Abstract
Activation of the cAMP/protein kinase A (PKA) second messenger cascade has been implicated in the induction of mechanical hyperalgesia by inflammatory mediators. We examined the role of this cascade in mechanical sensitization of nociceptive neurons that innervate the meninges, a process thought to be involved in the pathophysiology of headache syndromes such as migraine. Single unit activity was recorded in the trigeminal ganglion from 40 mechanosensitive dural afferents (conduction velocitity: 0.3–6.6 m s−1) and nine mechanically insensitive dural afferents (MIAs) (conduction velocitity: 0.3–2.8 m s−1) while stimulating the dura with a servo force-controlled stimulator or von Frey monofilaments, respectively. Local application to the dura of dibutyryl adenosine 3′,5′-cyclic monophosphate (dbcAMP, 100 μm), a stable membrane-permeant cAMP analogue, produced mechanical sensitization in the majority of mechanosensitive units (19/29, 66 %). Two distinct patterns of mechanical sensitization were observed. Thirty-eight per cent of the units exhibited only a decrease in threshold (TH group), while 28 % showed only an increase in suprathreshold responses (STH group). dbcAMP also induced mechanosensitivity in the majority of MIA units (6/9, 67 %). dbcAMP-induced sensitization was blocked by the PKA inhibitors, Rp-cAMP (1 mm) and H-89 (100 μm). A mixture of inflammatory mediators induced both components of sensitization in the majority of mechanosensitive units tested. However, in each unit, PKA inhibitors blocked only one of the two effects (either TH or STH). Units that were classified as TH or STH also differed in their baseline stimulus-response slopes, thresholds and conduction velocities. These findings implicate the cAMP-PKA cascade in sensitization of dural mechanonociceptors and suggest that this cascade may produce sensitization through at least two different mechanisms operating in separate neuronal populations.
Inflammation-induced sensitization of primary afferent mechanonociceptive neurons is thought to be a major contributor to states of pain hypersensitivity or mechanical hyperalgesia (Schmelz et al. 1994; Andrew & Greenspan, 1999a; Levine & Reichling, 1999). Although the term sensitization usually refers to a general increase in the level of excitability, at least two distinct response components have been reported following exposure of mechanonociceptors to an inflammatory milieu. Both mechanosensitive and mechanically insensitive (‘silent’) nociceptors were shown to respond to previously ineffective stimulus intensities, indicating decreased activation thresholds (Martin et al. 1987; Schaible & Schmidt, 1988; White et al. 1990; Davis et al. 1993; Wang et al. 1996; Ahlgren et al. 1997). In addition, other reports have indicated augmented responses to suprathreshold stimuli either concomitant with threshold changes (Su & Gebhart, 1998; Halata et al. 1999) or without a noticeable change in threshold (Cooper et al. 1991; Andrew & Greenspan, 1999a).
Behavioural studies have implicated the cAMP/protein kinase A (PKA) second messenger cascade as one of the mechanisms by which inflammatory mediators such as prostaglandin E2 (PGE2), serotonin (5-HT) and adenosine produce mechanical hyperalgesia (Ferreira & Nakamura, 1979; Taiwo et al. 1989, 1992; Taiwo & Levine, 1991; Ouseph et al. 1995; Aley & Levine, 1999; Cunha et al. 1999). Two electrophysiology studies of cutaneous C fibres have implicated the cAMP-PKA cascade in one component of mechanical sensitization, namely decreased threshold. Kress et al. (1996) showed that mechanical response thresholds were lowered by cAMP analogues, while Wang et al. (1996) showed that the threshold-lowering effects of PGE2 were blocked by cAMP/PKA inhibitors. Although Wang et al. (1996) also found sensitizing effects of PGE2 on suprathreshold responses, subsequent studies from the same laboratory found that PGE2 primarily augmented responses to stimulus intensities near the original response threshold without affecting responses to more intense (suprathreshold) stimuli (Ahlgren et al. 1997; Chen et al. 1999). These studies, employing von Frey hairs, point to a preferential involvement of cAMP/PKA in the threshold, rather than in the suprathreshold component of mechanical sensitization. By contrast, studies of sensitization to heat, which is more amenable to quantification than mechanical stimuli, implicated the cAMP-PKA cascade in the suprathreshold component of sensitization (Mizumura et al. 1993, 1996; Kress et al. 1996; Lynn & O'Shea, 1998). In addition, the studies of mechanical sensitization cited above were restricted to C fibres, leaving open the question of whether different sensitization patterns occur in Aδ nociceptors. In the present study, using an in vivo preparation of dural mechanonociceptors, we investigated whether different components of mechanical sensitization can be differentially modulated by activation of the cAMP-PKA cascade and whether the pattern of sensitization differs among different populations of neurons. We show that the cAMP-PKA cascade is capable of inducing both components of sensitization, namely reduced mechanical thresholds and increased responses to suprathreshold stimuli, albeit in distinct populations of neurons.
METHODS
Surgery and electrophysiological recording
Experiments were carried out on adult Sprague-Dawley male rats (350–450 g). The experimental protocol was approved by the institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. Rats were anaesthetized with urethane (2.0 g kg−1i.p.; Sigma Chemicals, St Louis, MO, USA) and throughout an experiment they were maintained under deep anaesthesia with supplemental injections of urethane as needed to maintain areflexia. At the end of the experiments, the animals were killed with an intra-sinus bolus injection of 1 m KCl.
The set-up for recording dural afferents has been described previously (Strassman & Raymond, 1999). Briefly, the anaesthetized rats were placed in a stereotaxic head-holder. A craniotomy was used to expose the left transverse sinus and the dura was bathed with a modified synthetic interstitial fluid (SIF, pH 7.2) comprising 135 mm NaCl, 5 mm KCl, 1 mm MgCl2, 5 mm CaCl2, 10 mm glucose and 10 mm Hepes. Tungsten microelectrodes were advanced through a second craniotomy into the left trigeminal ganglion. Dural afferent neurons in the ganglion were identified by their constant latency response to single shock stimulation of the dura overlying the ipsilateral transverse sinus (0.5 ms pulse, 5 mA, 0.5 Hz). The shortest latency site was identified as described previously (Strassman & Raymond, 1999). The response latency at this site was used to calculate conduction velocity, based on a conduction distance to the trigeminal ganglion of 12.5 mm. Neurons were classified as either C units (CV ≤ 1.5 m s−1) or Aδ units (CV > 1.5 m s−1). Action potentials were processed with a real-time waveform discriminator (SPS-8701, Signal Processing Systems, Prospect, South Australia, Australia) and acquired for on-line and off-line analysis with the Discovery data acquisition program (DataWave Technologies, Longmont, CO, USA).
