Abstract
The perifornical lateral hypothalamic area (PF-LHA) has been implicated in the control of several waking behaviours, including feeding, motor activity and arousal. Several cell types are located in the PF-LHA, including projection neurons that contain the hypocretin peptides (also known as orexins). Recent findings suggest that hypocretin neurons are involved in sleep-wake regulation. Loss of hypocretin neurons in the human disorder narcolepsy is associated with excessive somnolence, cataplexy and increased propensity for rapid eye movement (REM) sleep. However, the relationship of PF-LHA neuronal activity to different arousal states is unknown. We recorded neuronal activity in the PF-LHA of rats during natural sleep and waking. Neuronal discharge rates were calculated during active waking (waking accompanied by movement), quiet waking, non-REM sleep and REM sleep. Fifty-six of 106 neurons (53 %) were classified as wake/REM-related. These neurons exhibited peak discharge rates during waking and REM sleep and reduced discharge rates during non-REM sleep. Wake-related neurons (38 %) exhibited reduced discharge rates during both non-REM and REM sleep when compared to that during waking. Wake-related neurons exhibited significantly higher discharge rates during active waking than during quiet waking. The discharge of wake-related neurons was positively correlated with muscle activity across all sleep-waking states. Recording sites were located within the hypocretin-immunoreactive neuronal field of the PF-LHA. Although the neurotransmitter phenotype of recorded cells was not determined, the prevalence of neurons with wake-related discharge patterns is consistent with the hypothesis that the PF-LHA participates in the regulation of arousal, muscle activity and sleep-waking states.
The perifornical lateral hypothalamic area (PF-LHA) has been implicated in several physiological functions, including feeding, energy homeostasis, locomotor activity, cardiovascular regulation and sleep-wake control. Lesions of the PF-LHA and surrounding areas produce a syndrome of aphagia and adipsia (Teitelbaum & Epstein, 1962), akinesa (Levitt & Teitelbaum, 1975), sensory deficits (Marshall & Teitelbaum, 1974) and enhanced synchronization of the neocortical EEG (de Ryck & Teitelbaum, 1978; Shoham & Teitelbaum, 1982). Injections of neuropeptide Y into the PF-LHA increase feeding (Stanley et al. 1985) and administration of catecholamines into this region can suppress food intake (Leibowitz, 1986). Electrical stimulation of the PF-LHA evokes locomotor activity (Sinnamon et al. 1999), EEG activation (Krolicki et al. 1985) and increased blood pressure and heart rate (Stock et al. 1981).
The PF-LHA contains neurons that have widespread projections throughout the brain and spinal cord. One type of projection neuron contains the peptide melanin concentrating hormone (MCH). These neurons are principally localized in the PF-LHA and zona incerta (Bittencourt et al. 1992, 1998). Other PF-LHA projection neurons contain the hypocretin (Hcrt) peptides (also known as orexins). Hcrt-producing neurons are localized in the PF-LHA and dorsomedial hypothalamic nucleus (de Lecea et al. 1998; Peyron et al. 1998; Sakurai et al. 1998; Nambu et al. 1999). Although MCH- and Hcrt-immunoreactive neurons are intermingled within the PF-LHA, these peptides are not co-localized within individual neurons (Broberger et al. 1998; Peyron et al. 1998). Intracerebroventricular adminstration of both MCH and Hcrt promotes food intake (Qu et al. 1996; Sakurai et al. 1998) and food restriction results in elevated levels of mRNA for both families of peptide (Qu et al. 1996; Yamamoto et al. 2000).
The Hcrt peptides have recently been implicated in the regulation of arousal and in the neuropathology of narcolepsy. Narcolepsy is a neurological disorder characterized by excessive sleepiness and cataplexy (sudden loss of muscle tone precipitated by emotion; Siegel, 2000). A mutation of the Hcrt-2 receptor gene has been implicated in canine narcolepsy (Lin et al. 1999). Hcrt peptide knock-out mice exhibit cataplexy-like episodes, increased amounts of rapid eye movement (REM) and non-REM sleep and shorter waking episodes during the dark portion of the light-dark cycle (Chemelli et al. 1999). Hcrt mRNA is undetectable in the hypothalamus, pons and cortex of the brains of human narcoleptics (Peyron et al. 2000) and the number of Hcrt-immunoreactive neurons is reduced by 85–95 % in the hypothalamus of human narcoleptics (Thannickal et al. 2000).
Pharmacological studies support a role for Hcrt in the modulation of sleep-waking states. Intracerebroventricular infusion (Piper et al. 2000) or local microinjection of orexin into the target sites such as the preoptic area (Methippara et al. 2000) or locus coeruleus (Bourgin et al. 2000) promotes waking and suppresses REM sleep. Hcrt-1 application increases the discharge of rat locus coeruleus neurons in vitro (Hagan et al. 1999; Horvath et al. 1999). Immunoreactivity for c-fos protein is elevated in rat PF-LHA Hcrt and non-Hcrt neurons in response to systemic administration of the stimulant drug modafanil (Scammell et al. 2000), and in response to sustained wakefulness (Estabrooke et al. 2001).
The electrical activity of PF-LHA neurons during natural sleep and waking has not been described. The above findings led to the hypothesis that Hcrt-producing neurons are activated during wakefulness and deactivated during sleep. Here, we describe the frequency and distribution of sleep-wake-related discharge patterns of neurons recorded extracellularly within the field of Hcrt-immunoreactive neurons in the rat PF-LHA. Recorded neurons were grouped on the basis of the ratio of discharge rates during REM sleep and waking in anticipation of the possibility that a subset of PF-LHA neurons (specifically, those containing Hcrt) might exhibit differential activation during these two behavioural states.
