Abstract
To document the activity of cutaneous mechanoreceptors in the glabrous skin of the foot sole, tungsten microelectrodes were inserted through the popliteal fossa and into the tibial nerve of thirteen healthy human subjects. A total of 104 cutaneous mechanoreceptors were identified in the glabrous skin of the foot. This sample consisted of 15 slow adapting type I (14 %), 16 slow adapting type II (15 %), 59 fast adapting type I (57 %), and 14 fast adapting type II units (14 %). The location of the receptors and the outline of the receptive fields were determined by using nylon monofilaments perpendicularly applied against the surface of the skin. This revealed that the receptors were widely distributed without an accumulation of receptors in the toes. There were also larger receptive fields predominantly isolated on the plantar surface of the metatarsal-tarsal region of the foot sole. Furthermore, with the foot in an unloaded position, there was no background discharge activity in any of the cutaneous receptors in the absence of intentionally applied stimulation. These findings suggest that skin receptors in the foot sole behave differently from those receptors found on the glabrous skin of the hand. This may reflect the role of foot sole skin receptors in standing balance and movement control.
Somatosensory input from the lower limb has long been recognized as an important source of sensory information in controlling standing balance (Fitzpatrick et al. 1994; Allum et al. 1998). Although the specific source of this essential input remains to be determined, there are several classes of receptors in the lower limb that may provide feedback related to stance and movement. Proprioceptive information from muscle spindles in muscles from around the knee and ankle may code for the change in joint angle relative to the trunk (Ivanenko et al. 2000), while Golgi tendon organs may be responsible for force feedback about the loading of the body (Pearson, 1995). Finally, skin receptors in the foot sole are sensitive to contact pressures (Magnusson et al. 1990) and may be sensitive to potential changes in the distribution of pressure (Kavounoudias et al. 1998). Together, the integration of all these somatosensory inputs appears to provide important information about the body's position with respect to the supporting surface.
There are several lines of evidence in the recent literature that suggest a contributing role of cutaneous receptors from the foot sole in controlling standing balance. For example, mechanical stimulation of the plantar skin during quiet stance has been shown to evoke postural sway that is highly correlated with the cutaneous stimuli (Maurer et al. 2001). Reduction of this cutaneous information, either by cooling or placing a cuff on the leg, is associated with an increase in postural sway (Orma, 1957; Asai et al. 1992). Furthermore, compensatory stepping reactions to sudden postural perturbations are also affected by reduced plantar support information (Perry et al. 2000). Skin receptors may therefore be able to detect not only the movement of the centre of pressure as it moves towards the boundaries of the base of support, but may also be able to initiate postural reflexes that promote a more stable standing position (Do et al. 1990).
While the above studies illustrate the importance of cutaneous information in the control of standing balance, our knowledge of skin input from the foot sole is largely based on indirect evidence. On the other hand, microneurographic recordings from peripheral nerves have provided direct analysis of the functional properties of skin receptors in response to various stimuli (see Vallbo et al. 1979). To date, cutaneous afferent behaviour in humans has been examined in the face (Johansson et al. 1988), the upper limb (Johansson & Vallbo, 1983; Edin & Abbs, 1991; Edin, 1992; Vallbo et al. 1995), and the lower limb (Vallbo & Hagbarth, 1968; Ribot-Ciscar et al. 1989; Edin, 2001; Trulsson, 2001). However, the majority of the studies related to the lower limb have only examined the hairy skin of the calf and the border of the foot. Consequently, there is limited information about the characteristics of the mechanoreceptors specific to the foot sole.
Reports from other skin regions are often used to predict the properties of cutaneous mechanoreceptors in the foot sole. While it may be tempting to transfer the properties of skin receptors from different body regions to the foot, it may not be appropriate to do so. Firstly, anatomical differences exist between the glabrous and hairy skin types. There are also distinct differences in the relative distribution of receptors between skin regions (Vallbo et al. 1995), including a lack of fast adapting type II units in the face (Johansson et al. 1988) and a potential third type of slow adapting receptor in the thigh (Edin, 2001). In the light of these discrepancies, in order to discuss the role of cutaneous mechanoreceptors in bipedal stance further, it is essential to understand the distribution and behaviour of these sensory receptors in the foot sole of humans.
