Abstract
It has been known since 1851 that atmospheric oxygen is taken up by the human epidermis. The contribution to total respiration is negligible. Until now the significance for the local oxygen supply of the skin has remained unknown. With a newly developed sensor, the oxygen fluxoptode, it has become possible to make local measurements of the transcutaneous oxygen flux (tcJO2). In this study the sensor was calibrated so that absolute values of tcJO2 could be reported. At rest, tcJO2 was determined on normal, humidified skin on the volar forearm of 20 volunteers of different age groups. In order to evaluate the contribution of the blood flow to the oxygen supply of the skin, tcJO2 was recorded at the end of a 5 min suprasystolic occlusion of the forearm. At normal skin surface partial oxygen pressure (163 ± 9 Torr), tcJO2 was 0.53 ± 0.27 ml O2 min−1 m−2. A 5 min interruption of blood flow resulted in an increase of 9.5 ± 6.3 % in tcJO2. The value of tcJO2 was unaffected by the age of the subject. Published data on the oxygen diffusion properties of skin and simulations of intracutaneous profiles of oxygen partial pressure indicated that under these conditions, the upper skin layers to a depth of of 0.25–0.40 mm are almost exclusively supplied by external oxygen, whereas the oxygen transport of the blood has a minor influence. As a consequence, a malfunction in capillary oxygen transport cannot be the initiator of the development of superficial skin defects such as those observed in chronic venous incompetence and peripheral arterial occlusive disease.
The skin is the only organ besides the lungs that is directly exposed to atmospheric oxygen. Apart from the stratum corneum, oxygen is consumed in all layers of the epidermis and dermis. The oxygen demand is partially satisfied by the blood: the dermis exhibits a vasculature that is arranged in two tiers that are parallel to the skin surface. The superficial plexus between the papillary and the upper reticular dermis deep plexus in the lower reticular dermis are connected by perpendicularly orientated communicating vessels. Arcades of capillaries loop upwards into the papillae from the subpapillary plexus (Braverman, 1989). In contrast, the epidermis has no vasculature, but is exposed directly to the atmosphere. As early as 1851, Gerlach was able to show that human skin takes up oxygen from the atmosphere.
Local relative measurements of the changes in cutaneous oxygen uptake from the atmosphere, the so-called transcutaneous oxygen flux (tcJO2), have become possible with the development of an oxygen fluxoptode (Holst, 1994; Holst et al. 1995). Measurements of tcJO2 on the humidified skin of the volar forearm at normal skin temperature (33 °C) during artificially induced variations in blood perfusion have indicated the functional relevance of the external oxygen supply (Stücker et al. 2000a). The induction of hyperaemia in moist skin with a combination of nonivamide and nicoboxil resulted in a distinct decrease of tcJO2 to 70 % of the resting values. These experiments clearly demonstrated that the oxygen supply of the corium is a balance between oxygen transport by the blood and uptake from the atmosphere. If the oxygen supply from the blood increases, a lower tcJO2 suffices to cover the oxygen demand of the skin. Stopping capillary oxygen transport was compensated by an increase of tcJO2 of only 9 %. This indicates that under normal conditions a substantial part of the upper skin is supplied by direct oxygen uptake from the atmosphere. Until now it has not been possible to determine the thickness of the layer (T) that characterises the contribution of tcJO2 to the total skin oxygen supply.
In a theoretical analysis, Fitzgerald (1957) estimated a mean T of 48 μm, with a range of 34–84 μm, which would cover the main part or the whole of the epidermis. His calculations were based on data for the diffusion coefficient measured on the anterior abdominal wall of the frog following removal of the skin, because there were no comparable measurements on mammals. In fact, the true values for the oxygen permeability of skin tissue are an order of magnitude greater, whilst the oxygen consumption under normal conditions is about four times lower (actual data: 1470–2110 ml O2 m−3 min−1; Fitzgerald used 7800 ml O2 (ml tissue)−1 min−1). The approximate partial pressure of oxygen (PO2) of capillary blood, 95 Torr (1 Torr = 0.1333 kPa), was taken as the minimum PO2 of the skin. This is higher than the minimum value of 51 Torr that was measured in the skin in vivo using needle electrodes (Evans & Naylor, 1966a; Roszinski & Schmeller, 1995). A greater penetration depth T of the external oxygen is calculated using the latter values. Furthermore, Fitzgerald had to use data for the absorption of oxygen through the skin surface, which had a wide range of 0.4–2.9 ml O2 m−2 min−1. This was due to different measuring locations and temperatures, large measuring areas and poor sensitivity of the measuring devices (for example, changes in the oxygen absorption caused by increased or decreased blood flow could not be detected).
In 1987, Baumgärtl et al. measured the intracutaneous profile of PO2 directly with needle electrodes. The skin surface of the lower limb was covered by a film of water, which resulted in a reduced skin surface PO2 of 78 Torr. Furthermore, the needle puncture probably produced a local hyperaemia and increased the oxygen supply by the blood. Under these conditions, with reduced skin surface PO2 and hyperaemia, the PO2 profile had a distinct minimum at a depth of about 100 μm, roughly at the level of the capillary loops (Fig. 1). These invasive measurements demonstrated a penetration depth of atmospheric oxygen into the skin, double that of Fitzpatrick's estimated values. According to Fick's law of diffusion:
where JO2 is the oxygen flux, K the conductivity and ∂PO2/∂x the pressure gradient. These measurements show, therefore, that in the upper 100 μm at least, there can only be a diffusion of oxygen from the skin surface to that depth instead of from the blood to the skin surface.
Figure 1. Oxygen partial pressure (PO2) measured by a needle electrode inserted perpendicularly into the skin.
