Abstract
The venoarteriolar response causes vasoconstriction to skin and muscle via local mechanisms secondary to venous congestion. The purpose of this project was to investigate whether this response occurs through α-adrenergic mechanisms. In supine individuals, forearm skin blood flow was monitored via laser-Doppler flowmetry over sites following local administration of terazosin (α1-antagonist), yohimbine (α2-antagonist), phentolamine (non-selective α-antagonist) and bretylium tosylate (inhibits neurotransmission of adrenergic nerves) via intradermal microdialysis or intradermal injection. In addition, skin blood flow was monitored over an area of forearm skin that was locally anaesthetized via application of EMLA (2.5 % lidocaine (lignocaine) and 2.5 % prilocaine) cream. Skin blood flow was also monitored over adjacent sites that received the vehicle for the specified drug. Each trial was performed on a minimum of seven subjects and on separate days. The venoarteriolar response was engaged by lowering the subject's arm from heart level such that the sites of skin blood flow measurement were 34 ± 1 cm below the heart. The arm remained in this position for 2 min. Selective and non-selective α-adrenoceptor antagonism and presynaptic inhibition of adrenergic neurotransmission did not abolish the venoarteriolar response. However, local anaesthesia blocked the venoarteriolar response without altering α-adrenergic mediated vasoconstriction. These data suggest that the venoarteriolar response does not occur through adrenergic mechanisms as previously reported. Rather, the venoarteriolar response may due to myogenic mechanisms associated with changes in vascular pressure or is mediated by a non-adrenergic, but neurally mediated, local mechanism.
When venous pressure of a limb is elevated to pressures greater than 25 mmHg, either through venous stasis or through lowering the limb below heart level, muscle, subcutaneous and cutaneous vascular resistances increase within that region resulting in a reduction in blood flow of ∼40 % (Henriksen et al. 1973; Skagen & Bonde-Petersen, 1982; Andersen et al. 1986). Vasoconstriction during venous stasis and limb dependency has been termed the venoarteriolar response, since stretch receptors reported to be located in small veins are hypothesized to cause changes in arteriolar vascular tone ‘upstream’ of the vein (Henriksen, 1977). Henriksen and Sejrsen (Henriksen, 1977; Henriksen & Sejrsen, 1977) suggested that ∼45 % of the change in systemic vascular tone during upright tilt may be due to this response, with the remaining 55 % being due to central reflex mechanisms elicited via baroreceptors unloading.
Many studies have been performed to characterize the mechanisms of the venoarteriolar response. These studies revealed that the venoarteriolar response persists during acute spinal and sympathetic neural blockade proximal to the site of measurement (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Skagen & Bonde-Petersen, 1982; Hassan & Tooke, 1988; Vissing et al. 1997), in denervated skin flaps (Zoltie et al. 1989) and in areas distal to the lesion in spinal cord transection patients (Skagen et al. 1982; Andersen et al. 1986; Theisen et al. 2000). However, the venoarteriolar response is abolished by application of local anaesthetics at the site of measurement (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Hassan & Tooke, 1988; Vissing et al. 1997). Given these findings, the proposed pathway of this response suggests local neural impulses are responsible for the communication between the venous and arteriolar circulations, perhaps via the sympathetic axon reflex and that the central nervous system is not necessary to evoke the response (Henriksen, 1977; Moy et al. 1989).
To further identify the mechanisms responsible for the venoarteriolar response, Henriksen and Sejrsen (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977) locally administered phentolamine, a non-selective α-adrenoceptor antagonist, to identify whether the response was mediated by α-adrenoceptors. They reported that antagonism of α-adrenoceptors abolished the venoarteriolar response in skin (Henriksen & Sejrsen, 1976), subcutaneous tissue (Henriksen, 1977), and muscle (Henriksen & Sejrsen, 1977). However, in these studies very large doses of phentolamine were administered (1 ml of a 10 mg ml−1 solution) and data collection began 20 min after the local injection, which is likely to be an insufficient duration to eliminate vasodilatation associated with injection trauma (Andersson et al. 1995).