METHODS
Subjects
Thirty-one recording sessions were performed on thirteen healthy volunteers (7 males, 6 females) aged 22–50 years (mean 29.6 years). None of these individuals had any known neurological or motor disorders. The experimental protocol was explained and the subjects gave their written, informed consent to participate in this investigation. The clinical research ethics board at the University of British Columbia approved the following experimental procedures. All experimental procedures were carried out in accordance with the principles of the Declaration of Helsinki.
Experimental set-up
Subjects lay prone on an adjustable bed. In this position, both legs were extended and the test limb was stabilized on a support. The skin at the back of the knee was anaesthetized with a topical cream (Ametop, 4 % tetracaine), then cleaned with a 70 % isopropyl alcohol solution before electrode insertion. A surface-stimulating electrode was placed on the back of the knee to locate the approximate position of the tibial nerve. A Grass S48 Stimulator (Grass Instruments, Astro-Med Inc., West Warwick, RI, USA) delivered electrical pulses (1 ms duration) at a rate of 1 Hz. The twitch response of the triceps surae muscle group (elicited between 30 and 90 V) and the parasthetic sensation described by the subject were used to assess the location of the nerve. To locate the nerve subcutaneously, a sterile stimulating reference electrode (0.2 mm diameter, 55 mm length, standard profile tip, Fred Haer Inc., Bowdoinham, ME, USA) was inserted into the popliteal fossa over the predefined region while an electric current (1–15 V) was delivered intermittently. The location of the nerve was identified when a twitch was elicited at a level lower than 5 V, or a persisting parasthetic sensation was evoked signalling that the tip of the electrode had penetrated the neural sheath. A sterile recording microelectrode (0.2 mm diameter, 65 mm length, standard profile tip, Fred Haer Inc.) was inserted approximately 10 mm proximal and parallel to the stimulating electrode, which now functioned as a reference electrode. The impedance of the recording electrode in situ was between 50 and 360 kΩ (mean 150 kΩ) at 1 kHz.
Classifying cutaneous mechanoreceptors
The electrode was inserted into the tibial nerve at a variable depth across subjects (mean depth at 26 mm, range 15–37 mm). By manually manipulating the electrode within the nerve, single-unit recordings could be obtained. After isolating a recording, Semmes-Weinstein nylon monofilaments (Stoelting Co., Wood Dale, IL, USA) were used to classify the cutaneous afferents. The monofilaments were capable of applying 0.5–5000 mN of force against the skin. The location of the receptor terminal, or hotspot, was defined as the point of lowest mechanical threshold. A monofilament of four to five times the threshold force was used to outline the receptive field. To measure the size of the receptive field areas, transparent paper was placed against the skin of the foot sole. Receptive field size was calculated using an approach similar to that used for the glabrous skin of the human hand (Johansson & Vallbo, 1979).
Cutaneous afferents were classified as slow adapting when they responded continuously to maintained indentations. If the receptor responded only to the onset and removal of the stimulus, it was classified as fast adapting. Receptors were classified by type based on the following: slow adapting type I receptors had small receptive fields with multiple hotspots; slow adapting type II receptors had a single hotspot with large, obscure receptive fields and a higher skin stretch sensitivity; fast adapting type I receptors had small receptive fields with multiple hotspots; and fast adapting type II receptors had large, obscure receptive fields with a single hotspot and a higher sensitivity to vibration. Vibration sensitivity was assessed at 100 and 250 Hz with a dual-setting Panasonic electrical vibrator (commercially available, USA). For some receptors force sensitivity was measured on-line with a hand-held force transducer (1601 digital transducer, IITC Inc., Woodland Hills, CA, USA).