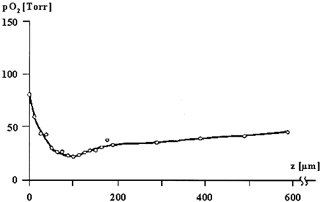
The depth z of the electrode is given in μm (skin surface at 0 μm). The skin surface was covered by a water film, resulting in a reduced skin surface PO2 (ssPO2) of 78 Torr. The PO2 profile has a distinct minimum at a depth of approximately 100 μm, roughly at the level of the dermo-epidermal junction (according to Baumgärtl et al. 1987). The needle puncture probably resulted in a local hyperemia. Under more physiological conditions, it is expected that the minimum would occur at a greater depth.
In this study, we have examined the importance of the cutaneous uptake of external oxygen for the skin supply by quantifying tcJO2 at a normal atmospheric PO2 using a new, highly sensitive, non-invasive measuring device, which only covers small homogeneous skin areas rather than the large, heterogeneous skin areas with varying skin thicknesses and different densities of skin adnexes of earlier studies. In contrast to these earlier studies, it has been possible on the one hand to quantify absolute values (rather than relative changes in oxygen) by using a newly developed calibration system and, on the other, to measure at a normal instead of reduced skin surface PO2 (Stücker et al. 2000a).
METHODS
Measurement of tcJO2
Measurements were carried out using an oxygen fluxoptode developed by our group (Holst, 1994; Holst et al. 1995). This device consists of three different layers in a sandwich arrangement (Fig. 2). The fluxoptode is applied to the skin surface and represents an artificial barrier to the external atmosphere. The upper polymer layer serves as a diffusion barrier with a defined oxygen conductivity, whilst the oxygen-sensing silicon layer (with a negligible oxygen conductivity) allows measurement of the skin surface PO2 (ssPO2). ssPO2 is lower than the external atmospheric value as a result of the decreased oxygen flux (JO2) through the diffusion barrier. By increasing the external PO2, ssPO2 can be varied until a stable value close to the normal atmospheric pressure is reached (Fig. 3C). At a given oxygen permeability P, the pressure difference (ΔPO2) between ssPO2 and the external PO2 is proportional to tcJO2:
| (1) |
It is thus possible to obtain an absolute value for tcJO2 if the oxygen permeability P of the diffusion barrier is known.
Figure 2. Cross section of the oxygen fluxoptode (according to Holst, 1994).
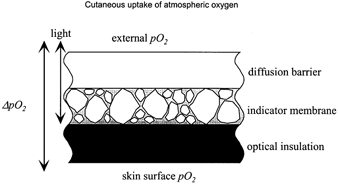
The oxygen flux through the diffusion barrier induces a pressure gradient, ΔPO2. The PO2 in the highly oxygen-permeable indicator membrane is measured with an optical oxygen indicator adsorbed onto silica gel particles. The external PO2 can be varied to adjust the ssPO2 close to the atmospheric value (‘normobaric conditions’).
Figure 3. Case example of a measurement of the transcutaneous oxygen flux (tcJO2; 24-year-old female volunteer, no. 6).
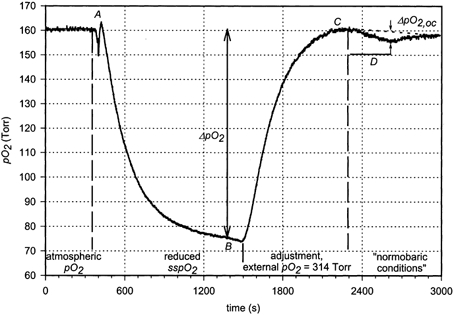
The normal atmospheric PO2 was recorded between 0 and 400 s. At A, the oxygen fluxoptode was applied to the volar forearm. ssPO2 reached a steady level at B, indicating a ΔPO2 of 85 Torr (equilibration time). Between points B and C, the external PO2 was increased to 314 Torr to adjust the ssPO2 to ‘normal atmospheric conditions’. In the time interval D, a suprasystolic occlusion was carried out, resulting in an increase in ΔPO2: ΔPO2 during occlusion (ΔPO2,oc) = 3.3 Torr. ΔPO2,oc was determined by subtraction of a linear baseline (dotted line).
Oxygen fluxoptode
In order to obtain constant diffusion properties, the oxygen fluxoptodes were produced in our laboratory according to a standardised protocol. A commercially available polymer membrane (PFA 6510, Nowofol, Siegsdorf, Germany) with a thickness d = 55 μm was used as the diffusion barrier. The value of P of this layer is given by:
| (2) |
where K is the oxygen conductivity, α is the oxygen solubility and D is the oxygen diffusion constant.
ssPO2 was measured optically using the oxygen indicator RuBiPy (Tris (2,2-bipyridyl) ruthenium (II) chloride hexahydrate; Strem Chemicals, Kehl, Germany) adsorbed onto silica gel particles, which were embedded in a silicone layer with a high oxygen conductivity (Wacker Chemie, Burghausen, Germany). A further, blackened silicone layer in direct contact with the skin served as optical insulation to suppress fluorescence artefacts from the skin (Fig. 2). The measuring principle is based on the reduction of the fluorescence lifetime (quenching) of the indicator by oxygen. If the indicator is excited harmonically, the phase shift (ΔΦ) between excitation and fluorescence intensity is a measure of the oxygen concentration at the indicator molecules. The calibration curve is non-linear and is described by eqn (3) (Holst, 1994):
| (3) |
The oxygen fluxoptode was calibrated daily by exposing the fluxoptode to water-saturated N2–O2 gas mixtures, the oxygen content of which varied from 0 to 20.95 %. The parameters A, B and C were determined by a least squares fit (SigmaPlot 2.01, Jandel Scientific, Erkrath, Germany). The in vitro accuracy of the determination of ΔPO2 was better than ± 0.2 Torr. A reproducibility of 5 Torr was obtained under our experimental conditions (Stücker et al. 2000a). The exponential equilibration time after changing the composition of the gas mixture was 87.9 ± 3.4 s (n = 8). No dependence on the oxygen concentration was observed. This value represents an upper limit, as the equilibration time was probably determined by the time course of the gas exchange within the volume of the calibration set-up.
