Abstract
Whole-cell patch-clamp recordings taken from guinea-pig duodenal myenteric neurones within intact ganglia were used to determine the properties of S and AH neurones. Major currents that determine the states of AH neurones were identified and quantified. S neurones had resting potentials of −47 ± 6 mV and input resistances (Rin) of 713 ± 49 MΩ at voltages ranging from −90 to −40 mV. At more negative levels, activation of a time-independent, caesium-sensitive, inward-rectifier current (IKir) decreased Rin to 103 ± 10 MΩ. AH neurones had resting potentials of −57 ± 4 mV and Rin was 502 ± 27 MΩ. Rin fell to 194 ± 16 MΩ upon hyperpolarization. This decrease was attributable mainly to the activation of a cationic h current, Ih, and to IKir. Resting potential and Rin exhibited a low sensitivity to changes in [K+]o in both AH and S neurones. This indicates that both cells have a low background K+ permeability. The cationic current, Ih, contributed about 20 % to the resting conductance of AH neurones. It had a half-activation voltage of −72 ± 2 mV, and a voltage sensitivity of 8.2 ± 0.7 mV per e-fold change. Ih has relatively fast, voltage-dependent kinetics, with on and off time constants in the range of 50–350 ms. AH neurones had a previously undescribed, low threshold, slowly inactivating, sodium-dependent current that was poorly sensitive to TTX. In AH neurones, the post-action-potential slow hyperpolarizing current, IAHP, displayed large variation from cell to cell. IAHP appeared to be highly Ca2+ sensitive, since its activation with either membrane depolarization or caffeine (1 mm) was not prevented by perfusing the cell with 10 mm BAPTA. We determined the identity of the Ca2+ channels linked to IAHP. Action potentials of AH neurones that were elongated by TEA (10 mm) were similarly shortened and IAHP was suppressed with each of the three Ω-conotoxins GVIA, MVIIA and MVIIC (0.3–0.5 μm), but not with Ω-agatoxin IVA (0.2 μm). There was no additivity between the effects of the three conotoxins, which indicates the presence of N- but not of P/Q-type Ca2+ channels. A residual Ca2+ current, resistant to all toxins, but blocked by 0.5 mm Cd2+, could not generate IAHP. This patch-clamp study, performed on intact ganglia, demonstrates that the AH neurones of the guinea-pig duodenum are under the control of four major currents, IAHP, Ih, an N-type Ca2+ current and a slowly inactivating Na+ current.
The aim of the present paper was to determine the electrotonic properties of myenteric neurones, using patch-clamp recording from non-dissociated myenteric neurones, with emphasis on the identification and quantitation of the ionic currents that modulate the resting membrane potential of AH neurones. Recordings were made with a technique we have recently developed for patch-clamp recording from intact ganglia (Kunze et al. 2000).
The electrophysiological properties of myenteric neurones in intact ganglia from the small intestine of the guinea-pig have been investigated previously by intracellular recordings in myenteric plexus/longitudinal muscle preparations. The first intracellular recording studies were performed using duodenal (Hirst et al. 1974) and ileal tissue (Nishi & North, 1973). In both of these parts of the intestine, the studies separated the myenteric neurones into two groups, S and AH neurones, terms that were introduced in 1974 for duodenal neurones (Hirst et al. 1974). S neurones were so named because they received prominent fast synaptic inputs, while AH neurones did not. AH neurones were so named because the action potential is followed by a long-lasting after-hyperpolarization (AHP). It was later demonstrated that both cell types receive slow EPSPs (Wood & Meyer, 1978; Johnson et al. 1980, 1981; Bornstein et al. 1984).
Correlations between electrophysiological characteristics and cell morphology have been made by intracellular recording using micropipettes filled with fluorescent dyes (e.g. biocytin or neurobiotin) in the ileum (Hodgkiss & Lees, 1983; Iyer et al. 1988; Bornstein et al. 1991) and the duodenum (Clerc et al. 1998). These and other studies showed that AH neurones comprise a single population with Dogiel type II morphology, and were later shown to be sensory, while S neurones were uniaxonal neurones (Bornstein et al. 1994). Motor neurones to muscle, secretomotor neurones and interneurones, of which there are four types in the myenteric ganglia of the guinea-pig small intestine (Costa et al. 1996; Furness, 2000) are S neurones.
At the present time, two different ionic currents are known to modulate the resting membrane potential of AH neurones directly: a K+ inward rectifier current (IKir), which has been described in both AH and S neurones, and a slow AHP current (IAHP), which is typical of the AH neurones. A cationic current activated by hyperpolarization (Ih) has been described in AH neurones (Galligan et al. 1990) and in a subpopulation of S neurones, the filamentous interneurones in the ileum (Song et al. 1997). This current has been shown to contribute to the membrane potential in other types of neurone (Doan & Kunze, 1999). In addition, a high-voltage-activated (HVA) Ca2+ current is activated during the action potential of AH neurones (Hirst et al. 1974) and less prominently in some filamentous interneurones (Song et al. 1997; Clerc et al. 1998). In AH neurones, but not in the filamentous interneurones of the duodenum (Clerc et al. 1998), this Ca2+ current indirectly controls the membrane potential by activating the IAHP.
Although described in a sharp electrode study (Galligan et al. 1990), the Ih has not been recognized in a recent patch electrode study of dissociated myenteric neurones (Zholos et al. 1999) in which only IKir was identified. We have found both currents in AH neurones and, because Ih is known to activate at potentials between −45 and −60 mV in other neuronal types (Pape, 1996), we investigated its possible contribution to the resting membrane potential. We have also detected a slowly inactivating Na+ current that was poorly sensitive to TTX and might modulate the resting membrane potential of these neurones.
The activation of IAHP is triggered by the opening of HVA Ca2+ channels whose identity is controversial (Baidan et al. 1992b; Furness et al. 1998; Starodub & Wood, 1999; Vogalis et al. 2001). Intracellular studies (Kunze et al. 1994; Furness et al. 1998) as well as a recent patch-clamp study (Vogalis et al. 2001) suggest that N-type but not L-type channels are involved, because the hump on the falling phase of the action potential of AH neurones persisted in the presence of nicardipine, but was attenuated by the N-type Ca2+ channel blocker Ω-conotoxin GVIA (Ω-CgTX GVIA). According to patch-clamp studies performed in dissociated myenteric neurones, the HVA Ca2+ current is suppressed by this conotoxin (Baidan et al. 1992b). In the case of rat myenteric neurones in cell culture, both L- and N-type Ca2+ channels contributed to the HVA Ca2+ current (Franklin & Willard, 1993). However, on the basis of the effect of Ω-CgTX MVIIC, which is a blocker of P- and Q-type Ca2+ channels, and to a lesser extent of N-type channels (Uchitel, 1997), Starodub & Wood (1999) concluded that AH neurones express mainly P/Q-type channels. Therefore, we performed a systematic pharmacological investigation to characterize the HVA Ca2+ channel types. In addition, we evaluated the sensitivity of IAHP to [Ca2+]i.
Some of the results presented here have been published in abstract form (Clerc et al. 2000).
METHODS
Preparation
Patch-clamp experiments were carried out on non-dissociated neurones, in intact ganglia of the myenteric plexus. All experiments were performed using 2- to 3-cm-long segments of the proximal duodenum excised from guinea-pigs (150–400 g) after they were stunned and decapitated. Guinea-pigs were Hartley strain from the inbred colony at the IFR ‘Sciences du Cerveau’, CNRS, Marseilles. All procedures were approved by the French Ministry of Agriculture and Fisheries and were in agreement with the European Communities Council Directive 86/609/EEC. The segments of duodenum were placed in a recording dish that had a transparent base and was filled with oxygenated Krebs solution (see below), which was kept at room temperature during dissection, but which was heated to 34 °C during recording. The myenteric plexus was exposed by dissecting away the mucosa, submucosal plexus and circular muscle, and the preparation was mounted in a transparent recording dish on the stage of an inverted microscope. The surface of one ganglion was exposed to 0.01–0.02 % protease type XIV (Sigma, St Louis, MO, USA) in physiological saline, and the upper surfaces of neurones were cleaned by sweeping with a hair over the ganglion (Gola & Niel, 1993; Kunze et al. 2000).
