Abstract
In vascular smooth muscle cells many agonists cause the release of Ca2+ ions from internal stores. An important problem concerns the mechanism by which the intracellular stores are refilled subsequent to depletion. In the present study, we describe the properties of a Ca2+-permeable non-selective cation channel current that is activated in rabbit portal vein myocytes by depletion of internal Ca2+ stores. Application of cyclopiazonic acid (CPA), which depletes internal Ca2+ stores, activated whole-cell currents that had a reversal potential (Er) of about +50 mV in 1.5 mm external Ca2+ (). In 0 mm , the currents were larger and Er was ∼0 mV. Application of CPA and caffeine during cell-attached recording activated single inward channel currents at negative potentials, which had a slope conductance of 2–3 pS and an Er of +20 mV. The slope conductance in 0 and 110 mm was 7 and 1.5 pS, respectively, and Er values indicated that these non-selective cation channels are highly permeable to Ca2+ ions. Bath application of the cell-permeant Ca2+ chelator, BAPTA-AM, also activated similar currents, indicating that these channels are not activated by Ca2+. Spontaneous channel currents with similar properties to store-operated channels were observed in some patches. Application of W-7, an inhibitor of the Ca2+-binding protein calmodulin, also activated similar Ca2+-permeable channel currents. In conclusion, it is demonstrated that agents that deplete Ca2+ stores and inhibit calmodulin binding activate Ca2+-permeable non-selective cation channel currents in rabbit portal vein myocytes. These channels may have an important role in vascular smooth muscle in providing an influx of Ca2+ to refill depleted internal Ca2+ stores and appear to possess different characteristics to store-operated channels described in other vascular smooth muscle preparations.
It is well recognised that agonist-induced contraction of vascular smooth muscle involves an increase in intracellular Ca2+ concentrations ([Ca2+]i) that is mediated partly by the release of Ca2+ from internal stores, probably the sarcoplasmic reticulum (SR). This mechanism is believed to involve G-protein-coupled receptors (e.g. α1-adrenoceptors) and the phosphoinositide system and results in the formation of inositol trisphosphate (IP3) that acts on the SR to release Ca2+ into the cytoplasm. The consequent rise in Ca2+ ions produces contraction via a direct action on contractile proteins and also by activating a Ca2+-activated Cl− conductance to produce depolarization, which opens voltage-dependent Ca2+ channels (Large & Wang, 1996). An intriguing problem concerning this mechanism is how the internal SR Ca2+ stores are refilled subsequent to agonist-induced depletion.
Agonist-induced influx of Ca2+ into a cell has been proposed to be important for refilling intracellular Ca2+ stores (Berridge, 1995). One explanation is that the influx of Ca2+ is mediated by the opening of Ca2+-permeable ion channels in the plasmalemma that are activated by depletion of internal Ca2+ stores. Hoth & Penner (1992), using a mast cell preparation, were the first to demonstrate the activation of an ionic current linked to the depletion of intracellular Ca2+ stores. This current was subsequently termed the Ca2+-release-activated Ca2+ current (ICRAC). The mechanisms that couple intracellular Ca2+ stores to ion channels are not yet known, although it has been suggested that the IP3 receptor and/or cytoplasmic factors are involved (Parekh & Penner, 1997).
In smooth muscle there have been few reports on the regulation of store-operated ionic currents or ion channels, possibly because these conductances are small or difficult to record. In mouse anococcygeus myocytes application of cyclopiazonic acid (CPA; an inhibitor of SR Ca2+-ATPase) and caffeine, which releases Ca2+ from internal stores, evoked a Ca2+-activated Cl− current (ICl(Ca)) and a smaller, sustained non-selective cation current (Wayman et al. 1996, 1999). These conductances were associated with, respectively, an initial transient rise in [Ca2+]i followed by a smaller sustained rise in [Ca2+]i. It was suggested that the initial transient rise in [Ca2+]i was due to release of Ca2+ from the SR, which activated ICl(Ca), whereas the sustained rise in [Ca2+]i was due to the influx of Ca2+ through Ca2+-permeable cation channels on the plasmalemma. More recently, store-operated non-selective cation currents have been recorded in vascular smooth muscle cells (mouse aortic myocytes, Trepakova et al. 2000, 2001; rabbit choroidal arteriolar smooth muscle cells, Curtis & Scholfield, 2001; human and rat pulmonary myocytes, Golovina et al. 2001).
In the present study, we investigated the properties of a Ca2+-permeable non-selective cation channel that is activated by depletion of internal Ca2+ stores in freshly dispersed rabbit portal vein smooth muscle cells. This channel sometimes opens spontaneously and may also be activated by calmodulin antagonists. In addition, this channel has several characteristics that distinguish it from the store-operated ion channels in other vascular preparations. A preliminary report of this work was presented to the Physiological Society (Albert & Large, 2001).
