Abstract
In order to elucidate the mechanisms underlying intracortical I-wave facilitation elicited by paired-pulse magnetic stimulation, we compared intracortical facilitation of I1-waves with that of I3-waves using single motor unit and surface electromyographic (EMG) recordings from the first dorsal interosseous muscle (FDI). We used a suprathreshold first stimulus (S1) and a subthreshold second stimulus (S2). In most experiments, both stimuli induced currents in the same direction. In others, S1 induced posteriorly directed currents and S2 induced anteriorly directed currents. When both stimuli induced anteriorly directed currents (I1-wave effects), an interstimulus interval (ISI) of 1.5 ms resulted in extra facilitation of the responses to S1 alone. The latency of this effect was equivalent to that of the I2-wave from S1. When S1 evoked posteriorly directed currents (I3-wave recruitment), facilitation occurred at a latency corresponding to the I3-wave from S1. This facilitation occurred at an ISI of 1.5 ms when both S1 and S2 flowed posteriorly, and at an ISI of approximately 3.5 ms when S1 was posteriorly and S2 was anteriorly directed. Based on these findings, we propose the following mechanisms for intracortical I-wave facilitation. When S1 and S2 induce currents in the same direction, facilitation is produced by summation between excitatory postsynaptic potentials (EPSPs) elicited by S1 and subliminal depolarization of interneurones elicited by S2 directly. When S1 and S2 induce currents in the opposite direction, facilitation is produced by the same mechanism as above or by temporal and spatial summation of EPSPs elicited by two successive stimuli at interneurones or corticospinal neurones of the motor cortex.
Paired-pulse magnetic stimulation techniques have been used to study the intracortical circuitry of the motor cortex in humans. There are several paired stimulation methods. Two of them have been used for studying inhibitory and facilitatory connections in the motor cortex at short interstimulus intervals (ISIs). When the first stimulus (S1) is subthreshold and the second (S2) suprathreshold, electromyographic (EMG) responses to both stimuli are smaller than the responses to S2 alone at short ISIs (1–5 ms; intracortical inhibition) and larger at longer ISIs (Kujirai et al. 1993). In contrast, when S1 is suprathreshold and S2 subthreshold, EMG responses to both stimuli can be larger than the control responses at ISIs of 1.3, 2.6 and 4.0 ms (Tokimura et al. 1996; Nakamura et al. 1997b; Ziemann et al. 1998; Rothwell, 1999). These two effects were not observed when S2 was a low intensity anodal electrical stimulus, which tends to evoke D-waves (direct waves: descending volleys produced by direct activation of pyramidal tract neurones), but were very clear when S2 was a magnetic stimulus that elicited I-waves (indirect waves: descending volleys produced by indirect activation of pyramidal tract neurones via presynaptic neurones). Based on these results, both effects were considered to be produced at the motor cortex. The latter effect has been termed ‘intracortical I-wave facilitation’ (Ziemann et al. 1998).
Several studies have shown that later I-waves are more affected by intracortical inhibition than early I-waves (Nakamura et al. 1997a; Hanajima et al. 1998; Di Lazzaro et al. 1998). I3-waves appear to be particularly susceptible to intracortical inhibition, whereas I1-waves are little affected (Hanajima et al. 1998). However, it remains to be determined whether there are differences in intracortical I-wave facilitation among different I-waves. In this paper, in order to clarify details of this effect, we studied intracortical I-wave facilitation of I1- and I3-waves using both single motor unit and surface EMG recordings.
METHODS
Subjects
Ten healthy volunteers (8 men and 2 women; 28–46 years old; height, 143–180 cm; weight, 45–95 kg) were studied. Written informed consent was obtained from all the subjects. Surface EMG recordings were done in all subjects. Single motor unit studies were performed in nine subjects, one subject (a professional pianist) declined to participate in this part of the study as it involved insertion of needle electrodes into the hand. The experiments were performed according to the Declaration of Helsinki and the procedures used were approved by the Ethics Committee of the University of Tokyo. No side effects were noted in any of the individuals.
Electromyographic recordings
Surface EMGs were recorded from the first dorsal interosseous muscle (FDI) with 9 mm diameter, Ag-AgCl surface cup electrodes, in all ten subjects. The active electrode was placed over the muscle belly, and the reference electrode over the metacarpophalangeal joint of the index finger. Responses were amplified with an amplifier (Biotop, GE Marquette Medical Systems Japan) through filters set at 100 Hz and 3 kHz, then recorded on a computer (Signal Processor DP-1200, GE Marquette Medical Systems Japan) on which a randomized conditional averaging was performed. Although surface EMG responses to less than 100 Hz are fairly powerful, we chose 100 Hz as the lower filter cut-off point as it reduces stimulus artifact due to magnetic stimulation. This filter setting has worked well in our experiments over the past 10 years and similar filters have been used in other laboratories. During the experiments subjects maintained a slight contraction of the right FDI (5–10 % of the maximum voluntary contraction), with the aid of an oscilloscope monitor.
