Abstract
Assessment of fluctuations in heart rate (HR) following a premature ventricular complex (PVC) is valuable for identifying patients at high risk of sudden cardiac death. We hypothesised that postextrasystolic potentiation is the main determinant of the regulation patterns of blood pressure (BP) and HR following a PVC. Twelve patients with idiopathic dilated cardiomyopathy (IDC) and 13 control subjects with single PVCs (comparable coupling intervals) were investigated. Non-invasive finger arterial BP and ECGs were analysed. Regulation patterns following a single PVC were quantified using the indices postextrasystolic amplitude potentiation (PEAP) and maximum turbulence slope of five consecutive mean BP values (MBP-TS), and compared with the HR turbulence parameters turbulence slope (HR-TS) and turbulence onset (HR-TO). PEAP was significantly higher in IDC patients compared to controls (48.7 ± 32.6 vs. 9.8 ± 5.4 %, P < 0.01), whereas MBP-TS was lower (0.97 ± 0.60 vs. 2.07 ± 1.04 mmHg BBI−1 (BBI, beat-to-beat interval), P < 0.05), as was HR-TS (8.46 ± 7.90 vs. 30.73 ± 22.90 ms BBI−1, P < 0.01). HR-TO was significantly higher in IDC patients (−0.56 ± 2.19 vs. −5.52 ± 4.13 %, P < 0.01). In addition, the regulation patterns of BP and HR following a single PVC differed significantly between IDC patients and controls. Specifically, we observed pronounced PEAPs in IDC patients. The baroreflex response initiated by the low pressure amplitude of the PVC was suppressed in IDC patients due to the augmented potentiation of the first postextrasystolic blood pressure. Furthermore, IDC patients displayed impressive postextrasystolic pulsus alternans phenomena, whereas healthy subjects exhibited a typical baroreflex pattern. The pulsus alternans phenomenon seems to be triggered by a PVC.
Premature ventricular contractions (PVCs) result in a deviation from the pressure set point of the baroreceptor reflex for at least one beat. The ejection of a lower stroke volume by the extrasystolic beat into the aorta induces a lower extrasystolic pulse wave amplitude and, thus, causes reduced distension of arterial pressoreceptors. The diastolic pressure falls due to a reduced filling of the arterial vascular compartment. Assuming that the premature beat is followed by a compensatory pause, postextrasystolic potentiation (PESP) (Yamazoe, 1987; Cooper, 1993) should occur. PESP is the phenomenon of augmented myocardial contractility immediately following a premature contraction and has been shown to be a predictor of myocardial viability and residual function in ischaemic heart disease (Dyke et al. 1974). The precise mechanism underlying PESP is unknown. It has been suggested that the augmented contractility might be due to the increased availability of intracellular Ca2+ (Suko et al. 1970), changes in preload and afterload (Sung et al. 1980) and activation of the sympathetic nervous system (Geschwind et al. 1984). Augmented myocardial contractility results in increased stroke volume and outflow velocity. Paradoxically, several studies have reported increased PESP in cardiomyopathic ventricles (Welch et al. 1989), which is in conflict with the fact that PESP is a marker of viable myocardium in coronary artery disease.
The regulation of postextrasystolic pressure may be influenced by two different effects. First, the pressure amplitude of the postextrasystolic beat compensates for the reduced diastolic starting pressure caused by the PVC; therefore, the changed baroreceptor response initiated by the PVC is abolished. Second, if the pressure amplitude of the postextrasystolic beat is less potentiated and does not compensate for the reduced diastolic starting pressure, the baroreflex response continues. Thus, both the extrasystolic and postextrasystolic pressure determine the regulation pattern following a PVC.
PVC response analysis has been applied successfully to risk stratification after myocardial infarction, to which the novel risk predictors turbulence onset and turbulence slope (Schmidt et al. 1999) were introduced. Davies and colleagues (Davies et al. 2001) assessed the heart rate turbulence slope (HR-TS) as well as the blood pressure turbulence slope (BP-TS) in patients with chronic heart failure. They calculated the baroreflex sensitivity (BRS) from the ratio of the HR-TS and BP-TS and found that HR turbulence is an effective measure of the baroreflex.