Signal analysis and processing
Tibial nerve recordings were amplified ×10 000–25 000 and band-pass filtered between 0.3 and 10 kHz (custom-built Yale microneurography amplifier). The neural data were converted (analog to digital) at a sample rate of 25–50 kHz (Spike2 and 1401-micro interface, Cambridge Electronics Design, UK). A Grass AM8 audio monitor (Grass Instruments) was used for audio presentation of the neural signal. single-unit spikes were captured and displayed on-line using an oscilloscope (20 MHz analog model 2522B, BK Precision, Placentia, CA, USA) with a 10 ms time base. Since the majority of action potentials encountered when using microneurography to record somatosensory afferent activity have an initial, positive double-peaked morphology that can change over time as the position of the recording electrode changes (Inglis et al. 1996), it is important to monitor the shape of all recorded units. Accordingly, action potential morphology was performed off-line using the Spike2 template matching software. This software allowed us to retrieve individual spikes under full visual control using waveform template matching.
Statistics
A one-way ANOVA was used to assess any difference in receptor thresholds between skin regions. A χ2 test was performed to examine the distribution of receptor types found in the hand versus the foot sole. Differences between the means were considered statistically significant at a level of P≤ 0.05.
Results
Multi-unit activity in the foot sole
Assessment of the multi-unit activity in the foot sole provided an outline of the fascicular innervation territories of the tibial nerve. Territories were defined as the cutaneous region that upon contact with the experimenter's hand could evoke a mass discharge of activity with a signal-to-noise ratio of at least 2 : 1. Multi-unit activity was evoked predominantly in the medial or lateral aspect of the foot, corresponding to the medial and lateral plantar nerve divisions in the tibial nerve. Although the size of the multi-unit territories varied between subjects, the locations of the fascicular fields were similar between subjects. Since only a small number of multi-unit fields were assessed in each subject, the data from all thirteen participants were used to map the innervation territories of the multi-unit recordings. Overall, nine regions were outlined on the foot sole based on the multi-unit activity observed in the subjects (Fig. 1). Similar to previous reports on multi-unit activity in the face (Johansson et al. 1988) there was no activity in the absence of any intentionally applied stimulation to the foot sole. Upon stimulation, there was a strong dynamic response with distinct on- and off-discharges during stimulus indentations.
Figure 1. Multi-unit innervation territories in the foot sole.
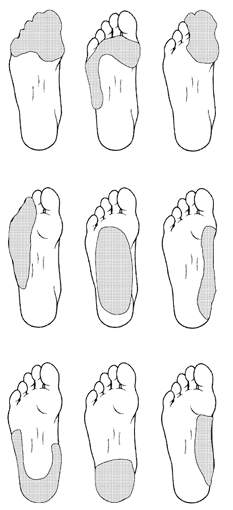
Fascicular receptive fields in the medial and lateral plantar nerves were mapped during multi-unit stimulation prior to single-unit recordings in all subjects. In the plantar surface of the foot, nine multi-unit regions were observed.
Single-unit nerve recordings
From the 126 consecutively recorded afferents, 106 were classified as cutaneous mechanoreceptors with 31 slowly adapting (SA) and 75 fast adapting (FA) units. The remaining 20 afferents were recorded from presumed muscle receptors, which will not be discussed further in this paper. While all four receptor types were present in the foot sole, certain aspects of the SA units made it difficult to classify the SA receptors into types II and I. Firstly, the receptive fields of the SAII units were similar to that of the SAI units. In addition, there was no apparent background discharge activity in any of the SAII receptors, one of several characteristics that have been used to distinguish between the two SA receptor types (Johansson & Vallbo, 1983). To resolve this discrepancy, we examined the discharge characteristics of the SA units in response to a maintained indentation. Since SAIs typically have an irregular firing pattern and SAIIs have a more regular firing rate, this SA behaviour (see Fig. 2) was used in conjunction with the number of hotspots, and the sensitivity to skin stretch to dissociate between SA afferent receptors.
Figure 2. Types of SA responses in the foot sole.
