Abstract
Modulation of non-monosynaptic excitation from ankle dorsiflexors to quadriceps (Q) motoneurones during human treadmill walking was investigated in 25 healthy human subjects. Stimulation of the common peroneal nerve (CPN) evoked a biphasic facilitation in the rectified and averaged (n = 50) Q electromyographic (EMG) activity between 0 and 100 ms after heel strike. Prior to heel strike, the stimulation had no effect on the Q EMG. The latency of both peaks in the response was too long to be explained by a monosynaptic pathway to Q motoneurones. During voluntary tonic co-contraction of Q and tibialis anterior (TA) while standing, only the first of the two peaks was evoked by the CPN stimulation despite a background EMG activity level in the Q and TA muscles corresponding to that observed 30–60 ms after heel strike during walking. Stimulation of cutaneous nerves did not evoke a similar biphasic facilitation in the Q motoneurones, which suggests that muscular afferents mediate the response. The second peak had a higher threshold than the earlier peak. During cooling of the CPN, the latency of the second peak was more prolonged than the latency of the earlier peak. This suggests that afferents of different diameters contributed to the two peaks. It is proposed that afferents from TA assist the contraction of Q during walking via spinal interneurones to stabilize the knee joint and maintain upright posture during walking.
Several studies have investigated the role of sensory feedback during human walking for the control of the muscle from which the afferents originate (Yang et al. 1991; Dietz, 1992; Sinkjær et al. 1996, 2000). However, muscle afferents project extensively also to motoneurones controlling muscles acting on other joints (Mao et al. 1984; Meunier et al. 1993; Simonetta-Moreau et al. 1999). It is a possibility that these heteronymous projections from muscles acting on one joint to muscles acting on other joints help to ensure that the muscles are activated optimally in relation to each other during standing and walking. However, little is known about the modulation of transmission during walking in such pathways.
Therefore, the aim of the present study was to investigate modulation of transmission in heteronymous pathways from ankle dorsiflexor muscles to knee extensor muscles during walking. This particular combination was chosen because it has recently been reported that stimulation of ankle muscle nerves in sitting human subjects may elicit heteronymous non-monosynaptic excitation of muscles acting on the knee. Two phases of excitation are seen, the first of which in all likelihood is mediated by group I afferents, whereas the second was suggested to be mediated by group II afferents (Marque et al. 1996; Chaix et al. 1997; Simonetta-Moreau et al. 1999). It has been suggested that the two phases of excitation are mediated by common lumbar interneurones located rostral to the motoneurones supplying ankle and knee muscles (Chaix et al. 1997). Such interneurones have also been described in the cat and it has been argued that they may be closely involved in the control of locomotion (Edgley & Jankowska, 1987; Edgley et al. 1988; Shefchyk et al. 1990; Perreault et al. 1995; Aggelopoulos et al. 1996). Investigation of the reflex effects evoked by ankle dorsiflexor afferents in knee extensor motoneurones therefore would be a reasonable starting point for investigating transmission in heteronymous reflex pathways during walking.
METHODS
The experiments were carried out on 25 healthy human subjects (aged 18–50 years). All subjects gave informed consent to the experimental procedure, which was approved by the appropriate local ethics committee (KF 01–055/98). All experiments conformed to the Declaration of Helsinki. In all subjects measurements were made from the right leg.
The experiments were made during walking on a treadmill (4 km h−1) or during standing on both legs in the upright position. In the latter case, the subjects were asked to perform a voluntary tonic co-contraction of the tibialis anterior (TA) and quadriceps (Q) muscles in the investigated leg. In order to be able to produce a sufficient amount of EMG activity in the muscles the right foot of the subject was strapped to the floor by a rigid band, so that the dorsiflexion was essentially isometric. The knee joint was maximally extended. The subjects were provided with visual feedback of the EMG level in the TA and Q muscles and it was ensured that the level of EMG activity in the two muscles corresponded to that measured in relevant phases during walking.
EMG recordings
EMG activity was recorded by bipolar surface electrodes (0.5 cm2 recording area, 1.5 cm inter-electrode distance), which were placed on the skin over the muscle bellies of the following muscles: (i) different heads of Q: vastus lateralis (VL), vastus medialis (VM) and rectus femoris (RF); (ii) TA; and (iii) soleus (Sol). The EMG activity was amplified (× 2000–10 000) and filtered (100 Hz to 1 kHz; rc-relay 6 dB) before being stored (sampling rate 2 kHz) on a personal computer for later off-line analysis (software package: Gilles Detillieux, University of Manitoba, Canada). The positions of the ankle, knee and hip joints were measured by goniometers (Penny & Gilles, Scotland).
Conditioning stimulations
The effect of stimulation of the CPN on Q motoneurones was investigated at different times of the walking cycle and during standing. During walking, the stimulation was triggered on the signal from a force-sensitive sensor placed under the heel of the investigated leg and a variable delay between the trigger signal and the stimulus was used in order to apply the stimulus at different times in the walking cycle.