Calibration of the oxygen fluxoptode and absolute determination of tcJO2
The oxygen permeability of the diffusion barrier was determined using a polarographic oxygen electrode constructed according to the principles described by Lübbers et al. (1969). In this case, a large circular platinum cathode (Ø 1 mm) and an annular Ag–AgCl anode were used. The surface of the electrode was protected mechanically by a cellophane membrane (Bamberger, Wuppertal, Germany; thickness 25 μm) with high oxygen permeability. The membrane to be tested was placed in close contact with the cellophane membrane and exposed to the air. At an appropriate electrode potential (600–750 mV), all oxygen molecules crossing the membrane and reaching the platinum surface of the electrode are reduced, transferring four electrons per molecule (Aiba et al. 1968). The resulting current (I) at atmospheric oxygen pressure (PO2,ATM) is:
| (4) |
where F is the Faraday constant and A is the electrode surface area (0.785 mm2). KTOTAL is the combined oxygen permeability of the cellophane and the membrane to be tested. The oxygen permeability of the fluxoptode membrane was obtained by measuring the permeability of the cellophane membrane separately.
Seven fluxoptode membranes were tested for the 20 investigations in this study. The mean oxygen conductivity of the diffusion barrier was K = 3.58 ± 0.14 × 10−7 ml O2 min−1 m−1 Torr−1. The thickness of the diffusion barrier for six of the optodes was 55 μm, with a tolerance of 1 μm. One membrane was exactly twice as thick, and although this led to a decreased permeability of the membrane, the transcutaneous oxygen uptake was unchanged compared to the other membranes.
Covering the electrode with the silicone layer of the fluxsoptode resulted in no measurable decrease in the polarographic current. This shows that this layer had no significant influence on the steady-state measurements of tcJO2.
Skin humidity
The skin humidity was quantified using a corneometer SM 825 PC (Courage & Khazaka, Cologne, Germany). This uses the dielectric constant as a measure of the water content of the skin tissue. The measuring depth is approximately 15 μm.
Skin perfusion
The time course of the perfusion during the experiment and the efficacy of a suprasystolic occlusion were assessed by monitoring the transcutaneous PO2 (tcPO2; TCM 3, Radiometer, Copenhagen, Denmark) and the laser Doppler flow (DRT 4, Moor Instruments, Axminster, UK). The tcPO2 and the laser Doppler sensor heads were positioned at a maximum distance of 10 mm from the fluxoptode. tcPO2 was recorded at a skin temperature of 37 °C (Huch et al. 1981) and laser Doppler flow at normal skin temperature (31–34 °C) using a wavelength of 780 nm.
Subjects
Measurements were carried out on the volar forearm of 20 volunteers who had no clinical history or physical evidence on examination of vascular diseases, diabetes mellitus or skin diseases in the area examined. In order to evaluate whether tcJO2 is influenced by age, the group comprised two subgroups with 10 volunteers younger than 30 years (23.7 ± 3.1 years, 6 females, 4 males) and 10 volunteers older than 70 years (77.8 ± 5.4 years, 7 females, 3 males). Informed consent to participate was obtained from all of the subjects. A histological examination was carried out on one subject for diagnostic reasons unrelated to this study. The study protocol was reviewed and approved by the Ethics Committee of Ruhr University, Bochum, and was carried out in accordance with the Helsinki guidelines.
Experimental design
The volunteers were acclimatised at a room temperature of 22–23 °C for 20–25 min lying in a comfortable supine position with the upper body slightly raised. Before applying the probe, the skin in the test area was cleaned with 63 % propanol (Cutasept, Bode Chemie, Hamburg, Germany). The superficial horny scales were removed by stripping 10 times with adhesive tape. Sejrsen (1968) demonstrated that the removal of the outer stratum disjunctum of the horny layer had no effect on the diffusion of xenon, but that by removing the stratum conjunctum and stratum granulosum (stripping 40–50 times), diffusion increased up to 40 times (skin temperature 33 °C at room temperature). At the beginning and end of the measurement, the tcJO2 sensor signal was recorded at normal atmospheric PO2 as a reference value. After humidifying the skin in the test area with 50 μl water, the measuring heads were fixed in position with adhesive rings. The temperature of the sensor heads was adjusted to 33 °C.
Figure 3 shows a typical example of the course of the tcJO2 measurement. In order to characterise the influence of changes of the skin perfusion on tcJO2 at normal ssPO2 a 5 min suprasystolic occlusion was carried out with a blood pressure cuff applied to the upper arm.
The skin humidity was measured at the start, after the stripping procedure and at the end of the measurement period. To differentiate between the changes in skin humidity due to the measuring procedure and those due to normal physiological alterations, corneometric measurements were also carried out on untreated skin in close proximity to the test area.
Statistics
Data are presented as means ± s.d. The data were tested for Gaussian distribution with the Kolmogorov-Smirnov test. The significance of any differences was tested with the t test (SPSS 8.0, SPSS, Chicago, IL, USA). The significance level was set at P = 0.05.