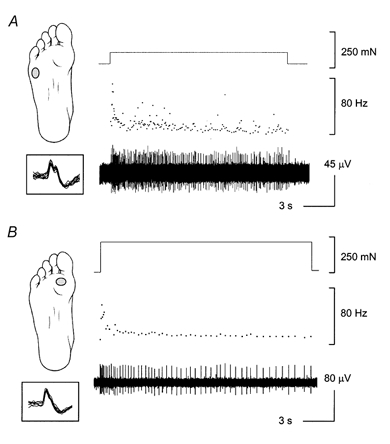
In response to a maintained force application, two slow adapting responses were observed in the foot sole. An example of the discharge activity and the location of the receptive fields for a SAI receptor (A) and a SAII receptor (B), as well as their adaptation properties are presented. Ten consecutive waveforms are aligned and overlapped to demonstrate that these were indeed single-unit recordings. It is important to note that there was no spontaneous activity in the absence of any intentionally applied stimulation in any of the cutaneous receptors in the foot sole.
Of the 106 single-unit recordings from cutaneous afferents, an overwhelming majority of these receptors was recorded from the glabrous skin of the foot sole (104 out of 106, 98 %). Only two afferents, one FAI and one FAII, had the receptor terminal on the hairy skin of the calf. These two units will not be discussed further in order to examine cutaneous mechanoreceptors exclusively located on the foot sole and therefore isolated to glabrous skin. Therefore, according to the classification criteria outlined in Methods, there were 15 SAIs (14 %), 16 SAIIs (15 %), 59 FAIs (57 %) and 14 FAIIs (14 %) documented in the glabrous skin of the foot. As estimated with the nylon monofilaments, the FAII units had the lowest median thresholds (5 mN), while the SAII units had the highest median thresholds (115 mN). Each of these afferents had a single hotspot, presumably corresponding to the location of the receptor terminal. There were approximately 4–6 hotspots measured in the fields of type I units. These afferents had intermediate thresholds, as the median values for the FAIs and SAIs were 12 and 36 mN, respectively.
The thresholds for activation of skin receptors in the foot sole were quite variable. Several FA units responded to stimuli as low as 0.5 mN, while a number of SA receptors were not activated until forces as high as 3000 mN were applied. Based on our sample, the receptor's threshold did not appear to be dependent upon the location of that receptor. That is, the mean thresholds for skin receptors in the toes (25 mN; range 0.5–150 mN), lateral foot (80 mN; range 0.5–750 mN) and heel (300 mN; range 0.7–3000 mN) were not found to be significantly different (P < 0.14).
Receptive field distributions and characteristics
The receptive fields of single-unit recordings were predominately isolated in the foot sole. Two of these units, one SAII and one FAII receptor, had initial threshold levels measured at approximately 3000 mN. The highest monofilament used in this study could exert 5000 mN of force. Because the receptive field is outlined with a monofilament of four to five times the initial threshold force, the receptive area for these two units could not be measured. Moreover, two FAII units had receptive field areas that extended from the glabrous skin onto the hairy skin of the calf, making it difficult to assess the size of the receptive fields. With the exception of these four units, Fig. 3 illustrates the position of the receptors and the measurable receptive fields specific to the plantar surface of the foot. There were a number of similarities between the receptive fields of the FAI, SAI and SAII units. These units had receptive fields that were typically round to oval in shape, with the point or points of highest sensitivity eccentrically located within the receptive field. Furthermore, the type I units exhibited a pattern in which the borders of the field were marked by the flexure lines in the skin. The median receptive field sizes for the FAI, SAI and SAII units were 38 mm2 (range 6–334 mm2), 71 mm2 (range 12–278 mm2) and 127 mm2 (range 44–296 mm2), respectively. In comparison, the FAII units were large and obscure in dimension. It was also very difficult to localize the location of the receptor or point of highest sensitivity. From the measurable FAII units, the median receptive field size was estimated at 284 mm2 (range 42–895 mm2) (see Table 1 for summary).
Figure 3. Distribution of cutaneous mechanoreceptors in the foot sole.