Electrical pulses (1 ms duration) were delivered to CPN through bipolar surface electrodes (1 cm diameter, inter-electrode distance ∼2 cm) placed at the level of the neck of the fibula. The site of stimulation was chosen so that the threshold for activation of TA was below the threshold for activation of the peroneal muscle group. It was also ensured that increasing the stimulus above motor threshold (MT) resulted in a steep increase in the motor (M) response in TA. The intensity of the stimulation was adjusted to evoke an M-response in TA, which was kept constant throughout the experiment. The size of the M-response was expressed as a percentage of the maximal M-response (Mmax), which was measured at the beginning and end of the experiment. The intensity of the stimulation was expressed in relation to the MT (× MT). In most of the experiments an intensity of 2 × MT was used. At this intensity the TA muscle was mainly activated, but it could not be avoided that the peroneal muscle group was also activated to some extent.
In experiments on five subjects, the cutaneous sensation (weak local and/or radiating paraesthesia) evoked by the CPN stimulation was mimicked by placing the bipolar surface electrodes approximately 5 cm distal to the neck of the fibula, thereby avoiding stimulation of the motor nerve supplying the TA muscle. It was checked that this stimulation did not evoke any response in the TA EMG. In addition, the radiating paraesthesia was reproduced by a stimulation of the superficial peroneal nerve (SPN) at the level of the ankle joint. The stimulus intensity was adjusted to imitate the sensation evoked by the mixed nerve stimulation.
Investigation of the effect of CPN stimulation on Q motoneurones
The effect of the CPN stimulation on Q motoneurones was investigated by stimulus-triggered averaging of the Q EMG activity. Stimuli of the CPN were given approximately every 2 s intermingled with recordings without stimulation during both walking and standing. The Q EMG activity was full-wave rectified using the CPN stimuli as trigger. The window used for the average lasted 150 ms and began 50 ms prior to the stimuli. Fifty traces with and without stimulation were averaged. The size of responses evoked in the Q EMG activity by the CPN stimulation was calculated as the area of the responses above the mean control EMG activity calculated from control trials without stimulation.
It may be difficult to determine if there is a difference between conditioned and control EMG activities because of the variability of EMG recordings. In our experiments, the CPN-evoked peaks were usually so clear (see Figs 1, 3, 5 and 6) that it was easy to determine if there was an effect or not. The beginning of the peaks evoked by the CPN stimulation and of the Q H-reflexes were calculated only on conditioned EMG and the areas of the two peaks (limited by the beginning and the end of each peak) were compared with the areas of the control EMG calculated in the same windows as the two peaks. The amount of facilitation results from the subtraction of the areas of conditioned and control EMGs expressed as a percentage of control EMG.
Figure 1. Recordings of the modulation of the angle joints, the ongoing EMGs and the CPN-induced facilitations in Q EMG activity during walking.
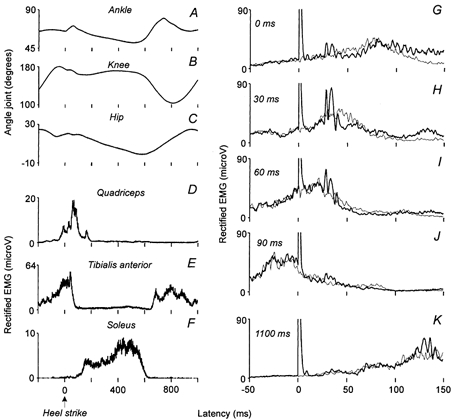
A–C, the variations of the angle of the ankle (A), of the knee (B) and of the hip (C) joints, expressed in degrees, are plotted against the latency (ms) after contact between the heel of the shoe and the treadmill (0 of the abscissa, Heel strike); the speed of the treadmill was set at 4 km h−1. D and E, the rectified EMG recordings (amplitude expressed in mV) of quadriceps (VL, D), tibialis anterior (TA, E) and soleus (Sol, F) muscles are also plotted against the latency (ms) after heel strike. G-K, the Q (VL) EMG activity conditioned by a CPN stimulation adjusted at 2.5 × MT (thick lines) and the control EMG (thin lines, average of 50 trials of each) are plotted against the latency (ms) after the stimuli (0 of the abscissa corresponds to the artefact of the stimulation).
Figure 3. Comparison of the effects induced by the CPN stimulation on Q EMG during walking and while standing.
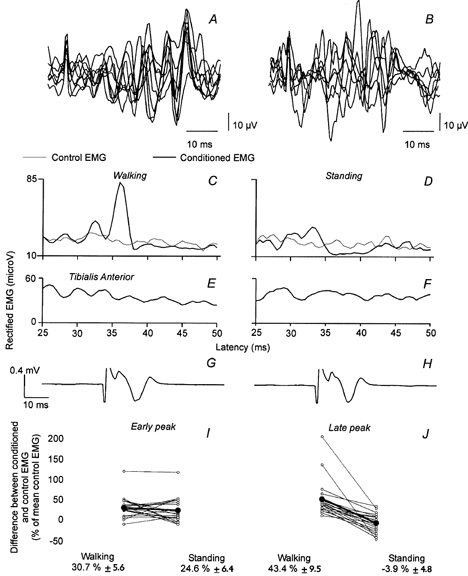
A-H, results obtained from one subject during the same experiment. A-B, superimposed traces (n = 10) of Q EMG following CPN stimulation 30 ms after heel strike (A) and during voluntary co-contraction of TA and Q while standing (B). C and D, control (thin line) and conditioned Q (VL) EMGs (thick line, 50 trials of each), plotted against the latency after the stimulation of the CPN (2.5 × MT), were recorded during walking when the stimuli were applied 30 ms after heel strike (C) and while standing up (D). E and F, the level of TA EMG activity during walking (E) and standing (F). G and H, M-response evoked in TA by the CPN stimulation during walking (G) and while standing (H). I and J, the difference between control and conditioned EMGs (expressed as a percentage of the mean control EMG) is shown for each subject investigated represented by ○ and the thin lines, for the early (I) and the late peak (J). The • and the thick lines represent the mean amount of facilitation induced by the CPN stimulation and the corresponding value (±s.e.m.) is written below.
