Abstract
Transepithelial anion secretion in many tissues depends upon the activity of basolateral channels. Using monolayers of the Calu-3 cell line, a human submucosal serous cell model mounted in an Ussing chamber apparatus, we investigated the nature of the K+ channels involved in basal, cAMP- and Ca2+-stimulated anion secretion, as reflected by the transepithelial short circuit current (Isc). The non-specific K+ channel inhibitor Ba2+ inhibited the basal Isc by either 77 or 16 % when applied directly to the basolateral or apical membranes, respectively, indicating that a basolateral K+ conductance is required for maintenance of basal anion secretion. Using the K+ channel blockers clofilium and clotrimazole, we found basal Isc to be sensitive to clofilium, with a small clotrimazole-sensitive component. By stimulating the cAMP and Ca2+ pathways, we determined that cAMP-stimulated anion secretion was almost entirely abolished by clofilium, but insensitive to clotrimazole. In contrast, the Ca2+-stimulated response was sensitive to both clofilium and clotrimazole. Thus, pharmacologically distinct basolateral K+ channels are differentially involved in the control of anion secretion under different conditions. Isolation of the basolateral K+ conductance in permeabilized monolayers revealed a small basal and forskolin-stimulated Isc. Finally, using the reverse transcriptase-polymerase chain reaction, we found that Calu-3 cells express the K+ channel genes KCNN4 and KCNQ1 and the subunits KCNE2 and KCNE3. We conclude that while KCNN4 contributes to Ca2+-activated anion secretion by Calu-3 cells, basal and cAMP-activated secretion are more critically dependent on other K+ channel types, possibly involving one or more class of KCNQ1-containing channel complexes.
Human airway serous cells, found within submucosal glands, are of fundamental importance in maintaining a sterile environment within the lung. In addition to secreting a variety of antimicrobial factors (Basbaum et al. 1990), they contribute to the quantity and composition of the airway surface liquid, both of which have been implicated in primary host defence mechanisms (Smith et al. 1996; Wine, 1999; Boucher, 1999). Serous cells are also the principal pulmonary site of expression of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel (Engelhardt et al. 1992; Jacquot et al. 1993), mutations in which cause cystic fibrosis (CF; Riordan et al. 1989). CFTR is a cAMP-dependent channel located in the apical membrane of a variety of epithelial cell types where it acts as the predominant Cl− conductance (Pilewski & Frizzell, 1999). Precisely how absence of functional CFTR in the airway leads to the devastating pulmonary pathology associated with CF remains unclear. However, the high degree of expression of CFTR in the serous cell makes it likely that the function of these cells is among the most affected in CF. Therefore, understanding fluid and electrolyte transport in serous cells provides important information as to the role of CFTR in normal airway physiology and may lead to meaningful insights into the pathogenesis of CF lung disease.
The Calu-3 cell line, originally derived from a lung adenocarcinoma (Shen et al. 1994), has been widely used as a model of the serous cell (Haws et al. 1994; Moon et al. 1997; Lee et al. 1998; Devor et al. 1999; Pilewski & Frizzell, 1999). Calu-3 cells express high levels of CFTR (Shen et al. 1994), form polarized monolayers with a transepithelial resistance of approximately 100 Ω cm2 (Shen et al. 1994), express markers of serous cell function such as lysozyme, lactoferrin and secretory leukocyte protease inhibitor (Finkbeiner et al. 1993) and demonstrate active transepithelial anion secretion in response to a number of pharmacological stimuli, including those which raise intracellular cAMP or Ca2+ concentrations (Shen et al. 1994; Moon et al. 1997). While it appears that the anion secreted may be either Cl− (Shen et al. 1994; Singh et al. 1997; Devor et al. 1999) or HCO3− (Illek et al. 1997; Lee et al. 1998; Devor et al. 1999) depending upon conditions, in both cases the final step appears to be electrodiffusional anion exit across the apical membrane via CFTR (Illek et al. 1997; Lee et al. 1998; Devor et al. 1999).
A number of studies have demonstrated that the rate of transepithelial anion secretion is dependent upon the activity of basolateral K+ channels, since exit of K+ across the basolateral membrane hyperpolarizes the cell, increasing the electrochemical driving force for anion efflux through open apical membrane channels (Smith & Frizzell, 1984; McCann & Welsh, 1990; Devor et al. 1996). A similar mechanism has been proposed in Calu-3 cells (Moon et al. 1997; Devor et al. 1999); however, despite this potentially important role, little is known about the nature of these basolateral K+ channels or their role in controlling transepithelial anion secretion. We therefore wished to investigate how different basolateral K+ channels may influence the cAMP- and Ca2+-mediated changes in transepithelial ion transport observed in Calu-3 cells. We investigated the effects of known epithelial K+ channel inhibitors on the Isc recorded across monolayers of Calu-3 cells under both basal and stimulated conditions. Furthermore, using RT-PCR we have identified a number of K+ channel genes and modifying channel subunits which may be integrally involved in anion secretion across these epithelial cells.
METHODS
Culture of Calu-3 cells
Cells were purchased from the American Type Culture Collection (Rockville, MD, USA) at passage 18. All experiments were performed on cells following an additional 5–12 passages (i.e. final passage number between 23 and 30). They were maintained in 1:1 Dulbecco's modified Eagle's medium-Ham's F-12 nutrient mixture supplemented with 10 % fetal bovine serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 0.25 μg ml−1 fungizone (all from Gibco BRL, Burlington, ON, Canada) and incubated at 37 °C in a humidified atmosphere containing 5 % CO2. For RNA extraction, cells were grown to confluence in 100 mm diameter Falcon tissue culture dishes (Becton Dickinson, Franklin Lakes, NJ, USA). For measurements of transepithelial short circuit current (Isc), cells were plated onto 12 mm diameter (1.1 cm2 surface area) Snapwell inserts (Corning Costar, Cambridge, MA, USA). Initially cells were grown submerged in culture medium. However after 3 days of culture, cells were grown at an air-liquid interface, i.e. medium was present only on the basolateral side of the inserts, as previously described (Singh et al. 1997; Moon et al. 1997). Culture medium was changed every 48 h, and the cells formed a confluent monolayer that held back fluid. Experiments were performed 10–26 days after establishment of the air-liquid interface.