Pilot studies in our laboratory indicated that large doses of phentolamine caused significant cutaneous vasodilatation in many subjects (unpublished observation). This observation, coupled with reports that the venoarteriolar response is attenuated when the cutaneous vasculature is vasodilated via local administration of sodium nitroprusside (Zoltie et al. 1989) and histamine (Henriksen & Sejrsen, 1976), raised the question of whether phentolamine-induced inhibition of venoarteriolar responses was due to a hyperaemic response associated with administration of large doses of phentolamine as opposed to α-adrenoceptor antagonism. Thus, the purpose of this study was to confirm the hypothesis that the cutaneous venoarteriolar response occurs through α-adrenoceptor mechanisms. This goal was accomplished by local delivery of drugs combined with laser-Doppler flowmetry to assess skin blood flow prior to and during the engagement of the venoarteriolar response via arm dependency.
METHODS
A total of 28 subjects (12 male, 16 females; age: 32 ± 2 years) participated in this study, although not every subject participated in each protocol. The number of subjects who participated in each protocol is indicated in the protocol description. Each subject was informed of the purpose and risks of this institutionally approved study before providing written consent. The consent form was approved by the Internal Review Board for Human Subjects at the University of Texas Southwestern Medical Center at Dallas and at the Presbyterian Hospital of Dallas and conforms to the Declaration of Helsinki.
Protocol 1: selective α-adrenoceptor antagonism
The goal of this protocol was to identify whether the cutaneous venoarteriolar response associated with arm dependency occurs via α1- and/or α2-adrenoceptors. Thirteen subjects participated in this protocol.
α1-Adrenoceptor mechanisms. Two microdialysis membranes were placed in the skin of the dorsal forearm. Skin blood flow over the microdialysis membranes was measured via laser-Doppler flowmetry (Perimed, Järfälla, Sweden). One membrane was perfused with a 1 mg ml−1 solution of the α1-adrenoceptor antagonist terazosin, while the adjacent membrane was perfused with the vehicle (Ringer solution). These membranes were separated by distance of 3–4 cm, which was sufficient to avoid migration of the drug from one membrane region to the adjacent region (Crandall et al. 1997). Following a 60–90 min period to allow the hyperaemic response associated with membrane placement (Anderson et al. 1994) to subside, as indicated via laser-Doppler flowmetry, a 5 min period of baseline data collection ensued. The venoarteriolar response was then engaged for 2 min and this perturbation was repeated 2–3 times; the responses measured in these trials were averaged. Following these procedures, to test the efficacy of terazosin, both microdialysis membranes were perfused with the α1-adrenoceptor agonist phenylephrine (1 μg ml−1).
α2-Adrenoceptor mechanisms
On a separate day, the same procedures as reported above were conducted, except that one membrane was perfused with the selective α2-adrenoceptor antagonist yohimbine (0.1 mg ml−1) prior to limb dependency. The adjacent membrane was perfused with Ringer solution. Following the arm dependency procedure, the efficacy of the yohimbine was then tested by perfusing both the yohimbine-treated and control sites with the selective α2-adrenoceptor agonist clonidine (2.7 μg ml−1).
Protocol 2: non-selective α-adrenoceptor antagonism
The goal of this protocol was to confirm prior findings (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977) that the venoarteriolar response was blocked by local administration of the non-selective α-adrenoceptor antagonist phentolamine. However, in contrast to the cited studies, substantially lower doses of phentolamine were administered. Eleven subjects participated in this protocol. In dorsal forearm skin, 50–100 μl of a 0.1 mg ml−1 phentolamine solution was injected intradermally. The same volume of the vehicle (saline) was injected a minimum of 3 cm from the phentolamine treated site. A minimum of 60 min elapsed between the time of these injections and the onset of data collection to allow the hyperaemic response associated with injection trauma to subside. Data collection began once skin blood flow returned to normal levels, as indicated from laser-Doppler flowmetry, with a 5 min period of resting baseline in which the forearm remained at heart level. The venoarteriolar response was then engaged for 2 min and this perturbation was repeated 2–3 times; the responses measured in these trials were averaged. To test the efficacy of the phentolamine treatment, each subject was then exposed to whole-body cooling by perfusing 10 °C water through a tube-lined suit worn by each subject.