Figure 5. Comparison of the effects induced by the CPN stimulation and pure cutaneous stimuli on Q EMG during walking.
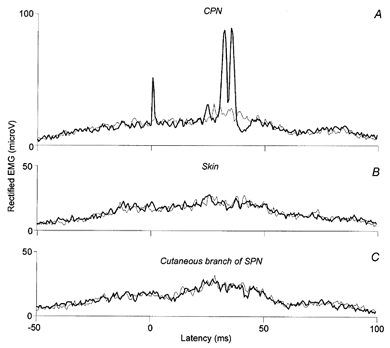
A-C, control (thin lines) and conditioned EMGs (thick lines, 50 trials of each), expressed in mV, are plotted against the latency (ms) in relation to the conditioning stimulations applied to the CPN adjusted at 2.5 × MT (A), to the skin where stimulating electrodes were 5 cm more distal than those of the CPN (B), and to the cutaneous branch of the SPN where stimulating electrodes were 32 cm more distal than those of the CPN (C). The intensity of the cutaneous stimuli was adjusted so as to imitate the cutaneous sensation produced by the mixed nerve stimulation.
Figure 6. Effect of cooling of the CPN on the early and the late peaks induced in Q EMG activity during walking.
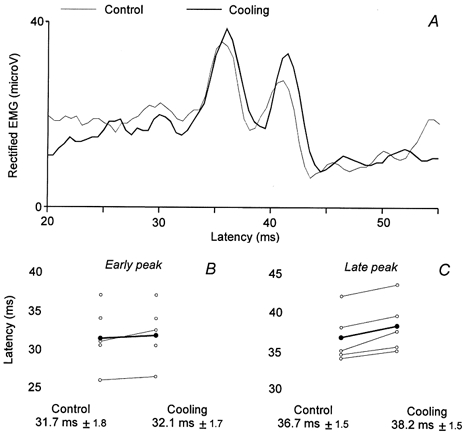
A, conditioned Q (VL) EMG by the CPN stimulation (2.5 × MT), plotted against the latency after the stimulation, was recorded during walking (40 ms after heel strike) in one subject before (control, thin line) and during (thick line, 50 trials of each) cooling. B and C, results obtained in all the subjects investigated this way (5, same legend as for Fig. 3I–J), the ordinate is the latency of the early peak (B) and of the late peak (C) observed before (control, on the left) and during (on the right) cooling. The mean latency (±s.e.m.) is written below each corresponding situation.
When decreasing the intensity of the CPN stimulation, the effects became smaller and it was more difficult to determine if there was an effect. In such cases, the same windows of analysis used for strong stimulation intensities were used.
Quadriceps H-reflexes
Quadriceps H-reflexes were evoked by stimulation of the femoral nerve (FN). The cathode (half ball with diameter of 2.5 cm) was placed in the femoral triangle, whereas the anode was placed on the back of the thigh. The H-reflex was used (in 20 out of 25 subjects) to calculate the latency of monosynaptic group I-mediated effects from the CPN stimulation on Q motoneurones. This calculation was based on the assumption that facilitatory effects in the Q EMG are mediated by the same motoneurones as the Q H-reflex. The efferent conduction time is thus the same for the Q H-reflex as for any facilitatory effects from the CPN. Assuming a similar conduction velocity in CPN and FN group I afferents, the longer latency of monosynaptic group I effects from the CPN onto Q motoneurones is only explained by the longer conduction distance from the site of CPN stimulation than from the site of FN stimulation. Based on measurements of the conduction velocity in I a afferents and the distance between the two sites of stimulation, the extra conduction time for the afferent volley in the CPN as compared with the afferent volley in the FN may be calculated. Adding this extra conduction time to the latency of the Q H-reflex gives the expected latency of group I monosynaptic effects in the Q EMG following CPN stimulation. From the difference between this expected monosynaptic latency and the actually observed latency, the segmental latency of the observed effect may be calculated. Similar calculations have been made in several previous publications (Marque et al. 1996; Chaix et al. 1997; Simonetta-Moreau et al. 1999).
Experiments with cooling and ischaemia
In order to determine which afferents were responsible for the effects evoked by CPN stimulation, the proximal part of the CPN was cooled in the popliteal fossa. In addition, inducing ischaemia in the leg blocked transmission in large diameter afferents.
Cooling
A cuff was placed around the knee and cold water (0 °C) was circulated through the cuff by an electrical pump. The effect of the CPN stimulation on Q EMG during walking was measured before, during 30–50 min of cooling and after 10–30 min of re-warming. Before cooling, the temperature of the skin in the popliteal fossa was 30–31 °C (room temperature: 22 °C), during cooling it dropped to 11 °C and after re-warming it was again 30–31 °C.