Measurement of transepithelial short-circuit current (Isc)
Calu-3 cells grown on Snapwell inserts were mounted in an Ussing chamber (World Precision Instruments (WPI), Sarasota, FL, USA), and the transepithelial potential difference clamped to zero using DVC-1000 voltage-clamp apparatus (WPI). The Isc was recorded using Ag-AgCl electrodes in 3 m KCl agar bridges. Data was sampled at 10 Hz and recorded to computer disk using custom-written software (courtesy of Dr A. S. French). Transepithelial resistance was calculated using Ohm's law by determining the change in current arising from applying 0.4 mV pulses every 50 s. Apical and basolateral solutions were maintained at 37 °C by heated water jackets, and were separately perfused and oxygenated with a 95 % O2 : 5 % CO2 mixture. The bath solutions were (mm): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, 10 glucose or mannitol held at pH 7.4 at 37 °C, when gassed with 5 % CO2. Glucose was present in the basolateral bath solution, but was substituted with mannitol in the apical solution to eliminate the activity of the Na+-glucose transporter (Singh et al. 1997). Monolayers were equilibrated in these buffers for 30 min before the experiment commenced.
Permeabilized monolayers
To investigate the activity of basolateral K+ channels in isolation, the apical membrane was permeabilized by the addition of 10 μm of the pore-forming antibiotic amphotericin B, (Sigma, Oakville, ON, Canada) in Cl−-free buffers. The following bath solutions were used. Apical (mm): 145 potassium gluconate, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 magnesuim gluconate, 4 calcium gluconate, 10 glucose, 10 Hepes. Basolateral (mm): 145 sodium gluconate, 3.3 NaH2PO4, 0.8 Na2HPO4, 1.2 magnesuim gluconate, 4 calcium gluconate, 10 glucose, 10 Hepes. Solutions were adjusted to pH 7.4 at 37 °C. By permeabilizing the apical membrane in the presence of these buffers, a mucosal to serosal K+ gradient is established and the Isc reflects K+ movement through basolateral K+ channels down a concentration gradient.
RNA extraction
Total RNA was extracted from Calu-3 cells using TRIzol reagent (Gibco). RNA was then DNase treated with RQ1 RNase-free DNase (Promega, Madison, WI, USA) and the product run on a 1 % agarose gel to check integrity. Two micrograms of DNase-treated RNA was then reverse transcribed using M-MLV reverse transcriptase (Gibco) in the presence of 5 mm dNTP and 1 μm oligo dT (Amersham Pharmacia, Baie d'Urfe, PQ, Canada) to produce cDNA.
Polymerase chain reaction
After reverse transcription, polymerase chain reaction (PCR) was performed to amplify DNA fragments using the primers and conditions described in Table 1. The primers and conditions used for KCNN4 and KCNQ1 have been described previously (Warth et al. 1999; Mall et al. 2000). Primers for KCNE1, KCNE2, KCNE3, KCNH2 and CFTR were designed using the published sequences for these genes available from the National Center for Biotechnology Information (NCBI). All custom primers were obtained from Gibco. PCR was performed using primer pairs at 10 μm with 2.5 units Taq polymerase (MBI Fermentas, Burlington, ON, Canada), 25 mm MgCl2 and 5 mm dNTP in a total reaction volume of 25 μl. PCR products were visualized by loading a 8 μl sample on a 1.5 % agarose gel containing 250 μg l−1 ethidium bromide, alongside a 100 bp DNA ladder (Gibco).
Table 1.
Primer sequences, expected size of the RT-PCR product, and PCR conditions for KCNE2, KCNE3, KCNQ1, KCNN4 and CFTR
| Primer | Expected size (bp) | Conditions (denaturation; annealing; extension) | |
|---|---|---|---|
| KCNE2 | 5′-TCTTCCGAAGGATTTTTATTACTTAT-3′ | 304 | 95 °C/1 min; |
| 5′-ACCATCCATGAGAACATTGGT-3′ | 58 °C/1 min; | ||
| 72 °C/2 min; 30 cycles | |||
| KCNE3 | 5′-TACCAATGGAACGGAGACCT-3′ | 264 | 95 °C/1 min; |
| 5′-GGGTCACTACGCTTGTCCAC-3′ | 58 °C/1 min; | ||
| 72 °C/2 min; 30 cycles | |||
| KCNN4* | 5′-CGGCGTCCTGCTCAACG-3′ | 337 | 95 °C/1 min; |
| 5′-CACCAGCAGGGCTGTGCAG-3′ | 62°C/30 s; | ||
| 72 °C/90 s; 30 cycles | |||
| KCNQ1† | 5′-TTCTGGATGGAGATCGTG-3′ | 738 | 95 °C/30 s; |
| 5′-GCCTTCCGGATGTAGATC-3′ | 63 °C/1 min; | ||
| 72 °C/1 min; 35 cycles | |||
| CFTR | 5′-CAAGGAGGAACGCTCTATCG-3′ | 326 | 95 °C/1 min; |
| 5′-ACGCCTGTAACAACTCCCAG-3′ | 58 °C/1 min; | ||
| 72 °C/2 min; 30 cycles |
DNA sequencing
To confirm the identity of the amplified PCR fragments, these products were isolated from the gel using the QIAquick gel extraction kit (Qiagen, Mississauga, ON, Canada). DNA was then ligated into the pGEM vector (Promega, Madison, WI, USA), propagated in E. coli strain JM109 and sequenced using the Sequenase DNA sequencing kit (USB, Cleveland, OH, USA).
Chemicals
Forskolin, clotrimazole, amphotericin B, 1-ethyl-2-benzimidazolinone (1-EBIO), 8-bromoadenosine 3′,5′-cyclic monophosphate (8-bromo-cAMP) and thapsigargin were obtained from Sigma Aldrich (Oakville, ON, Canada). Clofilium was purchased from RBI (Natick, MA, USA). Stock solutions were made up in either DMSO or ethanol (clotrimazole) so that the final bath concentration of DMSO was ≤ 0.1 %. Application of DMSO or ethanol alone had no effect upon the monolayers.
Statistics
All data are presented as means ± standard error of the mean. Differences between groups were tested for statistical significance using Student's t test, with significance determined as P < 0.05.