RESULTS
Case example
Figure 3 shows a typical course of a tcJO2 measurement. Within 16 min of applying the sensor to the volar forearm (A), the ssPO2 decreased from the normal atmospheric value to a steady-state value of 75 Torr (B), corresponding to a pressure difference across the diffusion barrier (ΔPO2) of 85 Torr.
After application of the oxygen fluxoptode, ssPO2 equilibrated exponentially during the period defined by a change in ΔPO2 from 75 to 100 % of the final value. In this case, an exponential fit revealed an equilibration time (τ) of 244 s, which is within the range of data published previously (Stücker et al. 2000a).
Increasing the external PO2 to 314 Torr resulted in an ssPO2 close to the atmospheric value (C, ‘normobaric conditions’). At this point, ΔPO2 was 154 Torr.
In order to examine the influence of an interruption of the blood supply under normobaric conditions, a suprasystolic occlusion was carried out for 5 min while the external PO2 of 314 Torr remained constant (D).
There was a considerable time lapse between the change of the external PO2 and the equilibration of the ssPO2 to the new steady state due to the equilibration time of the fluxoptode. It was usually not possible to wait until the signal was totally stable, and the steady-state value was therefore determined by subtracting a linear baseline (D, dotted line). In this experiment, a decrease in the ssPO2 of 3.3 Torr was observed at the end of the occlusion.
The oxygen permeability of the fluxoptode was 6.36 × 10−3 ml O2 m−2 min−1 Torr−1, yielding a tcJO2 of 0.54 ml O2 m−2 min−1 at the lower ssPO2 and 0.98 ml O2 m−2 min−1 under normobaric conditions at the skin surface. The increase in the tcJO2 induced by the occlusion was 0.021 ml O2 m−2 min−1.
Measurement of tcJO2 under normobaric conditions
After appropriate variation of the external PO2, an average ssPO2 of 163 ± 9 Torr was reached. The ΔPO2 across the membrane of 90 ± 37 Torr was measured (Table 1) under these conditions.
Table 1.
Measurements of transcutaneous oxygen flux (tcJO2; 33 °C, humidified skin)
| No pressure correction | Normal ssPO2 | |||||
|---|---|---|---|---|---|---|
| Volunteer | Age (years) | ssPO2 (Torr) | tcJO2 (ml O2 min−1 m−2) | ssPO2 (Torr) | tcJO2 (ml O2 min−1 m−2) | Occlusion ΔtcJO2 (ml O2 min−1 m−2) |
| 1 | 22 | 99 | 0.38 | 164 | 0.54 | 0.020 |
| 2 | 23 | 110 | 0.31 | 159 | 0.34 | 0.046 |
| 3 | 23 | 97 | 0.39 | 156 | 0.47 | 0.036 |
| 4 | 25 | 112 | 0.29 | 159.5 | 0.45 | 0.041 |
| 5 | 20 | 99 | 0.38 | 152 | 0.69 | 0.056 |
| 6 | 24 | 75 | 0.54 | 160 | 0.98 | 0.021 |
| 7 | 28 | 104 | 0.19 | 172 | 0.24 | 0.047 |
| 8 | 19 | 97 | 0.21 | 168 | 0.30 | 0.046 |
| 9 | 29 | 84 | 0.26 | 163 | 0.43 | 0.034 |
| 10 | 24 | 102 | 0.37 | 160 | 0.71 | 0.026 |
| 11 | 79 | 94 | 0.42 | 160 | 0.65 | 0.045 |
| 12 | 81 | 99 | 0.38 | 156 | 0.71 | 0.019 |
| 13 | 70 | 116 | 0.28 | 172 | 0.33 | 0.032 |
| 14 | 77 | 104 | 0.35 | 156 | 0.35 | 0.060 |
| 15 | 81 | 96 | 0.40 | 166 | 0.70 | 0.050 |
| 16 | 79 | 99 | 0.41 | 185 | 0.72 | 0.028 |
| 17 | 84 | 109 | 0.31 | 164 | 0.59 | 0.038 |
| 18 | 70 | 91 | 0.46 | 148 | 1.13 | 0.034 |
| 19 | 83 | 134 | 0.16 | 176 | 0.07 | 0.016 |
| 20 | 70 | 106 | 0.18 | 166 | 0.18 | 0.035 |
| Mean | 101.4 | 0.334 | 163 | 0.529 | 0.037 | |
| s.d. | 12.2 | 0.100 | 9 | 0.265 | 0.013 | |
tcJO2 is significantly reduced at lower values of skin surface partial pressures of oxygen (PO2).
After determination of the oxygen permeability of the fluxoptode, the transcutaneous oxygen uptake could be calculated using eqn (1). tcJO2 was found to be 0.529 ± 0.265 ml O2 m−2 min−1 (Fig. 4).
Figure 4. Comparison of the tcJO2 (33 OC, humidified skin) under normal (right) and reduced (left) ssPO2 (n = 20).
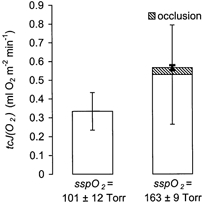
A 5 min suprasystolic occlusion resulted in only a small increase (shaded area).
Interruption of the blood supply
In this study the effect of an interruption of blood flow (ischaemia) was evaluated under normobaric conditions. On average, the laser Doppler flow decreased from 21.0 ± 7.8 arbitrary units (a.u.) to 5.0 ± 1.5 a.u. (P ≤ 0.0001). During the post-occlusional reactive hyperaemia it increased by 305 ± 165 % within 40.6 ± 52.2 s, followed by a decay to the baseline value (P ≤ 0.0001). The tcPO2 values were 7.1 ± 7.3 Torr at rest and 1.6 ± 2.4 Torr during suprasystolic occlusion (P ≤ 0.0001). The post-reactive hyperaemia represented a relative increase of 372 ± 336 % compared to the resting values (P ≤ 0.0001).