Ischaemia
The effect of the CPN stimulation on the Q EMG activity was measured while walking before, during and after ischaemia of the leg. Inflating a tourniquet (to above 240 mmHg) placed around the thigh above the knee induced ischaemia. Transmission in group I afferents was checked by monitoring a Sol H-reflex evoked by stimulation of the tibial nerve in the popliteal fossa (1 ms shock, anode placed above the patella), while the subject was sitting down. The time for measuring the effect of the CPN stimulation during ischaemia was decided from the decrease of the Sol H-reflex as a sign of blocked transmission in large diameter afferents. In all the experiments, the H-reflex began to decrease around 20 min after inflation of the tourniquet. The subjects were then able to walk for 2–3 min until there was also clear evidence of increasing block of transmission in motor axons. The tourniquet was then deflated and the measurements repeated again after 10 min with normal blood supply to the leg. Throughout the experiment, it was checked that the M-response in the TA EMG evoked by the CPN stimulation was constant.
Analysis and statistics
One-way ANOVA was used to determine whether the observed responses were significantly different from the background EMG activity. A paired Student's t test was used to compare the results obtained in the different motor tasks and to test the difference between the thresholds of early and late facilitations. The non-parametric Kruskall-Wallis test was used to compare the latency of the early and late facilitations before and during cooling of the CPN.
RESULTS
CPN stimulation induced a biphasic facilitation in Q EMG activity during walking
Figure 1A–F shows the angle of the ankle (A), knee (B) and hip (C) joints together with the Q (VL, D), TA (E) and Sol (F) EMG activities during treadmill walking at 4 km h−1. The zero of the abscissa corresponds to the time of heel strike. It is noticed that the Q muscle was active for a period from 100 to 200 ms before, until 100–150 ms after, heel strike. Figure 1G–K shows the effect of the CPN stimulation (2.5 × MT) on the Q (VL) EMG activity at different times in relation to heel strike. Although the M-response evoked in TA by the CPN stimulation was the same (70 % of Mmax) for all delays and although the background EMG activity in the Q muscle was comparable just before (i.e. 1100 ms delay, K) and after (i.e. 0 ms delay, G) heel strike, a response to the CPN stimulation was only observed in the latter case. The response consisted of a biphasic facilitation with latencies of 26 and 30.5 ms for the early and late peak, respectively. The response was largest at the 30 ms delay (H) and became gradually smaller at longer delays after heel strike (i.e. 60 and 90 ms, I-J).
In 21 out of the 25 investigated subjects, the CPN stimulation evoked a biphasic facilitation in the Q EMG activity immediately after heel strike, whereas no significant effect was observed just prior to heel strike in most subjects. In order to show the average size of the responses in the 21 subjects, we considered averaging the raw EMG values. However, this would not be valid, since the measured EMG activity varies from one subject to another depending on parameters such as the impedance of the recording electrode, the position of the electrode and the skin resistance. Furthermore, there is a considerable inter-individual variability in stride length and cycle duration and hence also in the time of maximal EMG activity in relation to heel strike. We therefore chose to express the size of the responses in the individual subjects in relation to the maximally recorded background Q rectified EMG activity during the gait cycle before the data were averaged. Thus, Fig. 2 shows the average amount of background EMG activity (triangles), the average amplitude of the early (filled circles) and late peak (open circles) in the 21 subjects, as a percentage of the maximal recorded rectified EMG activity. The size of the responses tended to vary with the background EMG level, but it should be noticed that significantly larger responses were observed at 30 and 60 ms than at 0 ms after heel strike, although there was no difference in the background EMG activity at these intervals (triangles). As seen in the figure, the two peaks of facilitation appeared and decreased in parallel, but in seven subjects the early peak had the same size regardless of the delay after heel strike, whereas the late peak was largest just after heel strike and decreased at longer delays as in the other subjects.
Figure 2. Modulation of the early and late peak during the gait cycle.
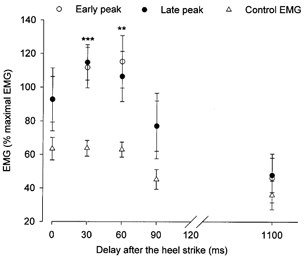
The mean amplitude of Q (VL) rectified EMG activity (21 subjects), expressed as a percentage of the maximal amplitude of the rectified EMG recorded during walking, is plotted against the delay after heel strike (ms). Circles represent the mean amplitude of the EMG conditioned by the CPN stimulation (2–2.5 × MT) corresponding to the early (•) and the late (○) peak. ▵, mean amplitude of the background EMG at the latency of the early and late peak. Vertical bars are the standard error of the mean (±s.e.m.). Statistical significance from the background EMG: *** P < 0.001; ** P < 0.01.