RESULTS
Effect of Ba2+ ions on anion secretion in Calu-3 monolayers
The basal Isc in intact monolayers of Calu-3 cells was 30.2 ± 1.6 μA cm−2 (range, 11.8–65.4 μA cm−2) with an initial resistance of 164.7 ± 7.4 Ω cm2 (n = 41). Basal Isc in these cells has previously been demonstrated to be almost exclusively accounted for by anion secretion (Singh et al. 1997). Basal Isc was decreased by the addition of the non-specific K+ channel inhibitor Ba2+ confirming the importance of K+ channels in transepithelial anion secretion. Furthermore, Ba2+ was more effective when applied to the basolateral, rather than the apical, side of the monolayer. When 5 mm Ba2+ was added to the basolateral membrane, basal Isc was inhibited by 77.1 ± 3.1 % (n = 5, Fig. 1A) whereas addition to the apical membrane inhibited only 15.7 ± 2.2 % (n = 6, Fig. 1B) of the Isc.
Figure 1. Inhibition of short circuit current (Isc) across Calu-3 monolayers by Ba2+.
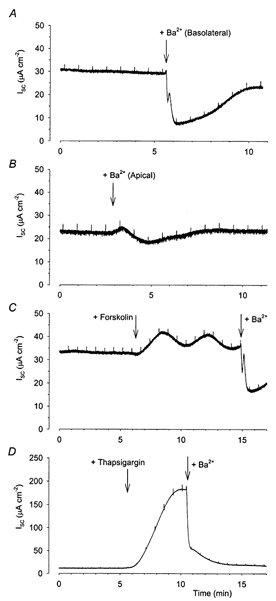
Basal Isc in Calu-3 monolayers was inhibited by the addition of 5 mm Ba2+, a non-specific inhibitor of K+ channels, to both the basolateral (A) and apical (B) membranes. Forskolin- and thapsigargin-stimulated Isc were also inhibited by basolateral application of Ba2+ (C and D). The transient inhibition frequently seen following the addition of Ba2+ was due to precipitation of Ba2+ from solution as previously reported (Moon et al. 1997).
Since basolateral K+ channels appear to play a more significant role than apical channels in the maintenance of basal Isc in Calu-3 cells, we further examined the role of basolateral K+ channels in cAMP- and Ca2+-stimulated anion secretion. The addition of 2 μm of the cAMP activator forskolin to both apical and basolateral sides of the monolayer resulted in an increased Isc (mean increase 23.2 ± 2.6 μA cm−2; range, 10.7–37.9 μA cm−2; n = 16, Fig. 1C), as previously reported (Shen et al. 1994). Further additions of forskolin had no effect upon the Isc recorded (results not shown). The addition of 300 nm thapsigargin to both sides of the monolayer resulted in a much larger increase in Isc (129.9 ± 20.8; range, 86.6–197.2 μA cm−2; n = 7, Fig. 1D), again confirming previous work (Moon et al. 1997). Thapsigargin produces a sustained increase in the intracellular Ca2+ concentration by inhibiting the sarcoplasmic reticulum Ca2+-ATPase. The increased Isc recorded from Calu-3 cells upon addition of forskolin and thapsigargin has previously been demonstrated to reflect increased anion secretion (Shen et al. 1997; Moon et al. 1997; Devor et al. 1999). Addition of Ba2+ to the basolateral side of the membrane inhibited both the forskolin- and thapsigargin-stimulated Isc, indicating a role for basolateral K+ channels in cAMP- and Ca2+-stimulated secretion (Fig. 1C and D).
Pharmacological characterization of basolateral K+ channels in intact Calu-3 monolayers
To investigate further the nature of the basolateral K+ channels responsible for maintenance of anion secretion, we examined the effects of the K+ channel inhibitors clofilium and clotrimazole. The addition of 100 μm clofilium, described at this concentration as an inhibitor of epithelial basolateral K+ channels in the rat trachea (Hwang et al. 1996), mouse trachea (Lock & Valverde, 2000) and mouse colon and nose (MacVinish et al. 1998), to the basolateral membrane significantly reduced the basal Isc (mean decrease of 7.9 ± 1.0 μA cm−2 corresponding to 27.2 ± 2.9 % of the baseline, n = 6; Fig. 2A and C). Addition of 30 μm of the antifungal agent clotrimazole, an inhibitor of Ca2+-activated K+ channels (Devor et al. 1996, 1999), to the basolateral membrane resulted in a much smaller decrease in basal Isc (2.3 ± 0.3 μA cm−2 or 11.3 % of the baseline, n = 8; Fig. 2B and C). A series of preliminary experiments confirmed that these concentrations of clofilium and clotrimazole were maximally effective, and that further addition of the agent produced no further inhibition (results not shown). These results suggest that clofilium-sensitive K+ channels play a larger role in maintaining basal Isc than clotrimazole-sensitive channels.
Figure 2. Inhibition of the basal Isc in Calu-3 cells by clofilium and clotrimazole.
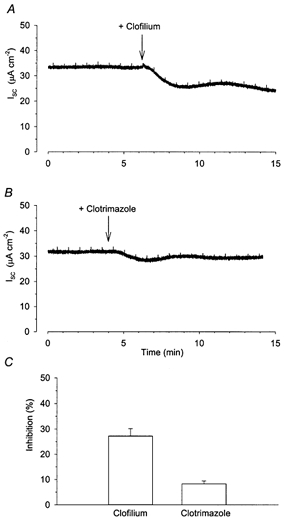
Addition of 100 μm clofilium to the basolateral membrane of Calu-3 cells reduced the basal Isc (A, n = 6). Addition of 30 μm clotrimazole to the basolateral membrane resulted in a much smaller decrease in basal Isc (B, n = 8). C shows the percentage of basal Isc inhibited by these agents.
cAMP stimulation
To characterize pharmacologically the basolateral K+ channel types involved in the cAMP secretory response, we next investigated the effects of clofilium and clotrimazole on the forskolin-stimulated Isc. In the first series of experiments (Fig. 3) cells were stimulated with 2 μm forskolin prior to the application of these different K+ channel blockers. The maximally effective concentration of clofilium (100 μm) caused a mean decrease in the forskolin-stimulated Isc of 21.1 ± 2.3 μA cm−2 (n = 8, Fig. 3A). In contrast, a maximally effective concentration of clotrimazole (30 μm) produced a much smaller reduction in the forskolin-stimulated Isc of only 3.5 ± 0.7 μA cm−2 (n = 8; Fig. 3B). As the amplitude of the response to forskolin was variable, 5 mm Ba2+ was added to the basolateral side of the monolayer at the end of the experiment and results expressed as the effect on the Ba2+-sensitive Isc. Following forskolin stimulation, clofilium blocked 56.2 ± 4.3 % (n = 7) of the Ba2+-sensitive Isc, while clotrimazole blocked only 16.7 ± 2.6 % (n = 8; Fig. 3C).