METHODS
Experimental subjects and surgical procedures
We used seven male Sprague-Dawley rats weighing 300–350 g, maintained on a 12 h:12 h light-dark cycle (lights on at 08.00 h) with food and water freely available. Unit recordings of suitable quality were obtained from five of the rats. All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and all procedures were reviewed and approved by the Internal Animal Care and Use Committee of the V.A. Greater Los Angeles Healthcare System.
Rats were surgically prepared for chronic recording of extracellular neuronal activity in the PF-LHA and electrographic assessment of sleep-waking states as described by Alam et al. (1997). In brief, neocortical EEG and dorsal neck EMG electrodes were implanted aseptically under anaesthesia (ketamine + xylazine, 80:10 mg kg−1, i.p.) for polygraphic determination of the sleep- waking state. A single barrel (23 gauge stainless steel tube) mechanical microdrive was implanted stereotaxically such that the tip rested 3 mm above the dorsal aspect of the PF-LHA (A, −2.9 to −3.3; L, 1.2; H, −4.8 to 5.0; Paxinos & Watson, 1998). Five pairs of microwires, each consisting of two 20 μm insulated stainless steel wires glued together except for 2.0 mm at the tip, were inserted into the barrel such that the tips projected into the PF-LHA.
Data acquisition
Experiments were carried out after allowing at least 7 days for recovery from surgery. During the recovery period, animals were placed for 2–3 h per day in an electrically shielded, sound attenuated recording chamber (temperature: 25 ± 2 oC) and acclimatized to the recording environment. On experimental days, recordings were performed during the light portion of the light-dark cycle between 09.00 h and 15.00 h. During the recording session, the microdrive was advanced by 25–30 μm steps until amplified microwire signals, as confirmed in oscilloscopic traces, showed the presence of action potentials with a signal to noise ratio ≥ 2.0. Microwire recordings were filtered between 10 Hz and 5 kHz. The sampling rate was 25 000 samples s−1. EEG and EMG recordings were digitized at a sampling rate of 220 samples s−1 and stored for subsequent off-line analysis of sleep and waking state-dependent unit activity.
Individual action potentials were sorted from filtered, amplified microwire recordings on the basis of spike shape parameters using Spike 2 software (Cambridge Electronic Design 1401, London, UK). Spike 2 software was used to produce templates based on waveform amplitude and shape for each distinct action potential present in the raw signal and to sort action potentials throughout the recording by matching them with these templates. Spike shapes were based on 135 analog to digital sampling points. All spikes that did not match existing templates were stored for subsequent off-line examination to ensure that such spikes were not incorrectly rejected. To minimize the possibility that spikes from multiple cells matched a single template, interspike interval histograms were constructed for each discriminated unit to verify that no intervals < 2 ms were recorded.
The discharge rate of each discriminated PF-LHA neuron was recorded through three to five stable sleep-wake cycles (∼60–180 min). After completion of these recordings, the microdrive was advanced again in an attempt to isolate additional units for study. This sequence was repeated 2–3 times during a 6 h period.
Histology
At the end of a recording session, animals were deeply anaesthetized (pentobarbital, 100 mg kg−1, i.p.,), injected with heparin (500 U, i.p.) and perfused transcardially with 30–50 ml of 0.1 m phosphate buffer (pH 7.4) followed by 300 ml of 4 % paraformaldehyde and 100 ml of 10 and 30 % sucrose in phosphate buffer. The brains were removed and equilibrated in 30 % sucrose solution and then freeze-sectioned at 40 μm thickness. Immunohistochemistry for Hcrt-1 was performed on every third section in three rats. Sections were treated with 0.6 % H2O2, rinsed in Tris-buffered saline (TBS) and incubated in blocking solution (0.2 % Triton X-100 and 4 % normal goat serum in TBS) for 2 h at 4 oC. The sections were then rinsed in TBS and incubated in the primary antisera, namely rabbit polyclonal anti-Hcrt-1 (Oncogene, USA) diluted 1:2000 in diluent (0.1 % Triton X-100 and 4 % normal goat serum in TBS) for 48 h at 4 oC. The sections were then rinsed in TBS and incubated in diluent containing goat anti-rabbit IgG (1:250, Vector Laboratories, USA) for 2 h and then rinsed and incubated in avidin-biotin peroxidase (1:100 in TBS, ABC Elite Kit, Vector Laboratories) for 2 h at room temperature. Sections were then rinsed in 0.05 m Tris buffer, treated for 5–10 min with 0.05 % diaminobenzidine (Vector Laboratories) and 0.001 % hydrogen peroxide in 0.05 m Tris buffer, and then rinsed in TBS. Staining was absent from control sections from each brain processed without primary antibody. The tracks of the microwire bundles were identified histologically and the locations of recorded neurons were reconstructed along each track (Fig. 1A). Individual microwires in a bundle could be separated by a maximum of 125 μm in laterality, depth or anterior-posterior plane. Because the location of individual microwires in a bundle could not be determined, the reconstructed locations of recorded neurons were subject to this potential error. The reconstructed locations of recorded neurons and Hcrt-1-immunoreactive neurons were plotted side by side on the same section to determine whether the locations of recorded neurons and Hcrt-1-immunoreactive neurons were congruent (Fig. 1B and C).
Figure 1. Distribution of Hcrt-immunoreactive neurons and locations of recorded neurons in the rat PF-LHA.