Electrophysiological recordings
Membrane current and voltage were measured with a List EPC7 amplifier using the whole-cell patch-clamp technique. Borosilicate glass capillaries were pulled in four steps with a P 87 Flaming-Brown puller (Sutter Instruments) to obtain micropipettes with a resistance of about 2–4 MΩ. A fairly strong pressure (60–80 hPa) was applied to the pipette in order to eliminate residual matter adhering to the nerve cell surface. The pressure was released when the pipette contacted the membrane and suction (10–20 hPa) was applied to form a gigaseal. The whole-cell configuration was achieved by subjecting the patch membrane to a slight suction. Current and voltage traces were low-pass filtered at 5 kHz, then digitized at 44 kHz, stored on a computer and subsequently analysed. Quasi-steady-state I-V relationships were obtained using slow voltage ramps rather than voltage steps. The voltage error due to uncompensated series resistance ranged from 1 to 3 mV. Values are expressed as means ± s.d. or s.e.m. (specified in the text).
Solutions
If not otherwise specified in the text and figure legends, solutions were as follows: Krebs solution (mm): 118 NaCl, 4.8 KCl, 1.0 NaH2PO4, 2.5 CaCl2, 1 MgSO4, 25 NaHCO3 and 11.1 d-glucose bubbled with a 95 % O2-5 % CO2 gas mixture (pH 7.4). Hyoscine (1 μm) and nicardipine (3 μm) were added to the superfusate to block spontaneous muscle movement. Ionic substitutions were achieved by isosmotically changing the [Na+] or [K+] of the following saline (mm): 145 NaCl, 4.8 KCl, 2.5 CaCl2, 1 MgSO4 and 10 Hepes. Na+ was replaced with either N-methyl d-glucamine (NMDG) or choline. The Hepes saline was also used when Cd2+ and Ba2+ were added. The patch pipettes were filled with a solution consisting of (mm): KCl 140, NaCl 4, CaCl2 1, MgCl2 2, Hepes 10, EGTA 2, GTP 0.2, (pH 7.3). Any changes in this standard filling saline are indicated in the text.
Cells were continuously superfused with the external solution (2 ml min−1). Fast local applications of drugs were performed by gravity from several reservoirs connected to a common outlet positioned as near as possible to the cell investigated. The solution to be applied was selected by activating solenoid valves. Salts were purchased from Prolabo (Lyon, France); the Ω-conotoxins MVIIA, MVIIC and GVIA were from Latoxan (Valence, France), and Ω-agatoxin IVA was from Peptides International (Louisville, KY, USA). All of the other chemicals were from Sigma (St Louis, MO, USA).
Criteria for identifying AH and S neurones
Some of the superficial neurones in the cleaned ganglia remained covered with glial cells, collagen fibres or basal lamina. Only recordings in which the seal resistance was larger than 5 GΩ were utilized. In a representative series of 20 experiments, the mean number of patch-clamped cells per ganglion was: glial cells, 0.65 (range 0–4); AH neurones, 2.6 (range 0–5); S neurones, 1.1 (range 0–4).
AH neurones had each of the following electrophysiological characteristics: a large action potential with a characteristic hump on the repolarizing phase (Fig. 1Aa), a long-lasting AHP (Fig. 12B) and a sag in the voltage response to hyperpolarizing current pulses (Kunze et al. 2000; see Fig. 4A). S neurones responded with an exponential hyperpolarization to small inward current pulses (see Fig. 2A). Their action potentials had no hump on the falling phase and were not followed by a long-lasting AHP.
Figure 1. Action potential characteristics of AH and S neurones.
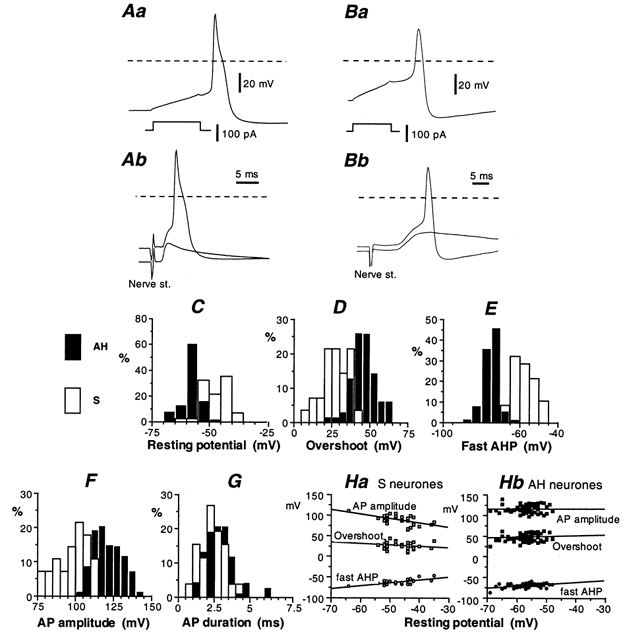
A and B, action potentials triggered by stimulating an AH neurone (Aa) and an S neurone (Ba) with an intracellular current pulse (lower trace in Aa and Ba) or applying an electrical shock (nerve stimulation, 0.1 ms, 10–20 V) to one of the nerves connected to the ganglion (Ab and Bb). In Ab, the action potential of the AH neurone was antidromically evoked. A non-invading electrotonic potential (‘A spike’; lower trace in Ab) was revealed by hyperpolarizing the cell. The nerve stimulation in Bb evoked a fast EPSP (lower trace) that was large enough to trigger an overshooting action potential in an S neurone. Hyperpolarization revealed the synaptic potential without the superimposed action potential (lower trace). Dashed lines show the 0 mV level. C, distribution of the resting potential of AH (filled columns) and S neurones (open columns). The resting potential was measured within 30 s after patch rupture and is not corrected for electrode tip potential. This histogram was derived from the results of 71 AH neurones and 38 S neurones. D-G, distribution of the action potential characteristics of AH neurones (filled columns) and S neurones (open columns). Same population as in C. The peak-to-peak action potential amplitude (F) was measured from the overshoot (D) to the after-hyperpolarization (AHP) immediately following the action potential repolarization (E). The action potential duration (G) was measured at half amplitude (∼ −20 mV). Ha and Hb, dependence of the action potential characteristics of S and AH neurones on the resting potential. In C-G, results are expressed as the percentage (%) of the cell population by bin (5 mV in C-F, 0.5 ms in G).
Figure 12. Block of the slow IAHP with Ω-CgTX MVIIA.
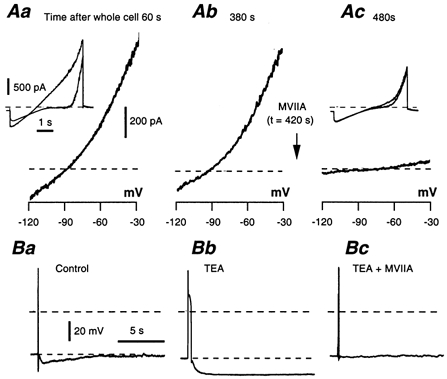
A, IAHP recorded as a difference current in an AH neurone 60 s (Aa) and 380 s (Ab) after breaking the membrane patch. Inset in Aa: two-ramp method applied from −120 to 0 mV. These successive controls were performed systematically to detect a possible spontaneous decrease in IAHP. Ac, same protocol applied 60 s after adding 0.5 μm Ω-CgTX MVIIA, which abolished IAHP. B, effect of 0.5 μm Ω-CgTX MVIIA on the slow AHP following the elongation of the action potential with TEA (10 mm). The slow AHP was abolished.
Figure 4. Rin of AH neurones.
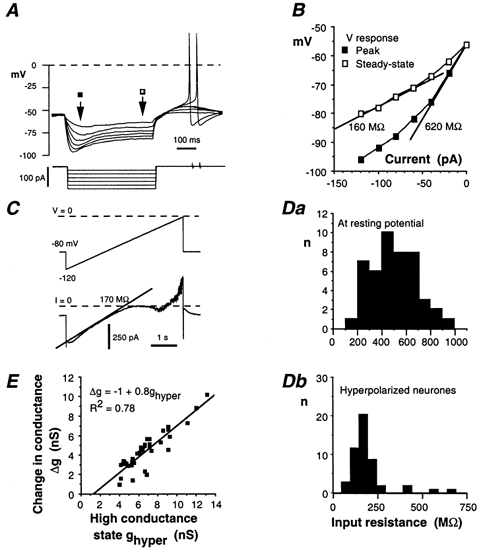
A, response of an AH neurone to 0.5 s hyperpolarizing current pulses incremented by 20 pA. B, I-V plot for the cell illustrated in A. Filled squares: relationship prior to Ih activation; open squares: relationship in presence of Ih. Slope resistances are measured from the straight lines shown on the figure. C, same neurone subjected to a slowly (24 mV s−1) rising voltage-ramp (voltage-clamp conditions) from −120 to 0 mV. The slope of the current trace at voltages positive to −90 mV corresponded to a Rin of 170 MΩ. D, distribution of the Rin in AH neurones. Da, neurones at the resting potential; Rin determined as shown in B (filled squares). Db, Rin upon activation of the h current (open squares in B). E, relationship between the high conductance state ghyper (nS) in hyperpolarized neurones (data are from Db) and the increase in conductance (Δg) that occurred when AH neurones were hyperpolarized from the resting level.