Comparison of the CPN-induced excitation of Q motoneurones during walking and tonic co-contraction of TA and Q
Figure 3A and B shows superimposed traces (n = 10) of unrectified Q EMG following CPN stimulation applied 30 ms after heel strike (Fig. 3A) and voluntary tonic co-contraction of TA and Q while standing (Fig. 3B). Figure 3C and D shows the averaged (n = 50) and rectified Q EMG from the same experiment. During walking the already described biphasic response was observed, but during standing the same stimulus produced a depression of the Q EMG activity at the latency of the later part of the response. Note also that the background EMG activity in the Q and TA muscles (Fig. 3E–F) was comparable in the two situations. The TA M-response was also kept constant throughout the experiment (75 % of Mmax, Fig. 3H–I).
Similar experiments were performed in 21 subjects (Fig. 3I and J; open circles). The early peak was larger during walking than during standing in 11 of the 21 subjects. However, for the population as a whole (filled circles) there was no statistically significant difference (I, size of peak during walking 30.7 ± 5.6 % as compared with 24.6 ± 6.4 % during standing). The late peak was in contrast much larger during walking than during standing in all subjects (43.4 ± 9.5 % vs. -3.9 ± 4.8 %, respectively) and the difference between the two motor tasks reached a highly significant level (P < 0.001). The statistical analysis was also made without the two subjects who showed the strongest difference between the two motor tasks and the difference was still significant (P < 0.001). It should be noticed that in 12 of the subjects the late peak of facilitation was replaced by an inhibition while standing up.
In the four subjects in whom there was evidence of monosynaptic I a facilitation in Q EMG from ankle dorsiflexors (see below) there was no statistically significant difference in the size of this facilitation between the two tasks.
Comparison of CPN-evoked excitation in different heads of the Q muscle
In six subjects, EMG recording was made from three different heads of the Q muscle (VL, VM and RF). In all subjects, the biphasic facilitation was observed in VL and RF, but for only one of the subjects was it observed in the VM EMG. For RF, which is both a hip flexor and a knee extensor, two bursts of EMG activity could be observed in three of the subjects during the walking cycle: in the swing phase and at the beginning of the stance phase. Consequently, the effects induced by the CPN stimulation in the RF EMG activity were compared when RF is of importance for the extension of the knee just after heel strike and when it is of importance for hip flexion during the swing phase. A biphasic facilitation was observed in the RF EMG activity just after the heel strike, whereas no effect was seen during the swing phase.
Central delay of the CPN-induced early and late peaks in Q EMG during walking
The onset latency of the CPN-induced facilitation in the Q EMG during walking (Table I, columns V and VII) was compared with the onset latency of the Q H-reflex (column II) in order to determine the central delay of the early and late part of the biphasic facilitation. This was possible in 20 out of the 21 subjects in whom the biphasic facilitation was observed. From the latency of the Q H-reflex, the conduction velocity of CPN I a afferents (on average around 68 m s−1 according to Meunier et al. (1993) and Nielsen & Kagamihara (1993)) and the distance between the sites where the FN and CPN were stimulated (column III), it was possible to calculate the shortest time required for group I a afferents activated by the CPN stimulation to reach Q motoneurones (monosynaptic latency, MS; column IV). This estimate was then compared with the actually observed latencies of the two peaks. For both peaks, it is evident that the latency far exceeds that of a monosynaptic I a pathway; the early peak appeared on average 4.4 ± 1.5 ms later than the MS latency (central delay, CD; column VI), whereas the second peak had an onset latency that was on average 9.0 ± 2.1 ms longer than the MS latency (CD, column VIII).
Table 1.
Characteristics of early and late peaks
| I | II | III | IV | V | VI | VII | VIII |
|---|---|---|---|---|---|---|---|
| Early peak | Late peak | ||||||
| Subject | H-reflex (ms) | D (cm) | MS (ms) | Lat. (ms) | CD (ms) | Lat. (ms) | CD (ms) |
| 1 | 16 | 46 | 22.8 | 26 | 3.2 | 30.5 | 7.7 |
| 2 | 21.5 | 49.5 | 28.8 | 32 | 3.2 | 35.5 | 6.7 |
| 3 | 17 | 45 | 23.6 | 26.5 | 2.9 | 31.5 | 7.9 |
| 4 | 16 | 46.5 | 22.8 | 30 | 7.2 | 35 | 12.2 |
| 5 | 22.5 | 45.5 | 29.2 | 35 | 5.8 | 40 | 10.8 |
| 6 | 20 | 46 | 26.8 | 34 | 7.2 | 40 | 13.2 |
| 7 | 24 | 53 | 31.8 | 36 | 4.2 | 41 | 9.2 |
| 8 | 21 | 51 | 28.5 | 33.5 | 5.0 | 37 | 8.5 |
| 9 | 24 | 46 | 30.8 | 33.5 | 2.7 | 38 | 7.2 |
| 10 | 22.5 | 46.5 | 29.3 | 33.5 | 4.2 | 38 | 8.7 |
| 11 | 23 | 45 | 29.6 | 34 | 4.4 | 37.5 | 7.9 |
| 12 | 24 | 48.5 | 31.1 | 34.5 | 3.4 | 37.5 | 6.4 |
| 13 | 19 | 42.5 | 25.3 | 30 | 4.8 | 33.5 | 8.3 |
| 14 | 22.5 | 45 | 29.1 | 34 | 4.9 | 40 | 10.9 |
| 15 | 27 | 47 | 33.9 | 37 | 3.1 | 44 | 10.1 |
| 16 | 26 | 52 | 33.6 | 36 | 2.4 | 40.5 | 6.9 |
| 17 | 15 | 42 | 21.2 | 27 | 5.8 | 31.5 | 10.3 |
| 18 | 18.5 | 47 | 25.4 | 31.5 | 6.1 | 38 | 12.6 |
| 19 | 22 | 47 | 28.9 | 32.5 | 3.6 | 37.5 | 8.6 |
| 20 | 21 | 42 | 27.2 | 32 | 4.8 | 34.5 | 7.3 |
| Mean | 4.4 ± 1.5 | 9.0 ± 2.1 | |||||
Column II, QH-reflex latency (ms); column III, distance (cm) between the CPN and the FN stimulating electrodes (cathode to cathode); columnIV, monosynaptic latency (MS, ms) resulting from the calculation of the time of arrival of CPN groupIa volley at the Q MN level by taking a conduction velocity of 68m s−1 (=0.1 × Col.III/0.68 + Col.II). Column V, latency (Lat., ms) of the early peak; columnVI, central delay (CD, ms) of the early peak; columnVII, latency (ms) of the late peak; column VIII, central delay (ms) of the late peak.