The present study was carried out to investigate the postextrasytolic regulation of BP and HR in patients with idiopathic dilated cardiomyopathy (IDC). We hypothesised that IDC patients and healthy subjects would exhibit different regulation patterns due to altered postextrasystolic BP potentiation.
METHODS
Patients
This investigation conforms with the principles outlined in the Declaration of Helsinki. Local ethics committee approval and informed consent of all subjects were obtained.
Twelve patients with IDC and 13 healthy patients (control group) were used in the study (Table 1). The healthy state of the controls was confirmed by history, physical examination and 12-lead ECG. The diagnosis of IDC was confirmed by cardiac catheterization and endomyocardial biopsy. In IDC patients, the mean left ventricular ejection fraction was 26.4 ± 12.3 %, mean New York Heart Association (NYHA) functional class was 2.6 (range I-IV) and mean left ventricular end-diastolic diameter (measured by echocardiography) was 70.1 ± 6.2 mm. All IDC patients received the best medical treatment, including treatment with angiotensin-converting enzyme (ACE) inhibitors and in seven cases β-adrenoceptor blockers (betablockers).
Table 1.
Characterisation of IDC patients and healthy subjects (Control)
| IDC | Control | |
|---|---|---|
| Number | 12 | 13 |
| Age (years) | 52.5 ± 11.7 | 50.4 ± 10.3 |
| Sex (m/f) | 8/4 | 9/4 |
| NYHA (I–IV) | 2.6 ± 0.8 | — |
| LVEF (%) | 26.4 ± 12.3 | — |
| LVEDD (mm) | 70.1 ± 6.2 | — |
| QRS (ms) | 144.0 ± 23.4 | 90.7 ± 12.5 |
All parameters are means ±s.d. Abbreviations: NYHA (I–IV), New York Heart Association functional class (range I–IV); LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; QRS, mean duration of the QRS complex.
Data acquisition and preprocessing
A high resolution (2000 Hz sampling frequency) ECG and non-invasive continuous BP recording (Imholz et al. 1998) were obtained simultaneously for 30 min in all subjects under standardised resting conditions (Fig. 1). BP was recorded with a Portapres (Model 2, Biomedical Instrumentation TPD-TNO, Amsterdam, The Netherlands). ECGs were examined by eye to detect single PVCs that were followed by fully compensatory pauses.
Figure 1.
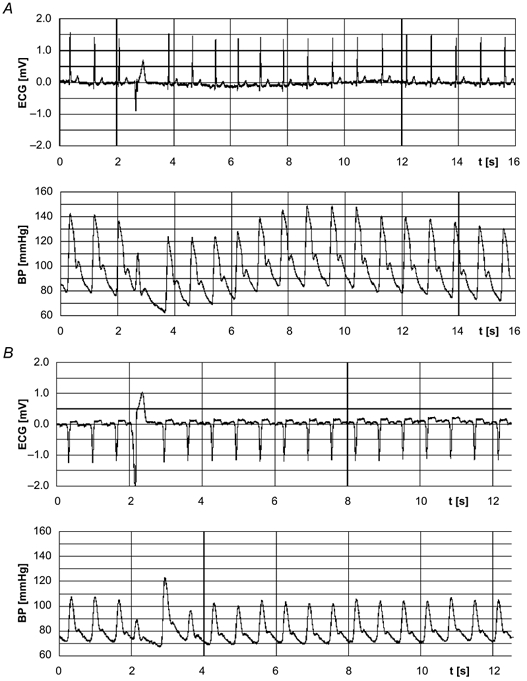
Non-invasively recorded arterial finger blood pressure (BP) curves and the respective ECGs with a single premature ventricular complex (PVC) in a control subject (A) and a patient with idiopathic dilated cardiomyopathy (IDC; B).
Data analysis
Systolic, mean and diastolic BP, BP amplitude and HR following a PVC were investigated in relation to reference values that represented the set point of the baroreflex regulation. The last normal BP value and beat-to-beat interval (BBI) prior to a PVC were the reference values. The relative deviation from the reference (expressed as a percentage) of the three beats preceding and 15 beats following the PVC was quantified (Fig. 2 and Fig. 4). These values were averaged for each subject in both groups. In total, we investigated 130 PVCs in 12 IDC patients (range: 1–36 PVCs per patient) and 30 PVCs in 13 control patients (range: 1–15 PVCs per patient).