Figure 2. Input resistance of S neurones.
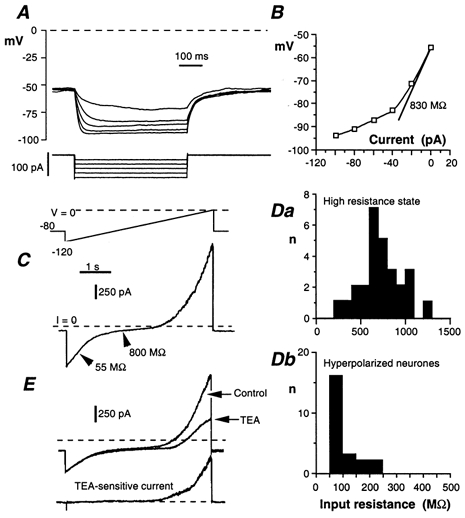
A, response of an S neurone to 0.5 s hyperpolarizing current pulses incremented by 20 pA. B, corresponding I-V plot measured at the end of the current pulse. Input resistance (Rin) at rest is given by the slope of the straight line. Reduced Rin at hyperpolarized potentials is due to the presence of an inward rectifier. C, S neurone subjected to a slowly rising (24 mV s−1) voltage ramp (voltage-clamp conditions) from −120 to 0 mV. S neurones had a high Rin between −90 and −40 mV. The impedance was considerably reduced by the activation of an inwardly rectifying K+ current at V < −90 mV and by a delayed rectifier at V > −40 mV. D, distribution of Rin of S neurones in the high-resistance state (Da) and upon activation of the inwardly rectifying current (Db). E, effect of TEA (10 mm) on the I-V curve. Same voltage programme as in C. Lower trace, difference current revealing the delayed rectifier.
AH neurones were also fired antidromically by applying a single electric shock (0.1 ms, 8–25 V) to one of the nerve fibre bundles joining the ganglion containing the nerve cell (Fig. 1Ab). The antidromic origin of the evoked action potential was confirmed by performing a collision test (not illustrated). Single electric shocks never induced any fast EPSP in AH neurones. In the case of S neurones, nerve stimulation generally resulted in an action potential superimposed on a fast excitatory synaptic potential. The synaptic potential was unmasked by moderately hyperpolarizing the cell (Fig. 1Bb).
RESULTS
Data were collected from 92 ganglia. In addition to the currents investigated in detail here, both S and AH neurones had a TTX-sensitive Na+ current (INaT), which participated in the generation of the action potential, an outwardly rectifying voltage-dependent and TEA-sensitive K+ current, and a transient A-type K+ current (Baidan et al. 1992a; Zholos et al. 1999; Hanani et al. 2000; Starodub & Wood, 2000; Vogalis et al. 2000). In both AH and S neurones, the inactivation of the A current was fast, 19–25 ms (n = 9) at −50 mV. Its voltage dependence (fitted to Boltzmann functions) had a sensitivity of 6.2 ± 0.9 mV, and a voltage at half activation of −78 ± 5 mV. These values are similar to those measured in cultured AH neurones (Vogalis et al. 2000). Therefore, at voltages positive to −60 mV, the A current played a limited role in shaping the action potential of AH and S neurones.
Action potential parameters
Immediately after the patch was disrupted, AH neurones had a resting potential of −57 ± 4 mV (mean ± s.d.), range −48 to −69 mV (n = 61). S neurones had a smaller resting potential to AH neurones, −47 ± 6 mV, range −36 to −64 mV (n = 35). The action potential was triggered with a 10-ms-duration depolarizing current pulse, the amplitude of which was adjusted so that the action potential was evoked after the pulse (Fig. 1Aa and Ba).
Action potential parameters, collected from 71 AH neurones investigated using the standard pipette saline, were (mean ± s.d.): overshoot, 46 ± 6 mV; maximum hyperpolarization of the fast AHP, −70 ± 5 mV; peak-to-peak amplitude, 115 ± 8 mV (amplitude from membrane potential, 102 ± 5 mV); duration (measured at half-amplitude) 2.8 ± 0.2 ms. The distribution of the AH action potential parameters are given in Fig. 1D-G (filled columns). The corresponding values for S neurones (n = 35) were: overshoot, 27 ± 9 mV; fast AHP, −62 ± 6 mV; peak-to-peak amplitude, 88 ± 12 mV (amplitude from membrane potential, 73 ± 12 mV); action potential duration, 2.2 ± 0.8 mV. The distribution of the S action potential parameters is given in Fig. 1D-G (open columns).
The relatively low action potential amplitude of S neurones was for a large part accounted for by the low resting polarization of these cells. When S neurones were progressively hyperpolarized by injecting an increasing inward current, the action potential overshoot increased and reached values comparable to those of AH neurones. The relationship between the cell polarization and the action potential characteristics of the S and AH populations shown in Fig. 1D-F are given in Fig. 1Ha and Hb, respectively.
Electrotonic properties of S neurones
Electrotonic properties were determined in either current-clamp or voltage-clamp conditions. The input resistance (Rin) was measured by injecting 0.5 s hyperpolarizing current pulses of increasing amplitude (from 20 to 120–160 pA). S neurones responded by a large hyperpolarization that tended to saturate at large current intensity (Fig. 2A). The corresponding I-V plot showed that the S neurones have a high Rin at voltages negative to −40 mV and that Rin was considerably reduced at voltages negative to −90 mV (Fig. 2B). The time constant (τ = 32 ± 4 ms, n = 18) of the exponential change in voltage in response to small (10 to −20 pA) current pulses yielded a membrane capacitance of 48 ± 3 pF.
The quasi steady-state I-V relationship obtained with slowly rising voltage ramps from −120 to 0 or +20 mV was S-shaped (Fig. 2C). From −90 to −40 mV, S neurones had a high Rin (713 ± 49 MΩ, n = 27; Fig. 2Da). On both sides of this range the membrane conductance increased. The conductance increase at V > −40 mV resulted from the activation of a TEA-sensitive delayed rectifier (Fig. 2E). The increase at V < −90 mV was due to the activation of a time-independent inwardly rectifying K+ current, IKir. The inward rectifier decreased Rin to 103 ± 10 MΩ (n = 23; Fig. 2Db). A prominent inward rectifier was present in 30 out of 32 S neurones. The IKir conductance, obtained by subtracting the conductance in the high-resistance state from that with IKir activated, had a mean value (± s.e.m.) of 9.5 ± 0.9 nS (range 3.1–17.4 nS).
The inward rectifier was blocked by adding 1–2 mm Cs+ (Fig. 3A) to the bath saline. As expected for an inward rectifier, the inward current was strongly dependent on the [K+]o of the bath saline for both its conductance and location on the voltage axis (Fig. 3B). The voltage at which this current vanished followed perfectly the change in K+ driving force derived from the Nernst relationship (Fig. 3C).
Figure 3. Properties of the inward rectifier of S neurones.
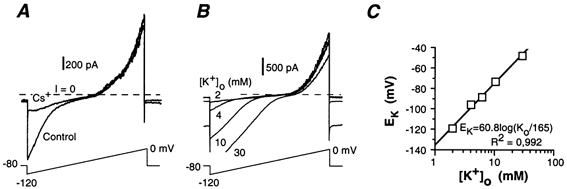
A, current response to depolarizing voltage-ramps in an S neurone in physiological saline (control) and in the presence of 2 mm extracellular Cs+. Cs+ abolished the increase in inward current at V < −90 mV. B, increase of the inwardly rectifying current by changing the [K+]o from 2 to 30 mm. C, plot of the threshold of the inwardly rectifying current, identified as the K+ reversal potential (EK), versus [K+]o. Data are from the experiment illustrated in B.
The high-resistance state was poorly dependent on [K+]o. In the experiment illustrated in Fig. 3B, the resting potential changed from −48 to −40 mV when [K+]o was increased from 2 to 30 mm. This voltage shift was much smaller than the value predicted (i.e. ΔV = 68 mV) for selective K+ conductance by the Nernst equation (Fig. 3C). This means that S neurones have a low resting K+ permeability.