At the end of the 5 min suprasystolic occlusion the ssPO2below the oxygen fluxoptode was decreased by 6.8 ± 3.3 Torr (measured as the distance to a linear baseline; C in Fig. 3, P ≤ 0.0001). This value indicates a mean increase in tcJO2 of 0.037 ± 0.013 ml O2 m−2 min−1. The occlusion resulted in a relative elevation of tcJO2 of 9.5 ± 6.3 % (P ≤ 0.05, Fig. 4).
The tcJO2 at a lower ssPO2
tcJO2 produced a decrease of ssPO2 below the oxygen fluxoptode to 101.4 ± 12.2 Torr (Fig. 4, left). These data correspond to a ΔPO2 across the diffusion barrier of 60.0 ± 11.9 Torr. This is equivalent to a tcJO2 of 0.334 ± 0.100 ml O2 m−2 min−1, representing 68 ± 17 % of the values determined under normobaric conditions (Fig. 4, right).
A mono-exponential equilibration time, τ, was found in 17 experiments. A multi-exponential time dependence was found in three experiments. These measurements were omitted because it was suggested that in these cases there was not an adequate airtight seal between the sensor and the skin surface. The mean exponential equilibration time was 413 ± 130 s.
Dependence on age
There was no significant difference in tcJO2 between the subgroups of young and old volunteers. Under normobaric conditions, tcJO2 was 0.515 ± 0.224 ml O2 m−2 min−1 (young volunteers) and 0.543 ± 0.313 ml O2 m−2 min−1 (old volunteers; P = 0.83). The relative increase during a suprasystolic occlusion was 9.1 ± 5.6 % and 9.9 ± 7.3 %, in the young and old subjects, respectively (P = 0.80).
Changes in the skin humidity
On average, at the beginning of the measurements the mean value of the skin humidity of untreated skin was 43 ± 11 a.u. (Fig. 5). After stripping the skin 10 times with adhesive tape, the skin humidity increased significantly to 49 ± 13 a.u. (P ≤ 0.0001). A value of 78 ± 24 a.u. was recorded at the end of the measurements, after removal of the oxygen fluxoptode. The humidity of untreated skin close to the test area was 63 ± 16 a.u. at this time. These numbers represent a relative increase of 86 ± 61 % in the test area and 51 ± 42 % in the reference area (P ≤ 0.0032).
Figure 5. Change of skin humidity during the investigations (n = 20).
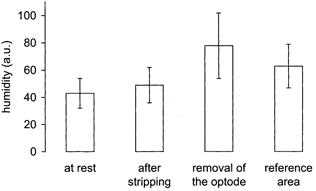
The skin humidity is given in arbitrary units (a.u.). An increase of humidity in the measurement area resulted from the stripping procedure and from the sealing of the skin surface by the oxygen fluxoptode. Measurements in the reference area showed a parallel increase of the humidity of untreated skin.
DISCUSSION
Contribution of tcJO2 to the oxygen supply of the whole organism
The transcutaneous oxygen uptake of 0.529 ± 0.265 ml O2 m−2 min−1, as determined in this study, is at the lower end of the wide range of 0.4–2.9 ml O2 m−2 min−1 quoted in the literature (see Fitzgerald, 1957). Assuming a body surface area of 1.7 m2, this yields a total transcutaneous oxygen uptake of 0.90 ± 0.45 ml O2 m−2 min−1, which represents only 0.4 % of the pulmonary oxygen uptake at rest (ca 230 ml O2 min−1; see Wade & Bishop, 1962). The contribution of tcJO2 to the oxygen supply of the whole organism is therefore negligible under normal conditions.
Estimation of the skin tissue supplied by tcJO2
The thickness of the skin tissue (T) that is supplied by external oxygen can be estimated if the whole amount of oxygen taken up from the atmosphere is consumed within the skin. Since in all the experiments in this study an increase of tcJO2 was observed during ischaemia, capillary oxygen removal from the skin is negligible. Furthermore, assuming a homogeneous oxygen consumption (V̇O2) in the upper skin layers, T is given by:
| (5) |
V̇O2 in skin tissue has been investigated in vivo by determination of the decrease in PO2 after stopping the oxygen supply. It was found to be strongly temperature dependent. At 43 °C V̇O2 = 4700 ml O2 m−3 min−1 was derived from tcPO2 measurements (Severinghaus et al. 1978; Stücker et al. 2000b). At about 35 °C, a oxygen uptake value of 1470 ml O2 m−3 min−1 has been measured with intradermal needle electrodes (Evans & Naylor, 1966b), whereas measurements on the stratum papillare without epidermis revealed a V̇O2 of 2110 ml O2 m−3 min−1 (blister base, Evans & Naylor, 1967). On the surface of the epidermis (blister lid) it was found to be 1990 ml O2 m−3 min−1 (Evans & Naylor, 1967).
With values between 1470 and 2110 ml O2 m−3 min−1, eqn (5) yields T = 251–360 μm. Since there is no oxygen consumption in the stratum corneum with a typical thickness of 15 μm, the total thickness of the skin supplied by external oxygen can be estimated to be 266–375 μm.
Comparison with a theoretical analysis
It is also possible to deduce tcJO2 and T from the diffusion properties of oxygen in skin tissue if the oxygen transport by blood is described by a model. Models examining the meaning of tcPO2 have been published (see Huch et al. 1981; Grossmann, 1982; Lübbers, 1994), but a similar analysis of the tcJO2 is lacking. The following assumptions are used to calculate intracutaneous PO2 profiles:
Under normal conditions the upper skin layers are supplied exclusively by the diffusion of oxygen from the atmosphere. This assumption is justified by the observation of only small changes of tcJO2 during occlusion.