METHODS
Cell isolation
New Zealand White rabbits (2–3 kg) were killed by an intravenous injection of sodium pentobarbitone (120 mg kg−1). The portal vein was removed and placed into standard physiological salt solution (PSS). Connective tissue and fat were dissected from the tissue before it was cut into strips and placed in Ca2+-free PSS. The tissue was dispersed in two sequential enzyme treatments. First, the strips of tissue were incubated in Ca2+-free PSS with 0.1–0.3 mg ml−1 protease type X (Sigma) for 5 min and then washed in Ca2+-free PSS. Second, the strips were incubated with 0.5–1.0 mg ml−1 collagenase type IV (Sigma) in 100 μm Ca2+ PSS for 10 min and then washed in 100 μm Ca2+ PSS. All enzyme treatments and wash procedures were carried out at 37 oC. After the enzyme treatments, the tissue strips were incubated in 100 μm Ca2+ PSS at room temperature (20–25 oC) for 10 min before the cells were dispersed in solution by gentle mechanical agitation of the tissue with a wide-bore Pasteur pipette. The cell suspension was then centrifuged (1000 r.p.m.) to form a pellet that was resuspended in 0.75 mm Ca2+ PSS. Cells were then plated onto glass coverslips and stored at 4 oC before use (1–6 h).
The standard PSS contained (mm): NaCl, 126; KCl, 6; CaCl2, 1.5; MgCl2, 1.2; glucose, 10 and Hepes, 11. The pH was adjusted to 7.2 with 10 m NaOH. Ca2+-free PSS, 100 μm Ca2+ PSS and 0.75 mm Ca2+ PSS had the same composition except that either Ca2+ was omitted or 1.5 mm CaCl2 was replaced by 100 μm CaCl2 and 0.75 mm CaCl2, respectively.
Electrophysiology
Whole-cell non-selective cation currents and single cation channel currents were recorded with a List L/M-PC patch clamp amplifier at room temperature using whole-cell recording and cell-attached patch configurations of the patch clamp technique (Hamill et al. 1981). Patch pipettes were manufactured from borosilicate glass and were routinely coated in Sylgard (Dow Corning, Germany) to reduce stray capacitance. Pipettes were fire polished to increase seal resistance, which was between 6 and 10 MΩ when filled with the standard internal patch pipette solution. To reduce ‘line’ noise, the recording chamber (∼150–200 μl volume) was perfused using two 10 ml syringes. One syringe was filled with external solution while the other was used to drain the chamber in a ‘push and pull’ manner. The external solution could be exchanged twice within 30–60 s. Whole-cell currents were evoked before drug application and during the peak response by applying voltage ramps from −150 to +100 mV (0.5 V s−1) every 10 s from a holding potential of 0 mV. The whole-cell currents were filtered at a bandwidth of 1 kHz (-3 dB, low pass 4-pole Bessel filter, List L/M-PC patch clamp amplifier) and sampled at 3 kHz. Leak-subtracted whole-cell currents were then created by subtracting an averaged current trace evoked before drug application from an averaged trace evoked at the peak of the response. At least three whole-cell currents were used to obtain an averaged trace. The current- voltage (I–V) relationship was then plotted and the reversal potential (Er) was calculated by interpolation. To evaluate the unitary single channel I–V characteristics, the membrane potential was stepped manually between −120 and +100 mV.
Single channel currents were recorded initially onto digital audiotape (DAT) using a CDATA digital tape-recorder (Cygnus Technology Inc., Delaware, PA, USA) at a bandwidth of 1 kHz (-3 dB, low pass 4-pole Bessel filter, List L/M-PC patch clamp amplifier) and a sample rate of 48 kHz. For off-line analysis single cation channel records were filtered at 100 Hz (-3 dB, low pass 8-pole Bessel filter, Frequency Devices model LP02, Scensys Ltd, Aylesbury, Bucks, UK) and acquired using a CED 1401plus interface and CED Patch and Voltage Clamp Software (Version 6.0, Cambridge Electronic Design Ltd, Cambridge, UK) at a sampling rate of 1 kHz. Data were captured with a Pentium (P5–100) personal computer (Gateway, Ireland).