Single motor units were recorded from the right FDI with a concentric needle electrode (Medelec, disposable type DML25). Signals were amplified through filters set at 100 Hz and 3 kHz. The subjects were instructed to fire the unit voluntarily at about 10 Hz with the aid of audiovisual feedback. Care was taken to record the same motor unit throughout a given experimental session by using an on-line oscilloscope monitor. Twenty-four motor units from nine subjects were studied. Post-stimulus time histograms (PSTHs) were constructed from single motor unit recording data under various conditions.
Stimulation
Transcranial electrical stimulation (TES) was performed with a high-voltage electrical stimulator (D180A; Digitimer, UK) in order to determine the D-wave latency for each muscle. Stimuli were given through two Ag-AgCl cup electrodes (9 mm in diameter) fixed to the scalp; the cathode was placed at the vertex and the anode over the hand motor area (about 5–6 cm lateral to the vertex). Electrical stimuli were given during a slight contraction of the target muscle. As a reference for the following experiments, D-wave latencies were measured from a trace of several superimposed electrical responses.
Transcranial magnetic stimulation (TMS) was performed with a Magstim 200 magnetic stimulator (Magstim Company, UK). A figure-of-eight-shaped coil (external diameter at each wing 9 cm) was placed over the hand motor area. Before the main experiments, we determined the current directions at which I1- or I3-waves were preferentially elicited in each subject, as described previously (Sakai et al. 1997). In this experiment, a figure-of-eight-shaped coil was placed over the hand motor area and held at eight different orientations, each separated by 45 deg. Surface EMG responses were recorded when the subjects made a constant voluntary contraction. In each stimulation condition, the intensity of stimulation was fixed so that responses of about 0.2 mV were elicited in the active FDI when given alone. Five trials were repeated for each condition. Sizes and latencies were measured from averaged responses in each condition, and compared for the different directions. Two current directions were chosen from these comparisons: the direction that was most effective for eliciting responses about 1.5 ms later (I1-waves) than responses evoked by TES (D-waves) and the direction that was most effective in producing responses about 4.5 ms later (I3-waves) than D-waves. Single motor unit studies confirmed the activation of a compatible single descending volley with these stimulation methods (Sakai et al. 1997; Hanajima et al. 1998). Anteromedially directed currents preferentially elicited I1-waves in one of ten subjects, and anteriorly directed currents in the remaining subjects. Posterolaterally directed currents preferentially produced I3-waves in one of ten subjects, posteriorly directed currents in seven and laterally directed currents in the remaining subjects. For descriptive purposes only, hereafter we refer to the direction for eliciting I1-waves as anterior, and that for I3-waves as posterior.
Paired-pulse stimulation for intracortical I-wave facilitation
Two successive stimuli separated by a short ISI were given. Both stimuli were given through the same figure-of-eight-shaped coil on the hand motor area by connecting two magnetic stimulators linked with a Bistim module (Magstim Company, UK). The intensity of the second stimulus (S2) was set below the threshold for an active target muscle and that of the first stimulus (S1) above the threshold. In some of the experiments, we also used an instrument that reversed the direction of the coil current (Magstim Company), in order to study the outcome in the case of oppositely directed S1 and S2. When this instrument was used with the Bistim module, it was possible to give two successive, oppositely directed stimuli that were separated by intervals of 3 ms or more through the same coil.
Depending on the current direction, three combinations of S1 and S2 were used. (1) A-A: both stimuli produced preferential activation of I1-waves, usually anteriorly directed currents. (2) P-P: both stimuli preferentially activated I3-waves, usually posteriorly directed currents. (3) P-A: the first stimulus evoked I3-waves and the second evoked I1-waves.
Surface EMG recordings
The threshold for each current direction was first determined using the averaged rectified EMGs for active muscles (average of at least 10 responses). The intensity of stimulation was changed in steps of 2 % of the maximum stimulator output. The threshold was defined as the lowest intensity that evoked a small response (∼50 μV) compared with the prestimulus background activity.
S1 was adjusted to evoke a response (control response) with an amplitude of approximately 0.2–0.4 mV peak to peak in the active FDI, which was 10–15 % above the threshold. Response latencies were measured from the superimposed responses. We confirmed that the latencies of control responses elicited by stimulation with the two selected currents were compatible with I1- or I3-waves. The intensity of S2 was 2, 7 or 12 % below the threshold for active muscles (−2, −7 and −12 %). A randomized conditioning-test design similar to that reported previously (Hanajima et al. 1996) was used. In short, various conditions (S1 or S2 given alone, or S2 preceded by S1 at various ISIs) were intermixed randomly in one block. Several blocks of trials were performed to investigate the complete time course of the studied effect. During this experiment the subjects maintained slight constant voluntary contraction of the target FDI (∼5 % of the maximum voluntary contraction), with the aid of an oscilloscope monitor.
