Abstract
Non-invasive magnetic resonance imaging (MRI) was used to characterize changes in left and right ventricular cardiac cycles following induction of experimental, streptozotocin (STZ)-induced, diabetes in male Wistar rats at different ages. The effects of the angiotensin-converting enzyme (ACE) inhibitor captopril upon such chronic physiological changes were then evaluated, also for the first time. Diabetes was induced at the age of 7 weeks in two experimental groups, of which one group was subsequently maintained on captopril (2 g l−1)-containing drinking water, and at 10 and 13 weeks in two further groups. The fifth group provided age-matched controls. All groups (each n = 4 animals) were scanned consistently at 16 weeks, in parallel with timings used in earlier studies that employed this experimental model. Cine magnetic resonance (MR) image acquisition provided transverse sections through both ventricles at twelve time points covering systole and most of diastole. These yielded reconstructions of cardiac anatomy used to derive critical functional indices and their dependence upon time following the triggering electrocardiographic R waves. The left and right ventricular end-diastolic (EDV), end-systolic (ESV) and stroke volumes (SV), and ejection fractions (EF) calculated from each, control and experimental, group showed matching values. This confirmed a necessary condition requiring balanced right and left ventricular outputs and further suggested that STZ-induced diabetes produced physiological changes in both ventricles. Absolute left and right ventricular SVs were significantly altered in all diabetic animals; EDVs and EFs significantly altered in animals diabetic from 7 and 10 but not 13 weeks. When normalized to body weight, left and right ventricular SVs had significantly altered in animals diabetic from 7 and 10 weeks but not 13 weeks. Normalized left ventricular EDVs were also significantly altered in animals diabetic from 7 and 10 weeks. However, normalized right ventricular EDVs were significantly altered only in animals made diabetic from 7 weeks. Diabetic hearts showed major kinetic changes in left and right ventricular contraction (ejection) and relaxation (filling). Both the initial rates of volume change (dV/dt) in both ventricles and the plots of dV/dt values through the cardiac cycle demonstrated more gradual developments of tension during systole and relaxation during diastole. Estimates of the derived left ventricular performance parameters of cardiac output, cardiac power output and stroke work in control animals were comparable with human values when normalized to both body (or cardiac) weight and heart rate. All deteriorated with diabetes. Comparisons of experimental groups diabetic from 7 weeks demonstrated that captopril treatment relieved the alterations in critical volumes, dependence of SV upon EDV, kinetics of systolic contraction and diastolic relaxation and in the derived indicators of ventricular performance. This study represents the first demonstration using non-invasive MRI of early, chronic changes in diastolic filling and systolic ejection in both the left and the right ventricles and of their amelioration by ACE inhibition following STZ-induction of diabetes in intact experimental animals.
The increased morbidity and mortality in diabetes result largely from its cardiovascular complications (Garcia et al. 1974; Paz-Guevara et al. 1975; Crall & Roberts, 1978; Kannel & McGee, 1979; Kannel, 1985; Stehouwer et al. 1997). The Framingham study reported substantially increased incidences of coronary artery disease (Kannel & McGee, 1979) and mortalities following acute myocardial infarction. The latter might reflect the higher incidence of the more extensive anterior myocardial infarction (Weitzman et al. 1982), post-infarction congestive cardiac failure and cardiogenic shock. However, diabetics also suffer a higher incidence of congestive heart failure that cannot be completely accounted for by the associated prevalence of coronary atherosclerosis or of hypertension or cardiac autonomic neuropathy (Kannel et al. 1974). Such findings suggest that distinct myocardial changes might produce a specific diabetic cardiomyopathic condition (Ledet, 1968, 1976; Goodwin & Oakley, 1972; Rubler et al. 1972; Hamby et al. 1974; Ledet et al. 1979; Bell, 1995).
Clinical studies using both invasive and non-invasive techniques have sought physiological evidence for such cardiac changes in diabetic patients. However, these variously reported normal (Airaksinen et al. 1984b) and abnormal systolic time intervals (Ahmed et al. 1975; Seneviratne, 1977; Shapiro et al. 1980, 1981a,b; Cellina et al. 1983; Uusitupa et al. 1983, 1985). Echocardiographic studies reported reduced (Airaksinen et al. 1984a, 1987), normal or modest increases (Shapiro et al. 1981a; Friedman et al. 1982) in left ventricular size. Prolonged isometric relaxation phases (Sanderson et al. 1978; Shapiro et al. 1980, 1981a,b; Shapiro, 1982; Airaksinen et al. 1984a) and depressed (Shapiro et al. 1981a,b, Uusitupa et al. 1985), normal (Airaksinen et al. 1984b) or enhanced (Thuesen et al. 1988) systolic function have also been reported in insulin-dependent diabetics with microvascular complications. Finally, radionuclide ventriculography studies variously reported reduced (Zola et al. 1986) or normal (Vered et al. 1984; Fisher et al. 1986; Arvan et al. 1988) resting ejection fractions.
Selected aspects of left but (not right) ventricular function have also been studied using conventional physiological techniques in chronic animal models in which disease timing and severity might be more readily controlled. Alloxan-induced diabetic dogs developed increases in left ventricular wall stiffness and end-diastolic pressures over an 11 month period (Regan et al. 1974). Isolated perfused hearts of diabetic rats showed decreased peak systolic pressures (Miller, 1979) and more rapid development of heart failure following severe global ischaemia (Feuvray et al. 1979) and of myocardial failure during late recovery from ischaemia (Hearse et al. 1975). They also demonstrate altered diastolic function that might reflect the significantly increased collagen content in their left ventricular free walls and septa (Riva et al. 1998). Isolated papillary ventricular muscles from diabetic rats showed delayed onsets and reduced rates of relaxation, depressed shortening velocities (Fein et al. 1980), increased times to peak tension and decreased sensitivities to extracellularly applied calcium and adrenaline (Warley et al. 1995).
The previous paper (Al-Shafei et al. 2002) introduced and validated magnetic resonance imaging (MRI) for non-invasive determinations of myocardial volume to submillimetre resolution in streptozotocin (STZ)-induced diabetic Wistar rats for the first time. MRI might thus offer useful methods of serially following chronic physiological changes in intact animal models of disease processes. Such an experimental approach could study animals at clearly defined stages following induction of experimental disease. The high soft tissue contrast due to the large differences in T1 and T2 relaxation times of myocardium, epicardial fat and blood would permit acquisition of high quality MR images. The present experiments accordingly extend the previous anatomical determinations and applied dynamic or cine magnetic resonance imaging with electrocardiographic gating to determine cardiac volumes critical to the left and right ventricular cycles in experimental and control rats following graded durations of diabetes. The rats were made diabetic at ages (7, 10 and 13 weeks) similar to those at which it was induced in earlier physiological studies of the same experimental diabetic model. Similarly, imaging took place at an age (16 weeks) largely in accord with the ages at which animals in the previous investigations were sacrificed for functional studies (13–17 weeks) The present MRI findings are therefore comparable with earlier physiological characterizations using conventional invasive techniques (see accompanying paper Al-Shafei et al. 2002).
Systematic sets of transverse cardiac images were acquired at a series of consistently chosen time points in the cardiac cycle and were used to reconstruct the critical volumes at close time resolution as well as identify reproducible kinetic changes. This animal MRI disease model was then applied to investigate the effect of the ACE inhibitor captopril on such cardiac changes in intact animals. It has been suggested that there exists an intracardiac renin- angiotensin system (Dostal et al. 1992a,b) which is activated in diabetes thus enhancing angiotensin II production (Rösen et al. 1995). The latter agent has been implicated in the pathogenesis of diabetic cardiomyopathy: it induces myocardial interstitial fibrosis through fibroblast proliferation (Schorb et al. 1993; Crabos et al. 1994; Matsubara et al. 1994; Schorb et al. 1994). STZ-diabetic rats show elevated ACE levels in their left ventricular tissue and a decreased left ventricular developed pressure; the latter changes are prevented by the ACE inhibitor enalapril (Goyal et al. 1998). This study accordingly represents the first demonstration by non-invasive MRI of early chronic changes in left and right ventricular diastolic filling and systolic ejection characteristics and of their amelioration by ACE inhibition following STZ-induction of diabetes in intact experimental animals.
METHODS
All procedures conformed to the Animal Scientific Procedures Act (1986) and are described in greater detail in the previous paper (Al Shafei et al. 2002). Briefly, twenty fully conditioned, healthy, pathogen-free male Wistar rats (6 weeks old; Harlan, UK), maintained under standard conditions, were divided into five experimental groups at 7 weeks old. Diabetes was induced in four groups (each n = 4 animals) at 7, 10 and 13 weeks. The rats were anaesthetized using 1–2 % halothane in oxygen, their blood glucose levels were checked. They were then given a single intraperitoneal injection of streptozotocin (STZ: 65 mg (kg body weight)−1; Sigma-Aldrich Co. Poole, Dorset, UK; see Junod et al. 1967, 1969). The STZ was dissolved in phosphate-buffered saline to which citric acid (10 g (100 ml)−1) was added to give a pH of 4.5. This precaution prevented the rapid inactivation of STZ at a neutral pH (see Junod et al. 1967). The control rats received sham injections of the citrated buffer when they were 7 weeks old. STZ (55–65 mg (kg body weight)−1) produces severe but stable diabetes eventually leading to structural and functional myocardial abnormalities in Wistar rats (Junod et al. 1967, 1969; Lopaschuk et al. 1983; Rodrigues et al. 1990, 1997; Warley et al. 1995).
A fourth diabetic group was treated with 2 g l−1 captopril (Sigma-Aldrich Co., Poole, Dorset, UK) in the drinking water (Dalton et al. 1997; Qi et al. 1999) following induction of diabetes at 7 weeks and the fifth (n = 4) provided age-matched controls for all the experimental groups. Hyperglycaemia with blood glucose levels > 13 mm ensued 48 h after STZ administration and remained at such levels even 2 weeks after injection. Body weight was recorded every 3 days and blood glucose every 2 weeks. All animals were scanned at 16 weeks so that all the diabetic rats were being compared with a single age and sex matched control group.
For MR imaging, rats were anaesthetized using 1–2 % halothane in oxygen, weighed and their systolic blood pressures measured non-invasively using a rat tail blood pressure monitor both before and after imaging sessions to confirm physiological stability. Electrocardiographic (ECG) monitoring used shielded subcutaneous electrodes and a Tektronix 2225 oscilloscope (Tektronix, Harpenden, Herts, UK). The cine imaging protocols were performed with the anaesthetized animal placed in a specially designed home-built half-sine-spaced birdcage radiofrequency (RF) probe unit contained within a cylindrical plastic holder fitted within a gradient set of internal diameter 11 cm. The RF probe unit was made up of a half-sine spaced birdcage RF probe of internal diameter of 4.5 cm with open ends, an RF shield consisting of a cylinder of copper gauze surrounding and sliding over the birdcage, a tuning capacitor and a coaxial cable to carry the RF (Ballon et al. 1990). The assembly included ECG leads, attachment plugs for the ECG leads and a unit to anchor anaesthetic delivery tubes near the nose of the animal. All experiments used a 2 T Oxford Instruments (UK) superconducting magnet with a horizontal internal bore of 31 cm. A gated cine protocol synchronized line acquisition to set times following alternate electrocardiographic R waves. This acquisition was then repeated at the same slice position at twelve equally incremented times through the cardiac cycle. This sequence in turn was repeated for each of the 128 lines to generate each 128 × 128 image, which itself was acquired twice for signal averaging. The preceding procedure was in turn repeated 12 times to obtain signal-averaged images for every one of the 12 contiguous transverse slices examined. Each imaging session therefore required (128 × 12 × 2 × 2) times the cardiac cycle duration. The effective repeat time (TR) was approximately 13 ms. The short echo time (TE) of 4.3 ms reduced motion artefacts and ensured good contrast between blood and myocardium.
