Abstract
The maitotoxin (MTX)-induced cationic current (Imtx) from human skin fibroblasts was characterized using the patch-clamp technique in whole-cell configuration. Under resting conditions (absence of MTX), the main current observed is produced by an outwardly rectifying K+ channel which is inhibited by 1 mm TEA. The current reversal potential was −86 mV (n = 12). MTX (500 pm) activated a current with a linear current–voltage relationship and a reversal potential of −10 mV (n = 10). Replacing the extracellular Na+ and K+ with N-methyl-d-glucamine (NMDG) caused a shift of the reversal potential to a value below −100 mV, indicating that Na+ and K+, but not NMDG, carry Imtx. Further ion selectivity experiments showed that Ca2+ carries Imtx also. The resulting permeability sequence obtained with the Goldman–Hodgkin–Katz equation yielded Na+ (1) ≈ K+ (1) > Ca2+ (0.87). The Imtx activation time course reflected the changes in intracellular Ca2+ and Na+ measured with the fluorescent indicators fura-2 and SBFI, respectively, suggesting that the activation of Imtx brings about an increment in intracellular Ca2+ and Na+. Reducing the extracellular Ca2+ concentration below 1.8 mm prevented the activation of Imtx and the increment in intracellular Na+ induced by MTX. Mn2+ and Mg2+ could not replace Ca2+, but Ba2+ could replace Ca2+. MTX activation of current in 10 mm Ba2+ was approximately 50 % of that induced in the presence of 1.8 mm Ca2+. When 5 mm of the Ca2+ chelator BAPTA was included in the patch pipette, MTX either failed to activate the current or induced a small current (less than 15 % of the control), indicating that intracellular Ca2+ is also required for the activation of Imtx. Intracellular Ba2+ can replace Ca2+ as an activator of Imtx. However, in the presence of 10 mm Ba2+ the activation by MTX of the current was 50 % less than the activation with nm concentrations of free intracellular Ca2+.
Maitotoxin (MTX), a water-soluble polyether obtained from the marine dinoflagellate Gambierdiscus toxicus, is a potent activator of Ca2+ influx in a wide variety of cells (Gusovsky & Daly, 1990). This toxin may be one of the molecules involved in the seafood poisoning known as ciguatera (Yasumoto et al. 1976).
Ciguatera is a severe health problem in many countries where seafood is an important part of the diet. There are more than 20 000 cases of this disease each year worldwide. However, it is believed that many more cases are not properly diagnosed or reported (Yasumoto & Satake, 1996). Elucidating the cellular and molecular mechanisms underlying MTX action may help to prevent or treat ciguatera poisoning.
MTX is a powerful tool for studying changes in Ca2+ homeostasis. The fact that MTX does not appear to have ionophoretic activity (Takahashi et al. 1983), and that this toxin can activate Ca2+ influx in practically all cells tested from a wide variety of organisms, suggests the presence of a highly conserved MTX receptor (Escobar et al. 1998).
Another feature of this toxin is its potency. MTX effects are observed with toxin concentrations ranging from picomolar to nanomolar. Recently, we reported that human skin fibroblasts are very sensitive to MTX. The influx of Ca2+ showed a half-activation constant of 450 fm (Gutierrez et al. 1997).
Although the mechanism by which MTX activates the influx of Ca2+ remains unsolved, it appears to involve the activation of a current (Imtx) through Ca2+-permeable cationic channels, as indicated by patch-clamp experiments performed on different cells and tissues (reviewed in Escobar et al. 1998). It is not yet clear whether the activation of this channel is direct, or mediated by a second messenger cascade.
The interactions between MTX and its putative receptor appear to be complex, since in some cells the presence of extracellular Ca2+ and/or Na+ is required for the activation of Imtx (Escobar et al. 1998). However, no detailed characterization of the cation requirements for the MTX effect has been reported to date.
In this study we have characterized Imtx from human skin fibroblasts utilizing the whole-cell configuration of the patch-clamp technique and fluorescent indicators to measure changes in the intracellular Ca2+ and Na+ concentrations in response to MTX. The results obtained indicate that MTX activates a cationic current with a linear current–voltage relationship. The time course of activation of Imtx is correlated with the changes in intracellular Ca2+ and Na+ as monitored with fluorescent indicators.
We have also characterized the ionic requirements for the MTX effect. We have found that in human skin fibroblasts extracellular Ca2+, but not Na+, is required for the activation of Imtx. Ca2+ is required only during the initial application of MTX; removal of this cation after the onset of the response did not eliminate Imtx.
Other divalent cations such as Mg2+ or Mn2+ cannot replace extracellular Ca2+, whereas Ba2+ can partially compensate for the absence of free extracellular Ca2+. In 10 mm Ba2+, MTX activates 50 % of the current obtained with 1.8 mm Ca2+.