ISIs between 0.6 and 2.4 ms (0.6, 0.8, 1.0, 1.2, 1.4, 1.5, 1.6, 1.8, 2.0, 2.2 and 2.4 ms) were used for the A-A and P-P combinations. For the P-A combination, ISIs between 3.0 and 5.4 ms (3.0, 3.2, 3.4, 3.6, 3.8, 4.0, 4.2, 4.4, 4.6, 4.8, 5.0, 5.2 and 5.4 ms) were used. Eight to ten responses were collected and averaged for each condition in which both stimuli were given, and 18 responses for the control condition in which the test stimulus was given alone. The amplitude of each single response in each condition was measured so that the amplitudes of the control and conditioned responses in the same block could be compared in each subject, using Student's t test corrected for multiple comparisons (Bonferroni's correction).
The ratio of the mean amplitude of the conditioned response to that of the control response was calculated for each condition in each subject. These individual mean ratios were then averaged to give a grand mean ratio. The time course of the effect was plotted with the grand mean ratio on the ordinate and the ISI on the abscissa. The time courses for different combinations of the conditioning and test stimuli were compared using a repeated measures analysis of variance (ANOVA) test and Tukey's method for post hoc analysis.
Single motor unit studies
The threshold of the first-recruited peak in the PSTH for each current direction was first determined by changing the intensity of stimulation in steps of 2 % of the maximum stimulator output. The threshold was defined as the lowest intensity that evoked a small peak in the PSTH. These thresholds were almost the same (difference < 5 % of maximum stimulator output) as those for surface EMG responses during a slight contraction.
The intensity of S1 was adjusted to produce 20–30 % firing probability, which was 10–15 % above the threshold, and S2 was set at 2 and 7 % below the threshold. Two kinds of PSTHs were recorded simultaneously. One was a PSTH evoked by S1 given alone (control PSTH); the other was a PSTH when both stimuli were given (conditioned PSTH). Control and conditioned trials were intermixed randomly by the computer until 100 trials had been collected for each condition. ISIs of 1.5 or 3.0 ms were used for the A-A and P-P combinations, because previous studies (Tokimura et al. 1996; Ziemann et al. 1998) and the present results from surface EMG recordings (see Results) showed that facilitation was very clear at around these two ISIs. For the P-A combination, an ISI of 3.5 ms was used because facilitation could be evoked at ISIs of approximately 3.5 ms in surface EMG recordings (see Results). A PSTH of unit discharges was constructed for each condition. The conditioned PSTH was compared with a control PSTH in the same session. These results were also compared between different combinations of stimulation currents.
RESULTS
Surface EMG recordings
Figure 1 shows an example of surface EMG responses in the P-P and A-A conditions. The top traces are control responses to S1 alone and have a peak-peak amplitude of ∼0.3 mV. The onset latency of the response elicited by a posteriorly directed current was 24.3 ms and corresponded to an I3-wave (left panel, top trace). An anteriorly directed S1 elicited a response with an onset latency of 20.9 ms, corresponding to an I1-wave (right panel, top trace). The intensity of S2 was fixed at 7 % below the threshold for the active FDI (−7 %). Responses to I3-waves were markedly enlarged when S2 was given at ISIs of 1.4, 1.6 and 1.8 ms (P < 0.05, Student's t test with Bonferroni's correction). Their onset latencies also corresponded to that of an I3-wave. The steep enlarged deflection at the onset of conditioned responses suggested that the earliest component of the response (I3-wave) was enhanced. In the case of responses to I1-waves (Fig. 1, right panel), the conditioned response was slightly larger than the control response only at an ISI of 1.6 ms (P < 0.05). The negative peak of the conditioned response at an ISI of 1.6 ms was later than the peak of the control response. Subtraction of the control response from the conditioned response at an ISI of 1.6 ms demonstrated that responses to combined stimulation became larger 1.7 ms later than the onset of the I1-wave. This could be compatible with an enhancement of I2-waves evoked by S1. A similar pattern was observed in all subjects.
Figure 1. Surface EMG responses from a single subject.
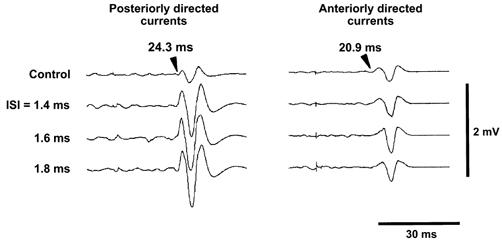
Responses were recorded from the right first dorsal interosseous muscle (FDI) with surface cup electrodes. Responses to posteriorly directed induced currents (I3-wave; left panel) and those to anteriorly directed induced currents (I1-wave; right panel) are shown. Control responses are shown at the top and responses to S1 followed by S2 with ISIs of 1.4, 1.6 and 1.8 ms below. The intensity of the conditioning stimulus was fixed at 7 % below the threshold for active muscles. Control responses were ∼0.3 mV in size. The onset latency of the control response to posteriorly directed induced currents was 24.3 ms, corresponding to an I3-wave, and that to anteriorly directed induced currents was 20.9 ms, corresponding to an I1-wave. Responses to I3-waves were much enlarged at all ISIs shown (Student's t test with Bonferroni's correction, P < 0.02). The shape of the enlarged responses (steep deflection at the onset of responses) suggests that the earliest component (I3-wave component) was enhanced. The amplitudes of responses to I1-waves were slightly increased at an ISI of 1.6 ms (Student's t test with Bonferroni's correction, P < 0.05). In this condition, enlargement occurred a few milliseconds later than the onset of responses, which indicates that enlargement was caused by an enhancement of I-waves occurring later than the I1-wave (see text).