Following imaging sessions, data were transferred from the MRI console to remote UNIX workstations. The borders of both ventricles in each transverse image slice were interactively defined using the blood-myocardial wall contrast to identify the endocardial borders and the myocardial wall-thoracic cavity contrast to draw the epicardial borders. The pixel numbers enclosed within each border were then converted into units of square millimetres. The epi- and endocardial borders of both ventricles were independently drawn 4 times for all the images of the 12 selected slices. Left and right ventricular epi- and endocardial volumes were then calculated.
RESULTS
The experiments: (1) characterized changes in both cardiac cycle volumes and derived cardiac functional characteristics in both the left and right ventricles following induction of experimental diabetes, over ages comparable to those employed in earlier invasive physiological studies (see Introduction; for references see Discussion). (2) They also provided indications of the effect of age of disease onset on such changes and (3) investigated how ACE inhibition influenced such changes in animals diabetic from 7 weeks, and scanned at 16 weeks. Accordingly, data was first analysed using a one-way analysis of variance (ANOVA) to compare results from control experimental groups not treated with captopril, but made diabetic from 7, 10 and 13 weeks. Tables 1–3 and Table 5 thus summarize results from rats with 3, 6 and 9 week histories of diabetes. A second one-way ANOVA was then applied to the control group and the two groups made diabetic from 7 weeks, one of which was treated with captopril (Tables 4 and 6). Demonstration of statistically significant differences then prompted pair-wise comparisons using Tukey's Honestly Significant Difference (HSD) test to a significance level of P < 0.05. Finally, statistical comparisons of functional parameters obtained from the left and right ventricles in each of the five experimental groups used Student's two-tailed paired t tests to a significance level of P < 0.05. All results are presented as means ± standard error of the mean (s.e.m.). The basic physiological parameters of body weight, blood glucose concentrations, heart rates and systolic blood pressures in both control and experimental groups agreed with earlier reports from this diabetic system (Pierce & Dhalla, 1981, 1983, 1985a,b; Afzal et al. 1988; Maeda et al. 1995; Shimabukuro et al. 1995; Hicks et al. 1998; Al-Shafei et al. 2002).
Table 1.
Diastolic and systolic volumes and ejection fractions (EF) of the left (LV) and right ventricles (RV) from cardiac cycles of the control and diabetic groups not treated with captopril
| Diabetic | |||||
|---|---|---|---|---|---|
| Control | 3 week | 6 week | 9 week | P | |
| LV end-diastolic volume (EDV) (μl) | 493.8 ± 12.1c, d | 461 ± 11.6c, d | 357.5 ± 11.1a, b, d | 285.8 ± 10.1a, b, c | < 0.001 |
| RV end-diastolic volume (EDV) (μl) | 472.3 ± 10.3c, d | 444.3 ± 11.1c, d | 361.3 ± 5.5a, b, d | 272.5 ± 11.1a, b, c | < 0.001 |
| LV end-systolic volume (ESV) (μl) | 176.3 ± 10.3 | 177.5 ± 4.8 | 180 ± 7.4 | 166.3 ± 5.2 | 0.572 |
| RV end-systolic volume (ESV) (μl) | 173.8 ± 12 | 172.5 ± 4.8 | 175 ± 6.5 | 162.5 ± 3.2 | 0.628 |
| LV stroke volume (SV) (μl) | 317.5 ± 4.8b, c, d | 283.5 ± 7.6a, c, d | 177.5 ± 4.3a, b, d | 119.5 ± 7.5a, b, c | < 0.001 |
| RV stroke volume (SV) (μl) | 298.5 ± 2.5b, c, d | 271.8 ± 6.4a, c, d | 186.3 ± 3.1a, b, d | 110 ± 8.4a, b, c | < 0.001 |
| LV ejection fraction (EF) (%) | 64.4 ± 1.3c, d | 61.5 ± 0.5c, d | 49.7 ± 0.7a, b, d | 41.7 ± 1.6a, b, c | < 0.001 |
| RV ejection fraction (EF) (%) | 63.3 ± 1.8c, d | 61.2 ± 0.2c, d | 51.6 ± 1.2a, b, d | 40.2 ± 1.6a, b, c | < 0.001 |
Animals were made diabetic from 13, 10 and 7 weeks and spared captopril treatment giving diabetic histories of 3, 6 and 9 weeks. All values are expressed as means ± standard errors of the mean (s.e.m.); n = 4. One-way analysis of variance (one-way ANOVA) was used to compare the control and the three untreated diabetic groups followed by Tukey's Honestly Significant Difference test for pair-wise multiple comparisons. A value of P < 0.05 was considered significant from
control
3
6
(untreated) 9 week diabetic groups.
Table 3.
Indices for the kinetics of left (LV) and right ventricular (RV) contraction and relaxation of the control and the three diabetic groups spared captopril treatment
| Diabetic | |||||
|---|---|---|---|---|---|
| Control | 3 week | 6 week | 9 week | P | |
| LV 25% SV and DFV (μl) | 79.4 ± 1.2b, c, d | 70.9 ± 1.9 a, c, d | 42.9 ± 0.9a, b, d | 28.6 ± 1.5a, b, c | < 0.001 |
| RV 25% SV and DFV (μl) | 74.6 ± 0.6b, c, d | 67.9 ± 1.6a, c, d | 44.6 ± 0.8a, b, d | 26.2 ± 2.1a, b, c | < 0.001 |
| LV time for 25% SV (ms) | 14.0 ± 0.8c, d | 13.8 ± 1.6c, d | 34.3 ± 2.7a, b | 37.3 ± 0.9a, b | < 0.001 |
| RV time for 25% SV (ms) | 14.0 ± 0.4c, d | 14.0 ± 1.0c, d | 34.8 ± 2.5a, b | 40.3 ± 0.8a, b | < 0.001 |
| LV time for 25% DFV (ms) | 11.3 ± 0.5 | 11.3 ± 0.6 | 12.8 ± 1.1 | 11.8 ± 1.0 | 0.578 |
| RV time for 25% DFV (ms) | 10.5 ± 0.6 | 10.3 ± 0.5 | 11.5 ± 0.9 | 11.5 ± 1.0 | 0.544 |
| LV rate of ejection during early systole (μl ms−1) | 5.7 ± 0.3c, d | 5.3 ± 0.5c, d | 1.3 ± 0.1a, b | 0.8 ± 0.03a, b | < 0.001 |
| RV rate of ejection during early systole (μl ms−1) | 5.3 ± 0.2c, d | 4.9 ± 0.3c, d | 1.3 ± 0.1a, b | 0.6 ± 0.05a, b | < 0.001 |
| LV rate of filling during early diastole (μl ms−1) | 7.1 ± 0.4c, d | 6.4 ± 0.5c, d | 3.4 ± 0.3a, b | 2.5 ± 0.2a, b | < 0.001 |
| RV rate of filling during early diastole (μl ms−1) | 7.2 ± 0.4c, d | 6.7 ± 0.5c, d | 3.9 ± 0.2a, b | 2.4 ± 0.3a, b | < 0.001 |
All values expressed as means ± s.e.m.; n = 4. One-way ANOVA was used in comparison of the control and the three untreated diabetic groups followed by Tukey's Honestly Significant Difference test for pair-wise multiple comparisons. A value of P < 0.05 was considered statistically significant.
Significantly different from the control group.
Significantly different from the 3-week diabetic group.
Significantly different from the 6-week diabetic group.
Significantly different from the 9-week untreated diabetic group.
Table 5.
Derived left ventricular indices of control and the three diabetic groups spared captopril treatment
| Diabetic | |||||
|---|---|---|---|---|---|
| Control | 3 week | 6 week | 9 week | P | |
| Left ventricular output (LVOP) (l min−1) | 0.102 ± 0.003b, c, d | 0.090 ± 0.003a, c, d | 0.050 ± 0.001a, b, d | 0.033 ± 0.002a, b, c | <0.001 |
| LVOP/body weight (l min−1 kg−1) | 0.291 ± 0.001b, c, d | 0.269 ± 0.005a, c, d | 0.176 ± 0.005a, b, d | 0.142 ± 0.007a, b, c | <0.001 |
| LVOP/heart weight (l min−1 g−1) | 0.12 ± 0.002b, c, d | 0.11 ± 0.002a, c, d | 0.06 ± 0.002a, b, d | 0.05 ± 0.002a, b, c | <0.001 |
| MLVP (W) | 0.033 ± 0.002c, d | 0.027 ± 0.001c, d | 0.012 ± 0.001a, b | 0.007 ± 0.001a, b | <0.001 |
| MLVP/body weight (mW g−1) | 0.093 ± 0.004c, d | 0.082 ± 0.005c, d | 0.044 ± 0.004a, b | 0.030 ± 0.004a, b | <0.001 |
| MLVP/Heart weight (W g−1) | 0.037 ± 0.002c, d | 0.032 ± 0.002c, d | 0.016 ± 0.001a, b | 0.010 ± 0.001a, b | <0.001 |
| MLVSW (J) | 0.006 ± 0.0003b, c, d | 0.005 ± 0.0002a, c, d | 0.003 ± 0.0001a, b, d | 0.001 ± 0.0001a, b, c | <0.001 |
| MLVSW/body weight (mJ g−1) | 0.017 ± 0.0005c, d | 0.016 ± 0.0009c, d | 0.009 ± 0.0006a, b, d | 0.006 ± 0.0007a, b, c | <0.001 |
| MLVSW/heart weight (J g−1) | 0.007 ± 0.0002c, d | 0.006 ± 0.0003c, d | 0.003 ± 0.0002a, b, d | 0.002 ± 0.0003a, b, c | <0.001 |
| Left ventricular systolic elastance (mmHg μl−1) | 0.82 ± 0.03 | 0.78 ± 0.06 | 0.63 ± 0.06 | 0.57 ± 0.09 | 0.05 |
| LVMV (μl)/LVEDV (μl) | 1.32 ± 0.02c, d | 1.38 ± 0.01c, d | 1.68 ± 0.03a, b, d | 1.88 ± 0.01a, b, c | <0.001 |
| RVMV (μl)/RVEDV (μl) | 0.43 ± 0.012c, d | 0.45 ± 0.007c, d | 0.50 ± 0.008a, b, d | 0.62 ± 0.009a, b, c | <0.001 |
MLVP, maximum left ventricular power; MLVSW, maximum left ventricular stroke work; MV, myocardial volume. All values expressed as means ± s.e.m.; n = 4. One-way ANOVA was used in comparison of the control and the three untreated diabetic groups followed by Tukey's Honestly Significant Difference test for pair-wise multiple comparisons. A value of P <0.05 was considered statistically significant. RVMV/RVEDV included for comparison with the corresponding left ventricular parameter.
Significantly different from the control group.
Significantly different from the 3-week diabetic group.
Significantly different from the 6-week diabetic group.
Significantly different from the 9-week untreated diabetic group.
Table 4.