Intracellular Ca2+ is also required for MTX action. When 5 mm BAPTA was included in the patch pipette, MTX failed to activate Imtx, indicating that nanomolar concentrations of free intracellular Ca2+ are also required for the activation of Imtx. As in the case of extracellular divalent cations, intracellular Mg2+ or Mn2+ cannot replace Ca2+. Only Ba2+ at high concentrations can partially compensate for the absence of Ca2+.
The results presented here, in combination with those found in the literature, indicate that MTX is a powerful and complex Ca2+-mobilizing agent.
METHODS
Solutions and reagents
All salts used are analytical grade (Sigma Chemical Co., St Louis, MO, USA). Ethyleneglycol-bis-(β-aminoethylether)N,N,N ′,N ′-tetraacetic acid (EGTA), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), bovine serum albumin (BSA) and trypsin A3 were purchased from the Sigma Chemical Co. Maitotoxin was purchased from Calbiochem. Fura-2 acetoxymethylester (fura-2 AM) was purchased from Molecular Probes. Maitotoxin was dissolved in a 10 mm Mes solution pH 5.6. For patch-clamp experiments, the extracellular solution contained (mm): 140 NaCl, 5 KCl, 1 MgCl2, 10 Hepes, 5 glucose and 2 CaCl2; pH 7.4. The CaCl2 was omitted in low Ca2+ solutions and in divalent cation experiments as indicated in the text and figure legends. The intracellular solution contained (mm): 145 potassium aspartate, 5 NaCl, 10 Hepes, 1 MgCl2 and 2 EGTA; pH 7.2. In experiments performed under low intracellular Ca2+ conditions, EGTA was replaced with 5 mm BAPTA. For intracellular Ca2+ and Na+ measurements, the extracellular solution contained (mm): 120 NaCl, 1.2 KH2PO4, 1.2 MgSO4, 4.75 KCl, 10 glucose, 20 Hepes, 1.8 CaCl2 and 0.05 % BSA; pH 7.2–7.4. In experiments with low extracellular Na+, this cation was replaced with N-methyl-d-glucamine (NMDG). To determine the relative ionic permeabilities for Ca2+versus Na+ and K+ the following extracellular solutions were used (mm): 145 KCl or NaCl, 10 Hepes, 5 glucose and 2 CaCl2; pH 7.4. When K+ was present in the extracellular solution, 2 mm TEA was also added to block K+ currents. The intracellular solutions contained (mm): 145 potassium or sodium aspartate, 10 Hepes and 2 EGTA; pH 7.2.
Cell culture
Human skin fibroblast cells were obtained from skin biopsies of healthy donors as previously described (Soto et al. 1996). Cells were given with the informed consent of the donors and all procedures were evaluated and approved by the ethics committee of the Universidad Nacional Autónoma de México and conformed to The Declaration of Helsinki. Cells were maintained in culture at 37 °C (8–13 passages) in a humidity controlled incubator with 5 % in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal bovine serum, 2 mm l-glutamine (Gibco), 100 μg ml−1 streptomycin and 25 ng ml−1 amphotericin B (Gibco).
Patch-clamp experiments
When monolayers reached confluency the cells were mechanically dispersed with a plastic pipette. Cells were placed on 35 mm Petri dishes in extracellular solution and mounted on the stage of a Nikon inverted microscope. The whole-cell configuration of the patch-clamp technique was used to study currents from single fibroblast cells under voltage-clamp (Hamill et al. 1981). Patch pipettes were made of 8161 glass (World Precision Instruments, Inc.) pulled to obtain pipette resistances in the range of 4–6 MΩ when measured in experimental solutions. Liquid junction potentials were compensated in experiments using NMDG-Cl solutions. The holding potential in all experiments was maintained at 0 mV. The cell capacitance varied between 8 and 12 pF. The AxoPatch 200A (Axon Instruments Inc., Foster City, CA, USA) was used as the patch-clamp amplifier. The reference electrode was an Ag–AgCl plug connected to the bath solution via a 100 mm KCl agar bridge. Voltage-clamp data were stored on tape and digitized later for analysis using the Digidata 1200 (Axon Instruments) analogue–digital interface and pCLAMP 6.0.4 software (Axon Instruments). The data were filtered at 10 kHz using a 4-pole Bessel filter (Axon Instruments) and digitized at 100 Hz. The binary data were converted to ASCII and multiple experiments were combined to obtain means ± standard deviation (s.d.) using the SigmaPlot 2.0 software (Jandel Corporation). In all the described experiments, n = number of experiments performed for each condition. All experiments were performed at room temperature (22–25 °C).
Determination of relative permeabilities
Ionic permeability ratios were calculated with a modified version of the Goldman–Hodgkin–Katz (GHK) equation (González-Perrett et al. 2001):
where V is the reversal potential (Erev), R is the the gas constant, T is the absolute temperature in kelvin, F is the Faraday constant and the ion is K+ or Na+.
Free divalent cation determinations
Free concentrations of Ca2+, Ba2+ and Mg2+ were determined using the program ‘Bound and Determined’ (BAD 4.35) as previously published (Brooks & Storey, 1992). Briefly, the free ion concentrations reported in this study were calculated by introducing the values of temperature, pH and concentrations of the buffers and all ions present in the experimental solutions into the BAD program.