Mean (± s.e.m.) time courses of the facilitatory effect elicited with different S2 are shown in Fig. 2. Responses to I3-waves, which were elicited by posteriorly directed stimuli, were enlarged at ISIs of 1.2–1.8 ms (P < 0.02, repeated measures ANOVA; ISI = 1.2, 1.4, 1.6 and 1.8 ms, Tukey's method; Fig. 2A). Responses to I1-waves produced by anteriorly directed stimuli were also enlarged at ISIs of 1.4–1.6 ms, when the intensity of S2 was 2 and 7 % below the threshold (P < 0.02, repeated measures ANOVA; Fig. 2B). The facilitation was greater for I3-waves than for I1-waves. In both cases, the stronger the second stimulus, the greater and longer the facilitation. The greatest facilitation occurred at ISIs of ∼1.5 ms.
Figure 2. Mean (± s.e.m.) time courses of the I-wave facilitation evaluated with surface EMG recordings.
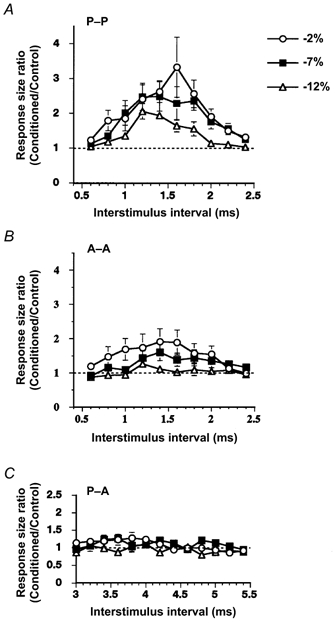
Abscissae indicate the ISIs, and ordinates the mean size ratios for all the subjects. Three time courses for different intensities of S2 (2, 7 and 12 % below the active threshold: −2, −7 and −12 %) are shown. A, S1 and S2 were magnetic stimuli inducing posteriorly directed currents in order to elicit preferentially I3-waves. At ISIs of 1.2–1.8 ms, the facilitatory effect was evoked with a conditioning stimulus of any intensity (P < 0.02). The stronger the conditioning stimulus (S2), the greater the facilitation. B, S1 and S2 were magnetic stimuli inducing anteriorly directed currents eliciting I1-waves. The facilitatory effect was evoked at ISIs of 1.4 and 1.6 ms when the intensity of the conditioning stimulus was fixed at 2 or 7 % below the threshold (P < 0.02). No facilitation was evoked with a conditioning stimulus set at 12 % below the active threshold. C, S1 was posteriorly directed and S2 anteriorly directed. Slight facilitation was evoked at ISIs of 3.6–4.0 ms by the conditioning stimulus at an intensity of 2 and 7 % below the threshold. The peak of facilitation appeared to be smoothed out because the best ISI for facilitation was different from subject to subject.
In the P-A condition, facilitation was evoked at ISIs of 3.6–4.0 ms in each subject. However, the amount of facilitation was less than that in the other two conditions and the ISI for the largest facilitation was different from subject to subject. The mean time course, therefore, showed only a slight facilitation (P < 0.05 for average (3.6–4.0 ms); Fig. 2C).
In order to examine whether this facilitation occurred at the cortical level, we studied the effect of replacing S2 with an electrical stimulus on responses to a posteriorly directed S1. No facilitation was evoked at ISIs of 1.0- 7.0 ms (data not shown). This indicates that no facilitation occurred even at ISIs compensated for the latency difference between I3- and D-waves (1.5 + 4.5 = 6.0 ms).
Single motor unit recordings
When S2 was set at 2 % below the threshold (−2 %), units other than the target unit were often activated in the conditioned trials when S1 and S2 were given together. This made it difficult for subjects to maintain the discharge of the selected single motor unit. Therefore, in the main set of experiments, we fixed the intensity of S2 at 7 % below the threshold (−7 %).
Control and conditioned PSTHs from the same unit are shown in Fig. 3A for the P-P combination. A posteriorly directed S1 evoked one peak at ∼4.5 ms later than that evoked by TES (the vertical dotted line indicates the latency of the peak produced by TES: D-wave; upper PSTH; firing probability, 20/100). This latency was appropriate for an I3-wave. A posteriorly directed S2 at an intensity of −7 % given 1.5 ms later than S1 facilitated the I3-peak (lower PSTH; firing probability, 31/100). Similar results were seen in all units studied. The mean (± s.e.m.) firing probability in the I3-peak was 0.20 ± 0.03 when S1 was given alone, and 0.36 ± 0.04 when S1 and S2 were given at an ISI of 1.5 ms. There was some facilitation even at an ISI of 3.0 ms (not shown), when the mean (± s.e.m.) firing probability in the I3-interval was 0.30 ± 0.08.