Basic physiological and MRI-derived parameters of the captopril-treated group
| Left ventricle | Right ventricle | |||
|---|---|---|---|---|
| Value | P | Value | P | |
| End-diastolic volume (EDV) (μl) | 346.3 ± 19.5*† | <0.001 | 322.5 ± 15.1*† | <0.001 |
| End-systolic volume (ESV) (μl) | 132.5 ± 9.7* | 0.015 | 123.8 ± 5.5*† | 0.004 |
| Stroke volume (SV) (μl) | 213.8 ± 10.1*† | <0.001 | 198.8 ± 10.5*† | <0.001 |
| Ejection fraction (EF) (%) | 61.8 ± 0.8† | <0.001 | 61.6 ± 0.8† | <0.001 |
| End-diastolic volume (EDV)/body weight (μl g−1) | 1.4 ± 0.06† | 0.005 | 1.3 ± 0.02† | <0.001 |
| End-systolic volume (ESV)/body weight (μl g−1) | 0.54 ± 0.02† | <0.001 | 0.50 ± 0.01† | <0.001 |
| Stroke volume (SV)/body weight (μl g−1) | 0.87 ± 0.04† | <0.001 | 0.80 ± 0.01† | <0.001 |
| 25% SV and DFV (μl) | 53.44 ± 2.5*† | < 0.001 | 49.7 ± 2.6*† | <0.001 |
| Time for 25% SV (ms) | 14.9 ± 0.9† | <0.001 | 15.8 ± 1.0† | <0.001 |
| Time for 25% DFV (ms) | 12.9 ± 0.7 | 0.075 | 11.9 ± 0.6 | 0.184 |
| Rate of ejection during early systole (μl ms−1) | 3.7 ± 0.3*† | <0.001 | 3.2 ± 0.2*† | <0.001 |
| Rate of filling during early diastole (μl ms−1) | 4.2 ± 0.1*† | <0.001 | 4.2 ± 0.4*† | <0.001 |
All values expressed as means ± s.e.m. (n = 4 animals). One-way ANOVA was used in comparison of the control and the two groups made diabetic from age 7 weeks (the group spared captopril treatment and the group treated with captopril) followed by Tukey's Honestly Significant Difference test for pair-wise multiple comparisons. A value of P <0.05 was considered statistically significant.
Significantly different from the control group.
Significantly different from the similarly diabetic group spared captopril treatment. The body weight-normalized values were calculated using the corresponding body weight of the anaesthetised rat.
Table 6.
Derived indices of the 9-week captopril-treated diabetic group
| Parameter | Value | P |
|---|---|---|
| Left ventricular output (LVOP) (l min−1) | 0.067 ± 0.005*† | <0.001 |
| LVOP/body weight (l min−1 kg−1) | 0.270 ± 0.013† | <0.001 |
| LVOP/heart weight (l min−1 g−1) | 0.11 ± 0.006† | <0.001 |
| Maximum left ventricular power (MLVP) (W) | 0.02 ± 0.003*† | <0.001 |
| MLVP/body weight (mW g−1) | 0.083 ± 0.008† | <0.001 |
| MLVP/heart weight (W g−1) | 0.034 ± 0.003† | <0.001 |
| Maximum left ventricular stroke work (MLVSW) (J) | 0.004 ± 0.0004*† | <0.001 |
| MLVSW/body weight (mJ g−1) | 0.016 ± 0.001† | <0.001 |
| MLVSW/heart weight (J g−1) | 0.006 ± 0.0005† | <0.001 |
| Left ventricular systolic elastance (mmHg μl−1) | 1.04 ± 0.04† | 0.001 |
| LVMV (μl)/LVEDV (μl) | 1.3 ± 0.04† | <0.001 |
| RVMV (μl)/RVEDV (μl) | 0.45 ± 0.015† | <0.001 |
All values expressed as mean ± s.e.m.; n = 4. One-way ANOVA was used in comparison of the control and the two groups diabetic from age 7 weeks followed (the group spared captopril treatment and the group treated with captopril) by Tukey's Honestly Significant Difference test for pair-wise multiple comparisons. A value of P <0.05 was considered statistically significant.
Significantly different from the control group.
Significantly different from the similarly diabetic group spared captopril treatment.
Transverse MRI cardiac sections
Figure 1 displays typical transverse sections through the widest regions of intact beating hearts in control rats (A), rats not treated with captopril but made diabetic from 13, 10 and 7 weeks (B-D) and captopril-treated diabetic rats (E). This gave experimental groups that could be conveniently described as having diabetic histories of 3, 6, 9 and 9 weeks, respectively; the groups are described as such in the remaining figures. These were selected from complete data sets themselves made up of 12 transverse contiguous slices of the same thickness. Each slice was positioned perpendicularly to the principal cardiac axis between the cardiac apex and aortic valve as far as possible following a consistent procedure for each experimental rat. This first positioned the cranio-caudal axis of the prone animal along the main magnetic field (B0) axis of the superconducting magnet, then obtained nine contiguous sagittal thoracic images using an expanded, 7 cm, field of view. That sagittal image most clearly representing the heart was then used to derive a series of contiguous transverse-coronal images. Finally, the clearest of the latter series was used to select a definitive set of typically 12 transverse cardiac sections entirely covering the left and right ventricles.
Figure 1. Typical transverse MR sections obtained from the heart of a normal control rat (A), and rats made diabetic from 13 (B), 10 (C) and 7 weeks (D) to give disease histories of 3, 6 and 9 weeks respectively, and a captopril-treated diabetic rat with a disease history of 9 weeks (E).
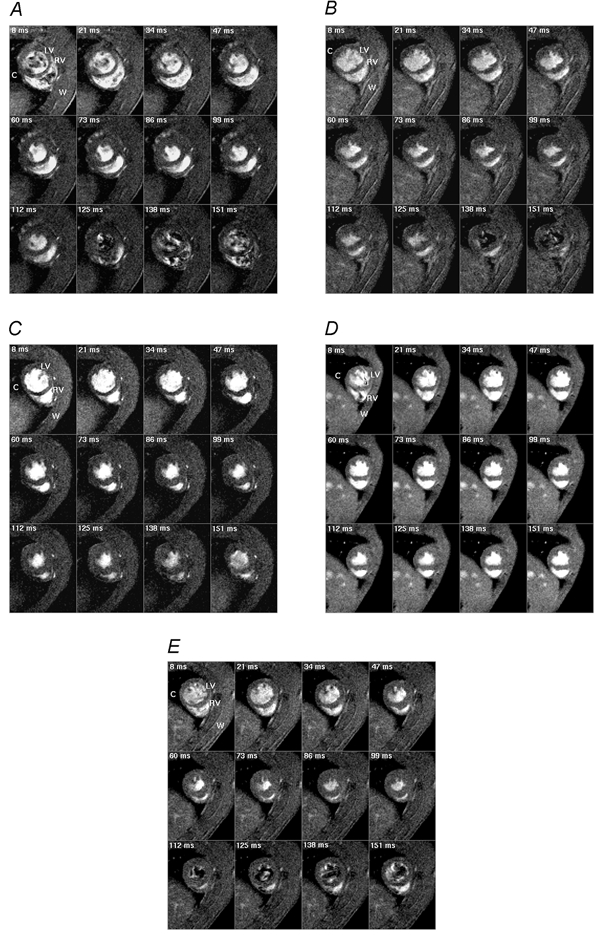
All rats were scanned at 16 weeks. The weight of the normal rat was 340 g and those of the diabetic rats were 230, 275, 330 and 245 g for the rat made diabetic at 7, 10 and 13 weeks and the captopril-treated rat, respectively. The heart rates were continuously monitored throughout imaging sessions; intrinsic heart rates were 315 ± 4 beats min−1 for the normal rat and 290 ± 4, 300 ± 6, 307 ± 4 and 300 ± 4 beats min−1 for the four diabetic rats, respectively. The sections were taken perpendicular to the principal cardiac axis at one spatial slice at typically twelve time points during the cardiac cycle. These time points are indicated in the upper left corner of each panel and correspond to the delay after the trigger, taken from the R wave of the electrocardiogram (ECG), at which the signal was acquired. Each image is the average of two signals obtained at corresponding points in the cardiac cycle following the R wave. LV and RV indicate left and right ventricles, respectively, and C and W indicate chest cavity and chest wall, respectively. Slice thickness was 1.50 mm for the normal control rat and 1.44 mm for the four diabetic rats. Field of view (FOV) was 5 cm for the normal control rat and 4.5 cm for the four diabetic rats. With an image matrix of 128 pixels × 128 pixels, the nominal in-plane resolution was approximately 351.6–390.6 μm pixel−1. The effective repeat time (TR) was approximately 13 ms and the echo time (TE) was 4.3 ms.
The MRI experiments thus provided complete sets of transverse cardiac sections through the cardiac cycle of both ventricles in all five experimental groups. These were first used to provide qualitative indications of ventricular geometry. The cine imaging protocol provided high quality anatomical images in all the experimental groups demonstrating blood as a bright intensity in a clear demarcation of myocardium from blood. The latter helped define the ventricular endocardial borders for clear estimations of end-diastolic and -systolic volumes. Both the epi- and endocardial borders of the left ventricles resembled human hearts in their circularly symmetrical transverse sections in all experimental groups. The quantitative measurements that followed accordingly treated the inter-ventricular septum as part of the left ventricle. In contrast, the right ventricles consistently resembled human right ventricles in their crescentic transverse sections and thinner walls.
In general, during systole and very early diastole, the cine cardiac imaging protocol demonstrates blood as a bright intensity in a clear demarcation of myocardium from blood. This results from inflow brightening (Pope & Yao, 1993) between successive excitations of the same slice at the 12 different time points that were studied in the cardiac cycle and the longer transverse relaxation time constant (T2) of blood than muscle (Beall et al. 1984), giving a reduced loss of transverse magnetization from blood during the used echo time. At the later time points in the cardiac cycle, i.e. later in diastole, there was little bright intensity in the left ventricles of the normal rats, those diabetic from 13 weeks, and the captopril-treated group that had been diabetic from 7 weeks. This may reflect turbulent flow of the blood entering the ventricle causing dephasing of the spins within the echo time. In contrast, for the rats diabetic from 7 and 10 weeks, blood showed as a bright intensity late in diastole. This could be interpreted in terms of much reduced flow rates during diastole as this could make the flow of blood less turbulent in these two experimental groups with less dephasing of spins within the echo time.
Secondly, Fig. 1 shows successive frames separated by 13 ms intervals; this close sampling permitted both the end-diastolic and -systolic points in the cardiac cycle to be clearly identified. The initial images were acquired 8 ms after the triggering R wave and demonstrate fully dilated end-diastolic ventricles. Systole then developed with wall thickening and luminal contraction in both ventricles in the normal animals (A), those diabetic from 13 weeks (B) and the captopril-treated diabetic rats (E). Both ventricles reached their end-systolic, minimal luminal cross-sectional areas synchronously at ∼100 ms after the trigger pulse. The rapid diastolic refilling of both ventricular cavities that followed was accompanied by a relative thinning of the ventricular walls. In contrast, the time course of myocardial thickening and luminal contraction in rats not treated with captopril, but made diabetic from 10 and 7 weeks, showed significant early systolic delays until approximately 34 ms: end-systole was not reached until ∼112 ms after the R wave trigger (C and D). Diastolic refilling was similarly retarded in these latter experimental groups.