A, photomicrograph of a representative coronal section through the PF-LHA showing the microwire tracts (long arrows) and Hcrt-1-immunoreactive neurons (short arrows). Scale bar, 100 μm. B, camera lucida drawing of a representative coronal section through the PF-LHA showing the distribution of Hcrt-immunoreactive neurons on the right side (•). The area delineated by the thin line indicates the highest density of Hcrt neurons in PF-LHA regions where neuronal recordings were performed. This area has been reproduced on the left side in B and C to indicate the location of recorded neurons with respect to Hcrt neurons. The locations of Type I wake-related (▴) and Type II wake-related (▵) neurons are plotted on the left side in B. Note that nearly all of the recorded wake-related neurons were localized in or immediately adjacent to the Hcrt-immunoreactive neuronal field. C, camera lucida drawing of the same section depicted in B, showing the distribution of wake/REM-related neurons (○) on the left side and REM-related neurons (*) on the right side. Abbreviations: 3V, third ventricle; f, fornix; opt, optic tract.
Data analysis
States of active waking (AW), quiet waking (QW), non-REM sleep and REM sleep were identified on the basis of EEG and EMG patterns using standard criteria (Timo-Iaria et al. 1970). Mean discharge rates of individual neurons during AW, QW, non-REM sleep and REM sleep were calculated from 40–300 s blocks of three to five stable recording episodes from each state. The discharge rate during REM sleep was calculated from all episodes that were longer than 20 s. Seven of 113 recorded neurons exhibited very slow firing (< 0.5 Hz) in all sleep-waking states and were excluded from subsequent analyses.
Preliminary examinations of PF-LHA unit activity revealed several types of sleep-wake discharge patterns among recorded neurons. Therefore, it was necessary to segregate neurons according to their sleep-wake discharge profile to permit meaningful statistical comparisons among groups of neurons. We decided to segregate neurons based on the value of the ratio of discharge rates during REM sleep vs. waking (REM/wake ratio). This parameter was selected because there is physiological evidence that Hcrt-producing neurons in the PF-LHA are activated during waking, but might exhibit suppression of discharge during REM sleep. Classification of neurons on the basis of the REM/wake ratio allowed us to distinguish neurons with this discharge pattern from those that are activated during both waking and REM sleep, a cell type commonly encountered in the lateral hypothalamus of cats and rats (Szymusiak et al. 1989; Krilowicz et al. 1994; Steininger et al. 1999).
Neurons were classified as wake related if the REM/wake ratio was < 0.5. Two types of wake-related neurons were defined, based on the magnitude of the suppression of discharge during REM sleep. Type I wake-related neurons displayed a REM/wake ratio ≤ 0.2. Type II wake-related neurons displayed a REM/wake ratio > 0.2 and < 0.5. Neurons were classified as wake/REM-related if the REM/wake ratio was ≥ 0.5 and < 1.5. Neurons were classified as REM related if the REM/wake ratio was ≥ 1.5. The effects of sleep-waking state on the discharge rate of each class of neuron were assessed with a one-way repeated measures ANOVA. The significance of differences between individual sleep-wake state means was evaluated with Newman-Keuls post hoc tests.
The duration of each discriminated action potential was calculated from averaged waveforms (based on 10–30 action potentials) to reduce noise. The duration of an action potential was measured from the initial inflection from baseline voltage to the point at which the after-potential returned to the baseline (see Figs 2C, 4C and 6C). The effects of cell classification on action potential duration were assessed with a one-way ANOVA followed by a Newman-Keuls post hoc test.
Figure 2. The discharge pattern of individual wake/REM-related neurons across the sleep-waking cycle.
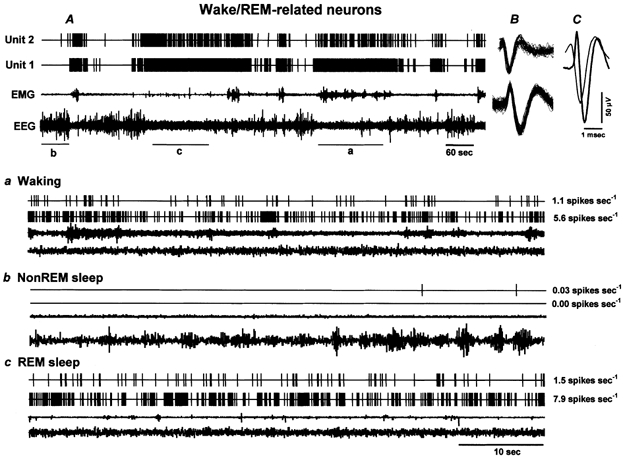
A, 15 min of continuous recording showing the discharge of two wake/REM-related neurons (Units 1 and 2) recorded simultaneously across the sleep-waking cycle. B, the superimposed waveforms of individual action potentials of the recorded neurons captured during five consecutive seconds along with the background signal variations. C, the averaged waveforms of the two action potentials recorded for five consecutive seconds. Note the difference in the spike shape and the duration of the action potentials, even though these neurons were recorded simultaneously from the same microwire. Lower panels illustrate expanded tracings (60 s) from A, showing the discharge patterns of the recorded neurons during waking (a), non-REM sleep (b) and REM sleep (c). The values to the right of each expanded tracing represent mean discharge rates (spikes s−1) for the respective neurons during the representative sections. These neurons exhibited high discharge rates during waking and REM sleep and dramatically reduced discharge rates during non-REM sleep. EEG, electroencephalogram; EMG, neck electromyogram.
Figure 4. Discharge patterns of wake-related neurons across the sleep-waking cycle.