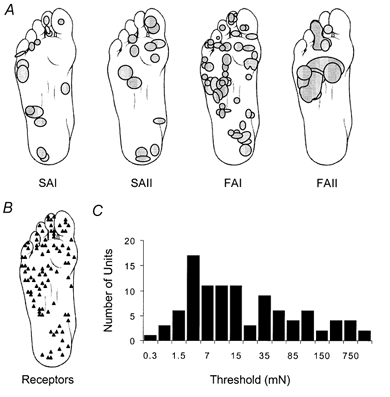
A, the receptive field for each receptor type in the foot sole is illustrated. The receptive field was outlined with a monofilament 4–5 times greater than the initial threshold value. B, the approximate position of the afferent unit in the foot sole for all receptor types is depicted. C, distribution of the total number of documented receptors and the accompanying threshold levels per unit in the foot sole (n = 104).
Table 1.
Profiles of cutaneous mechanoreceptors in the foot sole
| Receptive field size (mm2) | ||||||
|---|---|---|---|---|---|---|
| Type | Number | Per cent of total | Median threshold (mN) | Range (mN) | Median | Range |
| SAI | 15 | 14.4 | 35.6 | 4–744 | 70.9 | 11.8–277.5 |
| SAII | 16 | 15.4 | 115.3 | 36–2800 | 127.4 | 44.0–296.2 |
| FAI | 59 | 56.7 | 11.8 | 0.7–282 | 38.0 | 5.8–333.6 |
| FAII | 14 | 13.5 | 4.0 | 0.5–2800 | 284.2 | 41.7–1248.0 |
| Total | 104 | 100 | — | — | — | — |
The total number of units for each subpopulation, the threshold levels as estimated with calibrated nylon monofilaments, and the receptive field properties were calculated.
Response to mechanical indentations
Receptor response profiles were measured using a variety of innocuous stimuli that were applied to the unloaded foot in the horizontal plane. Directional sensitivity of SAII afferents has been well documented in the hand (Knibestöl, 1975; Johansson, 1978). The discharge activity of all sixteen SAII afferents in the foot sole was therefore assessed during periods of skin stretch in several directions. The force probe was placed perpendicular to the skin surface and could evoke skin stretch in several different directions. Before the application of the probe, there was no background discharge activity in any of these SA units. When the skin was stretched, magnitudes of skin stretch that did not cause any slips between the probe and the skin were used. Figure 4 shows example data for an SAII unit located in the heel. As the direction of skin stretch is altered in a clockwise manner from an anterior to a lateral direction, the number of corresponding action potentials increases for the same relative amount of skin stretch. Despite the fact that the sixteen receptors demonstrated a preferential skin strain axis, the orientation of this axis was not the same for all the SAII units. Although this preferential skin strain was clear, it was difficult to obtain an accurate assessment of the direction for each unit due to limited recording time.
Figure 4. Measuring directional sensitivity of the SAII receptor in the heel.
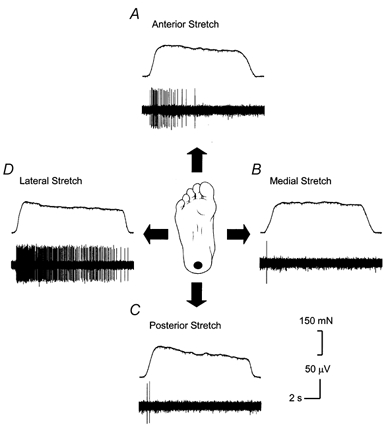
An example of directional sensitivity for one of the SAII receptors located in the heel is presented. A hand-held force transducer stretched the plantar skin of the foot in an attempt to ensure reproducible amounts of stretch. This figure shows the level of stretch applied to the skin and the corresponding discharge activity in response to anterior stretch (A), medial stretch (B), posterior stretch (C) and lateral skin stretch (D).