Figure 3. Post-stimulus time histograms (PSTHs) under different combinations of the first (S1) and second (S2) stimulus.
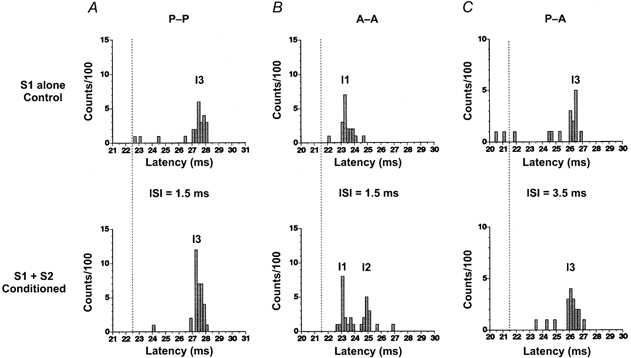
Upper panels, control PSTHs when S1 was given alone. Lower panels, conditioned PSTHs when S1 and S2 were given. ISIs were 1.5 ms in A and B, and 3.5 ms in C. Each PSTH was constructed from 100 trials. Abscissae show the latency after the test stimulus (S1). Vertical dotted lines indicate the latency of the D-wave in each subject. In A, both stimuli (S1 and S2) were posteriorly directed currents (P-P). In the control PSTH, a single peak was elicited 4.5 ms later than the D-wave (dotted line). This peak was compatible with an I3-wave. In the conditioned PSTH, the single peak of the I3-wave was enlarged by S2 and no later peaks were evoked. In B, S1 and S2 were anteriorly directed currents (A-A). In the control PSTH, the anteriorly directed stimulus produced a single peak 2 ms later than the D-wave. This peak corresponded to an I1-wave. When S2 was given 1.5 ms after S1 (conditioned PSTH), two peaks corresponding to the I1- and I2-wave from S1 were elicited. The size of the I1-peak of the conditioned PSTH was the same as that of the control PSTH. In C, S1 was a posteriorly and S2 an anteriorly directed current (P-A). In the control PSTH, the posteriorly directed stimulus produced a single peak 4.5 ms later than the D-wave (I3-wave). In the conditioned PSTH, the peak at the latency of the I3-wave from S1 was enlarged.
Figure 3B shows control and conditioned PSTHs in the A-A combination in another subject. An anteriorly directed test stimulus (S1) elicited one peak with a latency ∼1.5 ms later (I1-wave) than the D-wave (upper PSTH; firing probability, 17/100). When an anteriorly directed S2 (−7 %) was given 1.5 ms after S1, two peaks could be seen (lower PSTH). Their latencies corresponded to the original I1-wave from S1 (firing probability, 16/100) plus an additional I2-wave (firing probability, 11/100). The same effect was seen in all units studied. The mean (± s.e.m.) firing probability of the I1-peak obtained from all motor units was 0.19 ± 0.04 when S1 was given alone and 0.18 ± 0.05 when S1 was combined with S2 at an ISI of 1.5 ms (P > 0.1, Student's paired t test). That of the I2-peak was 0.20 ± 0.04. When the ISI was 3 ms there were no later peaks and the size of the I1 peak remained unchanged (firing probability, 0.20 ± 0.05; data not shown).
Figure 3C shows the control and conditioned PSTHs in the P-A condition in the same subject as in Fig. 3B. In the control PSTH (S1 alone), only one peak was evoked some 4.5 ms later (26.2 ms) than the D-wave (dotted line, upper PSTH; firing probability, 10/100). The same peak was enlarged by an anteriorly directed S2 at an ISI of 3.5 ms (lower PSTH; firing probability, 14/100). A similar facilitation was seen in all motor units studied. The mean (± s.e.m.) firing probability was 0.21 ± 0.07 when S1 was given alone and 0.37 ± 0.14 when both stimuli were given.
DISCUSSION
We studied intracortical I-wave facilitation in detail using single motor unit and surface EMG recordings. One of our aims was to investigate differences in the facilitation using different directions of stimulating current, which recruit different I-waves. Our new findings are summarized as follows. (1) In the P-P and A-A conditions, the largest I-wave facilitation occurred at ISIs of around 1.5 ms. In the P-P condition, facilitation affected the I3-peak produced by S1. In contrast, in the A-A condition, facilitation was produced by addition of an I2-peak to the original I1-peak evoked by S1. (2) The amount of facilitation was greater for I3-waves than for I1-waves. (3) In the P-A condition, I-wave facilitation was elicited at ISIs of ∼3.5 ms. Unfortunately, we could not determine what occurred at an ISI of 1.5 ms in this condition because we could not reverse coil currents at ISIs shorter than 3.0 ms.