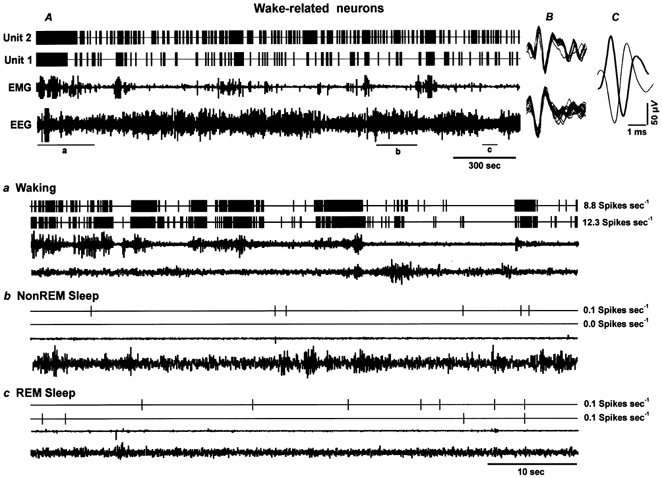
A, 66 min tracing showing continuous discharge of two Type I wake-related neurons (Units 1 and 2) recorded simultaneously across the sleep-waking cycle. The superimposed waveforms of individual action potentials captured during 5 s along with the background noise and their averaged waveforms are shown in B and C, respectively. Lower panels depict expanded tracings (60 s) showing discharge rates of these two neurons during waking (a), non-REM sleep (b), and REM sleep (c). Wake-related neurons exhibited highest discharge rates during AW, as indicated by desynchronized EEG and increased EMG activity, a dramatic decline in discharge rates during QW and a further decline during non-REM sleep and REM sleep. The values to the right of each expanded tracing represent the mean discharge rate during the representative sections of the tracing.
Figure 6. The discharge pattern of individual REM-related neurons across the sleep-waking cycle.
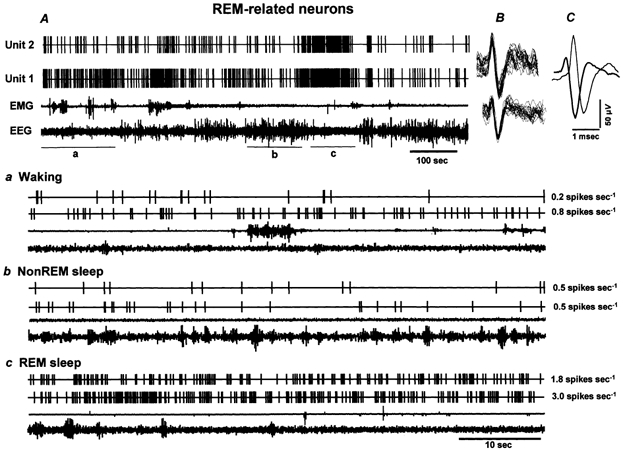
A, 15 min continuous recording showing the discharge of two REM-related neurons (Units 1 and 2) recorded simultaneously across the sleep-waking cycle. The superimposed waveforms of individual action potentials captured during 5 s for each recorded neuron along with background noise and their averaged waveforms are shown in B and C, respectively. Lower panels depict expanded tracings (60 s) from A, illustrating the discharge patterns of the two neurons during waking (a), non-REM sleep (b) and REM sleep (c). The values to the right of each expanded tracing represent mean discharge rates of the respective neurons during the representative sections. These neurons exhibited lower discharge rates during waking and non-REM sleep and increased discharge rates during REM sleep.
The relationship of neuronal discharge to muscle activity was examined for each class of neuron. For 60–120 min recording periods containing episodes of waking, non-REM and REM sleep, discharge rates were calculated for successive 5 s epochs. These values were paired with the amplified, rectified dorsal neck EMG during the same epoch. Thus, multiple pairs of discharge rate vs. EMG activity values were generated across all sleep-waking states. Simple linear regression was calculated for each of the classified neuronal types. To plot these data, values were grouped on the basis of EMG activity into 0.3 V bins (see Fig. 8).
Figure 8. Correlation of PF-LHA neuronal activity with muscle activity.
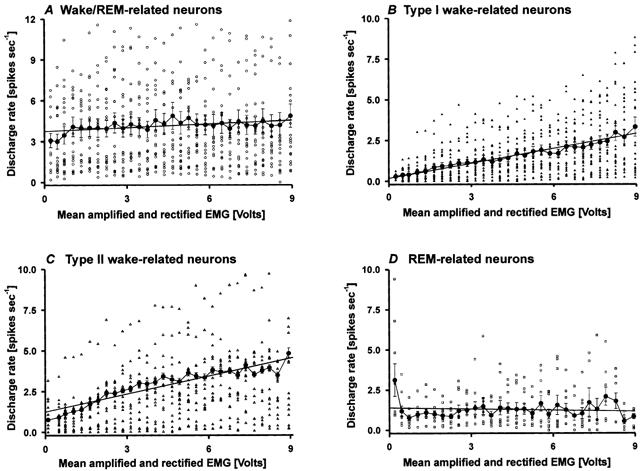
Correlation between discharge rate and EMG activity of wake/REM-related (A), Type I wake-related (B), Type II wake-related (C) and REM-related (D) neurons. Mean (± s.e.m.) discharge rates of individual neurons (large filled circles) are plotted against mean rectified and amplified EMG voltages calculated during successive 5 s epochs across 3–5 sleep-waking cycles. Discharge rates were divided into 0.3 V bins. The straight line in each graph represents the simple linear regression between discharge rate and EMG voltage. The discharge rates of Type I and II wake-related neurons (B and C) were strongly positively correlated with EMG voltage across all sleep-waking states. The discharge rates of a comparable number of wake/REM-related neurons (n = 23) and of REM-related neurons were not significantly correlated with EMG voltage.