Mechanical stimulation
Mechanical receptive fields of dural afferents were identified initially by stroking the dura with blunt forceps. The site of lowest mechanical threshold was determined using a calibrated set of von Frey (VF) monofilaments (0.03–6.9 g exerting 38–510 kPa, Stoelting, Chicago, IL, USA). Units that did not respond to the 510 kPa filament were deemed mechanically insensitive afferents (MIAs). Greater forces were not tested as they are potentially injurious and can induce subarachnoid and cortical bleeding.
For quantitative determination of mechanical stimulus-response functions, graded stimuli were applied to the dural surface at the lowest threshold site with a servo force-controlled mechanical stimulator (Series 300B Dual Mode Servo System, Aurora Scientific, Aurora, Ontario, Canada) (Khalsa et al. 1997). A flat-ended cylindrical plastic probe was attached to the tip of the stimulator arm. One of three probe diameters (0.5, 0.8 or 1.1 mm) was selected for each neuron, depending on the sensitivity of the neuron. The smallest probe was used unless the baseline threshold of the neuron was so low that responses were evoked even at the minimum setting of the stimulator (2 mN). In such cases, to deliver subthreshold stimuli, one of the larger probes was used (resulting in lower stimulus pressures). Stimulus intensity is reported in units of pressure or force per area (kPa, where 1 kPa = 1 mN mm2). Only one probe was used for each neuron. It should be noted that indentation of a soft tissue produces tension and shear, as well as compression, but that only the compressive component of the stimulus was under experimental control in this preparation (Khalsa et al. 1997).
Experimental protocols
Stimulus trials for testing mechanically sensitive units consisted of four ‘ramp and hold’ stimuli (rise time 100 ms, stimulus width 2 s, inter-stimulus interval 10 s), which included a subthreshold (S1), threshold (S2) and two suprathreshold (S3, S4) stimuli, delivered in ascending order (Fig. 1A). Stimuli that evoked one to two spikes were considered as threshold (S2). S1 stimuli were set at 40–50 % of S2 and usually did not evoke any response during baseline testing. These values were used based on previous studies that documented the range of threshold changes induced by PGE2 and 5-HT (Martin et al. 1988; White et al. 1990; Kress et al. 1996; Wang et al. 1996; Halata et al. 1999). S3 and S4 were usually 2 and 4 times stronger than S2, respectively. Although many dural afferent units are capable of firing more than 20 spikes s−1 in response to strong mechanical stimuli (Strassman et al. 1996; Bove & Moskowitz, 1997), we used S4 stimuli that evoked only 8–14 spikes s−1. Higher pressure stimuli were not used in order to remain within the linear part of the stimulus-response curve (Andrew & Greenspan, 1999b; Slugg et al. 2000). Stimulus trials were delivered repeatedly at a constant interval of either 2 or 4 min throughout the experiment. The intertrial interval was not changed once baseline testing started. A 10 s interval preceding S1 was used for measurement of spontaneous activity.
Figure 1. Mechanical stimulation and experimental protocols.
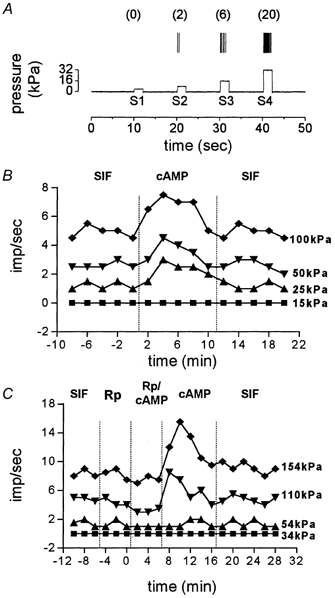
A, a representation of one experimental trial consisting of an ascending series of four 2 s ‘ramp and hold’ mechanical stimuli (S1–4) applied to the most sensitive spot of the receptive field with inter-stimulus intervals of 10 s. The 10 s of recording prior to S1 was used to measure spontaneous activity. The number of action potentials produced by each stimulus is given in parentheses. B, a representative experiment showing the protocol for testing the effect of dbcAMP on mechanical responsiveness. Baseline responses in the presence of SIF were followed by 5 trials in which dbcAMP (cAMP) was applied and another 5 trials of wash with SIF. Note the short duration of dbcAMP action. C, a representative experiment demonstrating the protocol for testing the effect of PKA blockade. The PKA inhibitors Rp-cAMPS or H-89 were applied first, followed by a mixture of dbcAMP and the inhibitor. dbcAMP was applied next for 10 min and followed by a wash period.
In all experimental protocols, baseline measurements of spontaneous and mechanically evoked activity were obtained prior to drug administration. Only units that exhibited consistent responses at all stimulus intensities in at least 3–4 consecutive baseline trials were tested further. These trials also served as vehicle controls, because the receptive field was bathed in SIF, which was the vehicle for all drugs except H-89 (see below). All chemical agents were applied topically to the dura. Two agents were tested for potential sensitizing effects, in separate neurons, namely (1) dibutyryl adenosine 3′,5′-cyclic monophosphate (dbcAMP, 100 μm), a stable membrane-permeant analogue of cAMP, and (2) a mixture (pH 7.2) of inflammatory mediators (IM) containing PGE2, 5-HT, bradykinin (100 μm each) and histamine (1 mm). Following baseline testing in SIF, a chemical agent was applied for 10–12 min, followed by a wash period with SIF (Fig. 1B).