Previous work (Tokimura et al. 1996; Nakamura et al. 1997b; Ziemann et al. 1998; Rothwell, 1999) suggested that I-wave facilitation occurs at a cortical level. In some previous experiments, the intensity of the second stimulus was fixed at 80 % of the threshold for relaxed muscles. This intensity was sometimes above the threshold for active muscles. In the present experiments, we set the intensity of the conditioning stimulus at 7 % below the threshold for active muscles. Since no descending volleys are elicited by such a low intensity conditioning stimulus, excitability changes should never occur at the spinal level, which strongly supports the notion that the facilitation occurs in the cortex. Our finding that an electrical conditioning stimulus did not evoke facilitation is consistent with previous reports (Tokimura et al. 1996; Nakamura et al. 1997b; Ziemann et al. 1998; Rothwell, 1999; Di Lazzaro et al. 1999) and also supports this notion.
The present PSTH results show that facilitation in the A-A condition is produced by an addition of later I-waves to those evoked by S1 alone, and are consistent with the previous report by Di Lazzaro et al. (1999). However, there is one discrepancy between our results and theirs. In conditioned trials, they measured the latency of descending volleys from S2, but we measured it from S1. Therefore, our I2-waves should be their I1-waves, our I3-waves their I2-waves and so on. They reported facilitation of I2- and I3-waves (our I3- and I4-waves), but we did not see any I3- or I4-waves in the A-A condition. It seems likely that differences in the intensity of S1 and S2 between these two studies are the reason for this difference. We used much lower intensities for S2 (7 % below the active threshold) than those used in their study (about the relaxed threshold). Higher conditioning stimuli may cause summation of the effects of S1 and S2 at several interneurones (see below) and be capable of producing several I-waves.
Another point that should be considered before discussion of possible mechanisms is why facilitation did not occur at an ISI of 3 ms in the A-A condition, although it was clear at that ISI in the P-P condition. Ziemann et al. (1998) reported that facilitation at an ISI of 1.5 ms was evoked by a low intensity S1, but that at an ISI of 3 ms was evoked only by a strong S1. In the A-A condition, we used lower intensities for S1 and S2 than in the P-P condition. This may explain why facilitation at an ISI of 1.5 ms was observed in both the P-P and A-A conditions, but at an ISI of 3 ms in only the P-P condition. The fact that we recorded from active muscles may also contribute to our failure to elicit facilitation at an ISI of 3 ms in the A-A condition since facilitation is smaller in active muscles (Ziemann et al. 1998).
The other point that should be considered before discussion of possible mechanisms for facilitation is which descending volleys are elicited by differently directed induced currents. Recordings of descending volleys (Di Lazzaro et al. 2001) have recently shown that preferential activation of different I-waves does not occur as often as reported by Sakai et al. (1997). These authors (Di Lazzaro et al. 2001) explained that the discrepancy probably occurred because they did not explore the eight different orientations of a coil in their experiments. They elicited surface EMG responses at the latency of an I3-wave by posteriorly directed induced currents in one of four subjects (subject 1) and no good late EMG responses in the other three subjects. In subject 1, the results of descending volleys were consistent with preferential activation of different I-waves proposed by Sakai et al. (1997). Because we carefully chose current directions for eliciting I1- and I3-waves and evoked EMG responses at the latency of I3-waves in all subjects, the descending volleys produced by the currents we selected should be the same as those seen in subject 1 of the study of Di Lazzaro et al. (2001). Therefore, hereafter we discuss the mechanisms of the I-wave facilitation based on our previous assumption that a certain I-wave is preferentially (not purely) produced by induced currents flowing in a certain direction (Sakai et al. 1997).
Based on our present results, we speculate that the mechanisms underlying intracortical I-wave facilitation are as follows. Preferential activation of an I1- or I3-wave does not mean that there is pure activation of one selected I-wave. Since stimulation at slightly higher intensities elicits several descending volleys (Hanajima et al. 1998; Di Lazzaro et al. 2001), we presume that some axons and neurones involved in producing other I-waves are also subliminally depolarized. Thus, in the P-P condition, a posteriorly directed test stimulus alone may subliminally depolarize motor cortical interneurones and their axons which are involved in the generation of several I-waves (I1, I2 and I3) even though it may only discharge I3-waves. A posteriorly directed S2 stimulus will also subliminally depolarize neurones and axons involved in the production of I1-, I2- and I3-waves. When S1 is given alone, some of the axons involved in producing I3-waves are discharged (Fig. 4A) and they induce excitatory postsynaptic potentials (EPSPs) at their target neurones (perhaps, for example, neurones involved in I2-wave production) ∼1.5 ms later. Some of the target neurones may be activated by these EPSPs, but some will be subliminally depolarized by their EPSPs and not discharged (Fig. 4B). If S2 is given 1.5 ms after S1, then it will probably be unable to discharge any axons activated by S1 since those axons will be in the refractory period (Ammasian et al. 1998). Any axons that were not activated by S1 should be discharged by S2 because S2 is weaker than S1. Therefore, we propose that S2 produces motor evoked potential (MEP) facilitation by directly activating axons or neurones that have been depolarized but not fired by S1. S2 may be able to produce direct depolarization of the subliminally activated target neurones of S1. Summation here could depolarize additional neurones to those activated by S1 alone and lead to facilitation (Fig. 4D). This summation most probably occurs at the initial segment or first node of neurones as proposed by other investigators (Amassian et al. 1990, 1998; Deletis et al. 2001). The latency of the effect would be the same as that of I3-waves activated by S1. Similar summation did not occur at the latency of I2-waves from S1. This result can be explained as follows. S1 preferentially activates axons and interneurones involved in producing I3-waves. None or very few of those involved in producing I2-waves are activated. Therefore, summation between S1 and S2 does not occur at the latency of I2-waves. We suggest that, if a stronger S1 that could elicit small I2-waves is used, then facilitation at I2-wave latency should occur when S2 is given.