Electrotonic properties of AH neurones
The electrotonic properties of AH neurones were determined as described for S neurones. The voltage response to hyperpolarizing current displayed a pronounced sag, even with small (20 pA) current pulses (Fig. 4A). This sag can be ascribed to the activation of a cationic h-type current (see below). Rin was obtained by plotting the peak of the voltage response versus injected current, and by taking the slope of the I-V curve extrapolated to zero current (Fig. 4B). This procedure avoided underestimation of Rin due to the activation of Ih during current injection. Activation of Ih, already present with currents as small as 20 pA, produced a large decrease in Rin when the current pulse was increased (open squares in Fig. 4B). This effect was quantified by subjecting the neurones to slowly rising voltage ramps (20–25 mV s−1) from −120 mV (Ih fully activated) to 0 mV, and by measuring the slope conductance at voltages positive to −90 mV (Fig. 4C). Voltages more negative than −90 mV activated an inward rectifier that was partly masked by the large Ih. This procedure gave values similar to those derived from the extrapolated steady-state I-V plot shown in Fig. 4B (170 MΩ compared to 160 MΩ).
The Rin of AH neurones displayed large variations, from 195 to 926 MΩ, with a mean ± s.e.m. of 502 ± 27 MΩ (n = 47; Fig. 4Da). In 53 out of 58 AH neurones, Rin fell upon activation of Ih (Fig. 4Db), on average to 194 ± 16 MΩ. Some of the variation of Rin might be attributable to the seal quality or, more probably, from variation in the level of Ih expression. There was indeed a strong correlation between the increase in input conductance (Δg) upon hyperpolarization (mostly attributable to Ih activation) and the level reached in the high-conductance state (Fig. 4E).
The cell capacitance was derived from the time constant of the initial exponential voltage change in response to small current pulses (5–10 pA). Its mean value was 61 ± 4 pF (n = 18).
Changing [K+]o from 2 to 30 mm increased the inwardly rectifying current as well as Ih, but did not significantly alter the slope conductance between −60 and −40 mV (Fig. 5A). In eight neurones, the mean resting potential changed from −58 mV at 2 mm [K+]o to −33 mV at 30 mm [K+]o, which was smaller than the value predicted for selective K+ conductance by the Nernst equation (see above; Fig. 5B) as well as the value measured when a pure K+ conductance (IAHP) is activated (Fig. 5B). Therefore, like S neurones, AH neurones exhibited a low permeability to K+ at rest.
Figure 5. Sensitivity of AH neurones to changes in [K+]o.
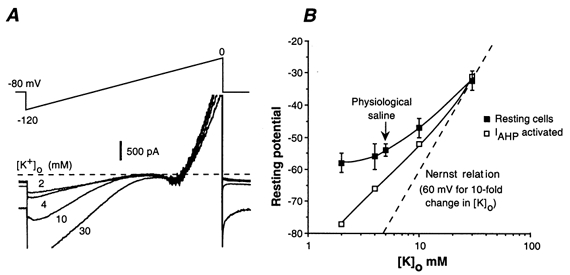
A, increase in IKir and Ih with elevated [K+]o (same conditions as in Fig. 3B). B, effects of changes in [K+]o on the membrane potential of AH neurones at rest (filled squares, n = 7) and when IAHP is activated (open squares) compared to the theoritical changes for a K+ conductance according to the Nernst relationship (dotted line). Open squares, example of the AHP sensitivity to changes in [K+]o.
Hyperpolarization-activated cationic h current in AH neurones
The sag in the voltage response of AH neurones to current pulses is characteristic of the presence of an h-type current. This current was not present in S neurones. The properties of Ih were determined in voltage-clamped neurones held between −40 and −50 mV and subjected to 1 s hyperpolarizing voltage pulses (Fig. 6A). This current had a null-current voltage (Eh) close to −40 mV. The maximal conductance (gh) reached at large polarization and the steady-state activation (a∞) were determined from the amplitude of the slowly activating h current, as shown in Fig. 6A, and the driving force V - Eh: Ih = gh × a∞ × (V - Eh). Except for a few neurones in which Ih was poorly present (5 of 58), most AH neurones had a prominent h conductance ranging from 2 to 8 nS (see below; Fig. 6B). The activation, a∞, was well approximated by the following equation:
(Fig. 6C) with the half-activation voltage being V0 = −72 ± 2 mV (n = 12), and the voltage sensitivity factor being p = 8.2 ± 0.7 mV, for an e-fold change in gh.
Figure 6. Properties of the cationic h current (Ih) of AH neurones.
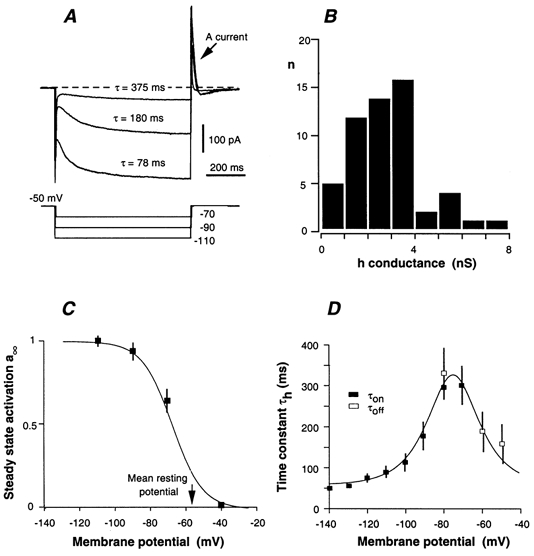
A, current response of an AH neurone to 1 s hyperpolarizing voltage pulses applied from −50 mV. Note the transient activation of a fast A-type K+ current immediately after the pulse. B, distribution of the maximal h conductance in 58 AH neurones. The h conductance was evaluated from the amplitude of the steady-state Ih and the Ih reversal potential, Eh = −40 mV. C, steady-state activation, a∞, of Ih. Data are from seven AH neurones. The curve was drawn according to the equation given in the text. D, voltage dependence of the time constant, τh of Ih changes. Data are from 14 AH neurones. τh was determined by fitting the current change to exponential curves during the hyperpolarizing voltage pulse (τon) and after the current pulse (τoff). The continuous curve was drawn according to the equation given in the text.
The h current developed almost monoexponentially with a time constant (τh) that decreased at large polarizations. The voltage dependence of τh was determined from both activation and deactivation time courses. The τh-V curve was bell-shaped (Fig. 6D). It was approximated by the following relationship:
V0 had the value determined above; a = 56 ± 8 ms; b = 537 ± 49 ms; r = 11.9 ± 1.8 mV.
The activation curve shows that Ih was activated by about 20 % (see Fig. 6C) at the mean resting potential of AH neurones (−57 mV). The maximal h conductance (gh) reached at large polarization was 2.85 ± 0.22 nS (n = 55), which means that Ih contributed 0.57 nS to the input conductance of resting AH neurones. This input conductance was 1.99 ± 0.11 nS. Therefore, the remaining background conductance, including the K+ conductance, would be about 1.42 nS. This estimate is supported by the following data. Cationic h currents are blocked by [Cs+]o (Pape, 1996). Adding 2 mm Cs+ to the bath saline fully and reversibly blocked Ih in AH neurones (Fig. 7Aa). In these conditions, the I-V relationship in the −100 to −40 mV range became almost linear, with a slope of 1.49 ± 0.13 nS (n = 14; Fig. 7Ab and Ba). Note that Cs+ also blocked IKir, which is visible at voltages negative to −100 mV in the instantaneous I-V plot shown in Fig. 7Ab (control curve at t = 0). As in S neurones, IKir was instantaneously activated, and was reversibly blocked by adding 2 mm Ba2+, while Ih persisted (Fig. 7C). The caesium-sensitive current in the voltage range −100 to −20 mV was divided by the cationic driving force (V - Eh), resulting in the activation curve shown in Fig. 7Bb. This method yielded results similar to that described above. Under current-clamp conditions, caesium-treated AH neurones responded to hyperpolarizing current pulses by an exponential hyperpolarization far larger than in control conditions, indicating an increase in Rin, which reached 0.7 GΩ (Fig. 7D). However, Cs+ treatment of neurones did not cause any change in resting membrane potential.
Figure 7. Contribution of Ih to the input conductance of AH neurones.