Figure 3. Effects of clofilium and clotrimazole on the cAMP-stimulated increase in Isc in Calu-3 cells.
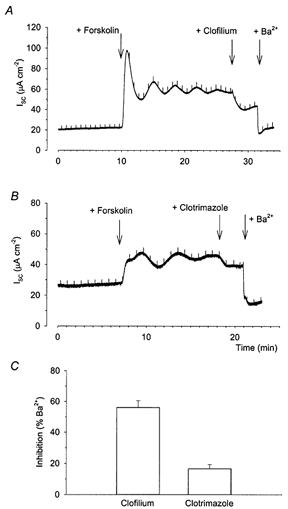
When Calu-3 monolayers were stimulated with 2 μm forskolin applied to both apical and basolateral sides of the membrane, the subsequent addition of 100 μm clofilium to the basolateral membrane reduced the Isc (A, n = 8). Addition of 30 μm clotrimazole to the basolateral membrane (B, n = 8) resulted in a much smaller decrease in Isc. At the end of every experiment 5 mm Ba2+ was added to the basolateral membrane, which further inhibited the Isc. C, reduction in Isc caused by clofilium and clotrimazole expressed as a percentage of the Ba2+-inhibitable Isc (as described in the text).
In an attempt to dissociate whether the decreases observed in forskolin-stimulated Isc with clofilium and clotrimazole (Fig. 3) were primarily due to inhibition of the response to forskolin or merely reflected a concurrent inhibition of the basal Isc (Fig. 1), a parallel series of experiments was performed in which the inhibitor was added prior to the forskolin stimulus (Fig. 4). When 100 μm clofilium was added to the basolateral membrane of the Calu-3 monolayer, the subsequent response to forskolin was almost completely abolished (Fig. 4B and D; the mean increase with forskolin addition was 2.1 ± 2.1 μA cm−2, n = 3). In contrast, pretreatment with clotrimazole did not significantly alter the response to forskolin (Fig. 4C and D; mean increase was 13.7 ± 4.9 μA cm−2, n = 3). This suggests that the forskolin-stimulated increase in Isc is more dependent upon the activity of clofilium-sensitive, rather than clotrimazole-sensitive, K+ channels.
Figure 4. Effect of prior treatment with clofilium or clotrimazole on the cAMP-stimulated increase in Isc in Calu-3 cells.
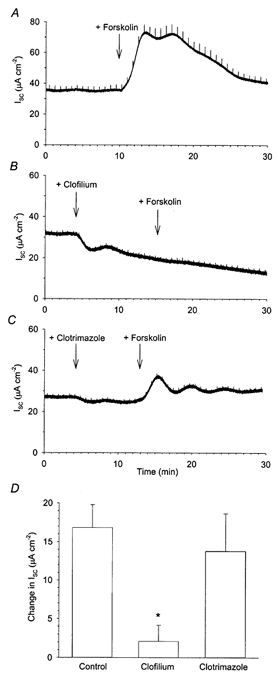
Addition of 2 μm forskolin to Calu-3 monolayers produced an increase in Isc (A, n = 16). When 100 μm clofilium was applied to the basolateral membrane prior to the forskolin stimulus, the increase in Isc was virtually abolished (B and D). In contrast, the increase in Isc recorded following the application of 2 μm forskolin when 30 μm clotrimazole had been pre-applied to the monolayers was not significantly different from the forskolin-alone response (* significantly different from forskolin stimulated as determined by Student's t test; P < 0.05).
Ca2+ stimulation
A corresponding series of experiments was then performed examining the effects of the above K+ channel blockers on the thapsigargin-stimulated Isc. Figure 5A shows a representative example of the large increase in Isc recorded when 300 nm thapsigargin was applied to a monolayer. Unfortunately, due to the transient nature of the response to thapsigargin stimulation, we determined that it was not feasible to quantify the inhibition of this response by subsequent addition of K+ channel blockers. For this reason, and to avoid ambiguity concerning the effects of blockers on basal versus Ca2+-activated Isc, clofilium and clotrimazole were applied prior to the thapsigargin stimulus.
Figure 5. Thapsigargin stimulation of Isc in Calu-3 monolayers.
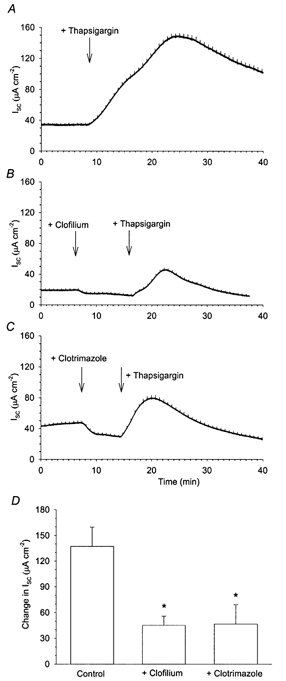
Addition of 300 nm thapsigargin to Calu-3 monolayers produced a large increase in Isc (A; n = 7). When 100 μm clofilium was applied to the basolateral membrane prior to the thapsigargin stimulus, there was a large increase in Isc, though it was significantly reduced from the thapsigargin-alone response (B and D). Addition of 30 μm clotrimazole prior to the thapsigargin stimulus also significantly reduced the amplitude of the thapsigargin response (C and D). * Significantly different from thapsigargin-stimulated Isc as determined by Student's t test (P < 0.05).
After the addition of clofilium or clotrimazole, (Fig. 5B and C) thapsigargin still induced a large increase in Isc, though it was significantly smaller than control. Following clofilium pretreatment, the mean increase in Isc was 45.2 ± 10.6 μA cm−2 (n = 3) while clotrimazole pretreatment reduced the thapsigargin-stimulated increase in Isc to 46.6 ± 10.6 μA cm−2 (n = 3). These results indicate that both clofilium- and clotrimazole-sensitive channels appear to contribute towards the thapsigargin-stimulated increase in Isc.