Mean unitary amplitudes were calculated from idealized traces of at least 10 s using the half-amplitude crossing method. I–V relationships, calculated from unitary amplitudes from an individual patch, were plotted and the unitary conductance and Er were calculated from the slope of the relationship using linear regression (Origin software, Microcal, USA). The values obtained from the individual patches were then used to calculate the mean slope conductance and mean Er values. In addition, I–V relationships were calculated from pooled single cation channel amplitudes. Single cation channel currents were not observed at membrane potentials positive to −20 mV in most solutions and, therefore, Er was estimated using extrapolation. Open time distributions were plotted (2–5 ms bins) and, where appropriate, were fitted with single or multiple exponential functions using the maximum likelihood method. Events lasting < 6.64 ms (twice the rise time for a 100 Hz (-3 dB) low pass filter) were excluded from analysis. In patches where the signal-to-noise ratio was exceptional high, the single channel currents were filtered off-line at 500 Hz (-3 dB, low pass 8-pole Bessel filter, twice the rise time, 2Tr = 1.33 ms) and sampled at 5 kHz. In such patches, the single channel current amplitudes and open lifetime distribution were not significantly different (P > 0.05) from those values determined using a 100 Hz (-3dB) low pass filter. Figure preparation was carried out using Origin software. Inward channel currents are shown as downward deflections and outward currents as upward deflections. Relative open probability (NPo) was calculated using the equation:
Solutions and drugs
The whole-cell K+-free external solution contained (mm): NaCl, 126; CaCl2, 1.5; Hepes, 10 and glucose, 11. The pH was adjusted to 7.2 with 10 m NaOH, which increased the external Na+ concentration by ∼5 mm. The external solution also contained 5 μm nicardipine, 100 μm DIDS and 100 μm niflumic acid. The whole-cell patch pipette solution contained (mm): CsCl, 18; caesium aspartate, 108; MgCl, 1.2; Hepes, 10; glucose, 11; BAPTA, 10 and CaCl2, 1. The concentration of free internal Ca2+ was approximately 14 nm (calculated using EQCAL software) and the pH was adjusted to 7.2 with Tris. Under these conditions, voltage-gated Ca2+, K+, swell-activated Cl− currents and Ca2+-activated conductances are abolished, allowing the recording of non-selective cation currents in isolation.
In the cell-attached patch experiments, the membrane potential was set at 0 mV by perfusing cells with a KCl external solution containing (mm): KCl, 126; CaCl2, 1.5; Hepes, 10 and glucose, 11. The pH was adjusted to 7.2 with 10 m NaOH. Nicardipine (3–5 μm) was included to prevent smooth muscle cell contraction by blocking Ca2+ entry through voltage-dependent Ca2+ channels. The standard 126 mm NaCl cell-attached patch pipette solution was K+ free and contained (mm): NaCl, 126; CaCl2, 1.5; Hepes, 10; glucose, 11; TEA, 10; 4-AP, 5; DIDS, 100 μm; niflumic acid, 100 μm and nicardipine, 3–5 μm; pH adjusted to 7.2 with NaOH (310 ± 5 mosmol l−1). In experiments carried out to study the relative permeability of channels to Ca2+ ions, NaCl, 126 mm and CaCl2, 1.5 mm were replaced by 110 mm CaCl2 in the pipette solution (450 ± 5 mosmol l−1). For the Ca2+-free pipette solution, CaCl2 was omitted and 1 mm BAPTA was added to chelate the Ca2+ and reduce the Ca2+ concentration to < 10 nm (calculated using EQCAL software). All drugs were purchased from Sigma (UK). All values are presented as means ± s.e.m. of n cells. Data were analysed with Student's t test and P < 0.05 was considered significant.
RESULTS
Depletion of internal Ca2+ stores activates a non-selective cation current using whole-cell recording
To investigate whether the depletion of internal Ca2+ stores activates a non-selective cation current in rabbit portal vein smooth muscle cells, we studied the effect of CPA, which inhibits SR Ca2+-ATPase, on whole-cell currents evoked by applying voltage ramps from −150 to +100 mV from a holding potential of 0 mV. In addition, 10 mm BAPTA was included in the patch pipette solution to further deplete the internal Ca2+ stores and prevent the activation of Ca2+-dependent conductances.
Figure 1A illustrates the mean time course of the CPA-evoked non-selective cation current at −50 mV. In 1.5 mm , bath application of 10 μm CPA evoked an inward cation current that had a mean peak amplitude of −12 ± 2 pA (n = 5) at −50 mV, a latency of 74 ± 12 s (n = 5) and a rise time of 62 ± 9 s (n = 5). In 0 mm , application of 10 μm CPA activated a cation current with a mean peak amplitude of −88 ± 14 pA (n = 5) at −50 mV, which was significantly (P < 0.05) larger than the mean peak amplitude in 1.5 mm (Fig. 1A). Figure 1B shows representative I–V relationships of the CPA-evoked cation currents recorded in either 1.5 or 0 mm . In 1.5 mm , the CPA-evoked cation current exhibited a markedly rectifying I–V relationship with inward rectification at membrane potentials more negative than −50 mV and outward rectification at potentials more positive than +50 mV. The CPA-evoked cation current had an Er of +52 ± 4 mV (n = 5). In 0 mm the I–V relationship of the CPA-evoked cation current was relatively linear between −150 and +100 mV with an Er of −5 ± 3 mV (n = 5, Fig. 1B). Figure 1C shows the mean I–V relationships of the CPA-evoked cation currents, which have been normalized to the mean amplitude in 1.5 mm at −50 mV to illustrate the potentiation of the whole-cell currents in 0 mm . The shift in the Er of the CPA-evoked cation current from about +50 mV to 0 mV on removing suggests that the cation channels are highly permeable to Ca2+ ions. In addition, the much larger whole-cell currents and changes in the characteristics of the I–V relationship in the absence of suggest that may inhibit whole-cell cation currents.