RESULTS
Anatomical location of recorded neurons
A total of 106 neurons recorded from dorsal to ventral passes through the PF-LHA were characterized by sleep and waking state-dependent discharge patterns. On the basis of the REM/wake discharge rate ratio, 56 neurons (53 %) were classified as wake/REM related, 24 (23 %) as Type I wake related, 16 (15 %) as Type II wake related and 10 (9 %) as REM related. A photomicrograph of a representative microwire pass in a Hcrt-immunostained PF-LHA section is shown in Fig. 1A. Figures 1B and C show the distribution of Hcrt-immunoreactive neurons in the vicinity of the microwire tracts and the location of each classified neuron. Hcrt-immunoreactive neurons were located in the perfornical lateral hypothalamus and dorsal medial hypothalamic area. The rostral-caudal plane of the recorded neurons varied by 0.1–0.4 mm through the PF-LHA but they have been superimposed on a single plane of the section illustrated in Fig. 1B and C. The different neuronal types encountered were not clearly segregated within the PF-LHA. Nearly all of the recorded neurons were located in the field of Hcrt-immunoreactive neurons.
The details of the discharge patterns and other characteristics of the different neuronal types encountered were as follows.
Sleep-waking state-related discharge patterns
Wake/REM-related neurons
This was the most frequently encountered class of neuron. Computer-generated tracings of the individual discharge patterns of two wake/REM-related neurons recorded simultaneously are shown in Fig. 2. The individual discharge rate (spikes s−1) of all the recorded neurons during AW, QW, non-REM sleep and REM sleep and their mean discharge rate as a group are shown in Fig. 3A and B, respectively. These neurons had a mean (± s.e.m.) REM/wake ratio of 0.87 ± 0.04 (range, 0.51–1.48). There was a significant overall effect of sleep- waking state on the discharge rate of wake/REM-related neurons (F3,55 = 31.2, P < 0.0001). The discharge rate in AW was significantly higher than in QW and non-REM sleep, but did not differ significantly from that in REM sleep (Fig. 3B). REM sleep rate was also significantly higher than rates during QW and non-REM sleep. The discharge rate during QW was significantly higher than non-REM sleep rate.
Figure 3. Discharge characteristics of wake/REM-related neurons.

Individual (A) and mean (± s.e.m.; B) discharge rates (spikes s−1) of wake/REM-related neurons during active waking (AW), quiet waking (QW), non-REM sleep and REM sleep. As a group, these neurons exhibited a significantly higher discharge rate during both AW and REM sleep when compared to QW and non-REM sleep. * P < 0.01. NS, not significantly different.
Type I wake-related neurons
Computer-generated tracings showing the individual discharge patterns of two simultaneously recorded Type I wake-related neurons are shown in Fig. 4. The discharge rate of individual Type I wake-related neurons and their mean discharge rate as a group are shown in Fig. 5A and B, respectively. Type I wake-related neurons exhibited a mean REM/wake ratio of 0.09 ± 0.01 (range, 0.003–0.200). There was a significant overall effect of sleep-waking state on discharge rates (F3,23 = 38.5, P < 0.0001). The discharge rate during AW was significantly higher than those in all other states. There were no significant differences in mean discharge rates during QW, non-REM sleep and REM sleep.
Figure 5. Discharge characteristics of Type I and II wake-related neurons.

Activity of Type I (A and B) and II (C and D) wake-related neurons during AW, QW, non-REM sleep and REM sleep, showing individual (A and C) and mean (± s.e.m.; B and D) discharge rates. Note that both types of neuron exhibited significantly higher discharge rates during AW compared to QW, non-REM and REM sleep. For both types of neuron, discharge rates during QW, non-REM and REM sleep were not significantly different. * P < 0.01.
Wake-related discharge patterns that exhibit near cessation of activity during REM sleep are characteristic of several monoaminergic cell groups (see Introduction). A comparison of Type I wake-related neurons recorded in PF-LHA with putative serotonergic ‘REM-off’ neurons recorded in dorsal raphe of rats (Guzman-Marin et al. 2000) is presented in Table 1. These two classes of neuron exhibited similar declines in discharge rate during REM sleep compared to AW (91.09 ± 1.56 vs. 92.58 ± 8.35 %). However, PF-LHA wake-related neurons exhibited a significantly higher discharge rate during AW and a significantly greater reduction in discharge rate from AW to QW compared to dorsal raphe neurons. Dorsal raphe neurons exhibited a significant reduction in discharge rate from non-REM to REM sleep, whereas PF-LHA neurons did not. In addition, action potential durations of dorsal raphe neurons were significantly longer.
Table 1.
Comparison of sleep-wake discharge of Type I wake-related neurons recorded in the PF-LHA and wake-related/‘REM-off’ neurons recorded in rat dorsal raphe nucleus
| Area | AW (spikes s−1) | QW (spikes s−1) | Non-REM sleep (spikes s−1) | REM sleep (spikes s−1) | Decrease from wake to REM sleep (%) | Decrease from AW to QW (%) | Spike width (ms) |
|---|---|---|---|---|---|---|---|
| Dorsal raphe (n = 24) | 1.09 ± 0.14 | 0.69 ± 0.12 | 0.34 ± 0.04 | 0.09 ± 0.02 | 92.6 ± 8.0 | 38.4 ± 4.7 | 2.88 ± 0.09 |
| PF-LHA (n = 24) | 4.54 ± 0.70* | 1.03 ± 0.31 | 0.29 ± 0.09 | 0.37 ± 0.10 | 91.1 ± 1.5 | 77.5 ± 4.2* | 2.23 ± 0.07* |
Data for dorsal raphe neurons are taken from Guzman-Marin et al. (2000). All values are means ± s.e.m.