Intracellular Ca2+ measurements
Confluent cells were mechanically dispersed with a plastic pipette after 2 min of incubation with 0.01 % trypsin solution and placed in extracellular solution to a final concentration of 1.5–2 × 106 cells ml−1 and incubated with 5 μm fura-2 AM for 45 min. After this, the cells were rinsed twice and incubated for 30 min in fura-2 AM-free solution. The cells were then rinsed 3–4 times and placed in a cuvette of an Aminco-Bowman series 2 luminescence spectrometer. The excitation wavelength was alternated between 340 and 380 nm every 2 s and the emission was collected at 510 nm. Each experiment was individually calibrated to obtain the maximum fluorescence after disrupting the cells with 0.1 % Triton X-100, and the minimum fluorescence obtained after buffering the Ca2+ in the solution with 20 mm EGTA. The values obtained with this procedure were used to calculate the intracellular Ca2+ concentration according to previously published equations (Grynkiewicz et al. 1985). Auto-fluorescence of cells not loaded with fura-2 AM was subtracted from each experiment.
Intracellular Na+ measurements
Confluent cells were rinsed with Hanks' balanced salt solution (HBSS) and incubated for 2 min in 0.01 % trypsin solution. After this, the cells were rinsed twice with extracellular solution and incubated for 45 min with extracellular solution containing 1 % pluronic acid and 25 μm SBFI AM (Molecular Probes). The cells were rinsed and maintained in SBFI AM-free solution for 15 min, and placed in a cuvette of an Aminco-Bowman series 2 luminescence spectrometer. The excitation wavelength was 358 nm and the emission at 500 nm was collected every 2 s. Na+ calibration curves were performed according to previously published procedures for each individual experiment (Harootunian et al. 1989; Zhao et al. 1995).
RESULTS
K+ currents present in non-stimulated human skin fibroblasts
First we measured the currents present in human skin fibroblasts under resting conditions (absence of MTX). Figure 1A illustrates the outwardly rectifying current observed in fibroblasts studied with the whole-cell configuration of the patch-clamp technique. This current showed a reversal potential of −86 mV (n = 12).
Figure 1. Basal K+ currents from human skin fibroblasts.
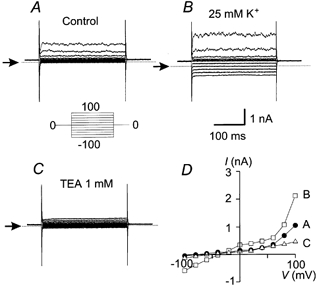
Representative currents elicited in response to voltage steps from −100 to +100 mV (increment 20 mV) obtained from non-stimulated human skin fibroblasts. A, basal conditions. B, after increasing the extracellular K+ concentration from 5 to 25 mm. C, after applying 1 mm TEA. D, I–V curves for the three conditions described: basal (•), after increasing extracellular K+ to 25 mm (□) and after addition of 1 mm TEA (▵). Representative traces are shown from one cell (n = 4). Arrows indicate zero current level. Holding potential, Vh = 0 mV.
Increasing the extracellular K+ from 5 to 25 mm caused a shift of the reversal potential towards more positive values. Under this condition the reversal potential observed was −40 mV, which is near the predicted potential by the Nernst equation for a K+ electrode (−44 mV).
The outwardly rectifying current is completely inhibited by 1 mm TEA as illustrated in Fig. 1C. Figure 1D illustrates the current–voltage (I–V) relationships obtained in resting conditions (•), after increasing the extracellular K+ to 25 mm (□) and after the addition of 1 mm TEA (▵).
These results indicate that under resting conditions, the main current present in human skin fibroblasts is produced by an outwardly rectifying K+ channel inhibited by TEA.
MTX activates a cationic current carried by K+, Na+ and Ca2+
The effect of 500 pm MTX on human skin fibroblast currents was measured. We have previously shown that at this toxin concentration, the maximal MTX response is achieved (Gutierrez et al. 1997). Interestingly, multiple applications of MTX up to a final concentration of 2 nm did not result in further changes in the amplitude of the MTX-induced current (data not shown). This result supports previously published data indicating that MTX has no ionophoretic activity.
After the addition of MTX, the activation of a linear current was observed when applying voltage ramps during a patch-clamp experiment in the whole-cell configuration (Fig. 2B). The linear current activated by MTX (Imtx) showed a reversal potential of −10 mV (n = 10, Fig. 2B). Figure 2C compares the control current (before addition of MTX, •) with the current evoked by MTX (Fig. 3C, □). Subtracting Imtx from the control produced the net current activated by MTX (Fig. 3C, ▴). Due to the large amplitude of Imtx in comparison with the control outwardly rectifying K+ current, the net current obtained after subtraction is very similar to Imtx but it reverses at a slightly more positive value (−7 mV, Fig. 3C).