-
Experiments using polarographic PO2 needle electrodes have revealed characteristics of the intracutaneous PO2 profiles: the PO2 decreases continuously from a maximum at the skin surface to the upper layers of the papillary dermis until reaching a minimum, followed by a slight increase in the deeper skin layers (Fig. 1, Baumgärtl et al. 1987). According to Fick's first law (JO2 =−K(∂PO2/∂x)), there is no oxygen flux (JO2) between the upper layer where the PO2 is decreasing and the skin tissue below the point at which the minimum is reached.
A mean intracutaneous PO2 of 51 Torr has been recorded at greater depths (Evans & Naylor, 1966a; Roszinski & Schmeller, 1995). We assume, therefore, that there is an intracutaneous PO2 minimum of 51 Torr. Its depth depends on the other parameters.
The oxygen permeabilities (K) are 3.7 × 10−7 ml O2 m−1 min−1 Torr−1 in the stratum corneum and 1.3 × 10−6 ml O2 m−1 min−1 Torr−1 in viable tissue (measured at 32 °C; Grossmann, 1982; for an overview see Huch et al. 1981).
As described above, a V̇O2 value of 1990 ml O2 m−3 min−1 is assumed for the epidermis (Evans & Naylor, 1967) and 1470 ml O2 m−3 min−1 for dermal tissue (Evans & Naylor, 1966b).
These assumptions allow an algebraic static solution of Fick's second law:
| (6) |
where cO2 is the concentration of dissolved oxygen). Equation (7) represents the general solution in a homogeneous tissue layer:
| (7) |
with the integration constants c1 and c2 (x-axis perpendicular to the skin surface). The application of Fick's first law yields JO2 at position x:
| (8) |
c1 and c2 result from the boundary conditions of each skin layer, starting from the PO2 minimum with PO2 = 51 Torr and JO2 = 0.
Trace A on Fig. 6 shows the PO2 profile derived according to the described assumptions. The skin tissue is supplied with external oxygen to a depth of 403 μm. The calculated tcJO2 is 0.58 ml O2 m−2 min−1. This value is in agreement with the data obtained from our experiments (0.529 ± 0.265 ml O2 m−2 min−1), indicating that the model represents a good description of the oxygen transport in the upper skin layers.
Figure 6. Theoretical estimation of the intracutaneous PO2 profile.
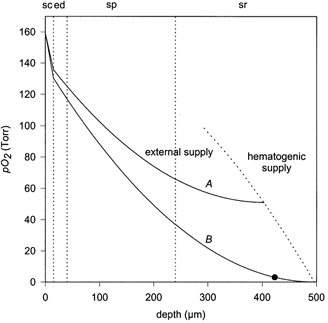
The PO2 minimum at 51 Torr (trace A) and at 0 Torr (trace B: suprasystolic occlusion) are shown. Below a critical PO2 of about 3 Torr, mitochondrial activity is reduced (Wilson, 1979). Skin surface at x = 0; sc: stratum corneum; ed: viable epidermis; sp: stratum papillare; sr: stratum reticulare. The area on the right side of the dotted line is supplied by blood. Temperature: 32 °C; oxygen permeabilities: 3.7 × 10−7 ml O2 m−1 min−1 Torr−1 (stratum corneum) and 1.3 × 10−6 ml O2 m−1 min−1 Torr−1 (viable layers); oxygen consumption = 1990 ml O2 m−3 min−1 (viable epidermis) and 1470 ml O2 m−3 min−1 (dermal tissue). • = 3 Torr.
Comparison with the experiments with needle electrodes
Baumgärtl et al. (1987) measured an intracutaneous PO2 profile in the lower leg using a polarographic needle electrode. A water film at the skin surface reduced the ssPO2 to 78 Torr. A minimum of PO2, = 22 Torr was observed at a minimum depth of 100 μm. Adaptation of eqn (7) to this curve (with the minimum at x = 0) yields (Fitzgerald, 1957):
| (9) |
This allows the depth T, which would be observed under normobaric conditions under the assumption of an unaltered minimal pressure, to be calculated:
| (10) |
The resulting value of 188 μm is still distinctly lower than the other estimates. Although this investigation was carried out in the lower leg, the difference cannot be attributed to regional variations. The ΔPO2 across the fluxoptode (measured without pressure correction) at the medial ankle (72.8 ± 12.3 Torr; Stücker et al. 2000a) is even greater than in the volar forearm (60.0 ± 11.9 Torr; this study). The difference might be explained by tissue damage due to the needle puncture and consequential hyperaemia.
Figure 7 correlates the different estimates of T with skin morphology. It shows that the whole epidermis and parts of the dermis are supplied by external oxygen from the air.
Figure 7. Penetration depth of atmospheric oxygen in the skin: different estimations.
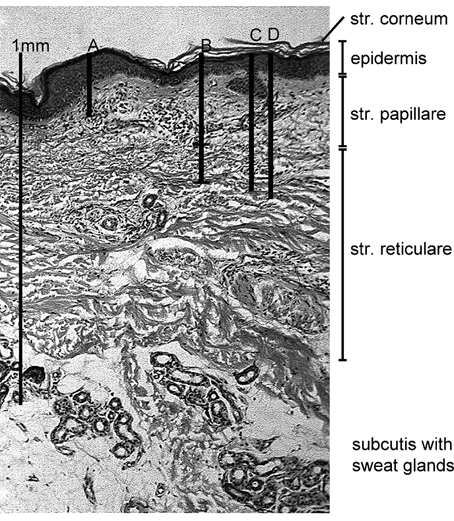
Histological section of healthy skin from the volar forearm (male subject, 64 years old) with different estimates of the thickness, T, of the skin layer that is supplied from the atmosphere. (Normal ssPO2, humid skin, skin temperature 33 °C). A: T = 188 μm. Extrapolation from data obtained from invasive experiments with PO2 needle electrodes (Baumgärtl et al. 1987). B: T = 266–375 μm. Estimation using the tcJO2 values determined in this study. C: T = 403 μm and D: T = 423 μm. Estimation of T using published data for the oxygen diffusion properties and oxygen consumption in skin tissue, assuming an intradermal PO2 of 51 Torr (C, normal conditions) and of 0 Torr (D, ischaemia).