Protocol 3: inhibition of adrenergic neurotransmission
The goal of this protocol was to identify whether the cutaneous venoarteriolar response was blocked when adrenergic neurotransmission was blocked by administration of bretylium tosylate (Haeusler et al. 1969; Kellogg et al. 1989). Eight subjects participated in this protocol. On dorsal forearm skin 100 μl of a 2 mg ml−1 bretylium tosylate solution was injected intradermally. The same volume of the vehicle (saline) was injected a minimum of 3 cm from the bretylium tosylate treated site. After administration of the drug and vehicle, and an appropriate period of time to allow the hyperaemic response to the injection trauma to subside, the same procedures outlined in protocol 2 were repeated.
Protocol 4 local anaesthesia and cutaneous vasoconstrictor responsiveness
The goals of this protocol were to confirm that local anaesthesia blocked the cutaneous venoarteriolar response and to identify whether the blockade of this response was due to impaired adrenergically mediated vasoconstriction associated with application of the topical anaesthetic. Eight subjects participated in this protocol. The topical anaesthetic EMLA (2.5 % lidocaine and 2.5 % prilocaine) was placed on the skin and was covered by a Tagaderm dressing for 90–120 min. After this period of time the dressing and EMLA cream were removed. The effectiveness of the cream was assessed via blockade of tactile sensation from that area. A laser-Doppler flow probe, housed by an iontophoresis chamber, was placed within the region of anaesthesia. A second laser-Doppler flow probe assembly was placed at an unanaesthetized area adjacent to the aforementioned site. The venoarteriolar response was then engaged for 2 min and this perturbation was repeated 2–3 times; the responses measured in these trials were averaged. Following the assessment of the venoarteriolar response, vasoconstrictor responsiveness was assessed at both the EMLA treated and untreated sites. To accomplish this objective, noradrenaline (norepinephrine) was administered either via iontophoresis (3.2 mg ml−1 in propylene glycol at 20 μA for 10 min) or via intradermal microdialysis (3.2 mg ml−1 in Ringer solution) at both sites. For the latter procedure, microdialysis probes were inserted in the skin prior to application of EMLA cream. There was no difference in response between these two methods of noradrenaline delivery, regardless of the site (i.e. control or EMLA sites), so responses from both methods were combined for each site.
Procedures
Each study was performed with the subject in the supine position and with the arm from which measurements were obtained at the level of the heart. The venoarteriolar response was engaged by the subject lowering his/her arm off the edge of the table such that the location of skin blood flow measurement was 34 ± 1 cm below heart level. This distance was identified as the average distance between the two laser-Doppler flow probes attached to the forearm relative to the mid-axillary line. The location of the treatment and control sites on the forearm (i.e. distal versus proximal placement) was randomized.
The microdialysis probes were constructed in our laboratory from a semipermeable cellulose membrane (18 000 MW cutoff; Spectrum, Houston, TX, USA) glued between two polyimide tubes and reinforced with a 51 μm diameter stainless steel wire placed in the lumen of the membrane and tubes (Crandall et al. 1997; Kellogg et al. 1998). The membrane window for each probe was 10 mm. The probes were placed by piercing a 25 gauge needle into the dermal space and then having the needle exit 20–25 mm away from the point of entry. The microdialysis probe was inserted through the lumen of the needle. The needle was then withdrawn, leaving the probe in place. After placement, the probes were perfused with Ringer solution (2 μl min−1). For each protocol, data collection did not begin until after skin blood flow had returned to normal levels following the hyperaemic response associated with probe placement or injection trauma, typically 60–90 min after probe placement or injection (Anderson et al. 1994). The absence of a hyperaemic response associated with these procedures was identified by laser-Doppler flowmetry.
Data collection and analysis
Skin blood flow was recorded throughout all experimental procedures at a sampling frequency of no less than 20 Hz via a commercial data acquisition system (Biopac, Santa Barbara, CA, USA). Data were averaged during the first minute prior to the onset of the venoarteriolar response and the last 30 s during the engagement of the venoarteriolar response. At each site, the change in skin blood flow from when the arm was at heart level to when the arm was lowered was statistically compared between control and drug treatment sites using Student's paired t test. Similarly, changes in skin blood flow during the cold stress and administration of drugs to test the efficacy of the blocking agents were also statistically analysed between sites using Student's paired t test. Data are expressed as means ± s.e.m. The level of statistical significance was set at P ≤ 0.05.