Ventricular volume curves
The closely sampled data sets were next used for three-dimensional reconstructions of endo-, epi-, and myocardial volumes for each of the 12 examined time points in the cardiac cycle for both ventricles; these were subsequently used additionally to determine the kinetics of such systolic and diastolic volume changes. The results of such quantitative analyses were then compared between different animals and experimental groups. Figure 2 and Figure 3 plot mean endo- (circles), epi- (squares), and myocardial (triangles) volumes for the normal rats (A), those made diabetic from 13 (B), 10 (C) and 7 weeks (D), as well the captopril-treated diabetic group (E). In all cases, the standard error bars are smaller than the size of the symbols and therefore could not be explicitly represented. The R wave trigger was followed by a prompt fall in both left and right endocardial volumes in the control rats, those diabetic from 13 weeks, and the captopril-treated group (Figs 2A, 2B, 2E, 3A, 3B and 3E). This was most striking in early systole; contraction subsequently decreased in rate. Both ventricles synchronously reached end-systole ∼100 ms after the trigger pulse. In contrast, endocardial volumes in the rats diabetic from 10 and 7 weeks but not treated with captopril (Figs 2C, 2D, 3C and 3D) declined more gradually and reached a later end-systole at ∼112 ms. In addition the diastolic filling of both their ventricles was considerably slower than in the control rats, those diabetic from 13 weeks and the captopril-treated group. These volume changes through the cardiac cycle took place despite constant left and the right ventricular myocardial volumes throughout the cardiac cycle.
Figure 2. Epi- (squares), endo- (circles) and myocardial (triangles) left ventricular (LV) volume curves.
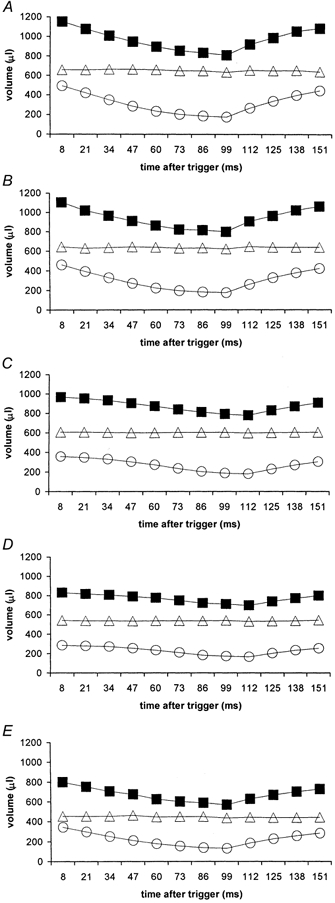
Epi-, endo- and myocardial left ventricular (LV) volume curves obtained from the transverse MRI images of the control group (A), and rats diabetic from 13 (B; n = 4), 10 (C; n = 4) and 7 weeks (D; n = 4), and the captopril-treated diabetic group (E; n = 4 rats) (disease durations 3, 6, 9 and 9 weeks, respectively). All the experimental animals were male Wistar rats aged 16 weeks at the time of scanning. The average body weight of the control group (n = 4 in each case) was 351.3 ± 9.7 g and those of the four diabetic groups were 235 ± 8.4, 282.5 ± 6.6, 335 ± 8.4 and 247.5 ± 15.5 g, respectively. The heart rate was continuously monitored throughout the imaging session giving average intrinsic heart rates of 322 ± 9 beats min−1 for the control rats and 280 ± 6, 280 ± 7, 318 ± 7, and 311 ± 10 beats min−1 for respective diabetic groups.
Figure 3. Epi- (squares), endo- (circles), and myocardial (triangles) right ventricular (RV) volume curves.
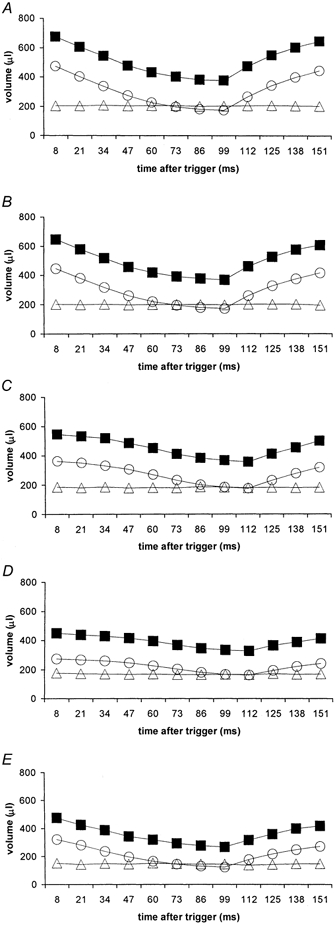
Epi-, endo- and myocardial right ventricular (RV) volume curves obtained from the transverse MRI images of the control group (A), and rats diabetic from 13 (B), 10 (C) and 7 weeks (D), and the captopril-treated diabetic group (E) (disease durations 3, 6, 9 and 9 weeks, respectively). Experimental details as summarized in the legend to Fig. 2.
Functional ventricular volumes and ejection fractions
Cardiac volumes at critical points in the cardiac cycle were now directly obtainable from the data plotted in Fig. 2 and Fig. 3. The maximum values of the endocardial volume curves at the end of ventricular diastolic filling prior to systole provided the end-diastolic volumes (EDVs) for each ventricle. The corresponding minima yielded the end-systolic volumes (ESVs) and subtracting the EDVs from the corresponding ESVs gave the stroke volumes (SVs). Table 1 summarizes the absolute values of these parameters for the left and right ventricles of the control and the three diabetic groups not treated with captopril. Table 2 normalizes these values to body weight so that they can be compared with earlier studies (see Introduction) that used conventional, rather than MRI, anatomical and physiological techniques. Table 4 summarizes the absolute and normalized values of the captopril-treated group and compares these values with those of the control group and the diabetic group spared captopril treatment that had been similarly diabetic from 7 weeks.
Table 2.
Diastolic and systolic volumes of the left (LV) and right ventricles (RV) of the control and the three diabetic groups spared captopril treatment normalized to their corresponding body weights
| Diabetic | |||||
|---|---|---|---|---|---|
| Control | 3 week | 6 week | 9 week | P | |
| LV end-diastolic volume/body weight (μl g−1) | 1.41 ± 0.01c, d | 1.38 ± 0.003c, d | 1.26 ± 0.02a, b, d | 1.22 ± 0.01a, b, c | <0.001 |
| RV end-diastolic volume/body weight (μl g−1) | 1.35 ± 0.01d | 1.33 ± 0.01d | 1.28 ± 0.02d | 1.16 ± 0.02,a, b, c | <0.001 |
| LV end-systolic volume/body weight (μl g−1) | 0.5 ± 0.02c, d | 0.53 ± 0.01c, d | 0.64 ± 0.01a, b, d | 0.71 ± 0.02a, b, c | <0.001 |
| RV end-systolic volume/body weight (μl g−1) | 0.49 ± 0.02c, d | 0.51 ± 0.01c, d | 0.62 ± 0.01a, b, d | 0.69 ± 0.01a, b, c | <0.001 |
| LV stroke volume/body weight (μl g−1) | 0.91 ± 0.02c, d | 0.85 ± 0.01c, d | 0.63 ± 0.01a, b, d | 0.51 ± 0.02a, b, c | <0.001 |
| RV stroke volume/body weight (μl g−1) | 0.85 ± 0.03c, d | 0.81 ± 0.003c, d | 0.66 ± 0.02a, b, d | 0.47 ± 0.03a, b, c | <0.001 |
The body weight-normalized diastolic and systolic volumes of the left and right ventricles of the experimental rats were calculated using their corresponding body weights determined while they were under anaesthesia. All values expressed as means ± s.e.m.; n = 4. One-way ANOVA was used in comparison of the control and the three untreated diabetic groups followed by Tukey's Honestly Significant Difference test for pair-wise multiple comparisons. A value of P <0.05 was considered statistically significant.
Significantly different from the control group.
Significantly different from the 3-week diabetic group.
Significantly different from the 6-week diabetic group.
Significantly different from the 9-week untreated diabetic group.
Ventricular end-diastolic volumes
Tables 1 and 4 indicate that diabetes decreased the EDV of both ventricles. The changes were significant in animals diabetic from 7 and 10 but not 13 weeks of age and persisted with captopril treatment. Thus, left ventricular EDVs decreased by 42.1, 27.6 and 6.6 % in animals diabetic from 7, 10 and 13 weeks, respectively, relative to control values. Captopril significantly reduced this decline to 29.9 %. Corresponding values for right ventricular EDVs were 42.3, 23.5 and 5.9 %, and 31.7 % with captopril treatment. Such EDVs showed similar trends when normalized to body weight (Tables 2 and 4). Values for the left (2.1 %) and right (1.5 %) ventricles were not significantly altered from the controls (changes in parentheses) in animals diabetic from 13 weeks nor were right ventricular EDVs in animals diabetic from 10 weeks (5.2 %). However normalized left (10.6 %) EDVs in animals diabetic from 10 weeks and left (13.5 %) and right (14.1 %) EDVs in animal diabetic from 7 weeks were significantly reduced from control values. In contrast the normalized left and right EDVs in the diabetic rats treated with captropril were insignificantly reduced (0.7 and 3.7 %) compared to the controls and were thus significantly higher than those of the corresponding diabetic rats spared captopril treatment.
Ventricular end-systolic volumes
In contrast to the marked EDV changes, Tables 1 and 4 demonstrate comparable left and right ventricular ESV values across groups of control and diabetic rats not treated with captopril. However, when normalized to body weight ESV values were significantly increased relative to their controls in animals diabetic from 7 and 10 but not 13 weeks (Tables 2 and 4). Thus normalized left ventricular ESVs were increased by 42, 28.0 and 6.0 % and right ventricular ESVs by 40.8, 26.5 and 4.1 % over the control group, respectively. In contrast, captopril-treated animals showed insignificant increases in normalized left (8 %) and right (2 %) ventricular ESV values over the control group.
Ventricular stroke volumes
Ventricular stroke volumes (SVs) were derived from the corresponding EDV and ESV values; left ventricular SVs significantly decreased by 62.4, 44.1 and 10.7 %, compared to control values, in animals diabetic from 7, 10 and 13 weeks respectively; the corresponding right ventricular changes were significant at 63.1, 37.6 and 8.9 %, respectively. Captopril treatment significantly relieved these changes with left and right ventricular SVs nevertheless decreased by 32.7 and 33.4 % compared with the control group. When normalized to body weight (Tables 2 and 4), left and right ventricular SVs differed significantly from control values in animals made diabetic from 7 and 10 but not 13 weeks. Thus, the left ventricular values decreased by 44.0, 30.8 and 6.6 % and the right ventricular SVs by 44.7, 22.4 and 4.7 %, respectively. However, captopril treatment restored values close to those shown by controls: normalized left and right ventricular SV were 4.4 and 5.9 % less than the respective control values.
Ventricular ejection fractions
Tables 1 and 4 express left and right ventricular ejection fractions, EFs, as the percentage ratio between the appropriate SVs and EDVs. EFs were decreased relative to control values in animals diabetic from 7 and 10 but not 13 weeks. Thus, left ventricular EFs were 35.2, 22.8 and 4.5 % and right ventricular EFs were 36.5, 18.5 and 3.3 % less than the control value, respectively. In contrast, captopril treatment significantly restored the left and right ventricular EFs to only 4.0 and 2.7 % less than control, respectively.