Figure 4. Our hypothesis for the mechanisms involved in intracortical I-wave facilitation.
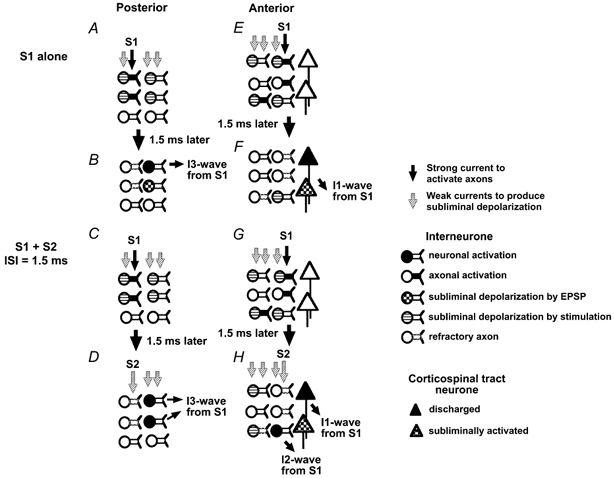
The proposed mechanisms for the P-P condition are shown on the left, and those for the A-A condition on the right. A, B, E and F show the generation of I-waves when S1 is given alone, and C, D, G and H the generation of I-waves when both S1 and S2 are given. In the P-P condition (left), a posteriorly directed S1 activates axons of motor cortical interneurones involved in production of I3-waves and subliminally depolarizes some interneurones. Approximately 1.5 ms later these axons induce excitatory postsynaptic potentials (EPSPs) at their target neurones (perhaps neurones involved in I2-wave production). Some of the target neurones are activated by these EPSPs (B, top interneurone), but some will be subliminally depolarized by their EPSPs and will not discharge (B, middle interneurone). If S2 is given 1.5 ms after S1 (D), there will probably be no resulting discharge of axons activated by S1 since those axons will be in the refractory period (D, top and middle axons, described by dotted lines). S2 may be able to produce direct depolarization of the subliminally activated target neurones of S1 (D, middle interneurone). Summation here could depolarize neurones additional to those activated by S1 alone and lead to facilitation. In this illustration, two interneurones take part in the generation of I3-waves in the S1 + S2 condition although only one interneurone does so when S1 is given alone. The latency of the effect would be the same as that of an I3-wave from S1. In the A-A condition (right), a suprathreshold S1 activates axons of motor cortical interneurones involved in I1-wave generation (E, top and middle interneurones) and a small number of interneurones involved in I2-wave production (bottom interneurone). They produce EPSPs at their target neurones (corticospinal tract neurones or interneurones for I1-waves) ∼1.5 ms later (F). Some corticospinal tract neurones are discharged and produce I1-waves (F, top corticospinal tract neurone), while others are subliminally depolarized but not discharged (F, bottom corticospinal tract neurone) and others are not influenced by S1. The S2 stimulus subliminally depolarizes interneurones especially those for I1-waves (H). When S1 and S2 are given at an ISI of 1.5 ms, axons of motor cortical interneurones involved in the generation of the I1-wave are activated in the same manner as when S1 is given alone (H, top corticospinal tract neurone). Summation between subliminal depolarization by EPSPs by S1 and direct depolarization by S2 induces no extra activation of corticospinal tract neurones (H, bottom corticospinal neurone) because small EPSPs are evoked at the corticospinal tract neurones due to their large size and slight direct depolarization is elicited in the corticospinal tract neurones due to their deep position. Because of the refractory period (dotted axons), S2 cannot activate any axons discharged by S1, but it could produce direct depolarization of some interneurones that had been subliminally facilitated by EPSPs released by S1. Summation occurs at the target interneurones of I2-waves (H, bottom row of interneurones), and it makes the peak with the latency compatible with I2-waves from S1.