P < 0.01 (independent two-tailed t test).
Type II wake-related neurons
The discharge rate of individual Type II wake-related neurons and their mean discharge rate as a group during AW, QW, non-REM sleep and REM sleep are shown in Fig. 5C and D, respectively. The mean REM/wake ratio was 0.35 ± 0.03 (range, 0.02–0.48). There was a significant overall effect of sleep- waking state on discharge rates (F3,15 = 8.1, P < 0.001). As in the case of Type I neurons, the discharge rate during AW was significantly higher than those in all other states and there were no significant differences in mean discharge rates during QW, non-REM sleep and REM sleep (Fig. 5D).
REM-related neurons
These neurons were the least frequently encountered type (10/106). Computer-generated tracings of individual discharge patterns from two REM-related neurons that were recorded simultaneously are shown in Fig. 6. The mean REM/wake ratio of REM-related neurons was 3.88 ± 1.10 (range, 1.99–12.97). Figure 7A and B shows the individual REM-related neuron discharge rates and their mean discharge rates during AW, QW, non-REM sleep and REM sleep. There was a significant effect of sleep-waking state on the discharge rates of these neurons (F3,9 = 8.3, P < 0.001). The discharge rate during REM sleep was significantly higher than those in all other states. There were no significant differences in mean discharge rates during AW, QW and non-REM sleep (Fig. 7B).
Figure 7. Discharge characteristics of REM-related neurons.
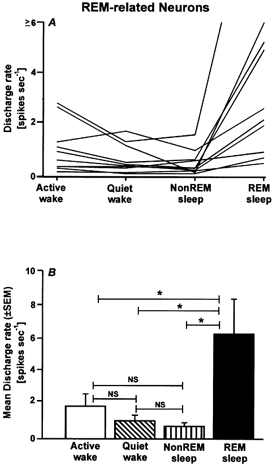
Individual (A) and mean (± s.e.m.; B) discharge rates of REM-related neurons during AW, QW, non-REM sleep and REM sleep. These neurons exhibited low discharge rates during AW, QW and non-REM sleep, with comparatively elevated discharge rates during REM sleep. * P < 0.01.
Action potential duration
Mean action potential duration was 1.84 ± 0.4 ms (range, 1.04–2.24 ms) for wake/REM-related neurons, 2.23 ± 0.08 ms (range, 1.6–3.12 ms) for Type I wake-related neurons, 2.24 ± 0.11 ms (range, 1.32–2.94 ms) for Type II wake-related neurons and 1.86 ± 0.07 ms (range, 1.44- 2.16 ms) for REM-related neurons. There was a significant effect of cell classification on action potential duration (F3,95 = 10.2, P < 0.0001). Mean durations for Type I and Type II wake-related neurons were significantly longer than for both wake/REM- and REM-related neurons (Newman-Keuls, P < 0.05), but did not differ significantly from each other. Mean action potential durations for wake/REM- and REM-related neurons did not differ significantly from each other.
EMG activity vs. discharge rate
Relationships between the discharge rate of individual neurons and changes in rectified EMG voltage occurring spontaneously across the sleep-waking cycle for each of the four classes of PF-LHA neuron are shown in Fig. 8. The discharge rate of Type I wake-related neurons showed a strong positive correlation with rectified EMG voltage across all sleep-waking states (r = 0.48, t = 6.43, P < 0.001), as was the case for type II wake-related neurons (r = 0.18, t = 2.95, P < 0.01). The discharge rates of a comparable number of wake/REM-related (n = 23) and of REM-related (n = 10) neurons were not correlated with EMG voltage (r = −0.0286, t = −0.410, P = 0.68, and r = −0.019, t = −0.597, P = 0.551, respectively).
DISCUSSION
The lateral hypothalamus has been implicated in several waking behaviours, including feeding, drinking and locomotor activity (Teitelbaum & Epstein, 1962; Sinnamon et al. 1999). This region has also been implicated in the maintenance of the waking state, i.e. in the behavioural and electrographic manifestations of arousal (de Ryck & Teitelbaum, 1978; Shoham & Teitelbaum, 1982). The unit recording studies carried out in the present study are consistent with a role for the lateral hypothalamus in one or more aspects of waking behaviour, because nearly all PF-LHA neurons that we examined were active during waking, but exhibited significantly reduced activity during non-REM sleep. Furthermore, we found that neurons in the PF-LHA that were active during waking were of two general types, namely those that exhibited similar levels of activation during waking and REM sleep and those that exhibited significantly reduced discharge rates during REM sleep compared to waking.
The discovery that neurons containing the hypocretin (or orexin) peptides are localized in the PF-LHA has generated considerable interest in this region of the hypothalamus (de Lecea et al. 1998; Sakurai et al. 1998). The available evidence suggests that activation of Hcrt neurons promotes waking, suppresses REM sleep and antagonizes loss of muscle tone during waking. Hcrt neurons exhibit c-fos activation in response to systemic administration of stimulatory drugs and in response to sustained waking (Scammell et al. 2000; Estabrooke et al. 2001). Central administration of Hcrt in rats acutely increases time spent awake and suppresses non-REM and REM sleep (Bourgin et al. 2000). Micro-perfusion of a Hcrt-2 receptor antagonist into the brainstem promotes REM sleep (Thakkar et al. 1999). Hcrt exerts excitatory effects on locus coeruleus neurons (Hagan et al. 1999; Horvath et al. 1999) and these neurons are normally silent during REM sleep (Aston-Jones & Bloom, 1981). Finally, loss of Hcrt-containing neurons in human narcolepsy (Peyron et al. 2000; Thannickal et al. 2000) and abnormalities in hypocretin receptors in animal models of narcolepsy (Chemelli et al. 1999; Lin et al. 1999) are associated with excessive sleepiness, reduced latencies to REM sleep onset and the intrusion of the muscle atonia of REM sleep into the waking state (known as cataplexy).