Figure 2. MTX activates a non-selective cationic current (Imtx).
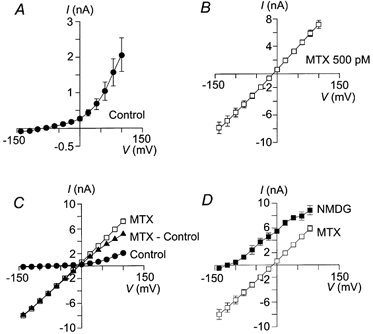
A, I–V curve of the basal K+ current present in human skin fibroblasts in the absence of MTX, the current reversal potential is −85.8 ± 4.1 mV (n = 12). B, I–V curve of the current activated by 500 pm MTX, the current reversal potential is −10.10 ± 6.4 mV (n = 10). C, comparison of the I–V curves under control conditions (•), total current after MTX application (control + MTX activated, □) and the net current activated by MTX (total – control, ▴). D, I–V curves for Imtx obtained with physiological extracellular solution (□) and with NMDG-Cl in the external medium (n = 4, ▪). In the presence of external NMDG the current reversal potential shifts to values more negative than −100 mV. In all the cases means ± s.d. are shown. Where not shown error bars are smaller than symbols. Vh = 0 mV.
Figure 3. Relative cationic permeability of Imtx.
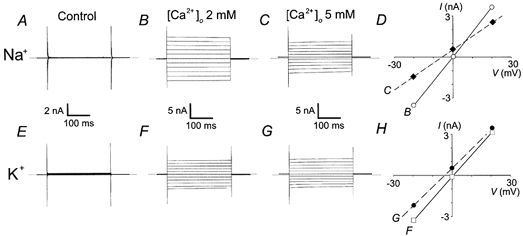
Representative experiments showing the shift in the reversal potential of Imtx induced after increasing extracellular calcium from 2 to 5 mm. A, control currents (absence of MTX) in 145 mm symmetrical Na+ produced by voltage steps from a holding potential of 0 mV to −100 mV and then to 100 mV in 20 mV steps. B, currents induced by 500 pm MTX in the presence of 2 mm extracellular Ca2+. C, same cell as in B after raising extracellular Ca2+ to 5 mm. D, current–voltage relationship showing the mean values (n = 4) of the currents in 145 mm Na+ and 2 mm Ca2+ (○—○) or 5 mm Ca2+ (♦—♦). E, control currents (absence of MTX) in 145 mm symmetrical K+ and 2 mm TEA produced by voltage steps from a holding potential of 0 mV to −100 mV and then to 100 mV in 20 mV steps. F, currents induced by 500 pm MTX in the presence of 2 mm extracellular Ca2+. G, same cell as in F after raising extracellular Ca2+ to 5 mm. H, current–voltage relationship showing the mean values (n = 5) of the currents in 145 mm K+ and 2 mm Ca2+ (□—□) or 5 mm Ca2+ (•—•). The voltage scales in D and H show only the data between −30 and +30 mV to evidence the shift in the reversal potential. Horizontal dotted lines indicate the zero current level.
To determine the ionic selectivity of Imtx, the cations from the extracellular bath solution were replaced with NMDG. Replacing the extracellular solution with NMDG-Cl resulted in a shift of the reversal potential of Imtx to a value more negative than −100 mV, indicating that Imtx carries cations but not NMDG (Fig. 2D). Under these experimental conditions, the calculated reversal potential for an anionic current according to the Nernst equation is near 0 mV.
Further ionic substitution experiments were performed to determine the relative permeability of Ca2+ with respect to K+ and Na+ (PCa/PK and PCa/PNa). Imtx was activated with [Ca2+]o = 2 mm and symmetrical intra- and extracellular concentrations of K+ or Na+ (145 mm). When K+ was present as the main cation, 2 mm TEA was added to the extracellular bath to inhibit the basal K+ current present in fibroblasts. When Imtx was fully activated, [Ca2+]o was raised to 5 mm and the change in the reversal potential (Erev) was monitored (Fig. 3). Addition of 5 mm Ca2+ with Na+ as the main cation present produced a shift in the Erev of −4.9 mV (Fig. 3D, n = 4). In a similar set of experiments with K+ as the main cation present, the shift in the Erev was −4.8 mV (Fig. 3H, n = 5). Calculating the relative permeabilities for each condition using a modified form of the GHK equation (Methods) resulted in PCa/PNa and PCa/PK = 0.87.
To evaluate the contribution of MTX to the changes in intracellular Ca2+ and Na+, cells were loaded with fluorescent indicators to measure the time course of changes in fluorescence. Whole-cell patch-clamp experiments were performed to follow the time course of Imtx activation.