Skin stretch generated by surface contact or movement that deforms the skin overlying a particular joint could also evoke responses in cutaneous afferents (Hulliger et al. 1979). Of the receptors that were isolated on or near the metatarsal-phalangeal joints, there were eleven prolonged recordings (> 25 min) that allowed us to assess the movement sensitivity of these units. These eleven receptors consisted of four FAIs, five SAIs and two SAIIs. Movement of the toes that was significant enough to cause a skin deformation of the receptive field elicited a response from the receptor. This response was observed in all eleven of these units. In all likelihood the remaining skin receptors, located near the toes, would have shown a similar response to toe movements if the recordings had been stable enough.
DISCUSSION
To examine the potential role of cutaneous mechanoreceptors in standing balance it is important to understand the characteristics of skin receptors in the plantar surface of the foot. Previous reports of the lower limb have only commented about the distribution and behaviour of mechanoreceptors isolated along the lateral border of the foot (Vedel & Roll, 1982; Ribot-Ciscar et al. 1989; Trulsson, 2001). Therefore the present study examined cutaneous activity in the tibial nerve, thereby recording skin receptors specific to the complete foot sole. The results of this study show that based on this sample: (1) a large percentage (73 out of 104, 70 %) of the skin receptors found in the foot sole are rapidly adapting with randomly distributed receptive fields, (2) skin receptors in the glabrous skin of the foot have elevated activation thresholds in comparison with the glabrous skin of the hand, and (3) there is an absence of background activity in any of the cutaneous mechanoreceptors with the foot in an unloaded position.
The present findings suggest that there are a number of differences between the skin of the foot sole and the glabrous skin of the hand. Despite the fact that there was a lower proportion of SA receptors documented in the foot (30 %) than in the hand (44 %; Johansson & Vallbo, 1979), there did not appear to be any significant differences between the percentages of units found in each of these regions. Other quantitative differences between the present findings and previously published reports include variations in the receptor activation thresholds. For instance, the median activation thresholds for the FAI and FAII units in the foot were 11.8 and 4.0 mN, respectively. Considerably lower thresholds were reported for the hand, as the median values for the FAIs and FAIIs were 0.58 and 0.54 mN (Johansson et al. 1980). This difference was even more pronounced between the SA receptors as there were much higher thresholds for SA units in the foot (SAI 35.6 mN and SAII 115.3 mN) than the SA units in the hand (SAI 1.3 mN and SAII 7.5 mN; Johansson et al. 1980). The elevated physiological thresholds for receptors are not surprising considering that psychophysical thresholds in the foot are also higher in comparison with thresholds in the hand (Weinstein, 1968). In all likelihood, the elevated thresholds in the foot resulted from an increased skin thickness in the foot in comparison with the hand.
The receptive field areas for units in the foot sole were also three times greater than the fields of units found in the hand (Johansson & Vallbo, 1980). However, unlike the hand, the positions of the receptive fields were randomly distributed throughout the plantar surface of the foot. There did not appear to be an accumulation of type I receptors in the toes, nor was there any preferred spatial distribution for receptors in the foot. In contrast, there is a proximodistal density gradient for receptors in the hand, including a congregation of type I receptors in the fingers (Johansson & Vallbo, 1979). The spatial arrangement of receptors in the palmar skin allows the hand to acquire information about skin deformation during object manipulation (Johansson & Vallbo, 1983). While cutaneous information from the plantar skin is also important, the foot is primarily involved in weight-bearing actions and would not require as high a level of acuity as that found in the hand.
In comparison with the cutaneous receptors in the glabrous skin of the foot sole, the tactile afferents innervating the lateral border of the foot and calf had lower force thresholds and smaller receptive fields (Trulsson, 2001). There also appears to be a higher proportion of SA receptors in the hairy skin of the leg including a third type of SA (type III) receptor in the thigh (Edin, 2001). However, the differences between skin receptors in the glabrous skin of the foot and the hairy skin of the leg are not surprising. Recently it has been shown that distinct differences exist between skin receptors in the hairy skin of the forearm with receptors in the glabrous skin of the hand (Vallbo et al. 1995). It is likely that similar differences exist in the lower limb. This may be due in part to the anatomical variations between the two skin types. The glabrous skin has tight connections to subcutaneous tissues that are absent in the hairy skin. Consequently, there is a greater degree of stretch in the hairy skin in response to joint movement. Because of this, it is believed that receptors in the hairy skin play a larger role in providing information on joint motion and position (Edin, 1992). Therefore, the mechanoreceptive afferents in the hairy skin of the calf (Trulsson, 2001) may be more suited to convey information about ankle orientation, while afferents in the glabrous skin of the foot would be more important for signalling the foot's contact with the ground.