Dependence of ventricular SV on EDV
Full assessment of systolic function would require SV to be measured systematically through a controlled range of EDVs but this was not possible in a non-invasive study. Nevertheless, Fig. 4A and B plot left and right ventricular SVs, respectively, of all the five experimental groups against their EDVs. First, these showed that EDV declined with diabetes in both ventricles. Secondly, the SV-EDV plots appeared to fall on two approximately linear functions, one formed by the data obtained from control animals and those made diabetic from 13 weeks and the other from animals diabetic from 7 and 10 weeks. This suggests that changes in left and right ventricular systolic function not explicable merely in terms of changes in diastolic properties had occurred in the animals made diabetic at 7 and 10 rather than at 13 weeks. Finally, captopril treatment shifted the data points from the values associated with the animals diabetic from 7 and 10 weeks towards the points formed by the control and the rats diabetic from 13 weeks suggesting that captopril at least partly reversed the latter abnormalities.
Figure 4. Left (LV) and right ventricular (RV) stroke volumes (SVs) versus end-diastolic volume (EDV).
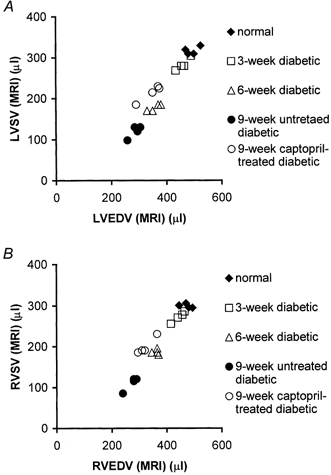
Comparative plots of MRI-measured left (A) and right (B) ventricular stroke volumes (SVs) and end-diastolic volumes (EDVs) of the five experimental groups. Data from rats diabetic from 7 and 10 weeks suggested impaired left and right ventricular diastolic and systolic function.
Matching of left and right ventricular volume measurements
Figure 5 compares MRI measurements of cardiac volumes in the right and left ventricles in order to assess the internal consistency of the data sets in each experimental group. It confirms statistically indistinguishable left and right ventricular EDVs, ESVs, SVs and EFs in each of the five experimental groups indicating a close matching of all these parameters in both control and diabetic rats. The equality of the left and right ventricular SVs in both normal and diabetic animals fulfils a necessary condition for the equality of their respective outputs. The matching of the remaining parameters of EDV, ESV, and EF suggests an involvement of both ventricles in the disease process.
Figure 5. MRI measurements of left and right ventricular volumes and ejection fraction (EF).
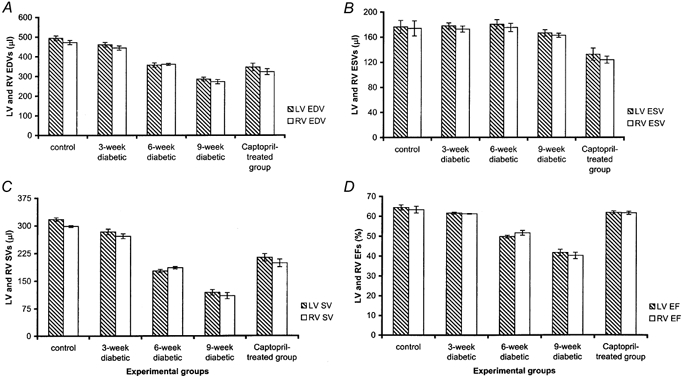
Comparative plots of left (LV) and right ventricular (RV) end-diastolic volumes (EDVs) (A), end-systolic volumes (ESVs) (B), stroke volumes (SVs) (C) and ejection fractions (EFs) (D) in the five experimental groups.
Dynamic systolic and diastolic indices
An inspection of Fig. 1 suggests that diabetes influenced the kinetics of ventricular contraction and relaxation in addition to the critical volumes above, and that captopril also influenced such changes. Tables 3 and 4 compare simple kinetic indices for systolic contraction and diastolic relaxation derived from the volume data. Firstly, the times required for the left and right ventricles to expel 25 % of their SVs during systole (25 % SV) were significantly increased in animals diabetic from 7 and 10 but not 13 weeks when compared to the control values; captopril reduced such changes. Such changes took place in the face of decreases in absolute SV values in the diabetic rats and their slower heart rates. Both these factors would potentially have permitted a more prolonged and therefore complete diastolic filling, particularly in view of similar times required for both ventricles to fill by 25 % of the SV during diastole (25 % DFV). Secondly, animals diabetic from 7 or 10 weeks both showed sharply reduced initial rates of systolic ejection in both ventricles relative to control values. Thirdly, such animals showed reduced initial rates of left and right ventricular diastolic filling compared with the control group. Finally, captopril treatment increased both these initial rates that nevertheless remained lower than their corresponding control values.
Time derivatives of left and right ventricular volume
Figure 6 systematically plots instantaneous rates of change, dV/dt, of left (filled bars) and right ventricular endocardial volumes (open bars). These were derived by comparing endocardial volumes measured at successive pairs of adjacent time points over the cardiac cycle, for each experimental group. The systolic dV/dt values over which ventricular volumes were decreasing are represented as negative whereas diastolic dV/dt values are represented as positive values. Left and right ventricular dV/dt values at each time point closely matched each other in all five experimental groups: both ventricles reached their end-systolic emptying and end-diastolic refilling limits at approximately the same time during the cardiac cycle. For example, end-systole corresponded to ∼100 ms after the R wave trigger in the control, the experimental group made diabetic from 13 weeks, and the captopril-treated group, and 112 ms in the groups diabetic from 7 and 10 weeks.
Figure 6. Plots of left and right ventricular dV/dt.
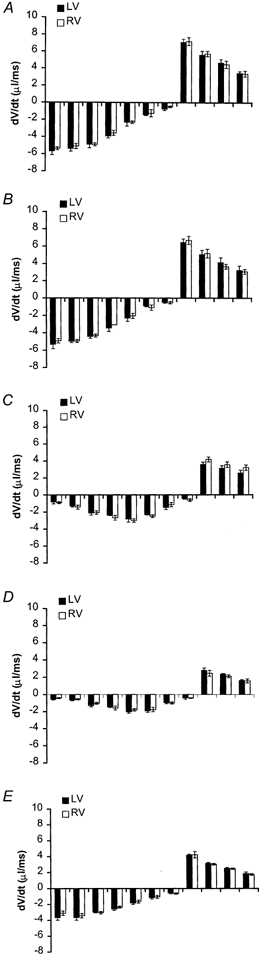
Block diagrams displaying left (filled bars) and right (open bars) ventricular volume changes with respect to time during the 12 studied time points through the cardiac cycle obtained from the normal control group (A), and rats diabetic from 13 (B), 10 (C) and 7 weeks (D), and the captopril-treated diabetic group (E) (disease durations 3, 6, 9 and 9 weeks, respectively). Each bar represents the average dV/dt ± s.e.m. between two consecutive time points through the cardiac cycle. Negative dV/dts represent contraction of the cardiac walls during systole and positive dV/dts represent their relaxation. The first bar represents dV/dt between the 1st and 2nd studied time points during the cardiac cycle with the first point timed typically 8 ms after the trigger pulse from the electrocardiographic R wave and 21 ms for the second point. The remaining bars represent dV/dts between volume points successively obtained 21, 34, 47, 60, 73, 86, 99, 112, 125, 138 and 151 ms following the R wave.
Systolic dV/dt values in both control and rats made diabetic from 13 weeks were greatest early in systole with diastolic dV/dt values greatest early in diastole. In both cases their values subsequently declined with time till end- systole or end-diastole, respectively (Fig. 6A and B). However, the systolic and diastolic patterns in the experimental groups diabetic from 10 and 7 weeks sharply contrasted with the controls (Fig. 6C and D). Their dV/dt values began gradually from small values in early systole, then only gradually increased in magnitude into the middle part of systole before finally declining. In contrast the diastolic dV/dt values were monotonic in time course, beginning from a maximum value early in diastole, then declining over time. Thus systolic dV/dt values in rats diabetic from 7 and 10 weeks only reached values comparable to the corresponding rates shown by the controls and animals diabetic from 13 weeks at around midsystole.
Captopril treatment restored the time dependence of dV/dt in both the left and right ventricles through both systole and diastole back to a normal pattern with contraction most rapid early in systole and relaxation most rapid early in diastole with both events synchronized in the left and right ventricles (Fig. 6E). However, the systolic and diastolic left and right ventricular dV/dt values were quantitatively smaller than those of the control group, though larger than rats that had been diabetic from 7 and 10 weeks.
Left ventricular output and power
The analysis of MRI data used here finally could estimate values for some derived indicators of cardiac performance and assess them through the diabetic process. These calculations used some of measurements made during the physiological monitoring associated with imaging. For example, Tables 5 and 6 demonstrate that left ventricular output fell with significant differences between controls and rats diabetic even from 13 weeks (11.8 %) and greater deteriorations in rats diabetic from 7 and 10 weeks (67.6 and 51.0 %, respectively). Captopril partially relieved such changes in diabetic rats but nevertheless left a 34.3 % reduction in output. When normalized to body weight the outputs were significantly decreased relative to control by 51.2, 39.5 and 7.6 % in animals diabetic from 7, 10 and 13 weeks, respectively. Normalizing to heart weight gave changes of 58.3, 50 and 8.3 %, respectively. In contrast, captopril-treated group showed decreases of only 7.2 and 8.3 % when cardiac output was normalized to body and heart weight, respectively.
An upper limit for left ventricular power (in W) was determined by multiplying cardiac output by systolic blood pressure (Tan, 1986). Tables 5 and 6 show that this significantly decreased in animals diabetic from 7 (78.8 %) and 10 (63.6 %) but not 13 weeks (18.2 %) of diabetes relative to control values. Captopril treatment significantly improved the power characteristics, reducing the deterioration in power to 39.4 % relative to control. Values normalized to body and heart weight similarly significantly decreased relative to control in animals diabetic from 7 (67.7 and 73.0 %) and 10 (52.7 and 56.8 %) but not 13 weeks (11.8 and 13.5 %, respectively). Captopril restored these normalized values leaving decreases of only 10.8 and 8.1 % relative to control.
Maximum left ventricular stroke work; systolic elastance
It was also possible to obtain derived variables describing systolic energy output. Maximum left ventricular stroke work was determined by multiplying stroke volume by systolic blood pressure (Tan, 1986) with subsequent normalization to either body or heart weight. Tables 5 and 6 show that this was significantly smaller than controls in animals diabetic from 7 (83.3 %), 10 (50.5 %) and 13 (16.7 %) weeks with the captopril-treated group showing a lower decrease of 33.3 %. When normalized to body and heart weights, stroke work was significantly reduced in animals diabetic from 7 (64.7 and 71.4 %, respectively) and 10 (47.1 and 57.1 %, respectively) but not 13 weeks (5.9 and 14.3 %, respectively). In contrast, values in the captopril-treated group (5.9 and 14.3 %, respectively) were statistically indistinguishable from controls.
Left ventricular systolic elastance (expressed in mmHg μl−1) was derived from the ratio of systolic blood pressure to the corresponding ESV in order to assess left ventricular contractility independent of left ventricle loading conditions or heart rate under some conditions (Tan, 1995). This provided a relatively insensitive measure distinguishing experimental groups in the absence of captopril treatment. Nevertheless, captopril treatment significantly restored elastance values in the diabetic rats to levels indistinguishable from those in the controls.