Figure 4 illustrates representative experiments of the time course of activation of Imtx in response to 500 pm MTX (Fig. 4A) compared to the changes in intracellular Ca2+ (Fig. 4B) and Na+ (Fig. 4C) as monitored by the fluorescent indicators fura-2 and SBFI, respectively. As illustrated in this figure, the time course of Imtx followed very closely the increment in intracellular Ca2+ and Na+ induced by MTX. This result along with the selectivity experiments previously shown strongly suggests that the increments in intracellular Ca2+ and Na+ are the result of the activation of Imtx. As we have previously shown, MTX does not induce release of Ca2+ from intracellular storage compartments in these cells (Gutierrez et al. 1997). Therefore, the changes in intracellular Ca2+ observed after MTX must result from Ca2+ influx.
Figure 4. Time course of Imtx and the increments in intracellular Ca2+ and Na+ concentrations.
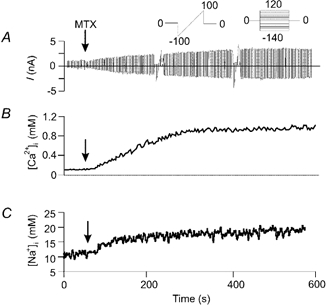
Concomitant measurements of current using patch clamp and intracellular Na+ and Ca2+ with fluorescent indicators. A, a typical experiment showing the time course of Imtx activation. The current was evoked with continuous ramps from −100 to +100 mV. The ramps were interrupted to perform voltage steps from −140 to +120 mV in increments of 20 mV (Vh = 0 mV) to obtain I-V curves. Voltage protocols shown in the inset. Vertical arrows indicate the time of addition of 500 pm MTX. B, time course of the changes in [Ca2+]i in response to MTX monitored with the fluorescent indicator fura-2. C, time course of the changes in [Na+]i monitored with the fluorescent indicator SBFI. Time scale is the same in all panels (shown at the bottom of the figure). Representative traces are shown from eight experiments.
Extracellular Ca2+ is required for the activation of Imtx
To investigate the dependence of extracellular Ca2+ on Imtx, we performed experiments in which the concentration of Ca2+ in the extracellular medium was modified.
Figure 5A illustrates the effect of 500 pm MTX on a cell bathed in the low Ca2+ extracellular solution. As shown in the figure, under these conditions MTX failed to activate Imtx. However, addition of 2 mm Ca2+ in the presence of MTX caused activation of Imtx (Fig. 5A, n = 5). To demonstrate that the current induced after Ca2+ application was not the result of Ca2+ re-addition, we performed experiments in which the extracellular Ca2+ was removed and later re-added in the absence of toxin (Fig. 5B). Under these conditions no current was observed until MTX was added.
Figure 5. Extracellular cation requirement for the activation of Imtx.
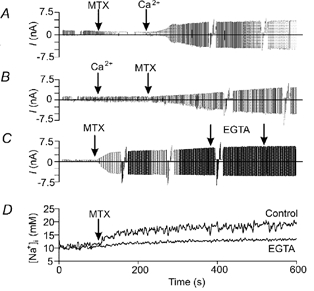
A, addition of MTX to the extracellular medium (in the absence of Ca2+) did not induce the activation of Imtx. The subsequent addition of Ca2+ (2 mm) resulted in a typical activation of Imtx. B, when 2 mm Ca2+ is added before MTX current activation is produced only after toxin application. C, the effect of extracellular Ca2+ appears to be an irreversible process, the repeated addition of EGTA (5 mm each occasion) after the activation of Imtx does not have any effect on the current. D, the rise in [Na+]i is not observed when extracellular Ca2+ is chelated with 5 mm EGTA prior to the addition of MTX. With 2 mm Ca2+ in the bath solution (Control), MTX induced typical increments in [Na+]i. The voltage ramp used in A is the same as that used in Fig. 3A. Representative traces are shown from five experiments for each condition.
Figure 5C shows that after Imtx is fully activated by the toxin, removal of extracellular Ca2+ has no effect on the current. This result indicates that Ca2+ is required to induce the current, but not to maintain Imtx, suggesting that Ca2+ is required for the association of MTX with its putative receptor.
Figure 5D shows that Ca2+ is required also for the increment in intracellular Na+ induced by MTX and measured with SBFI. This result further supports the hypothesis that the increments in intracellular Na+ are the result of Imtx activation.
Since Ca2+ is only required for the activation of Imtx, the relative permeability ratios for K+ and Na+ (PK/PNa) can be calculated once Imtx is fully activated by chelating extracellular Ca2+ with the addition of 5 mm EGTA to the bath solution. From these experiments the PK/PNa obtained was ∼1. The permeability ratios PCa/PNa and PCa/PK previously calculated (Fig. 3), give a selectivity of Imtx of Na+ (1) ≈ K+ > Ca2+ (0.87).
Extracellular Ca2+ cannot be replaced by other divalent cations for Imtx activation
Since extracellular free Ca2+ is required for the activation of Imtx, it was important to determine if other divalent cations could replace Ca2+ for the activation of Imtx. MTX failed to activate Imtx when Mg2+ (up to 10 mm) replaced Ca2+ in the extracellular solution (Fig. 6A). Similar results were obtained with Mn2+ (data not shown). MTX in the presence of 5 mm extracellular Ba2+ induced about 25 % of the current amplitude compared to 2 mm Ca2+(Fig. 6B).