RESULTS
Blockade of α1- and α2-adrenoceptors via microdialysis administration of terazosin (P = 0.48; Fig. 1) and yohimbine (P = 0.67; Fig. 2) did not affect the reduction in skin blood flow during the engagement of the venoarteriolar response. To test the efficacy of these drugs in blocking α1- and α2-adrenoceptors, their respective agonists, phenylephrine and clonidine, were administered through the appropriate microdialysis membrane. Both phenylephrine and clonidine cause a pronounced decrease in skin blood flow at the control site. However, these drugs did not cause skin blood flow to decrease at the sites previously treated with terazosin (Fig. 1) or yohimbine (Fig. 2), thereby demonstrating the effectiveness of blockade. Similarly, when the non-selective α-adrenergic antagonist phentolamine was administered (P = 0.39; Fig. 3), or when bretylium tosylate was administered (P = 0.09; Fig. 4), the reduction in skin blood flow during arm dependency was statistically similar between control and pharmacologically treated sites. Each subject was exposed to a cold stress to confirm that an appropriate dose of phentolamine and bretylium tosylate was administered. As illustrated in Fig. 3 and Fig. 4, these agents significantly attenuated the reduction in skin blood flow occurring during whole-body cooling, thereby confirming that the dose of phentolamine and bretylium tosylate administered blocked α-adrenoceptors non-selectively and blocked neural transmission from adrenergic nerves, respectively
Figure 1. Changes in skin blood flow during the venoarteriolar (VA) response and during local administration of phenylephrine at vehicle treated (open bars) and terazosin treated (grey bars) sites.
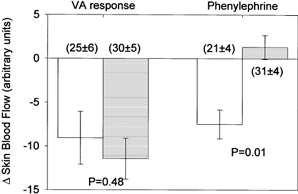
Inhibition of α1-adrenoceptors with terazosin did not significantly affect the decrease in skin blood flow associated with the venoarteriolar response. The lack of change in skin blood flow at the terazosin treated site during administration of the α1-adrenoceptor agonist phenylephrine demonstrates the efficacy of the dose of terazosin at blocking α1-adrenoceptors. Numbers in parentheses show skin blood flow prior to arm dependency and local administration of phenylephrine.
Figure 2. Changes in skin blood flow during the venoarteriolar (VA) response and during local administration of clonidine at the vehicle treated (open bars) and yohimbine treated (grey bars) sites.
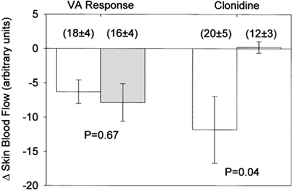
Inhibition of α2-adrenoceptors with yohimbine did not significantly affect the decrease in skin blood flow during the venoarteriolar response. The lack of change in skin blood flow at the yohimbine treated site during administration of the α2-adrenoceptors agonist clonidine demonstrates the efficacy of the dose of yohimbine at blocking α2-adrenoceptors. Numbers in parentheses show skin blood flow prior to arm dependency and local administration of clonidine.
Figure 3. Changes in skin blood flow during the venoarteriolar (VA) response and during whole-body cooling (cold stress) at the vehicle treated (open bars) and phentolamine treated (grey bars) sites.
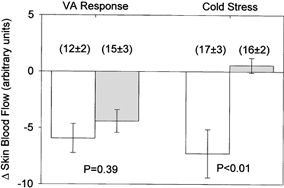
Non-selective inhibition of α-adrenoceptors with phentolamine did not significantly affect the decrease in skin blood flow during the venoarteriolar response. The lack of change in skin blood flow at the phentolamine treated site during whole-body cooling demonstrates the efficacy of the dose of phentolamine at blocking α-adrenoceptors. Numbers in parentheses depict skin blood flow prior to arm dependency and whole-body cooling.