Ventricular myocardial volume/end-diastolic volume ratios
Left ventricular myocardial volumes could also be normalized to their corresponding end-diastolic volumes rather than body weight as used above. The resulting values were greater the earlier the onset of diabetes with significant increases in animals diabetic from 7 (42.4 %) and 10 (27.3 %) but not 13 weeks (4.5 %). In contrast, values of the captopril-treated group were insignificantly decreased by 1.5 % over values of the control group. The right ventricles yielded similar findings.
Comparison of rat heart indices with human heart indices
The normal values in Table 5 could be compared with human values assuming a left ventricular output of 5.5 l min−1, a heart rate of 70 min−1, a heart weight of 300 g and a body weight of 70 kg (Tan, 1995). These indicate that left ventricular output and maximum power whether normalized to body- or heart weight were 4–4.5 times greater than the corresponding values in the normal human heart. However, similarly normalized values for stroke work were comparable: this is as expected if human and rat myocardium shared similar contractile characteristics.
DISCUSSION
The present study successfully applied magnetic resonance imaging (MRI) as a non-invasive physiological tool to detect and characterize alterations in both left and right ventricular volumes through their cardiac cycles that resulted from experimental diabetic cardiomyopathy in the widely accepted STZ-diabetic rat model. The experiments tested reports that associated diabetes with a specific cardiomyopathy that might explain frequencies of congestive cardiac failure in excess of those resulting from the increased prevalence of atherosclerosis, hypertension or autonomic pathology in human diabetes (Kannel et al. 1974).
Previous clinical physiological studies have yielded variable findings in diabetic patients (see below): studies in animal models permit more controlled analysis of the cardiac changes but these hitherto have used invasive physiological or in vitro techniques. Left ventricular diastolic compliance alters over 11 weeks in intact, mildly alloxan-diabetic dogs (Regan et al. 1974). Isolated perfused hearts from diabetic rats show decreased peak systolic pressures (Miller, 1979) and altered diastolic function (Riva et al. 1998) and a more rapid development of heart failure following severe global ischaemia (Hearse et al. 1975; Feuvray et al. 1979). Similarly, isolated diabetic rat papillary muscles show delayed onsets and rates of isometric relaxation, depressed velocities of isotonic shortening (Fein et al. 1980), increased times to peak isometric tension and 75 % relaxation and reduced sensitivities to extracellular calcium and adrenaline (Warley et al. 1995).
However, complete and detailed quantitative in vivo MRI measurements of cardiac cycle volume changes in both the right and the left ventricles have hitherto not been available in intact beating diabetic hearts. Yet, MRI has already proven useful for accurate and high-resolution measurements of anatomical and functional clinical parameters of human cardiac performance for which it is considered superior to other imaging modalities (Rehr et al. 1985; Higgins, 1986; Stratemeier et al. 1986; Caputo et al. 1987; Markiewicz et al. 1987; Pettigrew, 1989; Sechtem et al. 1987; Utz et al. 1987, 1988; Pflugfelder et al. 1989; Semelka et al. 1990). The present study utilized recent developments in system hardware and pulse sequences with their significant potential applications in MRI for chronic non-invasive physiological studies of animal models of common human cardiac pathology. In particular the use of cine MRI demarcated blood within the cardiac chambers and the myocardial wall particularly clearly. The resulting cardiac images were thus highly amenable to quantitative assessment of anatomical and functional characteristics (Rehr et al. 1985; Higgins, 1986; Stratemeier et al. 1986; Caputo et al. 1987; Markiewicz et al. 1987; Pettigrew et al. 1987; Sechtem et al. 1987; Utz et al. 1987, 1988; Pflugfelder et al. 1989; Semelka et al. 1990). The modifications applied in the present physiological studies to this end might conversely find application in clinical MRI practice.
The imaging techniques could be applied in animal studies that extended over weeks to quantify chronic cardiac changes (Wise et al. 1998). Experimental systems offer particular advantages for studying chronic physiological changes associated with human disease particularly when coupled with non-invasive imaging as opposed to invasive physiological measurements. Thus, the STZ-diabetic rat model permitted untreated disease of controlled severity that led to relevant cardiac pathology to be studied at well-defined time points permitting study over a manageable time scale. High MRI resolution and sensitivity permitted early detection of physiological changes in a minimized number of animals. The present experiments thus scanned groups of male Wistar rats at a consistent age, all age-matched to a single control group, that had been diabetic from 7, 10 and 13 weeks. All rats were scanned at 16 weeks. The findings obtained here are therefore comparable with earlier independent studies using the same model that used conventional invasive or ex vivo physiological techniques. These have included physiological studies of papillary muscle function (Fein et al. 1980; Fein & Sonnenblick, 1994; Warley et al. 1995), myocardial contraction and relaxation (Jackson et al. 1985; Afzal et al. 1988), diastolic and peak systolic pressures (Miller, 1979; Afzal et al. 1988; Shimabukuro et al. 1995; Rodrigues & McNeill, 1986; Paulson et al. 1987; Lopaschuk & Spafford, 1989; Rodrigues et al. 1988; Goyal et al. 1998) and heart rates (Jackson & Carrier, 1983; Afzal et al. 1988; Hicks et al. 1998). Despite their rapid heart rates, the cine MRI methods successfully imaged both ventricles at twelve time points which substantially covered the cardiac cycle and completely covered the ventricular anatomy. The subsequent image analysis derived their epi-, endo- and myocardial volumes over time; these included volumes defining critical points in the cardiac cycle, namely the left and right ventricular end-diastolic volumes (EDVs), end-systolic volumes (ESVs), stroke volumes (SVs), and ejection fractions (EFs). The data sets additionally permitted the rates of left and right ventricular contraction and relaxation to be determined giving both the initial rates of ejection and dilatation and plots of dV/dt through the cardiac cycle. These MRI measurements were expressed both as absolute values and were normalized to the corresponding body weights to permit comparison with earlier studies (Pierce & Dhalla, 1981, 1983, 1985a,b; Afzal et al. 1988; Maeda et al. 1995; Shimabukuro et al. 1995; Hicks et al. 1998).
The transverse cardiac MRI sections showed that both right and left ventricles reached end-diastole and -systole at synchronized times in both control and all diabetic groups. The left and right ventricular endocardial volume curves and their corresponding dV/dt plots (see below) similarly demonstrated very similar rates of systolic and diastolic volume change over time through the cardiac cycle of both ventricles in all rats studied. A similar correspondence between the right and left ventricles applied to the ventricular EDVs, ESVs, SVs and EFs. This resulted in an internally self-consistent data set that both satisfied the necessary condition for matched right and left ventricular outputs and that implicated both ventricles in the diabetic process.
The MRI studies demonstrated significant evidence of ventricular diastolic dysfunction in experimental diabetes, findings compatible with reported abnormalities in cardiac sarcoplasmic reticular function (Penpargkul et al. 1981; Lopaschuk et al. 1983; Ganguly et al. 1983) and of collagen accumulation in the myocardial interstitium (Regan et al. 1981). Firstly, both left and right ventricular end-diastolic volumes fell substantially below control values in rats diabetic from 7 and 10 weeks; values when normalized gave concordant results. Such results offer an improvement over the variable human echocardiographic findings of reductions (Airaksinen et al. 1984a, 1987), normal or modest increases in left ventricular size in diabetics (Shapiro et al. 1981a; Friedman et al. 1982). There are no existing reports whatsoever concerning right ventricular volumes in diabetes available for comparison. Secondly, initial diastolic filling rates, dV/dt, fell in both the left and right ventricles in rats diabetic from 7 and 10 weeks in agreement with echocardiographic findings of left ventricular diastolic dysfunction reflected in a prolonged isometric relaxation in diabetic patients (Sanderson et al. 1978; Shapiro et al. 1980, 1981a,b; Shapiro, 1982).
Experimental diabetes also produced significant systolic changes. Firstly, EFs in both right and left ventricles were markedly decreased compared to controls in rats diabetic from 7 and 10 weeks and SVs in rats diabetic from 13 weeks. Secondly, it is unlikely that such alterations in systolic function merely reflect altered diastolic properties. Thus, available plots of SV against EDV from control animals and animals diabetic from 13 weeks produced different functions from those obtained in animals diabetic from 7 and 10 weeks suggestive of at least some systolic dysfunction. Furthermore, captopril treatment shifted the data points back towards characteristics shown by the control group and the animals diabetic from 13 weeks. Thirdly, the systolic limbs of both the left and the right ventricular endocardial volume curves and their corresponding dV/dt values suggested additional, kinetic, changes in ventricular systole. In normal rats or those diabetic from 13 weeks, both left and right ventricular systolic ejection developed promptly generating their maximum dV/dt values followed by slower decreases in volume and declining dV/dt that continued into late systole. In contrast, systolic ejection when rats had been diabetic from 7 or 10 weeks began gradually, with small dV/dt values that developed slowly.
These results can be compared with earlier varying echocardiographic reports of depressed (Shapiro et al. 1981a,b; Uusitupa et al. 1985), normal (Airaksinen et al. 1984b), or enhanced left ventricular systolic function (Thuesen et al. 1988) in insulin-dependent patients developing microvascular complications. Radionuclide ventriculographic studies had reported significant reductions in left ventricular ejection fractions both at rest and with maximal exercise among diabetics with cardiac autonomic neuropathy (Zola et al. 1986), or only after exercise (Vered et al. 1984; Fisher et al. 1986; Arvan et al. 1988).
Additional cardiac performance characteristics were available with the aid of the measurements associated with physiological monitoring. Thus, both absolute and normalized values of cardiac output and cardiac power output fell sharply with earlier ages of disease induction, reflecting changes in both heart rate and stroke characteristics. These changes were most noticeably reflected in left ventricular stroke work whether expressed as absolute or normalized values, and to a smaller extent in values of systolic elastance. When compared with assumed normal human values these findings suggested a greater left ventricular output and maximum power in the rat when normalized to body- or heart weight. Nevertheless, allowing for the higher heart rates in rodents gave values consistent with fundamentally similar functional myocardial characteristics in human and rat hearts, a necessary condition for the use of the laboratory rat as an animal model of human cardiac disease.
The experimental system was finally used to evaluate the therapeutic effects of the ACE inhibitor, captopril upon MR assessments of the diabetic cardiac changes. Comparison using captopril-treated and control groups of rats diabetic from 7 weeks demonstrated significant effects upon such ventricular functional changes. Firstly, captopril ameliorated reductions in both the left and right ventricular EDVs. Secondly, captopril reversed some of the alterations in SV, EF as well as restored the relationship between SV and EDV, reflecting positive effects upon systolic function. Thirdly, captopril treatment partially restored both the reduced magnitudes and the delayed developments of the rates of systolic volume changes, dV/dt, towards normal values and patterns. Fourthly, captopril ameliorated changes in derived cardiac characteristics whether related to cardiac output, power, or diastolic or systolic characteristics. There is evidence for an intracardiac renin-angiotensin system (Dostal et al. 1992a,b) activated in diabetes with a resulting increased angiotensin II production (Rösen et al. 1995) inducing myocardial interstitial fibrosis through fibroblast proliferation (Schorb et al. 1993; Crabos et al. 1994; Matsubara et al. 1994; Schorb et al. 1994). STZ-diabetic rats show higher ACE levels in their left ventricular tissue and a decreased left ventricular developed pressure; the latter was prevented by the ACE inhibitor enalapril (Goyal et al. 1998). The present findings thus offer the first evidence in intact beating hearts that possible therapeutic benefits of such ACE inhibition merit further exploration in diabetic cardiac disease.