A separate experimental protocol was used for testing the ability of PKA inhibitors to block the sensitizing effects of dbcAMP or IM. The PKA inhibitors tested were adenosine 3′,5′-cyclic monophosphorothioate, 8-Bromo-Rp isomer (Rp-cAMPS, 1 mm) or N-[2-(p-bromocinnamyl-amino)ethyl]-5-iso-quinolinesulfonamide (H-89, 100 μm). Figure 1C demonstrates the sequence of drug applications in this protocol: (1) PKA inhibitor alone, (2) PKA inhibitor in combination with dbcAMP, (3) dbcAMP alone and (4) wash with SIF. An alternative protocol that involved attempting to reverse sensitization by applying the PKA inhibitors after the dbcAMP was not used, because dbcAMP-induced sensitization had a relatively short duration in most units, even in the continued presence of dbcAMP (see Fig. 1B). Therefore, it would have been difficult to distinguish between the simple decay of dbcAMP effects vs. true reversal of sensitization. A similar protocol was used to test for blockade of IM effects by PKA inhibitors. Since the IM effects were of longer duration, we tested whether IM effects can be reversed by combined application of IM and PKA inhibitors in some units.
VF filaments were used to test for the development of mechanosensitivity in response to dbcAMP in neurons that were initially classified as MIAs. The mechanical stimulator was not used for this, because it cannot be quickly repositioned for repeated testing of multiple sites, which is necessary when searching for the emergence of mechanosensitivity in MIAs. MIAs were selected for testing only if they responded to brief application of 50 mm KCl, indicating the presence of endings in the exposed dural area that were accessible to topically applied solutions (Strassman et al. 1996). Mechanical sensitivity was tested every 2 min following drug application, first with the 510 kPa filament and then (when units became responsive) with lower forces. For testing MIAs, threshold was defined as the lowest VF stimulus intensity that evoked a discharge in at least three of five consecutive stimuli (10 s inter-stimulus interval). Only one unit was tested in each animal.
Drugs
Bradykinin, PGE2, histamine, 5-HT, 4-AP, TEA (Sigma), dbcAMP and Rp-cAMPS (Calbiochem, San Diego, CA, USA) were dissolved in SIF. H-89 (Calbiochem) was first dissolved in DMSO (1 % in SIF) and further diluted in SIF (final DMSO concentration not exceeding 0.2 %). In three neurons tested during prolonged (30–60 min) exposure to 0.2 % DMSO, no effects on mechanosensitivity were observed.
Data analysis
The response to each mechanical stimulus was calculated by subtracting the mean spontaneous firing rate from the mean firing rate during the stimulus. The spontaneous firing rate for each trial was calculated from the 10 s interval preceding S1. The majority of units had low (< 0.5 Hz) or no spontaneous firing. Units with spontaneous activity (SA) greater than 1 Hz were not studied due to the greater difficulty in detecting changes in threshold.
A stimulus-response slope was calculated for each neuron by performing a linear regression of the threshold and suprathreshold responses (S2-S4). Following drug application, the firing of each unit was examined for both a decrease in mechanical threshold (as shown by a response to the initially subthreshold S1 stimulus) and an increase in threshold/suprathreshold responses (S2, S3, S4). Because the S1 stimulus was set at 40–50 % of the baseline threshold (S2), the appearance of an S1 response represents a threshold decrease of similar magnitude. Data are expressed as means ± s.e.m. Results were considered statistically significant at P < 0.05.
RESULTS
Dural afferent population
Forty mechanosensitive and nine mechanically insensitive dural afferent neurons were included in this study. The mechanosensitive neurons included 14 Aδ units (CV = 1.6–6.6 m s−1, median = 2.3 m s−1) and 26 C units (CV = 0.3− 1.5 m s−1, median = 0.7 m s−1). Thresholds were in the range of 7.2–121.7 kPa (median = 17.1 kPa) for Aδ units and 2.7–85.3 kPa (median = 23.4 kPa) for C units. The MIAs included one Aδ unit (CV = 2.8 m s−1) and eight C units (CV range = 0.3–1.0 m s−1, mean = 0.7 ± 0.1 m s−1).
dbcAMP induces two distinct patterns of mechanical sensitization
The effect of dbcAMP on mechanosensitivity was examined in 29 mechanosensitive dural afferent units. Two to 4 min following local application of dbcAMP (100 μm), mechanical sensitization was evident in 66 % (19/29) of the units (8/9 Aδ and 11/20 C units). Two distinct patterns of sensitization were observed (Fig. 2). Thirty-eight per cent (11/29, 3 Aδ and 8 C units) of the units exhibited a decrease in threshold, identified as a novel response to the previously subthreshold S1 stimulus, usually with 1–3 action potentials (threshold group, TH; Fig. 4A). Forty-five per cent (5/11) of those units also exhibited increased responses (of 113 ± 36 %) to the S2 stimulus. However, these units showed no increase in response to the suprathreshold stimuli (Fig. 4C). Twenty-nine per cent (8/29, 5 Aδ and 3 C units) of the units showed a different pattern of sensitization. Specifically, in these units (suprathreshold group, STH), only the S2, S3 and S4 responses were affected (Fig. 4D). Although responses were significantly higher for all three stimuli (176 ± 62 %, 119 ± 26 % and 50 ± 7 % for S2, S3 and S4 respectively), the effect on S4 was the weakest (P < 0.05, one-way ANOVA with Dunnett's post hoc test, S2–3 vs. S4). These units, however, showed no decrease in threshold, because they were still unresponsive to the S1 stimulus (Fig. 4B). The remaining units (10/29, 1 Aδ and 9 C units) were unaffected (non-affected group, NA). The duration of dbcAMP-induced sensitization in the TH and STH groups was short and rarely exceeded 10 min, even in the continued presence of dbcAMP (Fig. 1B). In most (24/29), dbcAMP did not produce any activation or change in SA. However, two Aδ and three C units underwent mild excitation, never exceeding 0.6 Hz (mean = 0.3, range = 0.2–0.6 Hz, data not shown).
Figure 2. Subdivision of dbcAMP-treated units.