Effect of 1-EBIO
To investigate the pharmacological specificity of clofilium and clotrimazole, 1-ethyl-2-benzimidazolinone (1-EBIO) was used as a stimulus for anion secretion. This agent has previously been reported to produce large increases in the Isc when applied to Calu-3 and human bronchial epithelial cells (Devor et al. 1999, 2000) by concurrently activating CFTR and basolateral Ca2+-activated K+ channels. Indeed, when 1 mm 1-EBIO was applied to intact monolayers, it caused a large sustained increase in Isc (190.6 ± 30.1 μA cm−2, n = 3; Fig. 6A). Application of 100 μm clofilium had no effect upon the response to 1-EBIO, while 30 μm clotrimazole almost completely abolished the secretory response (Fig. 6B; n = 3). This demonstrates that, at the concentrations used, clofilium has no effect upon the activity of 1-EBIO-sensitive Ca2+-activated K+ channels, while clotrimazole causes a near-total inhibition of these channels (Devor et al. 1999).
Figure 6. Clotrimazole, but not clofilium, inhibits the 1-EBIO stimulation of Isc in Calu-3 cells.
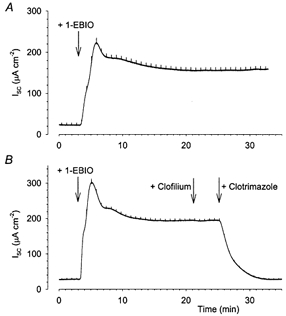
1-EBIO (1 mm) produced a large increase in Isc (A, n = 3), which was unaffected by the subsequent addition of 100 μm clofilium, but entirely abolished by the addition of 30 μm clotrimazole (B).
Isolation of basolateral K+ channel currents
To investigate further the nature of the basolateral channels involved in anion secretion from Calu-3 cells, a series of experiments examined the K+ conductance of the basolateral membrane in isolation. For these experiments, the apical membrane was permeabilized by the addition of 10 μm amphotericin B, in the presence of a mucosal-to-serosal K+ gradient and monolayers equilibrated for at least 20 min (see Methods). The basal Isc recorded under these conditions which presumably reflects basolateral K+ current was 5.6 ± 1.5 μA cm−2 (n = 12). The addition of 2 μm forskolin produced a small increase in the Isc (mean increase 2.5 ± 0.5 μA cm−2; n = 8 Fig. 7A). The subsequent addition of 100 μm 8-bromo cAMP, to increase the intracellular cAMP levels directly, had no further effect upon the Isc, indicating that the cAMP pathway was maximally stimulated (results not shown). Since the basal and forskolin-stimulated basolateral Isc were so small, it proved impossible to determine the effects of clofilium and clotrimazole on these currents. In contrast, the addition of 1 mm 1-EBIO produced a large increase in Isc (51.4 ± 8.6 μA cm−2, n = 3; Fig. 7B) which was insensitive to clofilium and almost entirely blocked by 30 μm clotrimazole (Fig. 7C).
Figure 7. Basolateral K+ conductances in permeabilized Calu-3 cells.
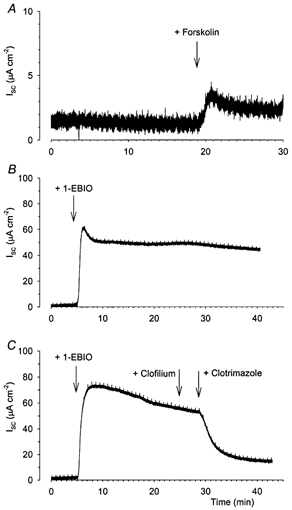
The basolateral K+ conductance in Calu-3 cells was isolated by permeabilizing the apical membrane. A small basal Isc was recorded under these conditions and the addition of 2 μm forskolin induced a small increase in Isc (A, n = 8) while the addition of 1 mm 1-EBIO induced a much larger increase in Isc (B, n = 3). This 1-EBIO-stimulated response in the permeabilized monolayers was unaffected by the addition of 100 μm clofilium, but entirely abolished by the addition of 30 μm clotrimazole (C).
Molecular characterization of the K+ channels in Calu-3 cells
By performing RT-PCR on total RNA extracted from cultures of Calu-3 cells, we were able to detect fragments of the K+ channel genes KCNN4 and KCNQ1, and the K+ channel subunit genes KCNE2 and KCNE3 (Fig. 8). Additionally we performed RT-PCR using primers specific for CFTR, known to be abundant in these cells. Fragments were not detected without reverse transcription (results not shown). Despite several attempts at a variety of PCR conditions, we were unable to detect KCNE1, or KCNH2. When bands were detected using RT-PCR, the DNA was excised, subcloned into the pGEM vector (Promega, Madison, WI, USA) and sequenced. Comparison of the sequenced product with published sequences (National Center for Biotechnology Information) confirmed the expression of KCNE2, KCNE3, KCNN4 and KCNQ1 in Calu-3 cells.
Figure 8. PCR analysis on RNA extracted from Calu-3 cells.
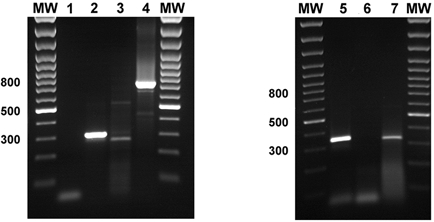
Transcripts were detected for KCNE2 (lane 2), KCNE3 (lane 3), KCNQ1 (lane 4), KCNN4 (lane 5) and CFTR (lane 7) but not KCNE1 (lane 1) or KCNH2 (lane 6).
DISCUSSION
Submucosal gland serous cells are the predominant site of CFTR expression in the human lung (Engelhardt et al. 1992; Jacquot et al. 1993). The fluid secreted from these cells is integrally involved in the primary host defence mechanisms of the lung, since it contains a host of potent antimicrobial factors (Basbaum et al. 1990) and also provides a low viscosity medium for effective mucociliary clearance of debris from the airways (Luk & Dulfano, 1983). It is a logical conclusion that the loss of functional CFTR Cl− channels which occurs in CF will have a profound effect upon the activity of the serous cell. Indeed, it has been proposed that it is the dysfunction of this cell type that is the primary trigger for the pathogenesis of CF lung disease (Jiang et al. 1997; Pilewski & Frizzell, 1999). Loss of CFTR from the serous cell could profoundly affect the quantity and/or the composition of glandular secretions, resulting in impaired host defence mechanisms. Knowledge of fluid and electrolyte movement in these cells is therefore important to improve our understanding of normal airway host defence and how this may be impaired in CF. The results of the present study demonstrate the importance of distinct basolateral K+ channels in controlling anion secretion from a well-characterized model of the serous cell, Calu-3 cells, under different conditions. Furthermore, we identify at the molecular level a number of different K+ channels and modifying subunits in Calu-3 cells which may account for the differential effects observed.