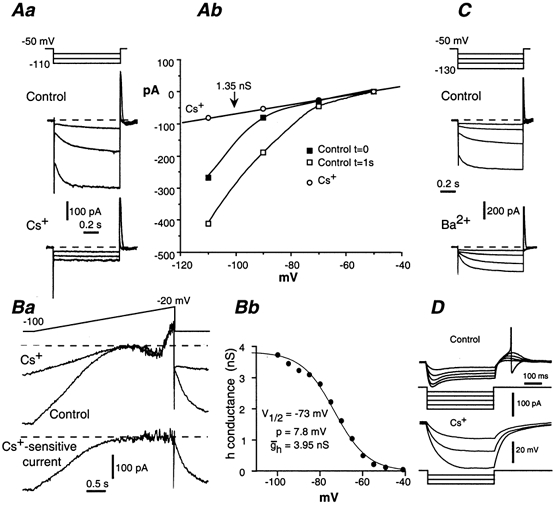
A and B, block of Ih with extracellularly applied Cs+ (2 mm). Aa, Ih induced by 1 s hyperpolarizing voltage pulses (incremented by 20 mV). Ab, corresponding I-V plot. In control conditions, currents were measured at the beginning (filled squares) and at the end (open squares) of the voltage pulse. Cs+ (circles) blocked both Ih and the inwardly rectifying K+ current, which is prominent at V < −90 mV. Ba, currents induced by slowly depolarizing the neurone (16 mV s−1) in control conditions and in the presence of Cs+. Lower trace, caesium-sensitive current obtained by subtracting the current trace in the presence of Cs+ to the control current. Bb, corresponding voltage dependance of the h conductance. C, insensitivity of Ih to Ba2+ (2 mm). In contrast to Cs+, Ba2+ did not affect the time-dependent Ih current, but blocked the time-independent inwardly rectifying K+ current. D, current-clamp conditions. Block of Ih with Cs+ increased the Rin of the neurone and abolished the sag in the voltage response to hyperpolarizing current pulses of increasing intensity (20 pA step).
Low-threshold sodium-dependent current in AH neurones
The quasi-steady-state I-V curve of AH neurones displayed a more or less pronounced inflexion at voltages positive to the resting potential (see Fig. 4C and Fig. 5A), or even a net inward current, as demonstrated in Fig. 7Ba, which suggests the presence of an inwardly flowing current. This current was insensitive to 0.5 mm Cd2+, indicating that it was not Ca2+ dependent, but was probably Na+ dependent. To isolate this current, part of the Na+ (125 mm) of the Hepes saline (145 mm Na+) was replaced with either 125 mm NMDG chloride (Fig. 8Aa) or 150 mm choline chloride. The difference current showed that both substitutes strongly decreased Ih and revealed the presence of an inwardly flowing sodium-dependent current present at voltages positive to the Ih activation range (Fig. 8Ab). Total blockade of Ih was obtained by adding 1–2 mm Cs+, which allowed the inwardly flowing sodium-dependent current to be better revealed (Fig. 8Ba and Bb). This Na+ current was present in all AH neurones studied (n = 38). It had a slow inactivation, the gradual onset of which was revealed by varying the speed of the voltage ramps from 50 to 10 mV s−1 (Fig. 8C). As a result, slowing the speed decreased the amplitude of the current. This current was voltage dependent. Its threshold was low (around −60 mV). Its peak amplitude was reached between −30 and −20 mV and varied strongly from cell to cell (311 ± 75 pA at 50 mV s−1; n = 13). The sodium-dependent current was not affected by a low concentration (100 nm) of TTX, which was sufficient to block the fast-inactivating Na+ current that participates in the generation of the action potential (INaT). However, this Na+-dependent current was attenuated by adding greater concentrations of TTX, 500 nm-2 μm (Fig. 8Da). This suggests strongly that there are different populations of Na+ channels for INaT and the slowly inactivating Na+ current. The slowly inactivating, low-threshold sodium-dependent current was absent in S neurones.
Figure 8. Low-threshold Na+ current in AH neurones.
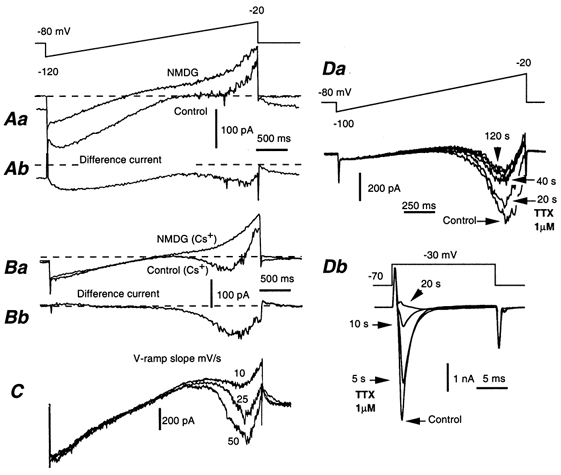
A and B, currents induced by slowly (25 mV s−1) depolarizing AH neurones according to the voltage programme shown in A, before (control) and after replacing 90 % of Na+ by N-methyl d-glucamine chloride (NMDG). Lower traces, difference currents showing the sodium-dependent currents. A, NMDG strongly depressed Ih and revealed the presence of an inward current at voltages more positive than −50 mV. B, same experiment performed in the presence of Cs+ (2 mm) in order to block Ih. NMDG suppressed a low-threshold, slowly inactivating sodium-dependent current (shown as a difference current Bb). This current results in a negative slope conductance in the control I-V curve as shown in Ba. C, AH neurone submitted to voltage ramps at different speeds (50, 25 and 10 mV s−1). As the ramp became slower, the amplitude of the sodium-dependent current decreased as a result of more effective inactivation. D, effect of TTX (1 μm) on the slowly inactivating Na+ current and INaT. Da and Db, experiments performed in the presence of 2 mm Cs+. Da, currents induced by depolarizing an AH neurone (40 mV s−1) with voltage ramps from −100 to −20 mV before (control) and 20, 40 and 120 s after bath application of 1 μm TTX. Db, current traces of INaT induced by applying a voltage pulse of 17.5 ms from −70 to −30 mV before (control) and 5, 10 and 20 s after bath application of 1 μm TTX. INaT was abolished in less than 20 s.
Slow IAHP
The IAHP is a slow, time- and voltage-independent, calcium-activated, potassium-selective current that produces the post-action-potential long-lasting hyperpolarization characteristic of AH neurones (Morita et al. 1982; Hirst et al. 1985b). The amplitude of IAHP was strongly dependent on the activation procedure. When IAHP was triggered by a depolarizing pulse, its amplitude and decay time, at a holding potential of −50 mV, increased with the duration of the pulse. Therefore, the presence and amplitude of IAHP were evaluated using a standard protocol. The neurones were subjected to two successive voltage ramps from −120 to 0 mV, spaced 2 s apart (Fig. 9Aa). The ramp duration was 5 s. The first ramp induced a Ca2+ entry that activated IAHP. This current was obtained by subtracting the current induced by the first ramp from the second (Fig. 9Ab). Successive activations of IAHP with voltage pulses or the two-ramp programme resulted in a progressive decline of the current, presumably by reducing the available intracellular Ca2+ pool, which takes part in activating IAHP. Therefore, a 5 min rest period was allowed between two successive activations.
Figure 9. Slow AHP current (IAHP) in AH neurones.
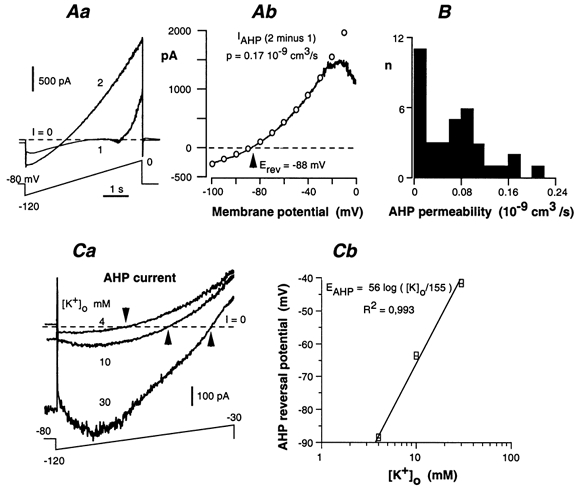
A, method for quantifying the slow IAHP. Aa, the neurone was depolarized with two successive voltage ramps from −120 to 0 mV separated by 2 s. The first ramp (trace 1) induced a Ca2+ entry that activated IAHP, which was prominent in trace 2. Ab, IAHP was obtained by subtracting the first current trace from the second. It was fitted to the Goldman-Hodgkin-Katz relationship (open circles) to determine the permeability (p). The current decrease that occurred at voltages positive to −20 mV actually resulted from the slow inactivation of the delayed-rectifier K+ current during the first ramp. B, distribution of the AHP permeability in 36 AH neurones. C, K+ dependence of the reversal potential of the AHP current. Ca, AHP currents at three different values of [K+]o. Experiment performed in the presence of 2 mm Cs+. Arrows indicate the reversal potential of the AHP current. The presence of Cs+ tended to block the inwardly flowing IAHP in a voltage-dependent manner (i.e. the block increased with the cell polarization). This effect was particularly prominent when the inward IAHP was increased with high [K+]o. Cb, semi-logarithmic plot of the AHP reversal potential versus [K+]o. Data from two neurones. The straight line has a slope of 56 mV for a 10-fold change in [K+]o.