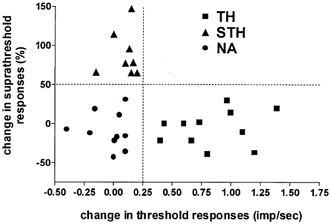
dbcAMP-treated units were divided into three groups based on whether they showed a decrease in threshold with no change in suprathreshold responses (TH group, ▪), an increase in suprathreshold responses with no change in threshold (STH group, ▴), or no effect (non-affected, NA group, •). No neurons showed both effects. Increases in suprathreshold responses are plotted on the ordinate as the mean percentage change in the response to the S3 and S4 stimuli. Decrease in threshold was detected by the appearance of a novel response to the initially subthreshold S1 stimulus. The absolute change in S1 response (calculated in spikes s−1) is plotted on the abscissa. As the S1 stimulus was set at about 50 % of the baseline threshold, the appearance of an S1 response represents a threshold decrease of at least 50 % (the minimum change that could be detected in this stimulus protocol).
Figure 4. Effect of PKA inhibitors (PKAI) on mechanical sensitization induced by dbcAMP.
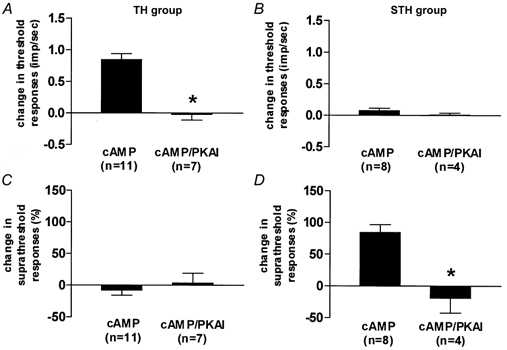
In TH units (A and C), the decrease in threshold (A) was blocked by PKAI. This group did not display any suprathreshold changes (C). In STH units (B and D), PKAI blocked the increases in suprathreshold responses. Threshold changes were not observed in this group (B). *P < 0.01, Mann-Whitney U test.
One Aδ (2.8 m s−1) and eight C units (CV range 0.3–1 m s−1, mean 0.6 m s−1) that were initially characterized as MIAs were tested for sensitization with dbcAMP. Four minutes following drug application, most of these units (6/9) developed mechanosensitivity, as shown by their responsiveness to the 510 kPa VF filament (Fig. 3). Continued exposure to dbcAMP typically produced further lowering of thresholds to as low as 177 kPa. Although thresholds increased after removal of the dbcAMP, units generally could still be activated with the 510 kPa VF filament up to 1 h later.
Figure 3. dbcAMP-induced sensitization of mechanically insensitive dural afferents (MIAs) is PKA dependent.
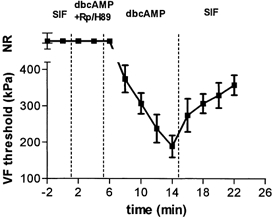
Time course of changes in von Frey threshold is plotted during dbcAMP application in the presence and absence of the PKA inhibitors (mean ± s.e.m of 6 units). Application of the PKA inhibitors, Rp-cAMPS (1 mm, n = 3) or H-89 (100 μm, n = 3), in combination with dbcAMP (100 μm, n = 8) did not affect any of the MIA units tested, whereas dbcAMP alone induced mechanosensitivity in 6/9 units. This sensitization was partially reversed by a subsequent wash with SIF. NR, no response.
dbcAMP produces mechanical sensitization via activation of PKA
To determine whether dbcAMP produces mechanical sensitization through activation of PKA, we tested whether its sensitizing effects could be blocked by two selective PKA inhibitors, Rp-cAMPS and H-89. In 16 of the neurons that were tested for sensitization with dbcAMP (see above), the dbcAMP was first applied in the presence of either Rp-cAMPS (n = 9) or H-89 (n = 7) and the effect was then compared with that produced subsequently by dbcAMP alone (Fig. 1C). When applied in the presence of the PKA inhibitors, dbcAMP had no significant effect on either threshold or suprathreshold responses (Fig. 4). Subsequent application of dbcAMP alone produced sensitization in 11 units (5 Aδ and 6 C units). As described above, dbcAMP-induced sensitization was expressed in individual units as either a reduction in threshold (n = 7) or an increase in suprathreshold responses (n = 4), but not both. Similarly, MIA units did not develop mechanosensitivity when tested with dbcAMP in the presence of Rp-cAMPS (n = 3) or H-89 (n = 3). Subsequent application of dbcAMP alone, however, induced the development of mechanosensitivity in 6/9 MIA units (e.g. Fig. 3).
Inflammatory mediators produce mechanical sensitization that is partially dependent on PKA activation
The effects of dbcAMP were compared to those of IM. The IM mixture included agents that are thought to promote nociceptor sensitization through activation not only of the cAMP-PKA cascade but also of other second messenger pathways. The IM produced sensitization in 82 % (14/17) of the units tested (4/7 Aδ and 10/10 C units). In contrast to the pattern of sensitization induced by dbcAMP, the majority of the IM-sensitized units (10/14) were sensitized to all stimuli (S1–4), and therefore exhibited both a decrease in threshold and an increase in suprathreshold responses. No units showed an exclusive sensitization to S1, but four units showed an increase only in their suprathreshold response (S2–4 or S3–4). In contrast to the transient nature of dbcAMP-induced sensitization, IM produced a sustained sensitization that was usually maintained throughout the application period (data not shown). The IM also produced significant excitation or increased SA in most (13/15) units (mean = 0.9 Hz, range = 0.2–1.4 Hz). The increases in SA might have obscured the changes in S1 in some units.
Thirteen of the units that were sensitized by IM were also tested for the effect of a PKA inhibitor, either Rp-cAMPS (n = 7) or H-89 (n = 6), applied in combination with IM, to determine whether IM-induced sensitization was PKA dependent. In eight units, the IM was applied first, followed by IM plus inhibitor, while five units were tested with the protocol described above for dbcAMP, in which the IM-inhibitor combination was applied first, followed by IM alone. Since results obtained with these two protocols were similar, they are described together. The PKA inhibitor produced suppression of IM-induced sensitization in 10 of the 13 units tested, but in each unit the sensitization was only partially blocked. In six units, the decrease in threshold was blocked while the increase in suprathreshold response remained (Fig. 5; TH group). In four units, the increase in suprathreshold response was blocked, while the decrease in threshold was still present (Fig. 5; STH group). These results indicate that there are two groups of neurons that exhibit an involvement of PKA in either the threshold or suprathreshold component of sensitization. These two groups appear to correspond to the TH and STH groups that were defined based on their pattern of dbcAMP-induced sensitization.