Figure 4. Changes in skin blood flow during the venoarteriolar (VA) response and during whole-body cooling (cold stress) at the vehicle treated (open bars) and bretylium tosylate treated (grey bars) sites.
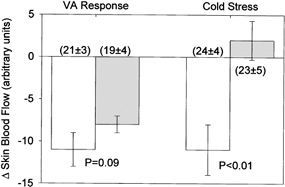
Inhibition of neural transmission of adrenergic nerves via bretylium tosylate administration did not significantly affect the decrease in skin blood flow during the venoarteriolar response. The lack of change in skin blood flow at the bretylium tosylate treated site whole-body cooling demonstrates the efficacy of the dose of bretylium tosylate at inhibiting adrenergic neural transmission. Numbers in parentheses show skin blood flow prior to arm dependency and whole-body cooling.
EMLA cream was applied to the skin to locally block cutaneous nerves. Each subject reported an absence of tactile sensation associated with 90–120 min application of EMLA cream. Decreases in skin blood flow associated with the venoarteriolar response were abolished at the EMLA treated site (Fig. 4). Given prior findings that lidocaine impairs calcium flux into the cytosol of smooth muscle (Fernandez del Pozo et al. 1997), it is possible that lidocaine-induced impairment of smooth muscle vasoconstrictor function was the mechanism for the venoarteriolar response being blocked at the EMLA site. To test this hypothesis, noradrenaline was delivered either via iontophoretic application or via intradermal microdialysis at both the control and EMLA treated sites. There was no difference in response between these two methods of noradrenaline delivery, regardless of the site, so responses from both methods were combined for each site. Local delivery of noradrenaline caused similar reductions in skin blood flow between the control and EMLA treated sites (P = 0.26; Fig. 4). These data confirm the hypothesis that local neural blockade abolishes cutaneous vasoconstriction associated with the venoarteriolar response.
DISCUSSION
The primary finding of the present study is that, in contrast to prior findings (Henriksen & Sejrsen, 1976), cutaneous vasoconstriction associated with the venoarteriolar response does not occur via α-adrenergic mechanisms. This conclusion was based upon the observation that cutaneous vasoconstriction during arm dependency was preserved in areas of skin treated with selective and non-selective α-adrenergic agonists, as well as in areas of skin treated with bretylium tosylate, which blocks neurotransmitter release from adrenergic nerves (Haeusler et al. 1969; Kellogg et al. 1989). Moreover, although application of a topical anaesthetic (EMLA) completely abolished the vasoconstrictor response associated with arm dependency, vasoconstriction in response to exogenous noradrenaline was not affected by local neural blockade. This observation suggests that the absence of vasoconstriction during engagement of the venoarteriolar response at the EMLA treated site was not due to impaired vasoconstriction of vascular smooth muscle.
In contrast to the present observations, prior studies indicated that vasoconstriction associated with the venoarteriolar response occurs through adrenergic mechanisms (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977). The dose of drug and the volume injected may be the primary reasons for differences in responses between the present and prior studies. For example, to identify the contribution of adrenergic receptors in mediating this response in skin, Henriksen and Sejrsen (Henriksen & Sejrsen, 1976) injected 1 ml of a 10 mg ml−1 solution of the non-selective α-adrenoceptor antagonist phentolamine into three subjects. Thus, not only was a large amount of drug delivered into the skin (10 mg), but also a large volume was injected (1 ml). In contrast, 5 to 10 μg of phentolamine were injected in the present study, which effectively blocked α-adrenergic vasoconstriction during a cold stress (see Fig. 3). In pilot studies in preparation for the present study, we found substantial and prolonged vasodilatation associated with administration of lower doses of phentolamine than that used by Henriksen & Sejrsen (1976). Moreover, it is unlikely that in the aforementioned study (Henriksen & Sejrsen, 1976) the hyperaemic response associated with the trauma of injecting 1 ml of solution into the skin subsided after the allotted 20 min (Anderson et al. 1994). These observations, coupled with findings that the venoarteriolar response is abolished when blood flow is elevated via local administration of histamine or sodium nitroprusside (Henriksen & Sejrsen, 1976; Zoltie et al. 1989), raises the question of whether responses abolished after phentolamine administration were due to hyperaemia associated with the concentration of drug and volume injected and not due to adrenoceptor antagonism. However, in the present study the doses of the pharmacological blocking agents administered did not significantly alter skin blood flow relative to the vehicle treated (i.e. control) sites prior to arm dependency (see Figs 1–5).