These estimated central delays are very similar to those found in sitting subjects for the early and late non-monosynaptic excitation of Q motoneurones presumably induced by group I and group II afferents from CPN, respectively (Marque et al. 1996; Chaix et al. 1997; Simonetta-Moreau et al. 1999).
It should be noted that in four subjects, CPN stimulation also induced a peak of facilitation at a very short latency in the Q EMG (see Fig. 5A). Based on the same considerations as before, an estimation of the central delay of this peak was made and a mean of −0.1 ± 0.4 ms (range −0.5 to 0.24) was found; the negative values indicate that the CPN-induced monosynaptic peak in Q EMG arrived earlier than the expected monosynaptic latency (column IV of Table 1). As the earliest disynaptic group I effects in humans start to manifest themselves at least 0.8 ms after the earliest monosynaptic I a excitation (Pierrot-Desseilligny et al. 1981; Day et al. 1984; Hultborn et al. 1987), it can be assumed that this very short latency peak probably reflects a monosynaptic excitation of Q motoneurones from ankle dorsiflexors.
Effects of varying the intensity of the CPN stimulation
In Fig. 4A, the sizes of the early and late peak of facilitation in the Q EMG activity are plotted against the intensity of the CPN stimulation. The early peak (open circles) appeared when the intensity of the CPN stimulation was around 0.9–1.0 × MT, whereas the late peak (filled circles) appeared around 1.2–1.4 × MT. At stimulation intensities of 1.1 and 1.2 × MT, only the early facilitation was observed. At these stimulation intensities, the two peaks thus had significantly different sizes (*P = 0.05 and 0.02, respectively). In 11 out of the 17 investigated subjects, the late peak had a higher threshold than the early peak and when pooling data from all investigated subjects, the difference between the threshold of the early and late peak reached a statistically significant level (B, 1.09 ± 0.05 vs. 1.31 ± 0.09 × MT, respectively, P = 0.02). Thus although the difference in threshold for the two peaks was not dramatic, it nevertheless supports the proposition that afferents of different diameter are responsible for the two peaks.
Figure 4. Threshold of the early and late facilitations induced in Q EMG during walking.
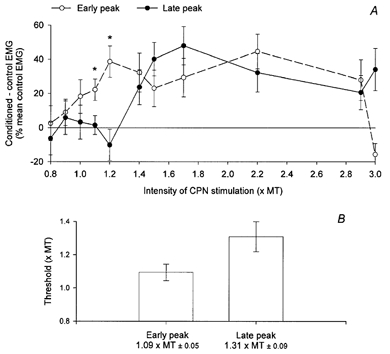
A, the difference (expressed as a percentage of the mean control EMG) between the area of the conditioned EMG by the CPN stimulation during walking (15 ms after heel strike) and the control EMG (50 trials of each) is plotted against the intensity of the CPN stimulation (× MT). ○, the amount of facilitation extracted from the area of the early peak, and • that from the late peak. Vertical bars are ±s.e.m. * Significant differences between the amplitude of the early and late peaks. B, the amplitude of the vertical columns represents the mean threshold (ordinate is the intensity of the CPN stimulation × MT) of the early (left) and of the late (right) peaks (±s.e.m.). The value (±s.e.m.) of the mean threshold for both peaks is written below each of the corresponding columns.
It is observed in Fig. 4A that the early peak of facilitation disappeared when the intensity of the CPN stimulation was increased to 3 × MT (which was Mmax in that case), whereas the late peak remained unchanged. A similar decrease of the early peak was observed in 6/10 of the subjects who have been investigated with maximal CPN stimulation. One possible explanation of this observation is that the CPN stimulation also activated inhibitory interneurones (Marchand-Pauvert et al. 1999), which prevents demonstration of the earlier excitation, as has also been demonstrated for inhibition of monosynaptic reflexes (Araki et al. 1960).