Figure 6. Extracellular divalent cations required for Imtx activation.
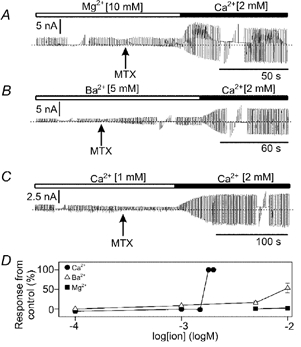
A, Mg2+ does not replace the action of Ca2+ in the activation of Imtx Only after the addition of Ca2+ was Imtx induced. B, Ba2+ was partially effective in replacing extracellular Ca2+ in the activation of Imtx. C, two extracellular concentrations of Ca2+ (1 and 2 mm). The activation of Imtx is observed only with 2 mm Ca2+. Bars above the current recordings show the cation present in the bath. The patch-clamp stimulation protocol shown here is the same as in Fig. 3A. D, data obtained from the experiments in (A–C) and at other concentrations given in the text. The maximum current activated in the presence of 2 mm [Ca2+]o is considered 100 % of the response. The symbols indicate mean and s.d. of n number of experiments for: Ca2+ (n = 25), Ba2+ (n = 17) and Mg2+ (n = 10). Where not shown error bars are smaller than symbols.
The percentage of current induced in the presence of Ba2+, Mg2+ and Ca2+ is compared in Fig. 6D. In 10 mm Ba2+, MTX induced about half the current amplitude activated in 2 nm Ca2+. The Ca2+ requirement reached a saturation point around 1.8 mm, and further additions of Ca2+ did not result in Imtx amplitude increments (Fig. 6D).
Intracellular Ca2+ is also required for the activation of Imtx
The requirement of intracellular Ca2+ for the activation of Imtx was also evaluated (Fig. 7). When 5 mm BAPTA was included in the pipette solution to chelate the Ca2+ in the intracellular medium, MTX failed to activate Imtx (Fig. 7B). These results were obtained in the presence of 2 mm extracellular Ca2+, indicating that free Ca2+ is required on both sides of the cell membrane for the activation of Imtx. Figure 7C shows the Imtx current density obtained with 1 mm free intracellular Ca2+ and in experiments with 5 mm BAPTA (no Ca2+ added). With BAPTA in the pipette, MTX induced 4.6 % of the current activated without BAPTA (control 1.05 ± 0.3 nA pF−1, BAPTA 0.05 ± 0.01 nA pF−1, n = 5).
Figure 7. Intracellular Ca2+ is required also for activation of Imtx.
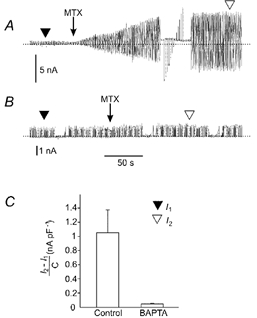
A, control experiment showing the activation of Imtx in response to 500 pm MTX with [Ca2+]o = 2 mm and [Ca2+]i = 1 μm. B, activation of Imtx when [Ca2+]o = 2 mm and [Ca2+]i is near zero with 5 mm BAPTA in the pipette solution. The voltage ramp used here is the same as in Fig. 3A. C, mean ± s.d. from experiments with or without BAPTA in the pipette solution. In BAPTA loaded cells only 4.57 % of the current obtained in control conditions was observed (n = 5). The values of the Imtx current were obtained by subtracting the basal fibroblast K+ current (I1, ▾) from the total current activated by MTX (I2, ▿, n = 10). The graph shows the values of the current corrected by capacitance (I2 – I1/C). The capacitance of the cells varied between 8 and 12 pF.
Intracellular Ca2+ can be replaced by Ba2+ but not by Mg2+
The ability of divalent cations other than intracellular Ca2+ as activators of Imtx was evaluated by including BAPTA (5 mm) in the pipette solution along with a fixed concentration of Mg2+ or Ba2+. The extracellular solution contained physiological concentrations of Ca2+ as described in Methods. The free intracellular concentration of Ca2+, Mg2+ and Ba2+ obtained under these conditions was calculated as described in Methods.
Only Ba2+ was capable of replacing Ca2+. However, about 10 times more Ba2+ was required. Even at concentrations as high as 8 mm intracellular Mg2+, MTX failed to induce Imtx (n = 5, data not shown).