Figure 5. Changes in skin blood flow during the venoarteriolar (VA) response and during local administration of noradrenaline (norepinephrine) at the vehicle treated (open bars) and EMLA treated (grey bars) sites.
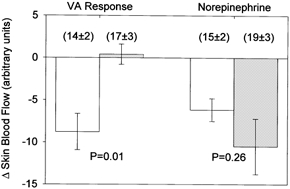
Application of the local anesthetic abolished the decrease in skin blood flow during the venoarteriolar response. However, similar decreases in skin blood flow between the sites during local administration of noradrenaline suggests the inhibition of the venoarteriolar response with EMLA application was not due to altered smooth muscle function (see text). Numbers in parentheses show skin blood flow prior to arm dependency and local administration of noradrenaline.
It is possible that differences in response upon engagement of the venoarteriolar response between the present and the prior study (Henriksen & Sejrsen, 1976) were related to the area of skin assessed and/or the method of measuring skin blood flow. In the present study, skin blood flow was assessed via laser-Doppler flowmetry from skin on the dorsal forearm, while in the cited study skin blood flow was assessed via gaseous 133Xe washout from the skin fold between the thumb and the forefinger (Henriksen & Sejrsen, 1976). Although both areas of skin would be considered non-glabrous and would probably exhibit similar vasodilator responses (Johnson et al. 1995), it remains unclear whether differences in vasoconstriction due to the venoarteriolar response between these areas are related to the location of skin measurement. This said, the majority of skin on the body has characteristics more like those of the the forearm than those of the skin fold area of the thumb. Thus, vascular resistance changes due to the venoarteriolar response from the forearm are probably more representative of responses from the general cutaneous circulation.
Similar to the findings of others (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Hassan & Tooke, 1988; Vissing et al. 1997), vasoconstriction associated with the venoarteriolar response was abolished following application of local anaesthetics. This observation led these investigators to suggest that the venoarteriolar response is neurally mediated. However, studies indicate that local anaesthetics, including lidocaine, impair vascular smooth muscle vasoconstriction (Altura & Altura, 1974; Wali, 1986), possibly through inhibiting calcium entry through voltage and receptor gated channels, as well as inhibiting calcium release from intracellular stores (Fernandez del Pozo et al. 1997). Thus, it is possible local anaesthetic inhibition of vasoconstriction associated with the venoarteriolar response was due to impaired calcium flux into the cell as well as impaired calcium release from intracellular stores. To address this hypothesis, we locally administered noradrenaline to both the control and locally anaesthetized areas. The justification for this procedure was that if noradrenaline-induced vasoconstriction was inhibited at that site, this would suggest that impaired vasoconstriction during the venoarteriolar response at the EMLA treated site was due to impaired vascular smooth muscle function. In contrast to this hypothesis, vasoconstrictor function was preserved at the EMLA treated site (see Fig. 5). Thus, it is unlikely that EMLA-induced inhibition of vasoconstriction with arm dependency was related to impaired smooth muscle function. Given these findings, it remains possible that vasoconstriction associated with the venoarteriolar response requires an intact local neural network as previously suggested by Henriksen et al. and others (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Hassan & Tooke, 1988; Vissing et al. 1997).
Data from the present set of experiments do not rule out the possibility that vasoconstriction associated with the venoarteriolar response is a myogenic response due to increases in arterial and venous pressures during limb dependency. It is interesting to note that the prevailing hypothesis regarding the mechanisms of the myogenic response suggests that vascular smooth muscle depolarization culminates in increased calcium entry via voltage-gated calcium channels and subsequent smooth muscle vasoconstriction (Davis & Hill, 1999; Hill et al. 2001). In the present study vasoconstriction during limb dependency was abolished by application of the local anaesthetic. If myogenic mechanisms are responsible for the venoarteriolar response, it is possible that in the present and prior studies (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Hassan & Tooke, 1988; Vissing et al. 1997) the absence of the venoarteriolar response at the anaesthetized site was a result of altering vascular smooth muscle depolarization and thus altered calcium mobilization. Conversely, data from the present experiments do not rule out the possibility that vasoconstriction associated with the venoarteriolar response is due to non-adrenergic but neurally mediated events and is unrelated to the myogenic response.