Based on this evidence, we hypothesized that Hcrt neurons should exhibit a wake-related discharge pattern, with peak discharge rates in the waking state and reduced rates during non-REM and REM sleep. Here, we report the presence of neurons with wake-related discharge patterns in regions of the rat PF-LHA that contain Hcrt-immunoreactive neurons. Type I wake-related neurons comprised 23 % of the total number of recorded neurons and Type II wake-related neurons accounted for an additional 15 %. Categorization of wake-related neurons as either Type I or II was based solely on the magnitude of the reduction in discharge rate during REM sleep compared to waking. Type I and II neurons exhibited similar patterns of neuronal discharge across the sleep-waking cycle (Fig. 5) and exhibited nearly identical action potential durations. Therefore, rather than constituting two physiologically distinct types of cell, Type I and II wake-related neurons may reflect two components of the same neuronal population.
For all of the PF-LHA cell types described here, changes in discharge rate during different waking behaviours and across the sleep-waking cycle may reflect changes in excitatory and/or inhibitory synaptic input to these cells, rather than endogenous pacemaker activity.
Based on our reconstructions of the microwire bundle tracks, almost all of the 106 neurons recorded in this study were located within the field of Hcrt-1-immunoreactive neurons. However, the extracellular recording method employed here precludes identification of the neurotransmitter phenotype of recorded neurons. Therefore, we were unable to determine whether Hcrt-producing neurons exhibit the wake/REM-related discharge pattern (Fig. 2 and Fig. 3), the wake-related discharge pattern (Fig. 4 and Fig. 5) or the REM-related discharge pattern (Fig. 6 and Fig. 7).
Kilduff & Peyron (2000) hypothesized that Hcrt-producing neurons exhibit activation during both waking and REM sleep, with diminished activity during non-REM sleep. We recorded several cells in the PF-LHA that exhibited a wake/REM-related discharge pattern. In fact, these were the most commonly encountered neurons, as is the case in more posterior regions of the lateral hypothalamus (Szymusiak et al. 1989; Krilowicz et al. 1994; Steininger et al. 1999). As a group, wake/REM-related cells exhibited a significant reduction in discharge rate during non-REM sleep compared to AW, QW and REM sleep and, therefore, fit the state-dependent discharge profile predicted by Kilduff & Peyron (2000) for Hcrt cells.
Regardless of which of the state-dependent discharge patterns defined here on the basis of the REM/wake discharge rate ratio is eventually found to be typical of Hcrt-producing neurons, it seems probable that Hcrt-producing neurons comprise only a subset of the neurons recorded in the present study. Hcrt-producing neurons in the PF-LHA are interspersed with other cell types, including neurons that contain MCH (Broberger et al. 1998; Peyron et al. 1998). Hcrt-producing neurons are outnumbered by non-Hcrt cells throughout the PF-LHA (Peyron et al. 1998). Therefore, assuming a random sampling of cell types in the present study, only a minority of cells that were recorded from microelectrode passes through this region would be Hcrt neurons. Given the relatively high percentage of wake-related neurons that we encountered in the PF-LHA (38 %), it seems plausible that this discharge pattern is not confined to PF-LHA cells with a single neurotransmitter phenotype.
Neurons with wake-related discharge patterns (also referred to as ‘REM-off’ neurons) have been most extensively characterized in brain regions that contain monoaminergic neurons. These include the serotonergic neurons in the dorsal raphe nuclei (McGinty & Harper, 1976; Jacobs & Fornal, 1999; Guzman-Marin et al. 2000), noradrenergic neurons of the locus coeruleus (Aston-Jones & Bloom, 1981) and histaminergic neurons in the tuberomammillary nuclei of the posterior hypothalamus (Vanni-Mercier et al. 1984; Steininger et al. 1999). Wake-related neurons recorded in the PF-LHA in the present study exhibit both similarities to and differences from the discharge patterns described for monoaminergic neurons. For example, the magnitude of suppression of discharge during REM sleep compared to AW was similar for PF-LHA Type I wake-related neurons and putative serotonergic neurons in the dorsal raphe nucleus (Table 1). However, the discharge of PF-LHA neurons was more strongly modulated from AW to QW than has been described for monoaminergic neurons in the dorsal raphe and other regions. Another characteristic of putative serotonergic neurons is a progressive decline in discharge rate from light to deep non-REM sleep and a further significant decrease in discharge rate from deep non-REM to REM sleep (Lydic et al. 1985; Guzman-Marin et al. 2000). However, PF-LHA wake-related neurons did not show a significant reduction in discharge rate from QW to non-REM sleep to REM sleep in the present study. Therefore, it appears that wake/REM-related neurons in the PF-LHA are more selectively active during AW than wake-related neurons recorded in monoaminergic brain regions.