Acknowledgments
The authors thank Dr Herchel Smith for his generous endowment, which supports the Herchel Smith Laboratory for Medicinal Chemistry. A.I.M.A-S. also thanks the Karim Rida Said Foundation for scholarship support. R.G.W. thanks the Wellcome Trust for his Research Training Studentship in Mathematical Biology. C.L-H.H., T.A.C. and L.D.H. acknowledge project grant funding form the BBSRC and Joint Research Equipment Initiative (JREI) support from the MRC. Special thanks are given to Mr. Simon Smith for technical assistance.
REFERENCES
- Afzal N, Ganguly PK, Dhalla KS, Pierce GN, Signal PK, Dhalla NS. Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes. 1988;37:937–942. doi: 10.2337/diab.37.7.936. [DOI] [PubMed] [Google Scholar]
- Ahmed SS, Jaferi GA, Narang RM, Regan TJ. Pre-clinical abnormalities of left ventricular function in diabetes mellitus. American Heart Journal. 1975;89:153–158. doi: 10.1016/0002-8703(75)90039-3. [DOI] [PubMed] [Google Scholar]
- Airaksinen KEJ, Ikäheimo M, Kaila J, Linnaluoto M, Takkumen J. Impaired left ventricular filling in young female diabetics. An echocardiographic study. Acta Medica Scandinavica. 1984a;216:509–516. doi: 10.1111/j.0954-6820.1984.tb05039.x. [DOI] [PubMed] [Google Scholar]
- Airaksinen KEJ, Ikäheimo M, Kaila J, Linnaluoto M, Takkunen J. Systolic time intervals and the QT-QS2 interval in young female diabetics. Annals of Clinical Research. 1984b;16:188–191. [PubMed] [Google Scholar]
- Airaksinen KEJ, Ikäheimo MJ, Linnaluoto MK, Huikuri HV, Takkumen JT. Increased left atrial size relative to left ventricular size in young women with insulin dependent diabetes: a pre-clinical sign of the specific heart disease of diabetes? Diabetes Research. 1987;6:37–41. [PubMed] [Google Scholar]
- Arvan S, Signal K, Knapp R, Vagnucci A. Subclinical left ventricular abnormalities in young diabetics. Chest. 1988;93:1031–1034. doi: 10.1378/chest.93.5.1031. [DOI] [PubMed] [Google Scholar]
- Ballon D, Graham MC, Midownik S, Koutcher JA. A 64 MHz half-birdcage resonator for clinical imaging. Journal of Magnetic Resonance. 1990;90:131–140. [Google Scholar]
- Beall PT, Amtey SR, Kasturi SR. NMR Data Handbook for Biomedical Applications. New York: Pergamon Press; 1984. [Google Scholar]
- Bell DS. Diabetic cardiomyopathy. A unique entity or a complication of coronary artery disease? Diabetes Care. 1995;18:708–714. doi: 10.2337/diacare.18.5.708. [DOI] [PubMed] [Google Scholar]
- Caputo GR, Tscholakoff D, Sechtem U, Higgins CB. Measurement of canine left ventricular mass by using MR imaging. American Journal of Roentgenology. 1987;148:33–38. doi: 10.2214/ajr.148.1.33. [DOI] [PubMed] [Google Scholar]
- Cellina G, Lo Cicero G, Brina A, Zanchetti A. Reversible alteration of myocardial function in gestational diabetes. European Heart Journal. 1983;4:59–63. doi: 10.1093/oxfordjournals.eurheartj.a061372. [DOI] [PubMed] [Google Scholar]
- Crabos M, Roth M, Hahn AW, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signalling systems and gene expression. Journal of Clinical Investigation. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crall FV, Jr, Roberts WC. The extramural and intramural coronary arteries in juvenile diabetes mellitus. Analysis of nine necropsy patients aged 19 to 38 years with onset of diabetes before age 15 years. American Journal of Medicine. 1978;64:221–230. doi: 10.1016/0002-9343(78)90049-9. [DOI] [PubMed] [Google Scholar]
- Dalton GR, Jones JV, Evans SJ, Levi AJ. Wall stress-induced arrhythmias in the working rat heart as left ventricular hypertrophy regresses during captopril treatment. Cardiovascular Research. 1997;33:561–572. doi: 10.1016/s0008-6363(96)00242-8. [DOI] [PubMed] [Google Scholar]
- Dostal DE, Rothblum KN, Chernin MI, Cooper GR, Baker KM. Intracardiac detection of angiotensinogen and renin: a localized renin-angiotensin system in neonatal rat heart. American Journal of Physiology. 1992a;263:C838–850. doi: 10.1152/ajpcell.1992.263.4.C838. [DOI] [PubMed] [Google Scholar]
- Dostal DE, Rothblum KN, Conrad KM, Cooper GR, Baker KM. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. American Journal of Physiology. 1992b;263:C851–863. doi: 10.1152/ajpcell.1992.263.4.C851. [DOI] [PubMed] [Google Scholar]
- Fein FS, Kornstein LB, Strobeck JE, Capasso JM, Sonnenblick EH. Altered myocardial mechanics in diabetes. Circulation Research. 1980;47:922–933. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- Fein FS, Sonnenblick EH. Diabetic cardiomyopathy. Cardiovascular Drugs and Therapy. 1994;8:65–73. doi: 10.1007/BF00877091. [DOI] [PubMed] [Google Scholar]
- Feuvray D, Idell Wenger JA, Neely JR. Effects of ischaemia on rat myocardial function and metabolism in diabetics. Circulation Research. 1979;44:322–329. doi: 10.1161/01.res.44.3.322. [DOI] [PubMed] [Google Scholar]
- Fisher BM, Gillen G, Lindop GBM, Dargie HJ, Frier BM. Cardiac function and coronary arteriography in asymptomatic type 1 (insulin-dependent) diabetic patients: evidence for a specific heart disease. Diabetologia. 1986;29:706–712. doi: 10.1007/BF00870280. [DOI] [PubMed] [Google Scholar]
- Friedman NE, Levitsky LL, Edidin DV, Vitullo DA, Lacina SJ, Chiemmongkolti P. Echocardiographic evidence for impaired myocardial performance in children with type 1 diabetes mellitus. American Journal of Medicine. 1982;73:846–850. doi: 10.1016/0002-9343(82)90775-6. [DOI] [PubMed] [Google Scholar]
- Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. American Journal of Physiology. 1983;244:E528–535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- Goodwin JF, Oakley CM. The cardiomyopathies. British Heart Journal. 1972;34:545–552. doi: 10.1136/hrt.34.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Satia MC, Bangaru RA, Gandhi TP. Effect of long-term treatment with enalapril in streptozotocin diabetic and DOCA hypertensive rats. Journal of Cardiovascular Pharmacology. 1998;32:317–322. doi: 10.1097/00005344-199808000-00021. [DOI] [PubMed] [Google Scholar]
- Hamby RI, Zoneraich S, Sherman S. Diabetic cardiomyopathy. Journal of the American Medical Association. 1974;229:1749–1754. [PubMed] [Google Scholar]
- Hearse DJ, Stewart DA, Chain EB. Diabetes and the survival and recovery of the anoxic myocardium. Journal of Molecular and Cellular Cardiology. 1975;7:397–415. doi: 10.1016/0022-2828(75)90046-2. [DOI] [PubMed] [Google Scholar]
- Hicks KK, Seifen E, Stimers JR, Kennedy RH. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. Journal of Autonomic Nervous System. 1998;69:21–30. doi: 10.1016/s0165-1838(98)00004-6. [DOI] [PubMed] [Google Scholar]
- Higgins CB. Overview of MR of the heart. American Journal of Roentgenology. 1986;146:907–918. doi: 10.2214/ajr.146.5.907. [DOI] [PubMed] [Google Scholar]
- Jackson CV, Carrier GO. Influence of short-term experimental diabetes on blood pressure and heart rate in response to norepinephrine and angiotensin II in the conscious rat. Journal of Cardiovascular Pharmacology. 1983;5:260–265. doi: 10.1097/00005344-198303000-00016. [DOI] [PubMed] [Google Scholar]
- Jackson CV, McGrath GM, Tahiliani AG, Vadalmudi R V S V, McNeill JH. A functional and ultrastructural analysis of experimental diabetic rat myocardium: Manifestation of a cardiomyopathy. Diabetes. 1985;34:876–883. doi: 10.2337/diab.34.9.876. [DOI] [PubMed] [Google Scholar]
- Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proceedings of the Society for Experimental Biology and Medicine. 1967;126:201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. Journal of Clinical Investigation. 1969;48:2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. American Heart Journal. 1985;110:1100–1107. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Hjotland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. American Journal of Cardiology. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Mcgee DL. Diabetes and cardiovascular disease. The Framingham study. Journal of the American Medical Association. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Ledet T. Histological and histochemical changes in the coronary arteries of old diabetic patients. Diabetologia. 1968;4:268–272. doi: 10.1007/BF01309899. [DOI] [PubMed] [Google Scholar]
- Ledet T. Diabetic cardiopathy. Quantitative histological studies of the heart from young juvenile diabetics. Acta Pathologica Microbiologica Scandinavica (A) 1976;84:421–428. [PubMed] [Google Scholar]
- Ledet T, Neubauer B, Christenson NJ, Lundback K. Diabetic cardiomyopathy. Diabetologia. 1979;16:207–209. doi: 10.1007/BF01221945. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Spafford M. Response of isolated working hearts to fatty acids and carnitine palmitoyltransferase I inhibition during reduction of coronary flow in acutely and chronically diabetic rats. Circulation Research. 1989;65:378–387. doi: 10.1161/01.res.65.2.378. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Tahiliani AG, Vadlamudi RV, Katz S, McNeill JH. Cardiac sarcoplasmic reticulum function in insulin- or carnitine-treated diabetic rats. American Journal of Physiology. 1983;245:H969–H976. doi: 10.1152/ajpheart.1983.245.6.H969. [DOI] [PubMed] [Google Scholar]
- Maeda CY, Fernandes TG, Lulhier F, Irigoyen MC. Streptozotocin diabetes modifies arterial pressure and baroreflex sensitivity in rats. Brazilian Journal of Medical and Biological research. 1995;28:497–501. [PubMed] [Google Scholar]
- Markiewicz W, Sechtem U, Higgins CB. Evaluation of the right ventricle by magnetic resonance imaging. American Heart Journal. 1987;113:8–15. doi: 10.1016/0002-8703(87)90003-2. [DOI] [PubMed] [Google Scholar]
- Matsubara H, Kanasaki M, Murasawa S, Tsukaguchi Y, Nio Y, Inada M. Differential gene expression and regulation of angiotensin II receptor subtypes in rat cardiac fibroblasts and cardiomyocytes in culture. Journal of Clinical Investigation. 1994;93:1592–1601. doi: 10.1172/JCI117139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TB., Jr Cardiac performance in isolated perfused hearts from alloxan diabetic rats. American Journal of Physiology. 1979;230:H808–812. doi: 10.1152/ajpheart.1979.236.6.H808. [DOI] [PubMed] [Google Scholar]
- Paulson DJ, Kopp SJ, Peace DG, Tow JP. Myocardial adaptation to endurance exercise training in diabetic rats. American Journal of Physiology. 1987;252:R1073–1081. doi: 10.1152/ajpregu.1987.252.6.R1073. [DOI] [PubMed] [Google Scholar]