Henriksen and Sejrsen (Henriksen, 1977; Henriksen & Sejrsen, 1977) hypothesized that ∼45 % of the increase in systemic vascular resistance during orthostasis was due to the adrenergically mediated venoarteriolar response (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Henriksen, 1991). Thus, according to their hypothesis, if a pathological or pharmacological state altered adrenergic neurotransmission and/or adrenoceptor responsiveness, the individual would be more susceptible to orthostatic intolerance due to the combined inhibition of the venoarteriolar response and efferent responses associated with central reflex mechanisms (i.e. baroreceptor unloading). Data from the present experiment suggests that impaired orthostatic tolerance in these individuals would occur through altered efferent responses to baroreceptor unloading and not through impaired venoarteriolar responses, since the venoarteriolar response would be preserved in these individuals.
In conclusion, the primary purpose of this project was to identify whether the venoarteriolar response occurs through α-adrenergic mechanisms. This was accomplished by local administration of pharmacological agents to block both pre- and postsynaptic α-adrenergic mediated responses, followed by engaging the venoarteriolar response via arm dependency. Despite prior findings to the contrary (Henriksen & Sejrsen, 1976), the venoarteriolar response was preserved when both selective and non-selective α-adrenergic antagonists were administered, as well as the administration of an agent that blocks neurotransmission from adrenergic nerves (i.e. bretylium tosylate). However, similar to the findings of others (Henriksen & Sejrsen, 1976, 1977; Henriksen, 1977; Hassan & Tooke, 1988; Vissing et al. 1997), application of a local anaesthetic to the skin abolished the response. Finally, application of the local anaesthetic did not alter cutaneous vasoconstriction due to exogenous administration of noradrenaline. Taken together these findings suggest that vasoconstriction associated with the venoarteriolar response does not occur through α-adrenergic mechanisms. Thus, the venoarteriolar response may be due to myogenic mechanisms associated with changes in vascular pressure, or is mediated by a non-adrenergic, but neurally mediated, response.
Acknowledgments
The authors would like to express their appreciation to Robyn Etzel, R.N. for her assistance with this project and to the subjects for their willing participation in the project. This research project was funded in part by grants from the National Aeronautics and Space Administration (NAG91033), the National Heart, Lung, and Blood Institute (HL-61388), and the Japan Society for the Promotion of Science (9808270).
REFERENCES
- Altura BM, Altura BT. Effects of local anesthetics, antihistamines, and glucocorticoids on peripheral blood flow and vascular smooth muscle. Anesthesiology. 1974;41:197–214. doi: 10.1097/00000542-197408000-00009. [DOI] [PubMed] [Google Scholar]
- Andersen EB, Boesen F, Henriksen O, Sonne M. Blood flow in skeletal muscle of tetraplegic man during postural changes. Clinical Science. 1986;70:321–325. doi: 10.1042/cs0700321. [DOI] [PubMed] [Google Scholar]
- Anderson C, Andersson T, Wårdell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. Journal of Investigative Dermatology. 1994;102:807–811. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- Andersson T, Wårdell K, Anderson C. Human in vivo cutaneous microdialysis: estimation of histamine release in cold urticaria. Acta Dermatovenereologica. 1995;75:343–347. doi: 10.2340/0001555575343347. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA, Johnson JM. Evidence of functional β-adrenoceptors in the cutaneous vasculature. American Journal of Physiology. 1997;273:H1038–1043. doi: 10.1152/ajpheart.1997.273.2.H1038. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiological Reviews. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Fernandez del Pozo B, Perez-Vizcaino F, Fernandez C, Zaragoza F, Tamargo J. Effects of several class I antiarrhythmic drugs on isolated rat aortic vascular smooth muscle. General Pharmacology. 1997;29:539–543. doi: 10.1016/s0306-3623(96)00517-4. [DOI] [PubMed] [Google Scholar]