The increased discharge rates of PF-LHA wake-related neurons during AW compared to QW prompted us to examine the relationship between neuronal discharge and muscle activity across each of the sleep and waking states. The discharge rates of both Type I and Type II wake-related neurons exhibited strong positive correlations with muscle activity (Fig. 8), whereas the discharge rates of the other two classes of neuron did not. The correlation between PF-LHA wake-related neuronal activity and muscle activity suggests a role for these cells in motor control, behavioural arousal and/or the regulation of muscle activity across the sleep-waking cycle.
Acknowledgments
This research was supported by the US Department of Veterans Affairs Medical Research Service and US National Institutes of Health grants MH 47480, MH 61354 and HL 60296.
REFERENCES
- Alam MN, McGinty D, Szymusiak R. Thermosensitive neurons of the diagonal band in rats: relation to wakefulness and non-rapid eye movement sleep. Brain Research. 1997;752:81–89. doi: 10.1016/s0006-8993(96)01452-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinepherine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. Journal of Neuroscience. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Frigo L, Rissman RA, Casatti CA, Nahon JL, Bauer JA. The distribution of melanin-concentrating hormone in the monkey brain (Cebus apella) Brain Research. 1998;804:140–143. doi: 10.1016/s0006-8993(98)00662-3. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. Journal of Comparative Neurology. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, De Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. Journal of Neuroscience. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. Journal of Comparative Neurology. 1998;402:460–474. [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ryck M, Teitelbaum P. Neocortical and hippocampal EEG in normal and lateral hypothalamic damaged rats. Physiology and Behavior. 1978;20:403–409. doi: 10.1016/0031-9384(78)90320-7. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. Journal of Neuroscience. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Marin R, Alam MN, Szymusiak R, Drucker-Colin R, McGinty D. Discharge modulation of rat dorsal raphe neurons during sleep and waking: effects of preoptic/basal forebrain warming. Brain Research. 2000;875:23–34. doi: 10.1016/s0006-8993(00)02561-0. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proceedings of the National Academy of Sciences of the USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. Journal of Comparative Neurology. 1999;415:145–159. [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacologyy. 1999;21:9–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends in Neurosciences. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Krilowicz BL, Szymusiak R, McGinty D. Regulation of posterior lateral hypothalamic arousal related neuronal discharge by preoptic anterior hypothalamic warming. Brain Research. 1994;668:30–38. doi: 10.1016/0006-8993(94)90507-x. [DOI] [PubMed] [Google Scholar]
- Krolicki L, Chodobski A, Skolasinska K. The effect of stimulation of the reticulo-hypothalamic-hippocampal systems on the cerebral blood flow and neocortical and hippocampal electrical activity in cats. Experimental Brain Research. 1985;60:551–558. doi: 10.1007/BF00236941. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Brain monoamines and peptides: role in the control of eating behavior. Federation Proceedings. 1986;45:1396–1403. [PubMed] [Google Scholar]
- Levitt DR, Teitelbaum P. Somnolence, akinesia and sensory activation of motivated behavior in the lateral hypothalamic syndrome. Proceedings of the National Academy of Sciences of the USA. 1975;72:2819–2823. doi: 10.1073/pnas.72.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, De Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. The time course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep-wake cycles. Experimental Brain Research. 1985;12:131–144. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- McGinty D, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Research. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Teitelbaum P. Further analysis of sensory inattention following lateral hypothalamic damage in rats. Journal of Comparative and Physiological Psychology. 1974;86:375–395. doi: 10.1037/h0035941. [DOI] [PubMed] [Google Scholar]
- Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. NeuroReport. 2000;11:3423–3426. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Research. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Medicine. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. European Journal of Neuroscience. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. Journal of Neuroscience. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Teitelbaum P. Subcortical waking and sleep during lateral hypothalamic ‘somnolence’ in rats. Physiology and Behavior. 1982;28:323–334. doi: 10.1016/0031-9384(82)90082-8. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Narcolepsy. Scientific American. 2000;282:58–63. [Google Scholar]
- Sinnamon HM, Karvosky ME, Ilch CP. Locomotion and head scanning initiated by hypothalamic stimulation are inversely related. Behavioral Brain Research. 1999;99:219–229. doi: 10.1016/s0166-4328(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Research Bulletin. 1985;14:521–524. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Research. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Stock G, Rupprecht U, Stumpf H, Schlor KH. Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. Journal of the Autonomic Nervous System. 1981;3:503–510. doi: 10.1016/0165-1838(81)90083-7. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Iriye T, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamic area of cats. Brain Research Bulletin. 1989;23:111–120. doi: 10.1016/0361-9230(89)90169-x. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Epstein A. Recovery of feeding and drinking after lateral hypothalamic lesions. Psychological Reviews. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Cape EG, Winston S, Strecker RE, McCarley RW. REM sleep enhancement and behavioral cataplexy following orexin (hypocretin)-II receptor antisense perfusion in the pontine reticular formation. Sleep Research Online. 1999;2:113–120. [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timo-Iaria C, Negrao N, Schmidek WR, Hoshino K, Demenezes CEL, Darocha TL. Phases and states of sleep in rat. Physiology and Behavior. 1970;5:1057–1062. doi: 10.1016/0031-9384(70)90162-9. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Jouvet M. Neurons specifiques de l'eveil dans l'hypothalamus posterior du chat. Comptes rendus Academie des Sciences. 1984;298:195–200. [PubMed] [Google Scholar]
- Yamamoto Y, Ueta Y, Serino R, Nomura M, Shibuya I, Yamashita H. Effects of food restriction on the hypothalamic prepro-orexin gene expression in genetically obese mice. Brain Research Bulletin. 2000;51:515–521. doi: 10.1016/s0361-9230(99)00271-3. [DOI] [PubMed] [Google Scholar]