Figure 5. Effect of PKA inhibitors (PKAI) on mechanical sensitization induced by a mixture of inflammatory mediators (IM).
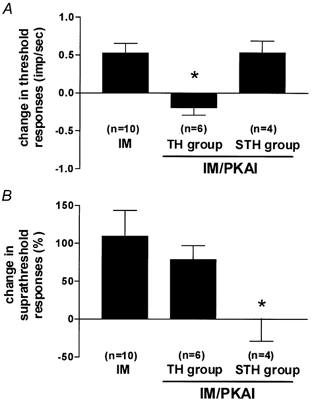
IM (n = 10) produced both decreases in threshold (A) and increases in suprathreshold responses (B). In TH units (n = 6), only decreases in threshold were blocked by PKAI. Conversely, in STH units (n = 4), only suprathreshold changes were affected. *P < 0.01, Mann-Whitney U test.
PKA-induced threshold and suprathreshold changes occur in distinct neuronal populations
We further examined whether the two distinct patterns of dbcAMP/PKA-mediated sensitization (represented by the TH and STH groups) were associated with neuronal populations that could be distinguished on the basis of other properties, such as conduction velocity, baseline stimulus-response slope, baseline mechanical thresholds or baseline SA. Slopes have been used as indicators of coding and adaptive properties in previous studies of cutaneous mechanonociceptors (Garell et al. 1996; Andrew & Greenspan, 1999b; Slugg et al. 2000). Figure 6 shows the baseline stimulus-response plots for units that were classified based on the pattern of dbcAMP-induced sensitization or the pattern of PKA-mediated inhibition (see Fig. 4 and Fig. 5). The STH group had significantly higher slopes, lower thresholds and faster conduction velocities than the TH group (P < 0.05 for all, Mann-Whitney U test; Fig. 7). Baseline SA was not statistically different between the two groups (data not shown).
Figure 6. Baseline stimulus-response plots of all dbcAMP and PKAI-affected IM-treated units.
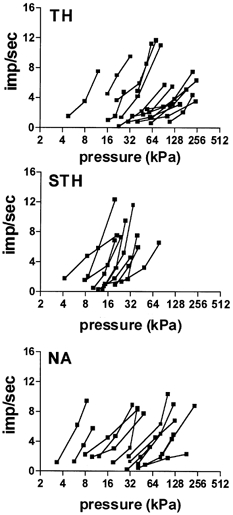
Units were subdivided based on the pattern of sensitization induced by dbcAMP (see text), or the pattern of inhibition induced by PKA inhibitors during IM-induced sensitization. TH, threshold affected; STH, suprathreshold affected; NA, not affected.
Figure 7. Comparison of the three groups (TH, STH and NA) on scatterplots of baseline stimulus-response slopes (A), baseline mechanical threshold (B) and conduction velocity (C).
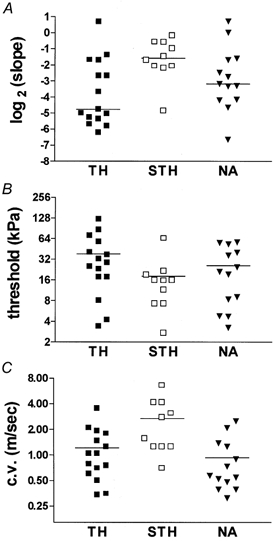
All 3 parameters were significantly different between the TH and STH groups (see Results). Horizontal lines depict the mean within a group.
DISCUSSION
Previous studies, carried out with von Frey hair stimulation, found evidence of a threshold-lowering effect of the cAMP-PKA cascade on mechanonociceptive cutaneous C fibres (Kress et al. 1996; Wang et al. 1996). In the present study, we used a force servo-controlled mechanical stimulator to examine sensitization in Aδ and C dural mechanonociceptors and found evidence that the cAMP-PKA cascade can produce both components of mechanical sensitization, namely decreased thresholds and increased suprathreshold responses. However, these two components were induced by dbcAMP in separate groups of neurons, which we have termed the TH and STH groups. Thus, each dbcAMP-sensitized neuron exhibited one or the other sensitization component, but not both. The two groups of neurons that were defined by their pattern of sensitization could also be distinguished on the basis of other neuronal properties. Specifically, the STH group had significantly steeper stimulus-response slopes, lower thresholds and faster conduction velocities than the TH group. It is noteworthy that the STH group is composed predominantly of Aδ fibres, which might account for the absence of suprathreshold sensitization effects in previous studies that were restricted to C fibres (Ahlgren et al. 1997; Chen et al. 1999). Our observations provide evidence that threshold and suprathreshold sensitivities can be modulated independently and suggest that there may be differences in the cellular mechanisms that underlie mechanical sensitization among different functional subtypes within the nociceptor population.
Selective effects on either the threshold or suprathreshold components of the stimulus-response curve have been observed in other studies of nociceptor sensitization. For example, a selective sensitizing action of cAMP on suprathreshold responses to heat stimulation, with no change in heat threshold, was found in cutaneous nociceptors (Kress et al. 1996), while a selective effect of inflammation on suprathreshold responses to mechanical stimulation, with little or no change in mechanical thresholds, was described for cutaneous and mucosal nociceptors (Cooper et al. 1991; Andrew & Greenspan, 1999a). However, little is known about the possible mechanisms by which threshold and suprathreshold response properties might be modulated independently.