- Haeusler G, Haefely W, Huerlimann A. On the mechanism of the adrenergic nerve blocking action of bretylium. Naunyn-Schmiedebergs Archives of Pharmacology. 1969;265:260–277. doi: 10.1007/BF01002340. [DOI] [PubMed] [Google Scholar]
- Hassan AA, Tooke JE. Mechanism of the postural vasoconstrictor response in the human foot. Clinical Science. 1988;75:379–387. doi: 10.1042/cs0750379. [DOI] [PubMed] [Google Scholar]
- Henriksen O. Local sympathetic reflex mechanism in regulation of blood flow in human subcutaneous adipose tissue. Acta Physiologica Scandinavica. 1977;(suppl. 450):1–48. [PubMed] [Google Scholar]
- Henriksen O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiologica Scandinavica. 1991;(suppl. 603):33–39. [PubMed] [Google Scholar]
- Henriksen O, Nielsen SL, Paaske WP, Sejrsen P. Autoregulation of blood flow in human cutaneous tissue. Acta Physiologica Scandinavica. 1973;89:538–543. doi: 10.1111/j.1748-1716.1973.tb05547.x. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Sejrsen P. Local reflex in microcirculation in human cutaneous tissue. Acta Physiologica Scandinavica. 1976;98:227–231. doi: 10.1111/j.1748-1716.1976.tb10299.x. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Sejrsen P. Local reflex in microcirculation in human skeletal muscle. Acta Physiologica Scandinavica. 1977;99:19–26. doi: 10.1111/j.1748-1716.1977.tb10347.x. [DOI] [PubMed] [Google Scholar]
- Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Arteriolar smooth muscle mechano-transduction: Ca(2+) signaling pathways underlying myogenic reactivity. Journal of Applied Physiology. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Pergola PE, Liao FK, Kellogg DL, Jr, Crandall CG. Skin of the dorsal aspect of human hands and fingers possesses an active vasodilator system. Journal of Applied Physiology. 1995;78:948–954. doi: 10.1152/jappl.1995.78.3.948. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. Journal of Applied Physiology. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. American Journal of Physiology. 1989;257:H1599–1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- Moy S, Opfer-Gehrking TL, Proper CJ, Low PA. The venoarteriolar reflex in diabetic and other neuropathies. Neurology. 1989;39:1490–1492. doi: 10.1212/wnl.39.11.1490. [DOI] [PubMed] [Google Scholar]
- Skagen K, Bonde-Petersen F. Regulation of subcutaneous blood flow during head-up tilt (45 °) in normals. Acta Physiologica Scandinavica. 1982;114:31–35. doi: 10.1111/j.1748-1716.1982.tb06948.x. [DOI] [PubMed] [Google Scholar]
- Skagen K, Jensen K, Henriksen O, Knudsen L. Sympathetic reflex control of subcutaneous blood flow in tetraplegic man during postural changes. Clinical Science. 1982;62:605–609. doi: 10.1042/cs0620605. [DOI] [PubMed] [Google Scholar]
- Theisen D, Vanlandewijck Y, Sturbois X, Francaux M. Blood distribution adaptations in paraplegics during posture changes: peripheral and central reflex responses. European Journal of Applied Physiology. 2000;81:463–469. doi: 10.1007/s004210050069. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Secher NH, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiologica Scandinavica. 1997;159:131–138. doi: 10.1046/j.1365-201X.1997.573344000.x. [DOI] [PubMed] [Google Scholar]
- Wali FA. Effects of local anaesthetics on responses of human saphenous vein and bovine coronary artery to neurotransmitters, acetylcholine, noradrenaline and 5-hydroxytryptamine. General Pharmacology. 1986;17:405–411. doi: 10.1016/0306-3623(86)90182-5. [DOI] [PubMed] [Google Scholar]
- Zoltie N, Young C, Faris I, Tan E. The veno-arteriolar reflex in free skin flaps. Clinical Physiology. 1989;9:183–188. doi: 10.1111/j.1475-097x.1989.tb00969.x. [DOI] [PubMed] [Google Scholar]