Figure 2. Postextrasystolic BP regulation patterns (mean deviation from reference for each group) for the control (top) and IDC (bottom) groups.
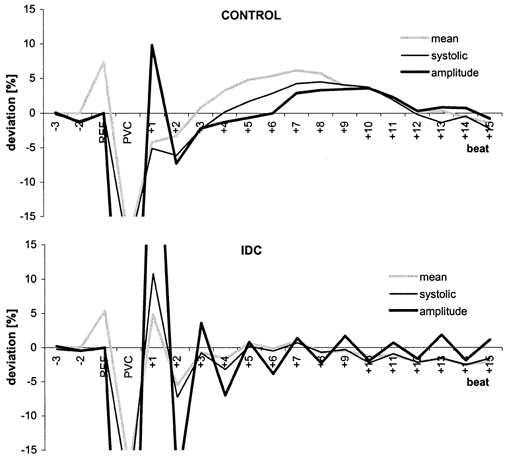
Beat−1 is the reference (REF) for systolic BP (thin black line) and BP amplitude (thick black line) and beat−2 is the reference for mean BP (grey line).
Figure 4. Postextrasystolic regulation patterns (mean deviation from reference for each group) of the systolic BP (SBP, grey line) and the heart rate (HR, black line) in controls (top) and IDC patients (bottom).
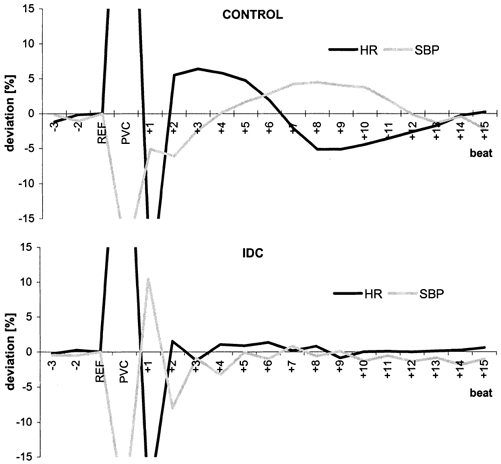
In controls, a typical baroreflex response was apparent, whereas in IDC patients, the baroreflex response was abolished due to the pronounced postextrasystolic potentiation (PESP).
To quantify the postextrasystolic regulation of the ECG and BP, we calculated four parameters, as follows. First, based on our hypothesis, we used the relative deviation of the first normal BP amplitude after a PVC to define the postextrasystolic amplitude potentiation (PEAP, as a percentage):
where AMP+1 is the first normal BP amplitude after a PVC and AMPREF is the last normal BP amplitude before the PVC.
Second, the turbulence onset (HR-TO) and turbulence slope (HR-TS) of the HR were calculated from the ECG as in Schmidt et al. (1999). HR-TO was defined as the difference between the first two BBIs following a PVC and the two BBIs immediately prior to the PVC. HR-TS was defined as the slope of the steepest regression line calculated over any sequence of five consecutive BBIs within the first 15 sinus rhythm intervals after a PVC.
Third, for BP analysis, we introduced a corresponding turbulence slope parameter, MBP-TS, that represented the maximum positive slope of a regression line fitted to five consecutive mean BP values within the first 15 sinus rhythm intervals after a PVC.
Only PVCs with fully compensatory pauses were included in the analysis. A PVC with a fully compensatory pause was defined as having a difference (Δt) of less than 150 ms between the sum of the extrasystolic (BBIPVC) and postextrasystolic (BBI+1) coupling intervals and the 2-fold cycle length of the reference value (BBIREF):
Validation of the non-invasive method
To validate the non-invasive method of measuring BP, simultaneous invasive and non-invasive BP recordings of 41 premature beats from 10 intensive care patients were analysed. For both methods, the mean values of systolic BP, mean BP, BP amplitude and diastolic BP were calculated and statistically compared.
Statistics
Student's two-tailed t test was used for statistical evaluation of differences between the groups and Student's paired two-tailed t test was used for within group comparisons. The Kolmogorov-Smirnov test was used to ensure that data were normally distributed. Using Pearson's correlation coefficients, we determined the correlation of the postextrasystolic response with clinical variables and the interdependency of BP and HR parameters. Univariate discriminant function analysis was applied to determine how effectively the groups were discriminated by our parameters.