IAHP displayed an outward rectification, as expected from the asymmetrical distribution of K+ ions. Accordingly, it was well fitted to the Goldman-Hodgkin-Katz (G-H-K) relationship:
where R, T, F, [K+]i and [K+]o (expressed in mmol cm−3) have their usual meaning, and p (in cm3 s−1) is the AHP-related K+ permeability of the neurone. In the experiment illustrated in Fig. 9Ab, a least-square program led to p = 0.17 × 10−9 cm3 s−1, and [K+]i = 146 mm. The AHP permeability deduced from the G-H-K relationship displayed large variations, ranging from almost zero (no IAHP) to 0.22 × 10−9 cm3 s−1 (Fig. 9B).
In physiological saline ([K+]o = 4.8 mm), IAHP reversed direction at voltages ranging from −86 to −95 mV (−89 ± 1 mV, n = 16). The ionic dependence of the IAHP reversal potential was evaluated by changing the K+ content of the bath saline (Fig. 9Ca). These experiments were performed in the presence of 2 mm Cs+ in order to block the h current, which was itself dependent on [K+]o and was prominent in the voltage range where IAHP reversed direction. The plot of the IAHP reversal potential against log[K+]o was linear, with a slope of 56 mV per decade, close to the theoretical value (59 mV) for a K+ selective current at 35 °C (Fig. 9Cb).
All of the above experiments were performed with the standard intracellular saline containing 2 mm EGTA. Although we did not perform a quantitative evaluation, the activation of IAHP was still present when EGTA was replaced with BAPTA and when the concentration of either chelator was increased to 10 mm. The AHP was abolished with 20–40 mm intracellular BAPTA. These facts indicate that the IAHP was highly Ca2+ sensitive. This conclusion is consistent with the effects of caffeine that we observed. In order to avoid a muscle Ca2+ release and subsequent contractions, caffeine (1 mm) was included in the pipette saline. With 10 mm BAPTA, dialysed caffeine activated an outward current that was detectable within 60 s after breaking the patch (Fig. 10A). The current developed progressively while the neurone hyperpolarized (inset in Fig. 10B). The activated current had the properties of IAHP: reversal potential near −90 mV and outward rectification (inset in Fig. 10A). IAHP peaked after 4–5 min dialysis, and then slowly decreased (Fig. 10B), while the neurone depolarized.
Figure 10. Activation of the slow AHP current with caffeine.
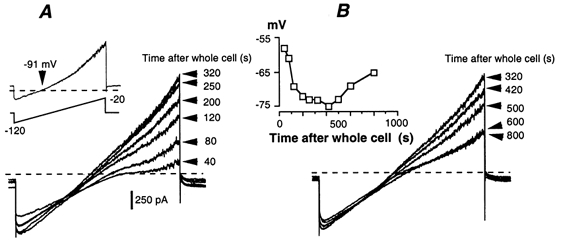
Caffeine (1 mm) and 10 mm BAPTA were included in the pipette saline. A, progressive activation of IAHP after breaking the patch membrane. IAHP was visualized with successive voltage ramps from −120 to −20 mV. Inset, caffeine-activated current obtained by subtracting the current after 320 s dialysis from the current recorded immediately after breaking the patch membrane (t = 40 s). B, spontaneous deactivation of the caffeine-induced AHP current. Inset, simultaneous changes in the neurone resting potential measured from the beginning of dialysis.
Ca2+ current in AH neurones
Experiments performed in order to determine the identity of HVA Ca2+ channels were made in the presence of the L-type calcium channel blocker nicardipine (3 μM).
In our first series of experiments, we tried to isolate the Ca2+ current by perfusing the cells with CsCl (replacing KCl in the patch saline), in order to block outward K+ currents. This procedure resulted in a large and fast increase in the action potential duration, which lasted up to 3–5 s after a few minutes of dialysis. Under these conditions, however, the cells could not be adequately voltage controlled (i.e. the Ca2+ current was triggered in an all-or-none manner).
We therefore used the shape of the action potential to detect the Ca2+ current contribution. This contribution was enhanced by adding TEA to the bath. The TEA concentration was set at 10 mm, which resulted in action potentials lasting 30–50 ms (Fig. 11). The highly specific blocker of N-type Ca2+ channels, Ω-conotoxin GVIA (Ω-CgTX GVIA) at 0.5 μm progressively decreased the action potential duration in less than 60 s (Fig. 11Ab). The same effect was obtained with another N-type channel blocker, Ω-CgTX MVIIA (0.5 μm) (Fig. 11Bc). With both blockers present, a hump persisted, indicating a residual Ca2+ current. This residual current was eliminated by 0.5 mm Cd2+ (Fig. 11Bc). This hump was not affected by the P/Q- and N-type channel blocker Ω-CgTX MVIIC (0.3–0.5 μm), added with the other toxins present (Fig. 11Ac and Bc). This blocker, however, had the same effect on the TEA prolonged action potential as Ω-CgTX GVIA and MVIIA (Fig. 11Bb), although its effect was very fast (steady-state blockade within 15 s at 0.3 μm). Here again, the current resistant to Ω-CgTX MVIIC was not affected by the other blockers. The P/Q-type channel blocker Ω-aga IVA (0.2 μM) had no effect on the TEA-induced prolongation of action potentials. The lack of additivity between the various blockers indicated that they acted on the same pool of Ca2+ channels. The high specificity of Ω-CgTX GVIA suggests strongly that these channels are mainly of the N-type.
Figure 11. Pharmacological identification of Ca2+ current of AH neurones.
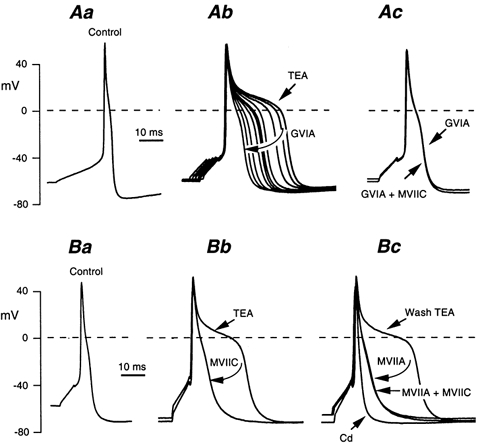
All experiments were performed in the presence of the L-type Ca2+ channels blocker nicardipine (3 μm). A, action potentials recorded in control saline (Aa), and after adding 10 mm TEA (Ab). The elongated TEA-treated action potential was progressively shortened in the presence of Ω-conotoxin (Ω-CgTX) GVIA (0.5 μm) over a period of 80 s (Ab). Adding Ω-CgTX MVIIC (0.5 μm) to the Ω-CgTX GVIA-containing saline had no additional effect on the action potential shape (Ac). B, same type of experiment using first Ω-CgTX MVIIC (Bb), then Ω-CgTX MVIIA (0.5 μm) after a 5 min wash period with the TEA saline (Bc), and finally Ω-CgTX MVIIA plus Ω-CgTX MVIIC (Bc). At the end of the experiment, the Ω-CgTX-resistant action potential elongation was abolished with 0.5 mm Cd2+.
IAHP, which in the present study was evaluated using the two-ramp method, was blocked by each of the three Ω-conotoxins (Fig. 12A) with the same time course as the decrease in action potential duration. Since the Ω-CgTX-sensitive Ca2+ current was investigated mainly in TEA-treated neurones, the same experiments were performed in the presence of 10 mm TEA. By itself, TEA did not prevent IAHP activation by either the voltage-ramp method described above or in response to spiking activity. It reduced, however, the peak of the fast AHP from −70.2 ± 1.5 mV to −66 ± 2 mV with 5 mm TEA, and to −63.4 ± 1.2 mV with 10 mm TEA (n = 13). These results are in agreement with those of Hirst et al. (1985b). The enhanced action potentials of TEA-treated neurones were followed by a large AHP, which reached −85 mV (Fig. 12Bb). Therefore, IAHP appeared to be poorly TEA-sensitive, at least at the concentrations used here. Even in the presence of TEA to maximize the involvement of the Ω-CgTX-resistant Ca2+ current, the three Ω-conotoxins fully blocked IAHP (Fig. 12Bc). Therefore, the Ω-CgTX-resistant Ca2+ current did not appear to trigger IAHP.
DISCUSSION
In this work we have evaluated the electrophysiological properties of myenteric neurones using the patch-clamp method applied to non-dissociated neurones within intact ganglia of a myenteric plexus/longitudinal muscle preparation.