The Calu-3 cell line has become a widely used and accepted model of the human serous cell (Haws et al. 1994; Shen et al. 1994; Singh et al. 1997; Devor et al. 1999; Pilewski & Frizzell, 1999). Calu-3 cells exhibit electrogenic transepithelial anion (Cl− and/or HCO3−) secretion under basal conditions (Singh et al. 1997) which is further stimulated by increases in intracellular cAMP or Ca2+ concentrations (Shen et al. 1994; Moon et al. 1997; Devor et al. 1999), consistent with the proposed value of these cells as models of serous cell secretion. Anion secretion occurs via cAMP-activated CFTR Cl− channels at the apical membrane (Haws et al. 1994; Moon et al. 1997; Illek et al. 1999), but also depends on the activity of K+ channels. Our finding that the non-specific K+ channel inhibitor Ba2+ produced a much larger decrease in basal Isc when applied to the basolateral versus the apical membrane (Fig. 1) confirms the importance of basolateral K+ channels in anion secretion. The opening of K+ channels presumably hyperpolarizes the cell, increasing the electrochemical driving force for anion secretion across the apical membrane. In our model system, whether these channels are located on the apical or basolateral membrane would have little effect on their ability to hyperpolarize the cell; however, our current understanding of transepithelial anion secretion in vivo is based upon a model in which K+ recycling across the basolateral membrane plays a central role in setting the electrochemical driving force for anion secretion (Smith & Frizzell, 1984; McCann & Welsh, 1990; Dawson & Richards, 1990; Cotton, 2000). While our results suggest that Calu-3 cells may possess some apical K+ channels, the basolateral K+ channels appear to play a much more important role, consistent with the prevailing model. Furthermore, it appears that pharmacologically distinct basolateral K+ channels differentially influence anion secretion under different conditions. Thus, the basal Isc is more sensitive to clofilium than clotrimazole (Fig. 2), the cAMP-stimulated Isc is almost completely abolished by clofilium but is clotrimazole insensitive (Fig. 4), while the Ca2+-stimulated Isc is partially blocked by both clofilium and clotrimazole (Fig. 5).
An important requirement for us to conclude that different K+ channels are responsible for the observed differential effects on basal, cAMP-and Ca2+-stimulated Isc is the pharmacological specificity of clofilium and clotrimazole. The specificity of these blockers at the concentrations used is demonstrated by their effects upon the 1-EBIO-stimulated response, resulting from the activation of basolateral Ca2+-dependent K+ channels as previously described by Devor et al. (1999). Thus, the response to 1-EBIO, both in intact (Fig. 6B) and permeabilized monolayers (Fig. 7C), is clofilium insensitive and almost entirely inhibited by 30 μm clotrimazole.
Despite the large role played by clofilium-sensitive K+ channels in supporting the basal (Fig. 2) and cAMP-stimulated (Fig. 3 and Fig. 4) Isc, only small basal and cAMP-activated basolateral K+ conductances could be identified in permeabilized monolayers (Fig. 7A). In contrast, a much larger 1-EBIO-activated K+ conductance, which was sensitive to clotrimazole but not clofilium, was observed under these conditions (Fig. 7B), suggesting that the majority of the basolateral K+ conductance can be accounted for by Ca2+-activated channels. Due to the small amplitude of the basal and cAMP-stimulated Isc, it was not possible to determine the pharmacological properties of the underlying K+ channels. However, despite its small size, this conductance would appear to play a pivotal role in transepithelial anion secretion, in particular that stimulated by cAMP. The observation by ourselves and others (Shen et al. 1994; Moon et al. 1997; Devor et al. 1999) that forskolin increases anion secretion in Calu-3 cells could theoretically result either from direct cAMP-stimulation of apical anion channels, or as an indirect consequence of increased K+ conductance enhancing anion efflux via already open channels. Calu-3 cells possess a significant resting Cl− conductance, identified as CFTR, which is further increased upon forskolin addition (Shen et al. 1994; Haws et al. 1994). We here identify that Calu-3 cells possess a significant resting basolateral K+ conductance that is also increased by forskolin. However, we are unable to determine whether the forskolin-stimulated increase in basolateral K+ conductance is sufficient or required for the forskolin-stimulated increase in Isc. Nevertheless, our finding that pretreatment with clofilium virtually abolishes the forskolin response in these cells (Fig. 4B) suggests that this basolateral K+ conductance, though small, does play a significant role in the forskolin-stimulated Isc. One interesting possibility is that cAMP causes a parallel increase in the apical anion and basolateral K+ conductances, maximizing the secretory response.
The differential effects of clofilium and clotrimazole suggests the presence of at least two distinct classes of basolateral K+ channels controlling anion secretion from Calu-3 cells. Using RT-PCR, we detected expression of the K+ channel genes KCNN4 and KCNQ1 in these cells (Fig. 8). KCNN4 encodes an intermediate-conductance Ca2+-activated K+ channel (Ishii et al. 1997; Joiner et al. 1997; Logsdon et al. 1997), activated by 1-EBIO and inhibited by clotrimazole (Jensen et al. 1998), and which has previously been suggested to form the basolateral Ca2+-activated K+ conductance of Calu-3 cells (Devor et al. 1999). KCNQ1 encodes the KvLQT K+ channel protein, which coassembles with the small accessory subunit minK (encoded by the KCNE1 gene) to form a slow voltage-gated cardiac K+ channel, mutated in long QT syndrome type 1 (Suessbrich & Busch 1999; Sanguinetti, 2000). KCNQ1 also coassembles with the related subunits MiRP1 (encoded by the KCNE2 gene) to form a constitutively open K+ channel (Tinel et al. 2000), and MiRP2 (encoded by KCNE3; Schroeder et al. 2000). Coassembly of KCNQ1 and KCNE3 gene products may form the cAMP-activated basolateral K+ channel of intestinal epithelial cells (Schroeder et al. 2000; Kunzelmann et al. 2001). We detected KCNE2 and KCNE3, but not KCNE1, in Calu-3 cells (Fig. 8), suggesting the possibility that Calu-3 cells may express different KCNQ1-containing K+ channel complexes with distinct biophysical properties. KCNQ1 has previously been suggested to contribute to airway epithelial basolateral K+ conductance (Mall et al. 2000) and forms channels which are sensitive to clofilium (Honoré et al. 1991; Suessbrich & Busch, 1999; Warth & Bleich, 2000). KCNE2 also coassembles with the cardiac K+ channel HERG (encoded by the gene KCNH2) to form a clofilium-sensitive, voltage-gated K+ channel (Suessbrich et al. 1997; Abbott et al. 1999). However, we were unable to detect KCNH2 in Calu-3 cells (Fig. 8). It must be stressed that our inability to detect KCNE1 or KCNH2 by RT-PCR, despite multiple attempts under different conditions, does not unequivocally rule out the possibility that these genes are expressed by Calu-3 cells. Furthermore, this study is by no means a comprehensive investigation of K+ channel expression in this cell line. For example, recent studies have indicated an extensive complement of K+ channel types and subunits in rat pituitary cells (Wulfsen et al. 2000) and it is not unlikely that K+ channels exist in Calu-3 cells in addition to those identified here. However, our positive identification of KCNE2, KCNE3, KCNN4 and KCNQ1, following isolation and partial sequencing of these gene fragments, demonstrates that these genes are expressed in Calu-3 cells, and that their gene products could potentially contribute to basolateral K+ conductances involved in the control of anion secretion.