RESULTS
Reference values and coupling intervals of PVC
Systolic BP and the BP amplitude of the reference beats (Table 2) were lower in IDC patients compared to controls (102.8 vs. 122.0 mmHg, P = 0.05; 42.9 vs. 59.0 mmHg, P = 0.04).
Table 2.
Reference values, blood pressure potentiation (PESP) and premature ventricular complex (PVC)characteristics in IDC patients and healthy subjects (Control)
| Measurements | IDC | Control |
|---|---|---|
| Reference | ||
| HR (bpm) | 77.4 ± 11.7 | 73.1 ± 11.1 |
| SBP (mmHg) | 102.8 ± 13.9 | 122.0 ± 22.6* |
| MBP (mmHg) | 74.8 ± 10.9 | 81.9 ± 15.9 |
| DBP (mmHg) | 66.4 ± 11.7 | 70.5 ± 13.2 |
| AMP (mmHg) | 42.9 ± 10.4 | 59.0 ± 12.6* |
| PESP | ||
| SBP+1 (%) | 10.77 ± 9.72 | −5.11 ± 1.92** |
| MBP+1 (%) | 4.83 ± 5.96 | −4.24 ± 2.38** |
| DBP+1 (%) | −0.09 ± 0.05 | −0.16 ± 0.04** |
| AMP+1 (PEAP)(%) | 48.70 ± 32.6 | 9.80 ± 5.54** |
| PVC | ||
| Coupling interval (s) | 0.55 ± 0.10 | 0.52 ± 0.08 |
| Compensatory pause (s) | 1.02 ± 0.19 | 1.13 ± 0.20 |
All parameters are means ±s.d. Abbreviations: HR, heart rate; SBP, systolic blood pressure; MBP, mean BP; DBP, diastolic BP; AMP, BP amplitude.
P <0.05
P <0.01.
The mean BP (74.8 vs. 81.9 mmHg, P = 0.30), mean reference HR (77.4 vs. 73.1 beats min−1 (bpm), P = 0.36), coupling interval (0.55 vs. 0.52 ms, P = 0.43) and duration of the compensatory pause (1.02 vs. 1.13 ms, P = 0.20) did not differ significantly between the two groups (Table 2).
The systolic and mean BP of PVCs were significantly (P < 0.001) lower than the reference values, both in IDC patients (−21.5 vs. −17.7 %) and in controls (−20.1 vs. −18.7 %).
PESP and turbulence analyses
The BP of the postextrasystolic beat differed significantly between groups (Table 2, Fig. 2). In comparison to the reference values, systolic and mean BP were higher in IDC patients (10.8 %, P = 0.006 and 4.8 %, P = 0.03) and lower in controls (−5.1 %, P = 0.001 and −4.2 %, P = 0.003). In controls, the group mean, like the individual measurements, revealed a lower systolic BP. In both groups, diastolic BP was only slightly below the reference values (IDC, −0.09 %; controls, −0.16 %; P < 0.01).
The four turbulence parameters, PEAP, MBP-TS, HR-TO and HR-TS, were influenced mainly by the postextrasystolic BP potentiation and differed significantly between the two groups. PEAP was significantly higher in IDC patients compared to controls (48.7 ± 32.6 vs. 9.8 ± 5.4 %, P < 0.01). MBP-TS was significantly lower in IDC patients (0.97 ± 0.6 vs. 2.07 ± 1.04 mmHg BBI−1, P < 0.05), as was HR-TS (8.46 ± 7.9 vs. 30.73 ± 22.9 ms BBI−1, P < 0.01). HR-TO was significantly higher in IDC patients compared to controls (−0.56 ± 2.19 vs. −5.52 ± 4.13 %, P < 0.01).
Each of the four turbulence parameters was correlated with the mean left ventricular end-diastolic diameter (Table 3). In addition, MBP-TS, HR-TO and HR-TS were also correlated with left ventricular ejection fraction (Table 3). None of the turbulence parameters was significantly correlated with clinical parameters, such as QRS duration, body surface area or patient age. Interestingly, PEAP was the only applied turbulence parameter that did not correlate with the others in both the IDC and control groups. The results of the univariate discriminant function analysis (Table 3) show that HR-TO discriminates best between normal and pathological regulation. However, PEAP is the only parameter that accurately classifies all healthy subjects (100 % positive predictive accuracy).