Interruption of the blood supply
During a 5 min suprasystolic occlusion, tcJO2 increased by 0.037 ± 0.013 ml O2 m−2 min−1, representing a relative increase of only 9.5 ± 6.3 %. The measurement of the tcPO2, representing the nutritive blood flow (Bongard & Bounameaux, 1993) proved the efficacy of this test: tcPO2 decreased from 7.1 ± 7.3 Torr to 1.6 ± 2.4 Torr. Due to the equilibration time of the fluxoptode of 413 ± 130 s, ssPO2 did not reach a stable equilibrium value during the limited period of suprasystolic occlusion (300 s). A further reason for the non-attainment of a steady state of the ssPO2 during the 5 min occlusion may be that the tissue oxygen saturation values did not approach a minimum value and therefore it is possible that not all the available oxygen had been unloaded from the haemoglobin within the microcirculation. As a consequence, the values of ΔtcJO2 determined may result in an underestimate of the contribution of oxygen by the blood. However, even the measurements with shorter equilibration times (e.g. the case example) showed only a minor increase in tcJO2 during suprasystolic occlusion. This indicates that in our measurements the capillary contribution to the oxygen supply of the corium is very limited.
In trace B of Fig. 6, a suprasystolic occlusion is simulated by assuming an intracutaneous PO2 of 0 Torr in deeper skin layers. Under this condition, tcJO2 increased by 24 %. Because this value was calculated assuming a steady-state situation, it agrees with the experimental data. Under the assumption of a minimal PO2 of 3 Torr, which is necessary to maintain mitochondrial activity (Wilson et al. 1979), tcJO2 can cover the oxygen demand of the upper 423 μm of the skin if blood perfusion is interrupted.
tcJO2 under hypobaric conditions
Without pressure correction, tcJO2 produced a ΔPO2 of 60 ± 12 Torr across the oxygen fluxoptode. This value is significantly lower than the previously published data. Using identical oxygen fluxoptodes, Stücker et al. (2000a) found values of 72.8 ± 12.3 Torr at the medial ankle and 81.8 ± 8.5 Torr on the abdomen. Such variations of tcJO2 could be the result of differences in the skin structure, skin perfusion or different adnex densities.
Skin humidity
The oxygen permeability of tissue is strongly dependent upon water content (Vaupel, 1976). During the investigations in this study, the humidity of the skin below the oxygen fluxoptode (measured with the Corneometer) increased significantly from 43 ± 11 a.u. to 78 ± 24 a.u. due to the stripping procedure, artificial humidification of the skin or just sweating. A lower JO2 is to be expected through dry skin. Nevertheless, the skin humidity in the measurement area did not differ greatly from the normal physiological range, as was shown by the comparison with the untreated reference area where values of 63 ± 16 a.u. were observed.
Age dependence
Skin in elderly people displays characteristic functional and structural alterations such as changes in permeability to drugs (Malkinson, 1958) and increased corneocyte surface (Marks, 1981). Effects such as a reduction in thickness and cell density of the corium, keratoses and elastoses are clearly related to long-term UV exposure (Balin & Lin, 1989; Yaar & Gilchrest, 1999) and should be absent in the volar forearm. No influence on the transepidermal oxygen uptake was found due to intrinsic alterations in aged skin, since the tcJO2 at this location proved to be unaffected by age.
Clincal relevance
In this study it has been shown that under normal conditions, atmospheric oxygen can supply the upper skin layers to a depth of 0.25–0.40 mm. This is 3–10 times deeper than has been calculated previously (Fitzgerald, 1957; Baumgärtl et al. 1987). The whole epidermis and the upper corium can therefore be supplied with oxygen from the atmosphere. For the first time, all of the data have been derived from non-invasive measurements in vivo in human skin instead of in vitro measurements in non-mammalian skin (Fitzgerald, 1957) or single invasive measurements (Baumgärtl et al. 1987). This may have significant consequences with regard to the treatment of lesions such as venous and ischaemic ulcers.
Acknowledgments
We wish to thank Courage and Khazaka GmbH, Cologne, Germany for providing the corneometer SM 825 PC, D. Seiler (Max-Planck-Institute) for the fabrication of the platinum electrode, S. Mischke (Max-Planck-Institute) for proof reading the manuscript and D. Pieck (Department of Dermatology) for assistance during the experiments. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, Al 389/4–1 and 4–2).