The large clotrimazole and 1-EBIO-sensitive, clofilium-insensitive, K+ conductance (Fig. 7) we observed is most probably accounted for by KCNN4 (Fig. 8) as previously proposed (Devor et al. 1999). This channel appears to play a role in Ca2+-activated secretion (Fig. 5) but does not appear to contribute to the cAMP-activated Isc (Fig. 4). We also demonstrated a second K+ conductance, carried by a clofilium-sensitive channel, which is involved in basal (Fig. 2) and cAMP-stimulated (Fig. 3 and Fig. 4) secretion and which interestingly also appears to contribute toward Ca2+-stimulated secretion (Fig. 5). Our finding that Calu-3 cells express KCNQ1 make this a possible candidate for forming the clofilium-sensitive basolateral K+ conductance, since KCNQ1 forms clofilium-sensitive basally active and/or cAMP-stimulated channels (Honoré et al. 1991; Suessbrich & Busch, 1999; Warth & Bleich, 2000; Schroeder et al. 2000; Tinel et al. 2000) Furthermore, we also find that Calu-3 cells express KCNE2 and KCNE3, both of which coassemble with KCNQ1 and regulate its biophysical properties (Schroeder et al. 2000; Tinel et al. 2000). Given the essential role of clofilium-sensitive K+ channels in the cAMP-stimulated Isc in Calu-3 cells (Fig. 5) it is tempting to suggest that KCNQ1-KCNE3 may serve a similar role in airway epithelia to that described in colonic epithelia (Schroeder et al. 2000), though we have no functional evidence of the role of KCNE2 or KCNE3.
Cultured submucosal gland cells secrete fluid in response to agents which stimulate cAMP- or Ca2+ signalling pathways (Yamaya et al. 1991). As previously described, we find that Calu-3 cells exhibit both cAMP and Ca2+-dependent increases in Isc (Figs 1, 3–5; Shen et al. 1994; Moon et al. 1997). The present work emphasizes the pivotal role of basolateral K+ channels in secretion by Calu-3 cells, and demonstrates clearly that distinct K+ channel types support secretion under different conditions. Ca2+-stimulated secretion is dependent, not only on the activity of a clotrimazole-sensitive channel, but also on additional K+ channels sensitive to clofilium. We believe our finding that Calu-3 cells express KCNN4 make this a strong candidate for a clotrimazole-sensitive channel. Conversely, cAMP-stimulated secretion is almost completely dependent on non-clotrimazole-sensitive channels, possibly formed by one or more KCNQ1-containing channels.
Loss of CFTR-mediated secretion from submucosal gland serous cells probably plays a fundamental role in pathogenesis of CF lung disease. Using a well-defined model of the human serous cell, we demonstrate that pharmacologically differential K+ conductances underlie basal, cAMP- and Ca2+-stimulated secretion and describe candidate genes that may be responsible for these channels. The co-ordinated activity of both apical CFTR and basolateral K+ channels is required for normal activity of these cells, thus strategies which aim to circumvent loss of functional CFTR in CF-affected epithelia must also consider the basolateral K+ conductance.
Acknowledgments
We are indebted to Ms Susan Burbridge for expert technical assistance, Dr Gabriel Bertolesi for help with the RT-PCR, Dr Andrew S. French for providing his software for voltage-clamp experiments, and Drs John W. Hanrahan, David H. Eidelman and Melanie Kelly for commenting on the manuscript. This work was supported by the Canadian Cystic Fibrosis Foundation (CCFF) and the Canadian Institutes for Health Research. P. L. is a CCFF scholar.