Table 3.
Univariate discriminant function analysis
| Sens | Spec | PPA | NPA | Class | |
|---|---|---|---|---|---|
| PEAP | 60 | 100 | 100 | 64 | 77 |
| MBP-TS | 80 | 57 | 73 | 67 | 71 |
| HR-TS | 92 | 54 | 65 | 88 | 72 |
| HR-TO | 92 | 77 | 79 | 91 | 84 |
All values are percentages. Abbreviations: Sens, sensitivity; Spec, specificity; PPA, positive predictive accuracy; NPA, negative predictive accuracy; Class, correct classification; MBP-TS, turbulence slope of the mean BP; HR-TS, turbulence slope of the HR; HR-TO, turbulence onset of the HR.
In the four IDC patients with a mean left ventricular end-diastolic diameter smaller than 70 mm, PEAP (14.9, 18.7, 16.1 and 18.7 %) was similar to the controls (9.8 ± 5.4 %). In these four patients, the mean left ventricular end-diastolic diameter was 63.3 ± 5.3 mm, compared to 74.2 ± 2.7 mm for the remaining IDC patients. In two of these four IDC patients the left ventricular ejection fraction was 50 %, whereas in IDC patients with a PEAP greater than 30 %, the left ventricular ejection fraction was < 32 %. Furthermore, the analysis of the betablocker-treated subgroup showed no substantial effects of betablockers on either PESP or the turbulence parameters (Fig. 3).
Figure 3. Influence of betablockers on PESP and turbulence parameters.
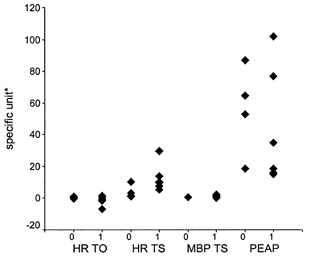
None of the four parameters was substantially affected by the betablockers. 0, no betablocker treatment; 1, betablocker treatment. * Units for the ordinate: turbulence onset of the HR (HR-TO), %; turbulence slope of the HR (HR-TS), ms BBI−1; turbulence slope of the mean blood pressure (MBP-TS), mmHg BBI−1; and postextrasystolic amplitude potentiation (PEAP), %.
The adaptation process of the systolic and mean BP and the BP amplitude triggered by the PVC is illustrated in Fig. 2, where BP amplitude had a pronounced alternans pattern in IDC patients. By contrast, a baroreflex-mediated BP regulation was apparent in the controls. The mean and systolic BP of the postextrasystolic beat in control patients were significantly lower than the reference values, but in IDC patients, these parameters were significantly higher than the reference values.
Figure 4 shows the postextrasystolic regulation patterns of the systolic BP and HR in both groups. In controls, the regulation lasted for ∼13–15 heart cycles. In IDC patients, the BP showed only slight beat-to-beat fluctuations, comprising an alternans pattern, whereas the HR was less influenced by the PVC and PESP.
Comparison of invasive and non-invasive measurements
The simultaneously measured non-invasive and invasive postextrasystolic BP regulation patterns of 10 intensive care patients with 41 single PVCs were found to be very similar. The small differences that occurred between the two curves were not significantly different (0.21 < P < 0.98).
DISCUSSION
We observed that PESP is associated with different patterns of HR and BP regulation in IDC patients and control subjects. In IDC patients, the baroreflex response evoked by the low pressure amplitude of the PVC was suppressed due to an abnormal postextrasystolic pressure response, whereas healthy subjects exhibited the typical baroreflex regulation pattern, lasting for about 13–15 heart cycles following a PVC. PEAP, a novel parameter for quantifying PESP in BP, had a positive predictive accuracy of 100 % of the controls.
The fast activation of the baroreflex is mediated by the parasympathetic limb of the autonomic nervous system, which adjusts the pressure on a beat-to-beat basis up to a HR of 80 bpm (de Boer et al. 1987). The vagal response to changes in BP starts within 0.5 s (Borst & Karemaker, 1983). The degree of activation of the pressoreceptors depends on the speed and amplitude of the arterial pressure increase and on the mean arterial pressure (Greger & Windhorst, 1996).