AH neurones had relatively broad action potentials with a mean half width of 2.8 ms. This figure compares well with those obtained with sharp electrodes: 2.8 ms in the duodenum (Clerc et al. 1998) and 2.1–2.7 ms in the ileum (Hirst et al. 1985b; Iyer et al. 1988; Brookes et al. 1995). The falling phase of the action potential consistently exhibited a hump (Clerc et al. 1998). Two inward currents have been shown to underlie the action potential in the soma, INaT and a Ca2+ current (Hirst et al. 1974; Hirst et al. 1985a). A significant difference from the published values, which were obtained with intracellular electrodes, concerns the action potential amplitude measured from the resting potential, 102 mV in this work compared to 72 mV in the duodenum (Clerc et al. 1998) and 71–87 mV in the ileum (Hirst et al. 1985b; Iyer et al. 1988; Brookes et al. 1995).
A similar observation applied to S neurones. The mean action potential amplitude we measured from the resting potential was 73 mV. Values derived from intracellular recordings ranged from 56 to 65 mV in the duodenum (Clerc et al. 1998) and 68 mV in the ileum (Iyer et al. 1988).
In view of the greater spike amplitude and of the larger Rin (see below), it was expected that patch electrodes would record a more hyperpolarized potential than sharp electrodes. The voltage recorded with patch electrodes is affected by a liquid-junction potential that is due to the low ionic strength of the pipette saline. According to Barry & Lynch (1991), the theoretical junction potential derived from absolute ionic mobilities of the pipette saline would be +4 to +5 mV. This evaluation underestimates the actual shift in voltage when the membrane patch is ruptured, since the cell interior contains large immobile anions. A direct comparison between the recordings of sympathetic neurones performed simultaneously with both types of electrode revealed a junction potential of +9 mV (Gola & Niel, 1993). Substraction of this value gives a mean resting potential of −66 mV for AH and −56 mV for S neurones.
Rin was substantially greater with patch electrodes compared to sharp electrodes, which confirms direct comparisons made in isolated myenteric neurones by Baidan et al. (1992a), who found that the Rin of a mixed sample of AH and S neurones was 1010 MΩ with patch electrodes and 111 MΩ with sharp electrodes. In AH neurones in intact ganglia we recorded an Rin of 502 MΩ, compared with average values of 92–190 MΩ with sharp electrodes in intact ganglia (Hodgkiss & Lees, 1983; Iyer et al. 1988; Christofi & Wood, 1994; Kunze et al. 1994; Brookes et al. 1995; Smith et al. 1999). Average values for S neurones in the guinea pig small intestine, recorded with intracellular electrodes, range from about 150 to 350 MΩ (Hodgkiss & Lees, 1983; Iyer et al. 1988; Bornstein et al. 1991; Kunze et al. 1994; Smith et al. 1999), although particular subgroups of neurones have different values, for example, longitudinal muscle motor neurones, which have very small cell bodies, have an Rin of over 500 MΩ (Smith et al. 1999). The value we obtained as an average over all S neurones, 713 MΩ, is 2–3 times the values measured with intracellular electrodes.
Ih was originally observed in guinea-pig AH myenteric neurones by Galligan et al. (1990) using a single-electrode voltage-clamp device. These authors found a half-activation at −85 mV and a voltage sensitivity of 10 mV. These values compare well with our data (half activation, −72 mV and voltage sensitivity, 8.2 mV) when the junction potential mentioned above is taken into consideration. The kinetics of activation and deactivation of Ih are strongly voltage dependent. Compared to the h-type currents described in several other cell types (Pape, 1996), Ih in AH neurones has relatively fast on/off kinetics.
In cultured myenteric neurones, Zholos et al. (1999) observed a time-dependent current activated by hyperpolarization, which closely resembles the Ih described here, particularly with regard to its kinetics. They ascribed this current to the activation of an inwardly rectifying K+ conductance. However, IKir are instantaneously activated by hyperpolarizing pulses. In addition, they are fully blocked by 2 mm Ba2+, whereas the current observed by Zholos et al. (1999) persisted in 5 mm Ba2+, although it was slightly decreased (see Pape, 1996, as well as our data for the effect of Ba2+ on Ih). The following observations support our identification of the current as Ih. First, Ih was present at the cell resting potential (i.e. at voltages much more positive than EK; −90 mV in standard conditions: see IAHP section). Second, reducing [K+]o from 5 to 2 mm did not affect the location of the activation curve, although it greatly reduced the size of the slowly activating component. Third, the sag in the voltage response to small current pulses appeared at voltages positive to EK, which could not be accounted for by a Kir current. Together, these data lead us to conclude that in addition to IKir, in situ as well as in culture, AH neurones do express Ih.
Blockade of Ih did not affect the resting membrane potential of AH neurones. This is in agreement with the data of Kilb & Luhmann (2000) obtained in the rat neocortex. As suggested by Galligan et al. (1990), the Ih contribution to the input conductance of AH neurones probably accounts for the relatively limited hyperpolarization upon activation of the slow IAHP. In our experiments, this is supported by the fact that even when a large IAHP was generated under voltage-clamp conditions, the AHP of the unclamped cells barely reached −80 mV, although the reversal potential of K+ ions was at −90 mV.
Our data provide evidence for the presence of an inward current activated from −60 mV in the myenteric sensory neurones. This current appears to be Na+ dependent because it was suppressed in the presence of NMDG. In addition, it was partly blocked by high concentrations of TTX (500 nm to 2 μm). The remaining current was not Ca2+ dependent because it persisted in the presence of Cd2+. In rat suprachiasmatic neurones, a low-threshold, partially TTX-resistant, inward current has also been described (Pennartz et al. 1997). The reduction in this low-threshold component of Na+ current when using slow instead of fast ramps suggests the involvement of a slowly inactivating rather than a persistent Na+ current (see Pennartz et al. 1997). Further experiments are needed to investigate the kinetics of this current. Persistent TTX-sensitive Na+ currents with voltage dependence similar to that described here have been observed in several preparations (Crill, 1996). Interestingly, as we observed, they were enhanced or presumably unmasked by increasing [K+]o (Somjen & Müller, 2000). It is possible that the low-threshold Na+ current identified in the present study is modulated by second-messenger systems, which might therefore affect neuronal excitability. Recently, such a modulation has been demonstrated to operate on a persistent Na+ current that was observed in the pyramidal neurones of the rat neocortex (Franceschetti et al. 2000).
The physiological significance of the inward rectifier observed in both S and AH neurones is not straightforward to deduce. Myenteric S neurones have a low resting potential, and receive mainly excitatory synaptic inputs. Therefore, S neurones have a low probability to enter the voltage region (around −90 mV) in which the inward rectifier may operate. This region corresponds to the limited window, positive to EK, which allows K+ ions to leave the cell. In the case of AH neurones, it can be hypothesized that IKir may act in synergy with IAHP to clamp the cell at a large polarization. This requires, however, a parallel reduction of Ih. Under these conditions, AH neurones would generate a long-lasting, almost permanent AHP, contributing to the low excitability state that AH neurones may enter. Although this idea has been put forward by Zholos et al. (1999), it still needs to be evaluated experimentally.
IAHP was highly variable in size and duration. A direct modulation of calcium-dependent K+ channels is possible. However, this variation is probably due to variations in [Ca2+]. We have shown directly that the IAHP amplitude progressively increased with the release of Ca2+ from intracellular stores by dialysing the neurone with 1 mm caffeine in the presence of a high concentration of BAPTA. In addition, a mechanism underlying the modulation of IAHP expression might be the blockade of Ca2+ channels upon synaptic activation, as has been suggested by Grafe et al. (1980).
Our data provide an unequivocal identification of HVA Ca2+ channels that activate the calcium-dependent K+ conductance that underlies the AHP. The absence of an effect of Ω-aga IVA on the action potential shows that these channels are not of the P/Q type, although Starodub & Wood (1999) suggested the existence of a P/Q-type current in cultured myenteric neurones. The strong decrease in duration of the action potential in the presence of Ω-CgTX GVIA and MVIIA, which have a high affinity for N-type channels, was never accentuated by adding Ω-CgTX MVIIC, which blocks both N- and P/Q-type channels. However, a small Ca2+ component of the action potential was resistant to all of the toxins. This suggests strongly that most of the HVA channels in guinea-pig myenteric sensory neurones belong to the N type. Abolition of the AHP with Ω-CgTX MVIIA demonstrated that N-type channels are the unique HVA Ca2+ channels, whose opening triggers the AHP, although a minor contribution of other HVA Ca2+ channels was suggested by previous data (Vogalis et al. 2001). This conclusion is strengthened by the fact that, in our experiments, the toxin was capable of blocking an AHP that was exaggerated by using 10 mm TEA to increase Ca2+ influx during the action potential. Our conclusion is consistent with the observation that myenteric sensory neurones express a high level of immunoreactivity to the α1 subunits of class B (N-type) but not of class A channels (P/Q-type; Kirchgessner & Liu, 1999).