DISCUSSION
Since the original finding showing that MTX may be one of the entities responsible for the seafood poisoning known as ciguatera, many attempts have been made to elucidate the molecular mechanism by which this toxin induces its effects. Evidence has accumulated in recent years indicating that MTX is a very powerful activator of Ca2+ influx in practically all cells tested. Due to the fact that MTX can activate Ca2+ influx in a wide variety of cells from different organisms, it was originally proposed that this toxin was acting as an ionophore (Gusovsky & Daly, 1990). However, several lines of evidence argue against the ionophoretic activity of MTX. Firstly, MTX has no effect on liposomes or mitochondria (Takahashi et al. 1983). Secondly, we have previously shown that trypsin treatment of human skin fibroblasts eliminates the effects of MTX on Ca2+ influx, suggesting that a trypsin-sensitive protein from the plasma membrane is mediating the effects of MTX on Ca2+ (Gutierrez et al. 1997). Thirdly, MTX cannot activate channels in cell patches in the inside-out configuration obtained from Madin-Darby canine kidney (MDCK) cells. However, activation of cationic channels can be achieved in patches in the outside-out configuration (Dietl & Völkl, 1994), showing that MTX can activate the channel only when applied to the extracellular surface of the membrane. Furthermore, MTX induced only one or two single channels over a wide range of toxin concentrations in patches obtained from guinea-pig single ventricular myocytes (Nishio et al. 1996). In this report we have found that the rise in intracellular Ca2+ and Na+ concentrations and the activation of Imtx by MTX saturate and occur at very low toxin concentrations. This type of behaviour is atypical for an ionophore. All these results strongly suggest that MTX is inducing Ca2+ and Na+ influx via activation of non-selective cationic channels such as the one shown in this study. These observations suggest that MTX selectively recognizes a target in the cell membrane, which opens the possibility that MTX could be acting through a widely distributed, highly conserved receptor or channel.
MTX effects on human skin fibroblasts
Human skin fibroblasts provide an excellent model to study the mechanism of action of MTX. These cells are highly sensitive to this toxin; the half-activation constant for Ca2+ mobilization is in the femtomolar range (Gutierrez et al. 1997). The results presented here show that MTX activates a non-selective cationic current (Imtx) in these cells. The activation of Imtx is correlated with the increments in intracellular Ca2+ and Na+. The activation of Imtx and the increments in intracellular Na+ induced by MTX require the presence of intracellular and extracellular free Ca2+.
Previous studies have also reported macroscopic and single-channel currents activated by MTX in different cellular types (Escobar et al. 1998; Schilling et al. 1999). In all of them MTX (1 pm to 30 nm) induced large voltage-independent currents similar to the one reported here. The relative permeability calculated in the present study for Imtx (Na+= K+ > Ca2+) is in agreement with previous reports showing the activation of non-selective cationic currents by MTX (reviewed in Escobar et al. 1998).
In a recent study using the SK45 fibroblast cell line, Schilling et al. (1999) described a dual effect of MTX. At low toxin concentrations (<2 nm), MTX activates non-selective cation channels (NSCCs); at higher toxin concentrations (>2 nm) a cytolytic/oncotic pore (COP) is activated. This COP becomes evident after 5 min of exposure to 2 nm MTX and permeates large molecules (<900 Da) such as fura-2 and NMDG. The MTX-induced COP resembles the large pore formed by stimulation of purinergic P2Z/P2X7 receptors (Schilling et al. 1999). The present report focuses on the effects of low MTX concentrations on ionic conductances and the changes in intracellular Ca2+ and Na+. Our results indicate that Imtx does not permeate fura-2, as intracellular Ca2+ increments reach a plateau phase after MTX stimulation (Fig. 4A and Gutierrez et al. 1997). If MTX promoted fura-2 leakage, a plateau phase would not be observed. Furthermore, we have previously shown that in human skin fibroblasts the increments in fluorescence promoted by increasing MTX concentrations saturate, arguing against the possibility that MTX is promoting fura-2 leakage from the cell. Finally, the shift that occurs in the reversal potential when the extracellular cations are replaced with NMDG indicates that this cation does not permeate the MTX-activated channel.
The activation of Imtx in human skin fibroblasts requires the presence of free Ca2+ in the intracellular and extracellular solutions. Extracellular Ca2+ is only required for the activation of Imtx, since removal of Ca2+ after Imtx activation does not affect the amplitude of the ionic current.
Ba2+ is an inefficient replacement for Ca2+ on both sides of the membrane, around 10 times the concentration being required to achieve the same activation of current. Other divalent cations do not replace Ca2+. In other cell types, Ca2+ is also required for Imtx activation but no dose–response curve for Ca2+ or any evidence about the role of other divalent cations has been presented to date (Gusovsky & Daly, 1990; Escobar et al. 1998; Weber et al. 2000). In guinea-pig single ventricular myocytes, Imtx is activated in the absence of free Ca2+ on both sides of the membrane (Nishio et al. 1996). This is one of few reports found in the literature where Ca2+ is not required for MTX activation.
There are several possible reasons for the requirement of free extracellular Ca2+ for Imtx activation. MTX may require extracellular Ca2+ to interact with its putative receptor in the plasma membrane. The hydrophobic region of MTX resembles that of gangliosides and it has been shown that the ganglioside GM1 inhibits some MTX effects (Konoki et al. 1999). Gangliosides aggregate in membranes, forming stable clusters (Thompson & Tillack, 1985). Extracellular Ca2+ may be required for the insertion of MTX into the plasma membrane.