Since the first postextrasystolic beat is so large in IDC patients that it compensates for the reduced stroke volume of the PVC and, therefore, exceeds the pressure set point (quantified by the reference measurements preceding the PVC) of the baroreflex, the reflex response to the extrasystolic pressure is abolished (Fig. 1B). Thus, extrasystolic and postextrasystolic pressure determine the baroreflex response following a PVC. Therefore, in the case of a pronounced PESP, the turbulence slope parameters are not suitable measures of BRS.
In control subjects, only a slight potentiation of postextrasytolic amplitude was observed, which did not compensate for the fall in diastolic pressure following a PVC (Fig. 1A). A decrease in extrasystolic pressure starts the sympathetically mediated baroreflex response. Sympathetic activation also causes an increase in arterial resistance, which predominantly increases diastolic BP with a regulation delay of 2–3 s. Taking the two beat latency into account, the sympathetic regulation lasted about 10 s (mean HR, 74 bpm; Fig. 4). This is in accordance with the delay of sympathetically mediated changes in HR and inotropy of ∼2 s with a time constant of about 10 s (de Boer et al. 1987). The two turbulence slope parameters MBP-TS (related to BP) and HR-TS (related to HR) exactly reflect and quantify this mechanism in healthy subjects.
The degree of the postextrasystolic BP potentiation appears to depend on the left ventricular end-diastolic diameter, because Pearson's correlation coefficient was significant between PEAP and the mean left ventricular end-diastolic diameter. In four patients with PEAP values similar to the controls, the left ventricular end-diastolic diameter was smaller than 70 mm; in two of these, the left ventricular ejection fraction was 50 %. These findings correspond to the observations of Yamazoe et al. (1987), who found that a residual left ventricular function in IDC patients can be estimated by the degree of PESP.
To identify patients at high risk of sudden cardiac death after acute myocardial infarction, two novel risk stratifiers, namely turbulence onset and turbulence slope of the HR, were introduced recently by Schmidt et al. (1999). These parameters quantify the behaviour of the instantaneous HR after a PVC. Because of the close interaction between HR and BP following a PVC, we developed additional parameters to characterise the regulation pattern of instantaneous BP after a PVC, namely the turbulence slope of the mean BP and PEAP. The turbulence slope parameters HR-TS and MBP-TS precisely reflect the baroreflex response after a PVC in control subjects, whereas PEAP quantifies the postextrasystolic amplitude potentiation. Mrowka et al. (2000) described a blunted arterial baroreflex that caused ‘pathological’ HR turbulence when studying the response of a physiological model involving baroreceptor feedback mechanisms to a PVC. They validated their findings on the HR series of two patients, but not on BP recordings.
The pulsus alternans implies that end-diastolic volume is altered, probably due to the Frank-Starling mechanism or an alternating contractility of the ventricle (Hada et al. 1982) caused by an increased availability of intracellular Ca2+ (Suko et al. 1970). Carlson & Rapaport (1984) described postextrasystolic and possibly sustained pulsus alternans as an inevitable consequence of HR and of the relationship between ejection and diastolic filling.
Interestingly, in many cases, alternans appears to be permanent, as reported by Schaefer et al. (1988). In most of our patients, the alternans could also be observed during long periods of sinus rhythm. The alternans seems to be triggered by the premature beat itself, which is in accordance with the findings of Davies et al. (2001). Otherwise, the alternating amplitude would be smoothed by the averaging procedure, as can be seen during the beats just prior to a PVC (Fig. 2).
Although we found in the present study that betablockers did not substantially affect the PESP or turbulence parameters, this needs to be validated. Additional effects of other drugs (e.g. atropine) on the PESP should be investigated in further studies. Furthermore, we should recognise that the approach used in the present study is only applicable to patients with single PVCs.
Despite these limitations, our analysis clearly shows that the pattern of BP and HR regulation following a single PVC is mainly influenced by the PESP of the BP amplitude and its influence on the activation of the baroreflex. Therefore, the method of analysis used in the present study might be a useful tool for non-invasive characterisation of different cardiac diseases and for risk stratification. This should be confirmed in future studies involving larger numbers of patients.