Effects of cutaneous stimulations
Figure 5A illustrates another subject in whom the CPN stimulation evoked a biphasic facilitation of the Q EMG activity during walking (peak latencies of 29.0 and 33.5 ms, respectively). When the cutaneous sensation evoked by the CPN stimulation was replicated either by stimulating the skin (B) or the cutaneous branch of the SPN at the level the ankle (C) a similar facilitation was not seen either in this subject or in any of the other four subjects tested this way. As pure cutaneous stimuli did not reproduce the effects produced by the mixed nerve stimulation, the early and late peaks evoked in Q EMG during walking by the CPN stimulation must be due mainly to the activation of muscular afferents.
Origin of the muscular afferents
Effects of cooling of the CPN
Animal experiments have shown that cooling a nerve produces a slowing of the conduction velocity of the nerve fibres. Effects mediated by small diameter afferents, such as group II afferents, will be more delayed than effects mediated by large diameter afferents, such as group I afferents (Paintal, 1965; Franz & Iggo, 1968). To investigate further whether different afferents were responsible for the two peaks of facilitation, the effect of the CPN stimulation in the Q EMG during walking was tested before and during cooling of the CPN in five subjects. Figure 6A shows the results obtained from one subject. In the control recording (thin line), the two peaks of facilitation appeared with latencies of 34 and 38 ms, respectively (CPN stimulation applied 40 ms after heel strike). During cooling (thick line), the latency of the early peak was unchanged, but the late peak was delayed by 1.5 ms. For all five subjects, the latency of the early peak was on average delayed by 0.4 ± 0.3 ms during cooling, whereas the late peak was delayed by 1.5 ± 0.3 ms (B and C). This difference reached a statistically significant level (P = 0.02).
Effects of ischaemia
Large diameter afferents, such as group I afferents, have been shown to be more sensitive to ischaemia than afferents with a smaller diameter (such as motor axons and group II afferents). It is therefore possible to block transmission in group I afferents selectively. In two subjects (the experiment was performed twice in one of them), the effect of CPN stimulation on Q EMG activity immediately after heel strike during walking was investigated with and without ischaemia of the leg. The efficiency of ischaemia was controlled by checking the amplitude of the Sol H-reflex and M-response at frequent intervals (initially every 5 min, then every minute and finally every 30 s) after the beginning of the ischaemic period. When the H-reflex began to decrease without any change in the M-response (around 20 min after initiation of ischaemia) the subject began walking on the treadmill and the effect of the CPN stimulation was investigated. In all three experiments the early and late peaks of facilitation in the Q EMG activity were abolished during ischaemia, despite intact transmission in motor axons as evidenced from the Sol and TA M-response (and the ability of the subject to generate EMG activity in the TA and Sol muscles during walking). This suggests that both the early and late peak depend on intact transmission in group I afferents.
DISCUSSION
The main finding of the present study is that stimulation of the CPN evoked a biphasic facilitation in the Q EMG at the beginning of the stance phase of walking, immediately after heel strike. A similar facilitation was not observed in the swing phase prior to heel strike. During standing, only the early peak of facilitation was present, whereas the later peak was often replaced by inhibition.
Origin of the biphasic facilitation
The failure of cutaneous stimulation to evoke a similar facilitation suggests that the afferents responsible for the biphasic facilitation originate in the ankle dorsiflexor muscles or tendons. The latency of the biphasic facilitation was clearly longer than expected for a monosynaptic group I reflex (which was in fact seen at an earlier latency in four subjects), which indicates that non-monosynaptic pathways were involved.
Since the two phases of the response had latencies comparable to that reported for (presumed) group I and group II effects from the CPN onto the Q H-reflex in resting subjects, we investigated whether there was evidence that different pathways contributed to the two phases of facilitation. The early peak of facilitation, indeed, had a lower threshold than the late peak, which suggests that afferents with different diameters contribute to the two peaks. Secondly, the late peak increased more in latency during cooling than the early peak, which is also consistent with the existence of two different pathways contributing to the peaks. Thirdly, only the early peak was seen during standing, whereas both peaks were seen during walking. Such a behaviour would be hard to explain, if different pathways did not contribute to the two phases of faciltiation.
Evidence for group I/group II pathways
It is one possibility, as has been suggested in sitting subjects, that common lumbar interneurones activated by group I and group II afferents, respectively, make a differential contribution to the two phases of facilitation (Marque et al. 1996; Chaix et al. 1997; Simonetta-Moreau et al. 1999). Indeed, the central delays for the two peaks of facilitation calculated during walking corresponded almost exactly to the central delays reported for sitting subjects (4.4 ± 1.5 and 9.0 ± 2.1 ms for the early and late peaks, respectively, in the present study as compared with 3.9 ± 0.3 and 10.0 ± 1.8 ms in the studies by Chaix et al. (1997) and Simonetta-Moreau et al. (1999)). Furthermore, although the change in latency of the late peak during cooling in the present study was less dramatic than that observed in the study by Simonetta-Moreau et al. (1999) (11 °C in our study as compared with 19 °C in their study), this difference may be explained by the better time resolution in their single motor unit study than in our surface EMG study. Finally, the appearance of the late peak only during walking is well in line with the suggestion from cat experiments that such lumbar group II interneurones may be part of the spinal network that generates the rhythmic activity underlying walking (Edgley & Jankowska, 1987; Edgley et al. 1988; Shefchyk et al. 1990; Perreault et al. 1995; Aggelopoulos et al. 1996). It should be noted that the observation that both peaks were abolished when transmission in group I afferents was blocked by ischaemia is not in conflict with this hypothesis, since the group II interneurones have been shown in the cat to receive substantial input from group I afferents.