REFERENCES
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SAN. MiRP1 forms Ikr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Basbaum CB, Berthold J, Finkbeiner WE. The serous cell. Annual Review of Physiology. 1990;52:97–113. doi: 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Molecular insights into the physiology of the ‘thin film’ of airway surface liquid. Journal of Physiology. 1999;516:631–638. doi: 10.1111/j.1469-7793.1999.0631u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton CU. Basolateral potassium channels and epithelial ion transport. American Journal of Respiratory Cell and Molecular Biology. 2000;23:270–272. doi: 10.1165/ajrcmb.23.3.f198. [DOI] [PubMed] [Google Scholar]
- Dawson DC, Richards NW. Basolateral K conductance in regulation of NaCl absorption and secretion. American Journal of Physiology. 1990;259:C181–195. doi: 10.1152/ajpcell.1990.259.2.C181. [DOI] [PubMed] [Google Scholar]
- Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and ΔF508 CFTR-expressing human bronchial epithelia. American Journal of Physiology - Cell Physiology. 2000;279:C461–479. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. American Journal of Physiology. 1996;271:L775–784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Lambert LC, Deluca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. Journal of General Physiology. 1999;133:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nature Genetics. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Finkbeiner WE, Carrier SD, Teresi CE. Reverse transcription-polymerase chain reaction (RT-PCR) phenotypic analysis of cell cultures of human tracheal epithelium, tracheobronchial glands, and lung carcinomas. American Journal of Respiratory Cell and Molecular Biology. 1993;9:547–556. doi: 10.1165/ajrcmb/9.5.547. [DOI] [PubMed] [Google Scholar]
- Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. American Journal of Physiology. 1994;266:L502–512. doi: 10.1152/ajplung.1994.266.5.L502. [DOI] [PubMed] [Google Scholar]
- Honoronoréeacute; E, Attali B, Romey C, Heurteaux C, Ricard P, Lesage F, Lazdunski M, Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO Journal. 1991;10:2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T, Suh D, Bae H, Lee S, Jung J. Characterization of K+ channels in the basolateral membrane of rat tracheal epithelia. Journal of Membrane Biology. 1996;154:251–257. doi: 10.1007/s002329900149. [DOI] [PubMed] [Google Scholar]
- Illek B, Tam AW-K, Fischer H, Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelium. Pflügers Archiv. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- Illek B, Yankaskas JR, Machen TE. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. American Journal of Physiology. 1997;272:L752–761. doi: 10.1152/ajplung.1997.272.4.L752. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proceedings of the National Academy of Sciences of the USA. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. European Respiratory Journal. 1993;6:169–176. [PubMed] [Google Scholar]
- Jensen BS, Strtrøbækoslash;btrøbækaelig;k D, Christophersen P, JØRGENSENoslash;rgensen T D, Hansen C, Silahtaroglu A, Olesen S-P, Ahring PK. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. American Journal of Physiology. 1998;275:C848–856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- Jiang C, Finkbeiner WE, Widdicombe JH, Miller SS. Fluid transport across cultures of human tracheal glands is altered in cystic fibrosis. Journal of Physiology. 1997;501:637–647. doi: 10.1111/j.1469-7793.1997.637bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang L-Y, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Hübner M, Schreiber R, Levy-Holzman R, Garty H, Bleich M, Warth R, Slavik M, Von Hahn T, Greger R. Cloning and functional expression of the rat colonic epithelial K+ channel KVLQT1. Journal of Membrane Biology. 2001;179:155–164. doi: 10.1007/s002320010045. [DOI] [PubMed] [Google Scholar]
- Lee MC, Penland CM, Widdicombe JH, Wine JJ. Evidence that Calu-3 human airway cells secrete bicarbonate. American Journal of Physiology. 1998;274:L450–453. doi: 10.1152/ajplung.1998.274.3.L450. [DOI] [PubMed] [Google Scholar]
- Lock H, Valverde MA. Contribution of the Isk (MinK) potassium channel subunit to regulatory volume decrease in murine tracheal cells. Journal of Biological Chemistry. 2000;275:34849–34852. doi: 10.1074/jbc.C000633200. [DOI] [PubMed] [Google Scholar]
- Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. Journal of Biological Chemistry. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- Luk CKA, Dulfano MJ. Effect of pH, viscosity and ionic strength on ciliary beating efficiency of human bronchial explants. Clinical Science. 1983;64:449–451. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- McCann JD, Welsh MJ. Basolateral K+ channels in airway epithelia. II. Role in Cl secretion and evidence for two types of K channel. American Journal of Physiology. 1990;258:L343–348. doi: 10.1152/ajplung.1990.258.6.L343. [DOI] [PubMed] [Google Scholar]
- MacVinish LJ, Hinkman ME, Mufti DA, Durrington HJ, Cuthbert AE. Importance of basolateral K+ conductance in maintaining Cl− secretion in murine nasal and colonic epithelia. Journal of Physiology. 1998;510:237–247. doi: 10.1111/j.1469-7793.1998.237bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Role of KvLQT1 in cyclic adenosine monophophate-mediated Cl− secretion in human airway epithelia. American Journal of Respiratory Cell and Molecular Biology. 2000;23:283–289. doi: 10.1165/ajrcmb.23.3.4060. [DOI] [PubMed] [Google Scholar]
- Moon S, Singh M, Krouse ME, Wine JJ. Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. American Journal of Physiology. 1997;273:L1208–1219. doi: 10.1152/ajplung.1997.273.6.L1208. [DOI] [PubMed] [Google Scholar]
- Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiological Reviews. 1999;79:S215–255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak J, Zelensli J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FC, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1072. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC. Long QT syndrome: ionic basis and arrhythmia mechanism in long QT syndrome type 1. Journal of Cardiovascular Electrophysiology. 2000;11:710–712. doi: 10.1111/j.1540-8167.2000.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Jensch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Shen B-Q, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. American Journal of Physiology. 1994;266:L493–501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- Singh M, Krouse ME, Moon S, Wine JJ. Most basal Isc in Calu-3 human airway cells is bicarbonate-dependent Cl− secretion. American Journal of Physiology. 1997;272:L690–698. doi: 10.1152/ajplung.1997.272.4.L690. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Smith PL, Frizzell RA. Cl secretion by canine tracheal epithelium. IV. Basolateral membrane K permeability parallels secretion rate. Journal of Membrane Biology. 1984;77:187–199. doi: 10.1007/BF01870568. [DOI] [PubMed] [Google Scholar]
- Suessbrich H, Busch AE. The IKs channel: coassembly of IsK (minK) and KvLQT proteins. Reviews of Physiology. Biochemistry and Pharmacology. 1999;137:191–226. doi: 10.1007/3-540-65362-7_6. [DOI] [PubMed] [Google Scholar]
- Suessbrich H, Schchönherrouml;nherr R, Heinemann SH, Lang F, Busch AE. Specificity of cloned cardiac K+ channel blockade by clofilium and its tertiary analog LY97241. FEBS Letters. 1997;414:435–438. doi: 10.1016/s0014-5793(97)01030-2. [DOI] [PubMed] [Google Scholar]
- Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO Journal. 2000;19:6326–6330. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth R, Bleich M. K+ channels and colonic function. Reviews of Physiology, Biochemistry and Pharmacology. 2000;140:1–62. doi: 10.1007/BFb0035550. [DOI] [PubMed] [Google Scholar]
- Warth R, Hamm K, Bleich M, Kunzelmann K, Van Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Archiv. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- Wine JJ. The genesis of cystic fibrosis lung disease. Journal of Clinical Investigation. 1999;103:309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfsen I, Hauber H-P, Schiemann D, Bauer CK, Schwarz JR. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. Journal of Neuroendocrinology. 2000;12:263–272. doi: 10.1046/j.1365-2826.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- Yamaya M, Finkbeiner WE, Widdicombe JH. Ion transport by cultures of human tracheobronchial submucosal glands. American Journal of Physiology. 1991;261:L484–490. doi: 10.1152/ajplung.1991.261.6.L485. [DOI] [PubMed] [Google Scholar]


