Scandinavian Journal of Clinical and Laboratory Investigation. 1992;52:725–730. doi: 10.3109/00365519209115518. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiological Reviews. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fink RH, Stephenson DG, Williams DA. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. Journal of Physiology. 1986;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi F, Martini F, Stendardi I, Matucci R, Zilletti L, Giotti A. Effect of taurine on calcium levels and contractility in guinea-pig ventricular strips. Biochemical Pharmacology. 1982;31:3181–3185. doi: 10.1016/0006-2952(82)90547-0. [DOI] [PubMed] [Google Scholar]
- Galler S, Hutzler C. Effects of free amino acids on tension generation by crustacean skinned muscle fibres. Pflügers Archiv. 1988;412:R82. [Google Scholar]
- Galler S, Hutzler C. Effects of taurine on Ca2+-dependent force development of skinned muscle fibre preparations. Journal of Experimental Biology. 1990;152:255–264. doi: 10.1242/jeb.152.1.255. [DOI] [PubMed] [Google Scholar]
- Gruener R, Markovitz D, Huxtable R, Bressler R. Excitability modulation by taurine. Transmembrane measurements of neuromuscular transmission. Journal of the Neurological Sciences. 1975;24:351–360. doi: 10.1016/0022-510x(75)90255-5. [DOI] [PubMed] [Google Scholar]
- Huxtable R, Bressler R. Effects of taurine on a muscle intracellular membrane. Biochimica et Biophysica Acta. 1973;323:573–583. doi: 10.1016/0005-2736(73)90165-x. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiological Reviews. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Progress in Neurobiology. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Iwata H, Obara T, Kim BK, Baba A. Regulation of taurine transport in rat skeletal muscle. Journal of Neurochemistry. 1986;47:158–163. doi: 10.1111/j.1471-4159.1986.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Kim BK, Baba A, Iwata H. Taurine transport in chronically stimulated fast- and slow-twitch muscles of the rat. Japanese Journal of Pharmacology. 1986;42:441–446. doi: 10.1254/jjp.42.441. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Chovan JP, Schaffer SW. Effect of taurine on calcium paradox and ischemic heart failure. American Journal of Physiology. 1981;240:H238–246. doi: 10.1152/ajpheart.1981.240.2.H238. [DOI] [PubMed] [Google Scholar]
- Kuo CH, Miki N. Stimulatory effect of taurine on Ca2+-uptake by disc membranes from photoreceptor cell outer segments. Biochemical and Biophysical Research Communications. 1980;94:646–651. doi: 10.1016/0006-291x(80)91281-4. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. Journal of Physiology. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG, Stienen GJ. Effects of osmolality and ionic strength on the mechanism of Ca2+ release in skinned skeletal muscle fibres of the toad. Journal of Physiology. 1993;464:629–648. doi: 10.1113/jphysiol.1993.sp019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MV, Alonso FJ, Martin del Rio R. Immunocytochemical localization of taurine in different muscle cell types of the dog and rat. Histochemical Journal. 2000;32:53–61. doi: 10.1023/a:1003910429346. [DOI] [PubMed] [Google Scholar]
- Nieminen ML, Tuomisto L, Solatunturi E, Eriksson L, Paasonen MK. Taurine in the osmoregulation of the Brattleboro rat. Life Sciences. 1988;42:2137–2143. doi: 10.1016/0024-3205(88)90128-2. [DOI] [PubMed] [Google Scholar]
- Perlman DF, Goldstein L. Organic osmolyte channels in cell volume regulation in vertebrates. Journal of Experimental Zoology. 1999;283:725–733. [PubMed] [Google Scholar]
- Pierno S, De Luca A, Camerino C, Huxtable RJ, Camerino DC. Chronic administration of taurine to aged rats improves the electrical and contractile properties of skeletal muscle fibers. Journal of Pharmacology and Experimental Therapeutics. 1998;286:1183–1190. [PubMed] [Google Scholar]
- Quesada O, Lu P, Sturman JA. Taurine distribution in different cat muscles as visualized by immunohistochemistry: changes with stimulus state. Cytobios. 1993;73:294–295. [PubMed] [Google Scholar]
- Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochemistry Journal. 1994;300:893–900. doi: 10.1042/bj3000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Mechanisms of ischemia-induced taurine release in mouse hippocampal slices. Brain Research. 1998;807:118–124. doi: 10.1016/s0006-8993(98)00793-8. [DOI] [PubMed] [Google Scholar]
- Schaffer SW, Ballard C, Azuma J. Mechanisms under-lying physiological and pharmacological actions of taurine on myocardial calcium transport. In: Huxtable RJ, Michalk D, editors. Taurine in Health and Disease. New York: Plenum Press; 1994. pp. 105–121. [Google Scholar]
- Schouboe A, Pasantes-Morales H. Role of taurine in cell volume regulation. Canadian Journal of Physiology and Pharmacology. 1992;70:S356–361. doi: 10.1139/y92-283. [DOI] [PubMed] [Google Scholar]
- Steele DS, Smith GL, Miller DJ. The effects of taurine on Ca2+ uptake by the sarcoplasmic reticulum and Ca2+ sensitivity of chemically skinned rat heart. Journal of Physiology. 1990;422:499–511. doi: 10.1113/jphysiol.1990.sp017997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. Journal of Physiology. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson EW. Activation of fast skeletal muscle: contribution of studies on skinned fibres. American Journal of Physiology. 1981;240:C1–19. doi: 10.1152/ajpcell.1981.240.1.C1. [DOI] [PubMed] [Google Scholar]
- Turner O, Phoenix J, Wray S. Developmental and gestational changes of phosphoethanolamine and taurine in rat brain, striated and smooth muscle. Experimental Physiology. 1994;79:681–689. doi: 10.1113/expphysiol.1994.sp003800. [DOI] [PubMed] [Google Scholar]