Another possibility is that the NSCC activated by MTX is Ca2+ dependent. Several NSCCs are activated by intracellular Ca2+ (Hofmann et al. 2000). More complex mechanisms could be envisioned involving calcium– calmodulin interactions or calcium-dependent phosphorylation processes.
Acknowledgments
We would like to thank A. Sampieri for her excellent technical assistance. We would like to thank Dr Mark West for comments on the manuscript. This work was supported by a grant from Consejo Nacional de Ciencia y Tecnologia (CONACyT) 25312-N to L.V. L.V. is an Alexander von Humboldt scholar. V.M.T. received a scholarship from the Centro Medico Siglo XXI (Instituto Mexicano del Seguro Social). This work is dedicated to the memory of our close friend and collaborator Dr Díaz de León.
REFERENCES
- Brooks SPJ, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Analytical Biochemistry. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- Dietl P, Völkl H. Maitotoxin activates a nonselective cation channel and stimulates Ca2+ entry in MDCK renal epithelial cells. Molecular Pharmacology. 1994;45:300–305. [PubMed] [Google Scholar]
- Escobar LI, Salvador C, Martínez M, Vaca L. Maitotoxin, a cationic channel activator. Neurobiology. 1998;6:59–74. [PubMed] [Google Scholar]
- González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Battelli M, Harris PC, Reisin IL, Arnaut MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proceedings of the National Academy of Sciences of the USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gusovsky F, Daly J. Maitotoxin: a unique pharmacological tool for research on calcium-dependent mechanisms. Biochemical Pharmacology. 1990;39:1633–1639. doi: 10.1016/0006-2952(90)90105-t. [DOI] [PubMed] [Google Scholar]
- Gutierrez D, León L, Vaca L. Characterization of the maitotoxin-induced calcium influx pathway from human skin fibroblasts. Cell Calcium. 1997;22:31–38. doi: 10.1016/s0143-4160(97)90087-7. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harootunian AT, Kao JP, Eckert BY, Tsien RY. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. Journal of Biological Chemistry. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. Transient receptor potential channels as molecular substrates of receptor-mediated cation entry. Journal of Molecular Medicine. 2000;78:14–25. doi: 10.1007/s001099900070. [DOI] [PubMed] [Google Scholar]
- Konoki K, Hashimoto M, Murata M, Tachibana K. Maitotoxin-induced calcium influx in erythrocyte ghosts and rat glioma C6 cells, and blockade by gangliosides and other membrane lipids. Chemical Research in Toxicology. 1999;12:993–1001. doi: 10.1021/tx990014m. [DOI] [PubMed] [Google Scholar]
- Nishio M, Muramatsu I, Yasumoto T. Na+-permeable channels induced by maitotoxin in guinea-pig single ventricular cells. European Journal of Pharmacology. 1996;297:293–298. doi: 10.1016/0014-2999(95)00751-2. [DOI] [PubMed] [Google Scholar]
- Schilling WP, Sinkins WG, Estacion M. Maitotoxin activates a nonselective cation channel and a P2Z/P2X7-like cytolytic pore in human skin fibroblasts. American Journal of Physiology. 1999;277:C755–765. doi: 10.1152/ajpcell.1999.277.4.C755. [DOI] [PubMed] [Google Scholar]
- Soto H, Massó F, Cano S, DíazDe León L. Effects of mebendazole on protein biosynthesis and secretion in human-derived fibroblast cultures. Biochemical Pharmacology. 1996;52:289–299. doi: 10.1016/0006-2952(96)00207-9. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tatsumi M, Ohizumi Y, Yasumoto T. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. Journal of Biological Chemistry. 1983;258:10944–10949. [PubMed] [Google Scholar]
- Thompson TE, Tillack TW. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annual Review of Biophysics and Biomolecular Structure. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Weber WM, Popp C, Clauss W, Driessche WV. Maitotoxin induces insertion of different ion channels into the Xenopus oocyte plasma membrane via Ca2+-stimulated exocytosis. Pflügers Archiv. 2000;439:363–369. doi: 10.1007/s004249900150. [DOI] [PubMed] [Google Scholar]
- Yasumoto T, Bagnis R, Vernoux JP. Toxicity study of surgeon fishes – II. Properties of the principal water-soluble toxin. Bulletin of the Japanese Society of Scientific Fisheries. 1976;42:359–365. [Google Scholar]
- Yasumoto T, Satake M. Chemistry, etiology and determination of methods of ciguatera toxins. Journal of Toxicology – Toxin Reviews. 1996;15:91–107. [Google Scholar]
- Zhao H, Xu X, Diaz J, Muallem S. Na+, K+, and H+/HCO3- transport in submandibular salivary ducts. Membrane localization of transporters. Journal of Biological Chemistry. 1995;270:19599–19605. [PubMed] [Google Scholar]