In the A-A condition, a suprathreshold S1 activates axons of motor cortical interneurones for I1-waves and some of those for I2-waves because anteriorly directed currents favour recruitment of I1-waves more than I2-waves, (Fig. 4E and F). The S2 stimulus also subliminally depolarizes interneurones for I1-, I2- and I3-waves, especially those for I1-waves. When S1 and S2 are given at an ISI of 1.5 ms, axons of motor cortical interneurones involved in the generation of the I1-wave from S1 are activated as usual (Fig. 4G and H). Because of the neuronal refractory period, S2 cannot activate any axons discharged by S1, but it could produce direct depolarization of some neurones that had been subliminally facilitated by EPSPs released by S1. If these neurones had been the target of I1-EPSPs (e.g. corticospinal neurones), then facilitation of the response to S1 + S2 would have occurred at I1 latency (from S1). This was not the case, facilitation occurred at I2 latency. One possible explanation for this phenomenon is that S2 is not able to produce or only slightly produces direct depolarization of the corticospinal neurones because they are in a deeper layer than the interneurones. Another possible reason for this phenomenon is that a greater depolarization should be produced by S2 in smaller interneurones than in larger corticospinal neurones as suggested by previous reports (Amassian et al. 1990, 1998; Deletis et al. 2001). From this we conclude that S2 facilitation occurs at those neurones responsible for I2-waves rather than those responsible for I1-waves (Fig. 4H).
In the P-A condition, we could only explore ISIs longer than 3.0 ms because of limitations in the design of the equipment used. In this situation, facilitation occurred only at the latency of the I3-wave from S1. The fact that smaller facilitation was evoked at ISIs of 3–4 ms in the P-A condition than in the P-P condition may be due to the fact that S2 was weaker in the P-A condition than in the P-P condition. We cannot make firm conclusions about the mechanisms involved in facilitation in the P-A condition because we have no data at an ISI of 1.5 ms. However, it seems likely that if the interneuronal systems for anteriorly directed currents and those for posteriorly directed currents are the same, a similar type of summation between EPSPs produced by S1 and direct depolarization elicited by S2 may occur at common interneurones. If both systems are completely or partly independent, summation of independent EPSPs occurs at either the common interneurones or the corticospinal neurones. The latter possibility has recently been suggested by Di Lazzaro et al. (2001). The interindividual variability of the best ISI for facilitation in this condition is probably due to variable latency differences between I1- and I3-waves from subject to subject.
Why were I3-waves more facilitated than I1-waves? I3-waves are also more influenced by the intracortical inhibition of the motor cortex than I1-waves (Hanajima et al. 1998; Di Lazzaro et al. 1999). We propose that I3-waves are more susceptible to cortical excitability changes than I1-waves, irrespective of whether this is inhibitory or facilitatory. This is partly because the generation of I3-waves may include more synapses in the motor cortex than the generation of I1-waves. If so, then the system producing I3-waves will be more easily modulated by cortical excitability than that for earlier I-waves. An additional fact is that S1 and S2 were stronger in the P-P condition than in the A-A condition because of the higher threshold for I3-waves. This might lead to greater subliminal depolarization of cortical neurones by S2, and contribute to the larger effect on I3-waves. If the motor cortical interneuronal circuits producing I1- and I3-waves are completely independent, the circuit responsible for I3-waves may be much more susceptible to cortical excitability than that for I1-waves.
Using PSTH studies, we have shown different patterns of facilitation of I1- and I3-waves. We propose that if both stimuli have the same direction, MEP facilitation is produced by S2 activating directly those interneurones at their initial segment or first node which have been trans-synaptically depolarized but not activated by S1.
Acknowledgments
Part of this work was supported by a grant from the Life Science Foundation of Japan and Research Project Grant-in-aid for Scientific Research No.12680768 from the Ministry of Education, Science, Sports and Culture of Japan. We are very grateful to Professor J. C. Rothwell (London, UK) for kindly editing our English and for helpful comments on this work.
REFERENCES
- Amassian VE, Quirk GJ, Stewart MA. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalography and Clinical Neurophysiology. 1990;77:390–401. doi: 10.1016/0168-5597(90)90061-h. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Rothwell JC, Cracco RQ, Maccabee PJ, Vergara M, Hassan N, Eberle L. What is excited by near-threshold twin magnetic stimuli over human cerebral cortex? Journal of Physiology. 1998;506.P:122–123P. [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Deletis VV, Isgum VV, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in electrical stimuli. Clinical Neurophysiology. 2001;112:438–444. doi: 10.1016/s1388-2457(01)00461-8. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturuno E, Pilato F, Insola A, Mazone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Experimental Brain Research. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Experimental Brain Research. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Massone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Experimental Brain Research. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. Journal of the Neurological Sciences. 1996;140:109–116. doi: 10.1016/0022-510x(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. Journal of Physiology. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Cortico-cortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1997a;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Trompertto A, Maruyama A, Rothwell JC. Very short facilitation in motor cortex produced by pairs of threshold magnetic stimuli. Electroencephalography and Clinical Neurophysiology. 1997b;103(suppl.):195. [Google Scholar]
- Rothwell JC. Paired-pulse investigations of short-latency intracortical facilitation using TMS in humans. In: Paulus W, Hallett M, Rossini PM, Rothwell JC, editors. Transcranial Magnetic Stimulation, Electroencephalography and Clinical Neurophysiology. suppl. 51. Amsterdam: Elsevier Science B.V.; 1999. pp. 113–119. [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-eight shaped coil. Experimental Brain Research. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalography and Clinical Neurophysiology. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. Journal of Physiology. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