REFERENCES
- Aiba S, Ohashi M, Huang SY. Rapid determination of oxygen permeability of polymer membranes. Industrial and Engineering Chemistry Fundamentals. 1968;7:497–502. [Google Scholar]
- Balin AK, Lin AN. Skin changes as a biological marker for measuring the rate of human aging. In: Balin AK, Kligman AM, editors. Aging and the Skin. New York: Raven; 1989. pp. 43–75. [Google Scholar]
- Baumgärtl H, Ehrly AM, Saeger-Lorenz K, Lübbers DW. Initial results of intracutaneous measurements of PO2 profiles. In: Ehrly AM, Hauss J, Huch R, editors. Clinical Oxygen Pressure Measurement. Berlin: Springer; 1987. pp. 121–128. [Google Scholar]
- Bongard O, Bounameaux H. Clinical investigation of skin microcirculation. Dermatology. 1993;186:6–11. doi: 10.1159/000247295. [DOI] [PubMed] [Google Scholar]
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. Journal of Investigative Dermatology. 1989;93:2S–9S. doi: 10.1111/1523-1747.ep12580893. [DOI] [PubMed] [Google Scholar]
- Evans NTS, Naylor PFD. Steady states of oxygen tension in human dermis. Respiration Physiology. 1966a;2:46–60. doi: 10.1016/0034-5687(66)90037-5. [DOI] [PubMed] [Google Scholar]
- Evans NTS, Naylor PFD. The dynamics of changes in dermal oxygen tension. Respiration Physiology. 1966b;2:61–72. doi: 10.1016/0034-5687(66)90038-7. [DOI] [PubMed] [Google Scholar]
- Evans NTS, Naylor PFD. The oxygen tension gradient across human epidermis. Respiration Physiology. 1967;3:38–42. doi: 10.1016/0034-5687(67)90021-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LR. Cutaneous respiration in man. Physiological Reviews. 1957;37:325–345. doi: 10.1152/physrev.1957.37.3.325. [DOI] [PubMed] [Google Scholar]
- Gerlach A. Über das Hautathmen. Archives of Anatomy and Physiology. 1851:431–479. [Google Scholar]
- Grossmann U. Simulation of combined transfer of oxygen and heat through the skin using a capillary-loop model. Mathematical Biosciences. 1982;61:205–236. [Google Scholar]
- Holst G. Fortschritt-Berichte VDI Reihe 17 Nr. Vol. 111. Düsseldorf: VDI Verlag; 1994. Entwicklung und Erprobung einer Sauerstoff-Flux-Optode mit einem Sauerstoff- Sensor nach dem Prinzip der dynamischen Fluoreszenzlöschung; pp. 1–105. [Google Scholar]
- Holst G, Köster T, Voges E, Lübbers DW. FLOX-an oxygen-flux-measuring system using a phase-modulation method to evaluate the oxygen-dependent fluorescence lifetime. Sensors and Actuators B. 1995;29:231–239. [Google Scholar]
- Huch R, Huch A, Lübbers DW. Transcutaneous PO2. New York: Thieme-Stratton; 1981. [Google Scholar]
- Lübbers DW. Microcirculation and O2 exchange through the skin surface. A theoretical analysis. Advances in Experimental Medicine and Biology. 1994;361:51–58. doi: 10.1007/978-1-4615-1875-4_6. [DOI] [PubMed] [Google Scholar]
- Lübbers DW, Baumgärtl H, Fabel H, Huch A, Kessler M, Kunze K, Riemann H, Seiler D, Schuchhardt S. Principle of construction and application of various platinum electrodes. Progress in Respiration Research. 1969;3:136–146. [Google Scholar]
- Malkinson FD. Studies on percutaneous absorption of 14C labelled steroid by use of the gas flow cell. Journal of Investigative Dermatology. 1958;31:19–28. doi: 10.1038/jid.1958.71. [DOI] [PubMed] [Google Scholar]
- Marks R. Measurement of biological aging in human epidermis. British Journal of Dermatology. 1981;104:627–633. doi: 10.1111/j.1365-2133.1981.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Roszinski S, Schmeller W. Differences between intracutaneous and transcutaneous skin oxygen tension in chronic venous insufficiency. Journal of Cardiovascular Surgery. 1995;36:407–413. [PubMed] [Google Scholar]
- Sejrsen P. Epidermal diffusion barrier to 133Xe in man and studies of clearance of 133Xe by sweat. Journal of Applied Physiology. 1968;24:211–216. doi: 10.1152/jappl.1968.24.2.211. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Stafford M, Thunstrom AM. Estimation of skin metabolism and blood flow with tcpO2 and tcpCO2 electrodes by cuff occlusion of the circulation. Acta Anaesthesiologica Scandinavica. Supplementum. 1978;68:9–15. doi: 10.1111/j.1399-6576.1978.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Stücker M, Altmeyer P, Struk A, Hoffmann K, Schulze L, Röchling A, Lübbers DW. The transepidermal oxygen flux from the environment is in balance with the capillary oxygen supply. Journal of Investigative Dermatology. 2000a;114:533–540. doi: 10.1046/j.1523-1747.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Stücker M, Falkenberg M, Reuther T, Altmeyer P, Lübbers DW. Local oxygen content in the skin is increased in chronic venous incompetence. Microvascular Research. 2000b;59:99–106. doi: 10.1006/mvre.1999.2202. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Effect of percental water content in tissue and liquids on the diffusion coefficients of O2, CO2, N2, H2. Pflügers Archiv. 1976;361:201–204. doi: 10.1007/BF00583467. [DOI] [PubMed] [Google Scholar]
- Wade OL, Bishop JM. Cardiac Output and Regional Blood Flow. Oxford: Blackwell; 1962. [Google Scholar]
- Wilson DF, Erecinska M, Drown C, Silver IA. The oxygen dependence of cellular energy metabolism. Archives of Biochemistry and Biophysics. 1979;195:485–493. doi: 10.1016/0003-9861(79)90375-8. [DOI] [PubMed] [Google Scholar]
- Yaar M, Gilchrest BA. Aging of skin. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, Fitzpatrick TB, editors. Fitzpatrick's Dermatology in General Medicine. 5. New York: McGraw-Hill; 1999. pp. 1697–1706. [Google Scholar]