- Paz-Guevara AT, Hsu TH, White P. Juvenile diabetes mellitus after forty years. Diabetes. 1975;24:559–565. doi: 10.2337/diab.24.6.559. [DOI] [PubMed] [Google Scholar]
- Penpargkul S, Fein F, Sonnenblick EH, Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. Journal of Molecular and Cellular Cardiology. 1981;13:303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- Pettigrew RI. Dynamic cardiac MR imaging. Techniques and applications. Radiologic Clinics of North America. 1989;27:1183–1203. [PubMed] [Google Scholar]
- Pflugfelder PW, Landzberg JS, Cassidy MM, Cheitlin MD, Schiller NB, Auffermann W, Higgins CB. Comparison of cine MR imaging with Doppler echocardiography for the evaluation of aortic regurgitation. American Journal of Roentgenology. 1989;152:729–735. doi: 10.2214/ajr.152.4.729. [DOI] [PubMed] [Google Scholar]
- Pierce GN, Dhalla NS. Cardiac myofibrillar ATPase activity in diabetic rats. Journal of Molecular and Cellular Cardiology. 1981;13:1063–1069. doi: 10.1016/0022-2828(81)90296-0. [DOI] [PubMed] [Google Scholar]
- Pierce GN, Dhalla NS. Mechanisms of defect in cardiac myofibrillar function during diabetes. American Journal of Physiology. 1985a;248:E170–175. doi: 10.1152/ajpendo.1985.248.2.E170. [DOI] [PubMed] [Google Scholar]
- Pierce GN, Dhalla NS. Mitochondrial abnormalities in diabetic cardiomyopathy. Canadian Journal of Cardiology. 1985b;1:48–54. [PubMed] [Google Scholar]
- Pierce GN, Dhalla NS. Sarcolemmal Na+-K+-ATPase activity in diabetic rat heart. American Journal of Physiology. 1983;245:C241–247. doi: 10.1152/ajpcell.1983.245.3.C241. [DOI] [PubMed] [Google Scholar]
- Pope JM, Yao S. Quantitative NMR imaging of flow. Concepts in Magnetic Resonance. 1993;5:281–302. [Google Scholar]
- Qi XL, Stewart DJ, Gosselin H, Azad A, Picard P, Andries L, Sys SU, Brutsaert DL, Rouleau JL. Improvement of endocardial and vascular endothelial function on myocardial performance by captopril treatment in postinfarct rat hearts. Circulation. 1999;100:1338–1345. doi: 10.1161/01.cir.100.12.1338. [DOI] [PubMed] [Google Scholar]
- Regan TJ, Ettinger PO, Khan MI, Jesrani MU, Lyons MM, Oldewurtel HA, Weber M. Altered myocardial function and metabolism in chronic diabetes mellitus without ischaemia in dogs. Circulation Research. 1974;35:222–237. [Google Scholar]
- Regan TJ, Wu CF, Yeh CK, Oldewurtel HA, Haider B. Myocardial composition and function in diabetes: the effect of chronic insulin use. Circulation Research. 1981;49:1268–1277. doi: 10.1161/01.res.49.6.1268. [DOI] [PubMed] [Google Scholar]
- Rehr RB, Malloy CR, Filipchuk NG, Peshock RM. Left ventricular volumes measured by MR imaging. Radiology. 1985;156:717–719. doi: 10.1148/radiology.156.3.4023232. [DOI] [PubMed] [Google Scholar]
- Riva E, Andreoni G, Bianchi R, Latini R, LuvaruvarÀagrave; G, Jeremic G, Traquandi C, Tuccinardi L. Changes in diastolic function and collagen content in normotensive and hypertensive rats with long-term streptozotocin-induced diabetes. Pharmacological Research. 1998;37:233–240. doi: 10.1006/phrs.1998.0290. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, Cam MC, Kong J, Goyal RK, Mcneill JH. Strain differences in susceptibility to streptozotocin-induced diabetes: Effects on hypertriglyceridemia and cardiomyopathy. Cardiovascular Research. 1997;34:199–205. doi: 10.1016/s0008-6363(97)00045-x. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, McNeill JH. Cardiac function in spontaneously hypertensive diabetic rats. American Journal of Physiology. 1986;251:H571–580. doi: 10.1152/ajpheart.1986.251.3.H571. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, Ross JR, Farahbakshian S, McNeill JH. Effects of in vivo and in vitro treatment with L-carnitine on isolated hearts from chronically diabetic rats. Canadian Journal of Physiology and Pharmacology. 1990;68:1085–1092. doi: 10.1139/y90-163. [DOI] [PubMed] [Google Scholar]
- Rodrigues B, Xiang H, McNeill JH. Effect of L-carnitine treatment on lipid metabolism and cardiac performance in chronically diabetic rats. Diabetes. 1988;37:1358–1364. doi: 10.2337/diab.37.10.1358. [DOI] [PubMed] [Google Scholar]
- Rösen R, Rump AF, Rösen P. The ACE-inhibitor captopril improves myocardial perfusion in spontaneously diabetic (BB) rats. Diabetologia. 1995;38:509–517. doi: 10.1007/BF00400718. [DOI] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grisham A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. American Journal of Cardiology. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Sanderson JE, Brown DJ, Rievellese A, Kohner E. Diabetic cardiomyopathy? An echocardiographic study of young diabetics. British Medical Journal. 1978;1:404–407. doi: 10.1136/bmj.1.6110.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circulation Research. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- Schorb W, Peeler TC, Madigan NN, Conrad KM, Baker KM. Angiotensin II-induced protein tyrosine phosphorylation in neonatal rat cardiac fibroblasts. Journal of Biological Chemistry. 1994;269:19626–19632. [PubMed] [Google Scholar]
- Sechtem U, Pflugfelder PW, Gould RG, Cassidy MM, Higgins CB. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163:697–702. doi: 10.1148/radiology.163.3.3575717. [DOI] [PubMed] [Google Scholar]
- Semelka RC, Tomei E, Wagner S, Mayo J, Kondo C, Suzuki J, Caputo GR, Higgins CB. Normal left ventricular dimensions and function: interstudy reproducibility of measurements with cine MR imaging. Radiology. 1990;174:763–768. doi: 10.1148/radiology.174.3.2305059. [DOI] [PubMed] [Google Scholar]
- Seneviratne BI. Diabetic cardiomyopathy: the preclinical phase. British Medical Journal. 1977;l:1444–1446. doi: 10.1136/bmj.1.6074.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LM. Echocardiographic features of impaired ventricular function in diabetes mellitus. British Heart Journal. 1982;47:439–444. doi: 10.1136/hrt.47.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LM, Howat AP, Calter MM. Left ventricular function in diabetes mellitus. I: Methodology, and prevalence and spectrum of abnormalities. British Heart Journal. 1981a;45:122–128. doi: 10.1136/hrt.45.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LM, Howat AP, Calter MM. Left ventricular function in diabetes mellitus: II: Relation between clinical features and left ventricular function. British Heart Journal. 1981b;45:129–132. doi: 10.1136/hrt.45.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LM, Leatherdale BA, Coyne ME, Fletcher RF, MacKinnon J. Prospective study of heart disease in untreated maturity onset diabetics. British Heart Journal. 1980;44:342–348. doi: 10.1136/hrt.44.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Shinzato T, Higa S, Nagamine F, Murakami K, Takasu N. Impaired mechanical response to calcium of diabetic rat hearts-Reversal by nifedipine treatment. Journal of Cardiovascular Pharmacology. 1995;26:495–502. doi: 10.1097/00005344-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovascular Research. 1997;34:55–68. doi: 10.1016/s0008-6363(96)00272-6. [DOI] [PubMed] [Google Scholar]
- Stratemeier EJ, Thompson R, Brady TJ, Miller SW, Saini S, Wismer GL, Okada RD, Dinsmore RE. Ejection fraction determination by MR imaging: comparison with left ventricular angiography. Radiology. 1986;158:775–777. doi: 10.1148/radiology.158.3.3945753. [DOI] [PubMed] [Google Scholar]
- Tan LB. Cardiac pumping capability and prognosis in heart failure. Lancet. 1986;2:1360–1363. doi: 10.1016/s0140-6736(86)92006-4. [DOI] [PubMed] [Google Scholar]
- Tan LB. The assessment of cardiac performance. In: Glasby MA, Huang CLH, editors. Applied Physiology for Surgery and Critical Care. London: Butterworth-Heinemann; 1995. pp. 160–169. [Google Scholar]
- Thuesen L, Christiansen JS, Mogenson CE, Henningen P. Cardiac hyperfunction in insulin-dependent diabetic patients developing microvascular complications. Diabetes. 1988;37:851–856. doi: 10.2337/diab.37.7.851. [DOI] [PubMed] [Google Scholar]
- Utz JA, Herfkens RJ, Heinsimer JA, Bashore T, Califf R, Glover G, Pelc N, Shimakawa A. Cine MR determination of left ventricular ejection fraction. American Journal of Roentgenology. 1987;148:839–843. doi: 10.2214/ajr.148.5.839. [DOI] [PubMed] [Google Scholar]
- Utz JA, Herfkens RJ, Heinsimer JA, Shimakawa A, Glover G, Pelc N. Valvular regurgitation: dynamic MR imaging. Radiology. 1988;168:91–94. doi: 10.1148/radiology.168.1.3380987. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Siitonen O, Aro A, Korhonen T, PyyÖRÄLÄouml;ryÖRÄLÄauml;lyÖRÄLÄauml; K. Effect of correction of hyperglycemia on left ventricular function in non-insulin-dependent (type 2) diabetics. Acta Medica Scandinavica. 1983;213:363–368. doi: 10.1111/j.0954-6820.1983.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Siitonen O, PyyÖRÄLÄouml;ryÖRÄLÄauml;lyÖRÄLÄauml; K, Länsimies E. Left ventricular function in newly diagnosed non-insulin dependent (type 2) diabetes evaluated by systolic time intervals and echocardiography. Acta Medica Scandinavica. 1985;217:379–388. doi: 10.1111/j.0954-6820.1985.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Vered A, Battler A, Segal P, Liberman D, Yerushalmi Y, Berezin M, Neufeld HN. Exercise-induced left ventricular dysfunction in young men with a symptomatic diabetes mellitus (diabetic cardiomyopathy) American Journal of Cardiology. 1984;54:633–637. doi: 10.1016/0002-9149(84)90263-7. [DOI] [PubMed] [Google Scholar]
- Warley A, Powell JM, Skepper JN. Capillary surface area is reduced and tissue thickness from capillaries to myocytes is increased in the left ventricle of streptozotocin-diabetic rats. Diabetologia. 1995;38:413–421. doi: 10.1007/BF00410278. [DOI] [PubMed] [Google Scholar]
- Weitzman S, Wagner GS, Heiss G, Haney TL, Slome C. Myocardial infarction site and mortality in diabetes. Diabetes Care. 1982;5:31–35. doi: 10.2337/diacare.5.1.31. [DOI] [PubMed] [Google Scholar]
- Wise RG, Huang CL-H, Gresham GA, Al-Shafei AIM, Carpenter TA, Hall LD. Magnetic resonance imaging analysis of left ventricular function in normal and spontaneously hypertensive rats. Journal of Physiology. 1998;513:873–887. doi: 10.1111/j.1469-7793.1998.873ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola B, Kahn JK, Juni JE, Vinik AI. Abnormal cardiac function in diabetic patients with autonomic neuropathy in the absence of ischemic heart disease. Journal of Clinical Endocrinology and Metabolism. 1986;63:208–214. doi: 10.1210/jcem-63-1-208. [DOI] [PubMed] [Google Scholar]