In conclusion, our results extend knowledge of the types of currents expressed in the myenteric sensory neurones of the guinea-pig. We have shown that Ih is a major conductance of myenteric sensory neurones, despite the fact that this conductance was not recognized in a recent patch study. In addition, we have discovered the presence of a low-threshold sodium-dependent current that is poorly sensitive to TTX. Finally, we have provided unambiguous evidence that N-type Ca2+ channels are the unique HVA channels that trigger the AHP.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (CNRS, France), the National Health and Medical Research Council of Australia (grant 96323) and by Australia-France exchange grants from the Programme International de Coopération Scientifique (PICS, France) and the International Researcher Exchange Scheme (IREX, Australian Research Council).
REFERENCES
- Baidan LV, Zholos AV, Shuba MF, Wood JD. Patch-clamp recording in myenteric neurones of guinea-pig small intestine. American Journal of Physiology. 1992a;262:G1074–1078. doi: 10.1152/ajpgi.1992.262.6.G1074. [DOI] [PubMed] [Google Scholar]
- Baidan LV, Zholos AV, Wood JD. Properties of calcium currents determined by patch-clamp recording in myenteric neurones from adult guinea-pig small intestine. Gastroenterology. 1992b;102:A420. [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. Journal of Membrane Biology. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB, Lees GM. Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurones of guinea-pig small intestine. Journal of Physiology. 1984;351:313–325. doi: 10.1113/jphysiol.1984.sp015247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. Journal of Neuroscience. 1991;11:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WAA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? Journal of the Autonomic Nervous System. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Song ZM, Ramsay GA, Costa M. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. Journal of Neuroscience. 1995;15:4013–4022. doi: 10.1523/JNEUROSCI.15-05-04013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL, Wood JD. Electrophysiological subtypes of inhibitory P1 purinoceptors on myenteric neurones of guinea-pig small bowel. British Journal of Pharmacology. 1994;113:703–710. doi: 10.1111/j.1476-5381.1994.tb17050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc N, Furness JB, Bornstein JC, Kunze WAA. Correlation of electrophysiological and morphological characteristics of myenteric neurones of the duodenum in the guinea-pig. Neuroscience. 1998;82:899–914. doi: 10.1016/s0306-4522(97)00318-7. [DOI] [PubMed] [Google Scholar]
- Clerc N, Gola M, Rugiero F, Furness JB, Kunze WAA. Properties of patch clamped myenteric sensory neurons in intact ganglia. Autonomic Neuroscience: Basic and Clinical. 2000;82:65. [Google Scholar]
- Costa M, Brookes SJH, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annual Review of Physiology. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Doan TN, Kunze DL. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. Journal of Physiology. 1999;514:125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones. Journal of Physiology. 2000;528:291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JL, Willard AL. Voltage-dependent sodium and calcium currents of rat myenteric neurones in cell culture. Journal of Neurophysiology. 1993;69:1264–1275. doi: 10.1152/jn.1993.69.4.1264. [DOI] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. Journal of the Autonomic Nervous System. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurones of the intestine. Progress in Neurobiology. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Tatsumi H, Shen K-Z, Surprenant A, North RA. Cation current activated by hyperpolarization (Ih) in guinea pig enteric neurones. American Journal of Physiology. 1990;259:G966–972. doi: 10.1152/ajpgi.1990.259.6.G966. [DOI] [PubMed] [Google Scholar]
- Gola M, Niel JP. Electrical and integrative properties of rabbit sympathetic neurones re-evaluated by patch clamping non-dissociated cells. Journal of Physiology. 1993;460:327–349. doi: 10.1113/jphysiol.1993.sp019474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P, Mayer CJ, Wood JD. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. Journal of Physiology. 1980;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M, Franke M, Hartig W, Groshe J, Reichenbach A, Pannicke T. Patch-clamp study of neurones and glial cells in isolated myenteric ganglia. American Journal of Physiology. 2000;278:G644–651. doi: 10.1152/ajpgi.2000.278.4.G644. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurones in the myenteric plexus of the duodenum in the guinea-pig. Journal of Physiology. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Johnson SM, van Helden DF. The calcium current in a myenteric neurone of the guinea-pig ileum. Journal of Physiology. 1985a;361:297–314. doi: 10.1113/jphysiol.1985.sp015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Johnson SM, van Helden DF. The slow calcium-dependant potassium current in a myenteric neurone of the guinea-pig ileum. Journal of Physiology. 1985b;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss JP, Lees GM. Morphological studies of electrophysiologically-identified myenteric plexus neurones of the guinea-pig ileum. Neuroscience. 1983;8:593–608. doi: 10.1016/0306-4522(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Iyer V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T. Electrophysiology of guinea-pig myenteric neurones correlated with immunoreactivity for calcium binding proteins. Journal of the Autonomic Nervous System. 1988;22:141–150. doi: 10.1016/0165-1838(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Katayama Y, Morita K, North RA. Mediators of slow synaptic potentials in the myenteric plexus of the guinea-pig ileum. Journal of Physiology. 1981;320:175–186. doi: 10.1113/jphysiol.1981.sp013942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Katayama Y, North RA. Slow synaptic potentials in neurones of the myenteric plexus. Journal of Physiology. 1980;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Characterization of a hyperpolarization-activated inward current in Cajal-Retzius cells in rat neonatal neocortex. Journal of Neurophysiology. 2000;84:1681–1691. doi: 10.1152/jn.2000.84.3.1681. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Differential localization of Ca2+ channel alpha 1 subunits in the enteric nervous system; presence of alpha 1B channel-like immunoreactivity in intrinsic primary afferent neurones. Journal of Comparative Neurology. 1999;409:85–104. doi: 10.1002/(sici)1096-9861(19990621)409:1<85::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Bornstein JC, Furness JB, Hendriks R, Stephenson DSH. Charybdotoxin and iberiotoxin but not apamin abolish the slow after-hyperpolarization in myenteric plexus neurones. Pflügers Archiv. 1994;428:300–306. doi: 10.1007/BF00724511. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurones in the guinea-pig intestine respond differentially to deformation. Journal of Physiology. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, North RA, Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. Journal of Physiology. 1982;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. Journal of Physiology. 1973;231:471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H-C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurones. Annual Review of Physiology. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Bierlaagh MA, Geurtsen AMS. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. Journal of Neurophysiology. 1997;78:1811–1825. doi: 10.1152/jn.1997.78.4.1811. [DOI] [PubMed] [Google Scholar]
- Smith TK, Burke EP, Shuttleworth CW. Topographical and electrophysiological characteristics of highly excitable S neurones in the myenteric plexus of the guinea-pig ileum. Journal of Physiology. 1999;517:817–830. doi: 10.1111/j.1469-7793.1999.0817s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG, Müllerüller M. Potassium-induced enhancement of persistent inward current in hippocampal neurons in isolation and in tissue slices. Brain Research. 2000;885:102–110. doi: 10.1016/s0006-8993(00)02948-6. [DOI] [PubMed] [Google Scholar]
- Song AM, Brookes SJH, Ramsay GA, Costa M. Characterization of myenteric interneurons with somatostatin immunoreactivity in the guinea-pig small intestine. Neuroscience. 1997;80:907–923. doi: 10.1016/s0306-4522(96)00605-7. [DOI] [PubMed] [Google Scholar]
- Starodub AM, Wood JD. Selectivity of omega-CgTx-MVIIC toxin from Conus magnus on calcium currents in enteric neurones. Life Science. 1999;64:PL305–310. doi: 10.1016/s0024-3205(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Starodub AM, Wood JD. A-type potassium current in myenteric neurons from guinea pig small intestine. Neuroscience. 2000;99:389–396. doi: 10.1016/s0306-4522(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Uchitel OD. Toxins affecting calcium channels in neurones. Toxicon. 1997;35:1161–1191. doi: 10.1016/s0041-0101(96)00210-3. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Hillsley K, Smith TK. Diverse ionic currents and electrical activity of cultured myenteric neurones from the guinea pig proximal colon. Journal of Neurophysiology. 2000;83:1253–1263. doi: 10.1152/jn.2000.83.3.1253. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Furness JB, Kunze WAA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. Journal of Neurophysiology. 2001;85:1941–1951. doi: 10.1152/jn.2001.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Wood JD, Meyer CJ. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflügers Archiv. 1978;374:265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]
- Zholos AV, Baidan LV, Starodub AM, Wood JD. Potassium channels of myenteric neurones in guinea-pig small intestine. Neuroscience. 1999;89:603–618. doi: 10.1016/s0306-4522(98)00337-6. [DOI] [PubMed] [Google Scholar]