However, there is at least one other possible explanation for our data. In the cat, it has been demonstrated that the autogenetic I b inhibition of extensor motoneurones, which is seen at rest, is replaced by excitation during walking (see Hultborn et al. 1998; McCrea, 1998). The interneurones in the I b excitatory pathway described by Hultborn et al. (1998) has also been shown to be part of the spinal rhythm-generating network (Conway et al. 1987). Our observation that the CPN stimulation evoked a weak inhibition of the Q EMG activity in several subjects during voluntary tonic co-contraction of TA and Q while standing, but a strong facilitation in the early stance phase, may reflect a similar reflex reversal. The different threshold of the two peaks and their different change in latency during cooling only suggest that the afferents responsible for the late peak have a smaller diameter than those inducing the early peak, but we cannot conclude with any certainty whether the late peak is caused by group I b or group II afferents. However, since our findings share several common features with the biphasic facilitation observed in sitting subjects by Chaix et al. (1997) and Simonetta-Moreau et al. (1999), we tend to believe that lumbar neurones activated by group I and group II afferents are involved, as evidenced in those studies.
Functional considerations
The fact that the late peak of facilitation was only seen during walking in the period immediately after heel strike suggests that transmission in the pathway responsible for the facilitation is selectively increased at this time. Since the facilitation was not seen at comparable levels of Q EMG activity during voluntary tonic contraction in standing subjects, it seems most likely that it reflects increased excitability of the interneurones in the pathway during walking. Regardless of the underlying pathway responsible for the response, this is consistent with cat experiments, which have suggested that populations of interneurones, activated by group I and group II afferents, are part of the spinal circuitry involved in the generation of the basic rhythmic activity during locomotion (Edgley et al. 1988; Shefchyk et al. 1990; Perreault et al. 1995; Hultborn et al. 1998).
From a functional point of view, the appearance of the facilitation immediately after heel strike makes sense since the weight of the body is shifted to the leg that is about to begin the stance phase at this time. Strong contraction of the Q muscle at this time is of importance for stabilization of the knee joint in order to support the weight of the body. Our data suggest that positive feedback carried by ankle dorsiflexor muscle afferents may help in ensuring this stabilizing contraction.
Acknowledgments
The authors wish to express their gratitude to Professor E. Pierrot-Deseilligny for reading and commenting upon the manuscript. They would also like to thank Professor Hans Hultborn for valuable discussions and comments during the study. This work was supported by The Danish Society for Multiple Sclerosis and the Danish Research Council. V.M.P. was supported by a grant from INSERM.
REFERENCES
- Aggelopoulos NC, Bawa P, Edgley SA. Activation of midlumbar neurones by afferents from anterior hindlimb muscles in the cat. Journal of Physiology. 1996;497:795–802. doi: 10.1113/jphysiol.1996.sp021810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Eccles JC, Ito M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. Journal of Physiology. 1960;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix Y, Marque P, Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M. Further evidence for non-monosynaptic group I excitation of motoneurones in the human lower limb. Experimental Brain Research. 1997;115:35–46. doi: 10.1007/pl00005683. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Day BL, Marsden CD, Obeso JA, Rothwell JC. Reciprocal inhibition between the muscles of the human forearm. Journal of Physiology. 1984;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiological Reviews. 1992;72:33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. Journal of Physiology. 1987;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Shefchyk S. Evidence that mid-lumbar neurones in reflex pathways from group II afferents are involved in locomotion in the cat. Journal of Physiology. 1988;403:57–71. doi: 10.1113/jphysiol.1988.sp017238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DN, Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. Journal of Physiology. 1968;199:319–345. doi: 10.1113/jphysiol.1968.sp008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard J-P, Brownstone R, Fedirchuk B, Shomburg ED, Enriquez-Denton M, Perreault M-C. How do we approach the locomotor network in the mammalian spinal cord? Annals of the New York Academy of Sciences. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Neuronal basis of afferent-evoked enhancement of locomotor activity. Annals of the New York Academy of Sciences. 1998;860:216–225. doi: 10.1111/j.1749-6632.1998.tb09051.x. [DOI] [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Experimental Brain Research. 1984;56:341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to human thigh motoneurones. Journal of Physiology. 1999;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Pierrot-Deseilligny E, Simonetta-Moreau M. Evidence for excitation of the human lower limb motoneurones by group II muscle afferents. Experimental Brain Research. 1996;109:357–60. doi: 10.1007/BF00231793. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous I a connections in the human lower limb. Experimental Brain Research. 1993;96:534–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. Differential projection of the sural nerve to early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiologica Scandinavica. 1993;147:385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Block of conduction in mammalian myelinated nerve fibres by low temperatures. Journal of Physiology. 1965;180:1–19. [PMC free article] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. Journal of Physiology. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Experimental Brain Research. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, McCrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Experimental Brain Research. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. Journal of Physiology. 1999;517:287–300. doi: 10.1111/j.1469-7793.1999.0287z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. Journal of Physiology. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. Journal of Neurophysiology. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Experimental Brain Research. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]