In general, potential targets for the actions of sensitizing agents on primary afferent nociceptors include both sensory transduction elements and voltage-gated ion channels that are involved in action potential generation. Sensitizing actions on the transducer for noxious heat, VR1, have been described (Tominaga et al. 1998; Premkumar & Ahern, 2000), but the identity of the transducer(s) for mechanical nociception has not yet been established and no sensitizing actions that are specifically targeted to the transduction process of mechanical nociception have been identified. However, sensitizing actions on voltage-gated ion channels, which would be expected to affect all sensory modalities, have been described, including several actions that are mediated by the cAMP-PKA cascade. Based on studies in dissociated dorsal root and nodose ganglion cells, the cAMP-PKA cascade has been implicated in mediating at least three of the modulatory actions of PGE2 on voltage-gated ion channels that could contribute to increased excitability in nociceptors. These are (1) enhancement of the TTX-resistant Na+ current that is expressed exclusively in a subpopulation of small diameter sensory neurons with nociceptor characteristics (England et al. 1996; Gold et al. 1998), (2) suppression of the sustained (delayed rectifier) outward K+ current that is thought to modulate the firing threshold (England et al. 1996; Evans et al. 1999) and (3) enhancement of Ih, the hyperpolarization-activated cation current that is thought to facilitate repetitive firing (Ingram & Williams, 1996).
Are there any known cellular mechanisms that could account for the differential effects of cAMP on the TH and STH groups in the present study? In particular, are there any known mechanisms of sensitization that could result in a selective action on either the threshold or suprathreshold components of the stimulus-response curve? There are membrane currents that are thought to regulate the capacity for repetitive firing without affecting firing threshold. Although the issue has not been examined directly, modulation of such currents might be expected to exert a selective effect on the suprathreshold portion of the stimulus-response curve without changing the response threshold. Two examples of such currents are Ih, which is thought to enhance repetitive firing, and the transiently activated outward K+ current, IA, which is thought to suppress repetitive firing and, thus, enhance spike adaptation (Stansfeld et al. 1986; Rudy, 1988). Ih is positively coupled to adenylate cyclase in a variety of cell types. Thus, cells with a large Ih might be expected to exhibit a high baseline capacity for repetitive discharge, as well as a further enhancement of this capacity in response to cAMP-PKA cascade activation. These features could be viewed as consistent with the high baseline slopes (which require higher discharge rates) and the suprathreshold sensitization pattern of the STH neurons. By contrast, IA was not markedly affected by PGE2 (Nicol et al. 1997), which is an activator of the cAMP-PKA cascade. Cells with a large IA might be expected to exhibit a low capacity for repetitive firing, which would not be strongly influenced by cAMP-PKA cascade activation. These features would be consistent with the low baseline slope and absence of suprathreshold sensitization found in the TH neurons.
Such an explanation for the difference in TH and STH properties would require that Ih and IA be segregated into distinct neuronal populations. Such segregation was described recently. Under a classification system for nociceptive dorsal root ganglion cells based on current signatures, Type 2 neurons exhibit a large IA with no Ih, whereas Type 4 neurons exhibit a large Ih with no IA (Scroggs et al. 1994; Petruska et al. 2000). Furthermore, these two classes of cell exhibit a size difference that is consistent with the difference in conduction velocity between TH and STH neurons. Specifically, Type 2 neurons are small (25–32 μm), which is consistent with a predominantly unmyelinated cell population, whereas Type 4 neurons are medium-sized (33–40 μm), which is indicative of a population with somewhat more rapid conduction. Although the TH and STH populations did not show a strict segregation by fibre class (Aδ vs. C fibres), they nevertheless exhibited a significant difference in conduction velocity that paralleled the size difference of Type 2 and 4 neurons. Therefore, both the current signature and the size differences between Type 2 and 4 neurons are suggestive of a possible congruence with the TH and STH populations of the present study.
Another difference between the two cell types that could influence sensitization patterns is the presence of TTX-resistant Na+ currents in Type 2, but not in Type 4, neurons (Cardenas et al. 1997). The cAMP-PKA cascade lowers the activation threshold of the TTX-resistant Na+ current (England et al. 1996; Gold et al. 1998), which might result in a decrease in the response thresholds of Type 2 neurons. Such an action might account for the decrease in threshold that is observed in TH, but not STH, neurons, with any concomitant increases in the suprathreshold responses of TH neurons being blocked by the excessively large IA.
In addition to possible differences in voltage-gated currents, differences in mechanotransduction might also contribute to the different patterns of sensitization exhibited by TH and STH neurons. In a study that bypassed the transduction process by injecting current into dorsal root ganglion cells, Gold et al. (1996) found that PGE2 evoked an increase in suprathreshold responses that occurred either alone or together with a decrease in threshold, but almost never found an effect on threshold alone. This observation suggests indirectly that modulation of the mechanotransduction process might be of particular importance for producing selective sensitizing effects on threshold alone. More direct investigation of possible modulatory effects on mechanotransduction in nociceptors is limited by the lack of information on the mechanosensitive membrane elements. A putative mechanosensory Na+ channel, BNaC1-α, has been identified. This channel is expressed exclusively in dorsal root ganglion cells and is selectively transported to peripheral terminals of mechanosensory neurons (Garcia-Anoveros et al. 2001). However, the neuronal distribution of this channel appears to be more indicative of a role in the transduction of non-noxious, rather than noxious, mechanical stimuli.
The sensitizing effects of dbcAMP on mechanosensitive dural afferents that we observed in the present study were of relatively short duration. While the current study is the only one to document the time course of cAMP-mediated actions on mechanonociceptors, Lopshire & Nicol (1997, 1998) observed a similarly brief time course for PGE2-induced sensitization of the capsaicin response in dorsal root ganglion cells. These studies also showed that the rapid recovery from sensitization was mediated by activation of the nitric oxide (NO)-cyclic GMP pathway. Although behavioural studies have revealed that the effects of cAMP-PKA cascade activation on mechanical hyperalgesia are longer lasting (Taiwo et al. 1989), the time course of these effects might not necessarily be strictly correlated with the sensitization of mechanonociceptors. For example, there is considerable evidence that changes in central excitability also contribute to mechanical hyperalgesia. It is also intriguing to note that the dural MIA neurons in our study were sensitized by dbcAMP for a much longer period than the mechanosensitive neurons. This raises the possibility that cAMP/PKA-induced mechanical hyperalgesia may largely be due to sensitization of MIA neurons and, only to a lesser extent, of mechanosensitive neurons.