In this study, the foot was not subjected to any form of significant loading, as the primary purpose was to assess the innervation features of the glabrous skin of the foot. To make the complete foot sole available for manipulation and probing, the dorsum of the foot had to be supported with the foot sole itself unrestrained. In this position, there was no background discharge activity in any of the cutaneous mechanoreceptors encountered. The presence of a background discharge has often been used as a criterion to help dissociate between SA receptor types in the hand (Knibestöl, 1975). However, if the skin on the hand was manipulated to remove any pre-existing stretch, the majority of SAII receptors lost their background discharge (Johansson, 1978). We suggest that the lack of spontaneous activity in the tibial nerve is probably not a unique attribute of skin receptors in the foot sole. Instead, the fact that there was no background activity in a natural, unloaded position suggests that any activity from skin receptors in the foot sole may be important for signalling that the foot is in contact with the supporting surface (Kavounoudias et al. 1998). The wide dispersal of receptors throughout the foot sole would ensure that skin receptors would be able to code for contact pressures, and hence the position of the foot with the ground. Interestingly, there were a limited number of units documented in the longitudinal arch. If a preferential distribution exists, the accumulation of receptors in the anterior aspect of the foot, the lateral border and the heel would correspond to the critical regions of the foot that take up the majority of the body's weight in loaded conditions (Perry et al. 2000).
The present study is the first to examine the location and distribution of cutaneous mechanoreceptors in the glabrous skin of the foot. Initial reports about skin receptors in the glabrous skin of the hand indicated that it was largely comprised of SA receptors (Knibestöl, 1973, 1975). However, it was later shown that there was a larger proportion of FA receptors in the hand than previously indicated (Johansson & Vallbo, 1979). While the lack of spontaneous activity in any of the cutaneous mechanoreceptors from the foot sole may have caused an underestimation of the number of SAII units, ultimately this number would have been low since SAII units are not very common (Johansson & Vallbo, 1979). Moreover, for any given recording session the number of SA receptors never outnumbered the number of FA units documented across all subjects. Therefore, we feel that the relative percentage of FA to SA receptors documented in this study is reflective of the overall distribution of receptors in the foot sole, and may reflect the need for a high dynamic sensitivity in balance control. At present it is unclear how skin receptors may respond to partial loading of the foot or the changes that might occur in receptor behaviour with a more physiological load such as half body weight. Consequently this study is the first of a series of investigations to explore the specific role of cutaneous mechanoreceptors in the foot sole in standing balance.
Acknowledgments
The authors would like to thank Dr S. C. Gandevia and Dr D. Burke for comments on a draft of this manuscript. The Natural Sciences Engineering Research Council of Canada (J. T. I.) and an NSERC Studentship (P. M. K.) supported this study.