Acknowledgments
This work was partly supported by grants from the Federal Ministry of Education, Science, Research and Technology (BMBF 13N7720) and the Deutsche Forschungsgemeinschaft (DFG vo505–2/3).
REFERENCES
- Borst C, Karemaker JM. Time delays in the human baroreceptor reflex. Journal of the Autonomic Nervous System. 1983;9:399–409. doi: 10.1016/0165-1838(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Carlson CJ, Rapaport E. Postextrasystolic pulsus alternans and heart rate. American Journal of Physiology. 1984;246:245–249. doi: 10.1152/ajpheart.1984.246.2.H245. [DOI] [PubMed] [Google Scholar]
- Cooper MW. Postextrasystolic potentiation. Do we really know what it means and how to use it? Circulation. 1993;88:2962–2971. doi: 10.1161/01.cir.88.6.2962. [DOI] [PubMed] [Google Scholar]
- Davies LC, Francis DP, Ponikowski P, Piepoli MF, Coats AJ. Relation of heart rate and blood pressure turbulence following premature ventricular complexes to baroreflex sensitivity in chronic congestive heart failure. American Journal of Cardiology. 2001;87:737–742. doi: 10.1016/s0002-9149(00)01493-4. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. American Journal of Physiology. 1987;253:H680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Dyke SH, Cohn PF, Gorlin R, Sonnenblick EH. Detection of residual myocardial function in coronary artery disease using post-extra systolic potentiation. Circulation. 1974;50:694–699. doi: 10.1161/01.cir.50.4.694. [DOI] [PubMed] [Google Scholar]
- Geschwind HJ, Lhoste F, Scriven AJ, Dhainaut JF, Sabatier C, Laurent D. Sympathetic nervous system activation in postextrasystolic potentiation: role of catecholamine release in enhancement of ventricular function. Journal of the American College of Cardiology. 1984;4:216–225. doi: 10.1016/s0735-1097(84)80205-3. [DOI] [PubMed] [Google Scholar]
- Greger R, Windhorst U. Comprehensive Human Physiology – From Cellular Mechanisms to Integration. New York and Heidelberg: Springer Verlag; 1996. pp. 1897–1901. [Google Scholar]
- Hada Y, Wolfe C, Craige E. Pulsus alternans determined by biventricular simultaneous systolic time intervals. Circulation. 1982;65:617–626. doi: 10.1161/01.cir.65.3.617. [DOI] [PubMed] [Google Scholar]
- Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovascular Research. 1998;38:605–616. doi: 10.1016/s0008-6363(98)00067-4. [DOI] [PubMed] [Google Scholar]
- Mrowka R, Persson PB, Theres H, Patzak A. Blunted arterial baroreflex causes ‘pathological’ heart rate turbulence. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2000;279:R1171–1175. doi: 10.1152/ajpregu.2000.279.4.R1171. [DOI] [PubMed] [Google Scholar]
- Schaefer S, Malloy CR, Schmitz JM, Dehmer GJ. Clinical and hemodynamic characteristics of patients with inducible pulsus alternans. American Heart Journal. 1988;115:1251–1257. doi: 10.1016/0002-8703(88)90017-8. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schomig A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- Suko J, Ueba Y, Chidsey CA. Intracellular calcium and myocardial contractility. II. Effects of postextrasystolic potentiation in the isolated rabbit heart. Circulation Research. 1970;27:227–234. doi: 10.1161/01.res.27.2.227. [DOI] [PubMed] [Google Scholar]
- Sung CS, Mathur VS, Garcia E, de Castro CM, Hall RJ. Is postextrasystolic potentiation dependent on Starling's law? Biplane angiographic studies in normal subjects. Circulation. 1980;62:1032–1035. doi: 10.1161/01.cir.62.5.1032. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Smith ML, Rea RF, Bauernfeind RA, Eckberg DL. Enhancement of sympathetic nerve activity by single premature ventricular beats in humans. Journal of the American College of Cardiology. 1989;13:69–75. doi: 10.1016/0735-1097(89)90551-2. [DOI] [PubMed] [Google Scholar]
- Yamazoe M. Response of the left ventricle in idiopathic dilated cardiomyopathy to postextrasystolic potentiation. American Heart Journal. 1987;113:1449–1456. doi: 10.1016/0002-8703(87)90661-2. [DOI] [PubMed] [Google Scholar]