In contrast to the pattern of sensitization produced by dbcAMP, the IM mixture produced both a decrease in threshold and increased suprathreshold responses in most sensitized neurons. The more complete pattern of sensitization produced by IM compared to cAMP/PKA might be explained by the combined activation of other second messenger cascades in addition to PKA. Of the agents in the IM mixture, PGE2 and 5-HT are thought to activate PKA (Taiwo et al. 1989, 1992; Cunha et al. 1999). However, the sensitizing effects of PGE2 are also probably mediated through local formation of NO (Aley et al. 1998). In addition, bradykinin probably activates PKC (Burgess et al. 1989; Cesare & McNaughton, 1996) and both bradykinin and histamine can stimulate the release of NO (Clough, 1999). Activation by IM of both PKA and non-PKA-dependant mechanisms is consistent with our finding that in each IM-sensitized neuron, only one of the two sensitization components was blocked by the PKA inhibitor. In addition, this finding suggests that the non-PKA-dependant mechanisms alone (operating in the presence of the PKA inhibitors) are capable of producing only one of the two components of sensitization. Therefore, it appears that neither the PKA nor the non-PKA mechanisms alone are sufficient to produce both components of sensitization. Our results raise the possibility that the combined activation of PKA- and other second messengers may be necessary to produce a leftward shift of the stimulus-response function throughout its entire range. A possible cellular correlate of this is the finding that combined activation of both PKA and PKC is required for maximal enhancement of the TTX-resistant sodium current in dorsal root ganglion cells (Gold et al. 1998).
In the present study, the induction of spontaneous activity was a prominent feature of sensitization induced by IM, but not dbcAMP, and was not blocked by PKA inhibitors. This is consistent with some previous reports that spontaneous activity is increased by activation of PKC, but not PKA (Leng et al. 1996). Activation of PKC can cause heat sensitization (Cesare & McNaughton, 1996; Leng et al. 1996; Cesare et al. 1999), which could result in spontaneous discharge if the response threshold to heating is decreased to a level below the tissue temperature (Reeh & Petho, 2000).
Cyclic AMP/PKA-mediated sensitization of mechanonociceptors, as well as recruitment of afferents that initially were mechanically insensitive, could contribute to the threshold and suprathreshold components of mechanical hyperalgesia. Sensitization of dural afferents, in particular, has been postulated to play a role in headache syndromes, such as migraine (Wolff, 1963; Strassman et al. 1996). At present, the most effective anti-migraine agents are the triptans, which are potent agonists at 5-HT1B/D receptors. Activation of these receptors has been found in a variety of cell types to lower intracellular cAMP levels through the inhibition of adenylate cyclase, which is distinct from the 5-HT4-mediated activation of adenylate cyclase (Hen, 1992). Our findings in the present study of the role of cAMP in dural afferent sensitization suggest that inhibition of adenylate cyclase in the mechanosensitive endings of dural afferents is a potential mechanism for the therapeutic action of triptans in migraine.
Acknowledgments
The authors thank Bernard J. Ransil for statistical advice, Peter Grigg for help with the methodology for mechanical stimulation, and Rami Burstein and Geoffrey Bove for valuable comments on the manuscript. This work was supported by the National Headache Foundation and by the National Institute of Neurological Disorders and Stroke, Grant NS-32534.
REFERENCES
- Ahlgren SC, Wang JF, Levine JD. C-fiber mechanical stimulus-response functions are different in inflammatory versus neuropathic hyperalgesia in the rat. Neuroscience. 1997;76:285–290. doi: 10.1016/s0306-4522(96)00290-4. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. Journal of Neuroscience. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Mccarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. Journal of Neuroscience. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. Journal of Neurophysiology. 1999a;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics. Journal of Neurophysiology. 1999b;82:2641–2648. doi: 10.1152/jn.1999.82.5.2641. [DOI] [PubMed] [Google Scholar]
- Bove GM, Moskowitz MA. Primary afferent neurons innervating guinea pig dura. Journal of Neurophysiology. 1997;77:299–308. doi: 10.1152/jn.1997.77.1.299. [DOI] [PubMed] [Google Scholar]
- Burgess GM, Mullaney I, McNeill M, Dunn PM, Rang HP. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. Journal of Neuroscience. 1989;9:3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Del Mar LP, Cooper BY, Scroggs RS. 5HT4 receptors couple positively to tetrodotoxin-insensitive sodium channels in a subpopulation of capsaicin-sensitive rat sensory neurons. Journal of Neuroscience. 1997;17:7181–7189. doi: 10.1523/JNEUROSCI.17-19-07181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proceedings of the National Academy of Sciences of the USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tanner K, Levine JD. Mechanical sensitization of cutaneous C-fiber nociceptors by prostaglandin E2 in the rat. Neuroscience Letters. 1999;267:105–108. doi: 10.1016/s0304-3940(99)00345-6. [DOI] [PubMed] [Google Scholar]
- Clough GF. Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo. Journal of Physiology. 1999;516:549–557. doi: 10.1111/j.1469-7793.1999.0549v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BY, Ahlquist M, Friedman RM, Labanc J. Properties of high-threshold mechanoreceptors in the goat oral mucosa. II. Dynamic and static reactivity in carrageenan-inflamed mucosa. Journal of Neurophysiology. 1991;66:1280–1290. doi: 10.1152/jn.1991.66.4.1280. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Teixeira MM, Ferreira SH. Pharmacological modulation of secondary mediator systems-cyclic AMP and cyclic GMP-on inflammatory hyperalgesia. British Journal of Pharmacology. 1999;127:671–678. doi: 10.1038/sj.bjp.0702601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. Journal of Neurophysiology. 1993;69:1071–1081. doi: 10.1152/jn.1993.69.4.1071. [DOI] [PubMed] [Google Scholar]