REFERENCES
- Allum JH, Bloem BR, Carpenter MG, Hulliger M, Hadders-Algra M. Proprioceptive control of posture: a review of new concepts. Gait and Posture. 1998;8:214–242. doi: 10.1016/s0966-6362(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Asai H, Fujiwara K, Toyama H, Yamashina T, Tachino K, Nara I. The influence of foot soles cooling on standing postural control analyzed by tracking the center of foot pressure. In: Woollacoot M, Horak F, editors. Posture and Gait: Control Mechanisms. II. Eugene, OR, USA: University of Oregon Books; 1992. pp. 151–154. [Google Scholar]
- Do MC, Bussel B, Breniere Y. Influence of plantar cutaneous afferents on early compensatory reactions to forward fall. Experimental Brain Research. 1990;79:319–324. doi: 10.1007/BF00608241. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. Journal of Neurophysiology. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB. Cutaneous afferents provide information about knee joint movements in humans. Journal of Physiology. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. Journal of Neurophysiology. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Rogers DK, McCloskey DI. Stable human standing with lower-limb muscle afferents providing the only sensory input. Journal of Physiology. 1994;480:395–403. doi: 10.1113/jphysiol.1994.sp020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin AE, Vallbo AB. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. Journal of Physiology. 1979;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JT, Leeper JB, Burke D, Gandevia SC. Morphology of action potentials recorded from human nerves using microneurography. Experimental Brain Research. 1996;110:308–314. doi: 10.1007/BF00228561. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Lacquaniti F. Influence of leg muscle vibration on human walking. Journal of Neurophysiology. 2000;84:1737–1747. doi: 10.1152/jn.2000.84.4.1737. [DOI] [PubMed] [Google Scholar]
- Johansson RS. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. Journal of Physiology. 1978;281:101–123. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Trulsson M, Olsson KA, Westberg KG. Mechanoreceptor activity from the human face and oral mucosa. Experimental Brain Research. 1988;72:204–208. doi: 10.1007/BF00248518. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. Journal of Physiology. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Spatial properties of the population of mechanoreceptive units in the glabrous skin of the human hand. Brain Research. 1980;184:353–366. doi: 10.1016/0006-8993(80)90804-5. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensory cording in the glabrous skin of the human hand. Trends in Neurosciences. 1983;6:27–32. [Google Scholar]
- Johansson RS, Vallbo AB, Westling G. Thresholds of mechanosensitive afferents in the human hand as measured with von Frey hairs. Brain Research. 1980;184:343–351. doi: 10.1016/0006-8993(80)90803-3. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a ‘dynamometric map’ for human balance control. NeuroReport. 1998;9:3247–3252. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- KnibestÖl M. Stimulus-response functions of rapidly adapting mechanoreceptors in the human glabrous skin area. Journal of Physiology. 1973;232:427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnibestÖl M. Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. Journal of Physiology. 1975;245:63–80. doi: 10.1113/jphysiol.1975.sp010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson M, Enbom H, Johansson R, Pyykko I. Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Acta Oto-Laryngolica. 1990;110:182–188. doi: 10.3109/00016489009122535. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Bolha B, Hlavacka F. Human balance control during stimulation of the plantar soles. Neuroscience Letters. 2001;302:45–48. doi: 10.1016/s0304-3940(01)01655-x. [DOI] [PubMed] [Google Scholar]
- Orma EJ. The effects of cooling the feet and closing the eyes on standing equilibrium. Different patterns of standing equilibrium in young adult men and women. Acta Physiologica Scandinavica. 1957;38:288–297. doi: 10.1111/j.1748-1716.1957.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Proprioceptive regulation of locomotion. (Review) Current Opinion in Neurobiology. 1995;5:786–791. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Research. 2000;877:401–406. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Vedel JP, Roll JP. Vibration sensitivity of slowly and rapidly adapting cutaneous mechanoreceptors in the human foot and leg. Neuroscience Letters. 1989;104:130–135. doi: 10.1016/0304-3940(89)90342-x. [DOI] [PubMed] [Google Scholar]
- Trulsson M. Mechanoreceptive afferents in the human sural nerve. Experimental Brain Research. 2001;137:94–102. doi: 10.1007/s002210000649. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Experimental Neurology. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. (Review) Physiological Reviews. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. Journal of Physiology. 1995;483:783–795. doi: 10.1113/jphysiol.1995.sp020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel JP, Roll JP. Response to pressure and vibration of slowly adapting cutaneous mechanoreceptors in the human foot. Neuroscience Letters. 1982;34:289–294. doi: 10.1016/0304-3940(82)90190-2. [DOI] [PubMed] [Google Scholar]
- Weinstein S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: Kenshalo DR, editor. The Skin Senses. Springfield, IL, USA: Thomas; 1968. pp. 195–222. [Google Scholar]