- England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. Journal of Physiology. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. Journal of Physiology. 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Nakamura M. I-Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979;18:179–190. doi: 10.1016/0090-6980(79)90103-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Samad TA, Woolf CJ, Corey DP. Transport and localization of the deg/enac ion channel bnac1alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. Journal of Neuroscience. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garell PC, McGillis SL, Greenspan JD. Mechanical response properties of nociceptors innervating feline hairy skin. Journal of Neurophysiology. 1996;75:1177–1189. doi: 10.1152/jn.1996.75.3.1177. [DOI] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. Journal of Neuroscience. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Cooper BY, Baumann KI, Schwegmann C, Friedman RM. Sensory nerve endings in the hard palate and papilla incisiva of the goat. Experimental Brain Research. 1999;129:218–228. doi: 10.1007/s002210050892. [DOI] [PubMed] [Google Scholar]
- Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends in Pharmacological Sciences. 1992;13:160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. Journal of Physiology. 1996;492:97–106. doi: 10.1113/jphysiol.1996.sp021292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa PS, Lamotte RH, Grigg P. Tensile and compressive responses of nociceptors in rat hairy skin. Journal of Neurophysiology. 1997;78:492–505. doi: 10.1152/jn.1997.78.1.492. [DOI] [PubMed] [Google Scholar]
- Kress M, Rodl J, Reeh PW. Stable analogues of cyclic AMP but not cyclic GMP sensitize unmyelinated primary afferents in rat skin to heat stimulation but not to inflammatory mediators, in vitro. Neuroscience. 1996;74:609–617. doi: 10.1016/0306-4522(96)00181-9. [DOI] [PubMed] [Google Scholar]
- Leng S, Mizumura K, Koda H, Kumazawa T. Excitation and sensitization of the heat response induced by a phorbol ester in canine visceral polymodal receptors studied in vitro. Neuroscience Letters. 1996;206:13–16. doi: 10.1016/0304-3940(96)12414-9. [DOI] [PubMed] [Google Scholar]
- Levine JD, Reichling DB. Peripheral mechanisms of inflammatory pain. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1999. pp. 59–84. [Google Scholar]
- Lopshire JC, Nicol GD. Activation and recovery of the PGE2-mediated sensitization of the capsaicin response in rat sensory neurons. Journal of Neurophysiology. 1997;78:3154–3164. doi: 10.1152/jn.1997.78.6.3154. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. Journal of Neuroscience. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B, O'Shea NR. Inhibition of forskolin-induced sensitisation of frog skin nociceptors by the cyclic AMP-dependent protein kinase A antagonist H-89. Brain Research. 1998;780:360–362. doi: 10.1016/s0006-8993(97)01360-7. [DOI] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Goetzl EJ, Levine JD. Leukotriene B4 decreases the mechanical and thermal thresholds of C- fiber nociceptors in the hairy skin of the rat. Journal of Neurophysiology. 1988;60:438–445. doi: 10.1152/jn.1988.60.2.438. [DOI] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Mizumura K, Koda H, Kumazawa T. Augmenting effects of cyclic AMP on the heat response of canine testicular polymodal receptors. Neuroscience Letters. 1993;162:75–77. doi: 10.1016/0304-3940(93)90563-z. [DOI] [PubMed] [Google Scholar]
- Mizumura K, Koda H, Kumazawa T. Opposite effects of increased intracellular cyclic AMP on the heat and bradykinin responses of canine visceral polymodal receptors in vitro. Neuroscience Research. 1996;25:335–341. doi: 10.1016/0168-0102(96)01056-5. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR. Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. Journal of Neurophysiology. 1997;77:167–176. doi: 10.1152/jn.1997.77.1.167. [DOI] [PubMed] [Google Scholar]
- Ouseph AK, Khasar SG, Levine JD. Multiple second messenger systems act sequentially to mediate rolipram-induced prolongation of prostaglandin E2-induced mechanical hyperalgesia in the rat. Neuroscience. 1995;64:769–776. doi: 10.1016/0306-4522(94)00397-n. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. Journal of Neurophysiology. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Reeh PW, Petho G. Nociceptor excitation by sensitization: a novel hypothesis, its cellular and molecular background. In: Saade NE, Apkarian AV, Jabbur SJ, editors. Pain and Neuroimmune Interactions. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 9–19. [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Schmidt RF. Excitation and sensitization of fine articular afferents from cat's knee joint by prostaglandin E2. Journal of Physiology. 1988;403:91–104. doi: 10.1113/jphysiol.1988.sp017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Ringkamp M, Handwerker HO, Torebjork HE. Sensitization of insensitive branches of C nociceptors in human skin. Journal of Physiology. 1994;480:389–394. doi: 10.1113/jphysiol.1994.sp020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Todorovic SM, Anderson EG, Fox AP. Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. Journal of Neurophysiology. 1994;71:271–279. doi: 10.1152/jn.1994.71.1.271. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli. Journal of Neurophysiology. 2000;83:2179–2191. doi: 10.1152/jn.2000.83.4.2179. [DOI] [PubMed] [Google Scholar]
- Stansfeld CE, Marsh SJ, Halliwell JV, Brown DA. 4-Aminopyridine and dendrotoxin induce repetitive firing in rat visceral sensory neurones by blocking a slowly inactivating outward current. Neuroscience Letters. 1986;64:299–304. doi: 10.1016/0304-3940(86)90345-9. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA. Electrophysiological evidence for tetrodotoxin-resistant sodium channels in slowly conducting dural sensory fibers. Journal of Neurophysiology. 1999;81:413–424. doi: 10.1152/jn.1999.81.2.413. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. Journal of Neurophysiology. 1998;80:2632–2644. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Heller PH, Levine JD. Mediation of serotonin hyperalgesia by the cAMP second messenger system. Neuroscience. 1992;48:479–483. doi: 10.1016/0306-4522(92)90507-x. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang JF, Khasar SG, Ahlgren SC, Levine JD. Sensitization of C-fibres by prostaglandin E2 in the rat is inhibited by guanosine 5′-O-(2-thiodiphosphate), 2′,5′-dideoxyadenosine and Walsh inhibitor peptide. Neuroscience. 1996;71:259–263. doi: 10.1016/0306-4522(95)00429-7. [DOI] [PubMed] [Google Scholar]
- White DM, Basbaum AI, Goetzl EJ, Levine JD. The 15-lipoxygenase product, 8R,15S-diHETE, stereospecifically sensitizes C-fiber mechanoheat nociceptors in hairy skin of rat. Journal of Neurophysiology. 1990;63:966–970. doi: 10.1152/jn.1990.63.5.966. [DOI] [PubMed] [Google Scholar]
- Wolff HG. Headache and Other Head Pain. New York: Oxford University Press; 1963. [Google Scholar]