Figure 1. CPA activates a non-selective cation current in rabbit portal vein smooth muscle cells recorded with the whole-cell method.
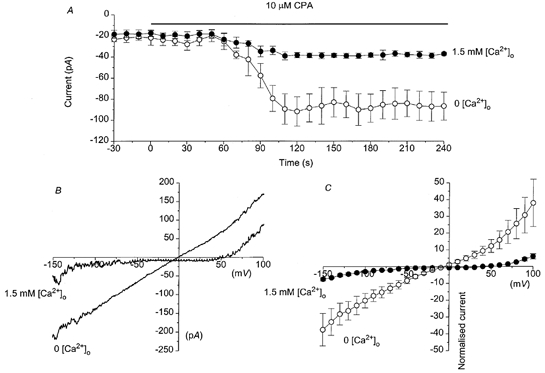
A, the mean time course of the CPA-evoked cation currents recorded at −50 mV in either 1.5 mm (•) or 0 mm (○) . Each data point is the mean of at least 4 cells. B, examples of I–V relationship of the CPA-evoked cation currents recorded in either 1.5 mm or 0 . Note the larger CPA-evoked current, linear I–V relationship and more negative reversal potential (Er) in 0 mm compared to 1.5 mm . C, the mean I-V relationships of the CPA-evoked cation currents in 1.5 mm (•) or 0 mm (○) . Each point is the mean of 5 cells. The plots have been normalized to the amplitudes of the whole-cell cation currents in 1.5 mm at −50 mV (= −1 on y-axis).
Agents that deplete internal Ca2+ stores activate inward ion channels in cell-attached patches
To study the properties of the CPA-evoked non-selective cation current at the single channel level, we investigated the effect of CPA and caffeine, which releases Ca2+ from internal Ca2+ stores, on quiscent cell-attached patches. Figure 2A illustrates a typical record of single inward channel activity (downward deflections) in a cell-attached patch in response to bath application of 10 μm CPA using the standard (126 mm NaCl with 1.5 mm Ca2+) patch pipette solution. Application of 10 μm CPA evoked currents that could only be recorded at patch potentials at, or more negative to, −40 mV. At potentials positive to −40 mV, channel currents could not be resolved, even at large positive potentials (e.g. +100 mV) at which there would be a large driving force for current flow (see later). The channel currents were observed ∼1–3 min after application of CPA and did not noticeably run down during the course of the cell-attached patch recordings (up to 30 min). The amplitude of the CPA-evoked channel currents was ∼0.2 pA at −80 mV (Fig. 2A). Figure 2B shows the I–V relationship of the CPA-evoked channel currents illustrated in Fig. 2A. The currents behaved ohmically between −120 and −40 mV and the slope conductance between these two potentials was 2.1 pS. Since channel currents were not observed at potentials positive to −40 V, it was not possible to obtain a precise estimate of Er; nevertheless, the extrapolated Er in Fig. 2B was +23 mV. In six patches, CPA-evoked channels had an unitary conductance of 2.5 ± 0.3 pS and an extrapolated Er of +16 ± 5 mV (Table 1).
Figure 2. Application of CPA or caffeine activates single channel currents in cell-attached patches from rabbit portal vein smooth muscle cells.
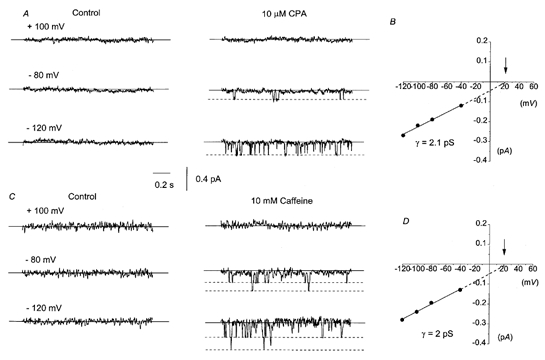
A, using 126 mm NaCl with 1.5 mm Ca2+ patch pipette solution, bath application of 10 μm CPA activated single channel currents in a patch that had not previously shown single channel activity. Note that inward currents are represented as downward deflections and that there is no CPA-evoked single channel activity at +100 mV. Continuous lines indicate the closed level, whereas dashed lines indicate open levels. B, the I–V relationship of the CPA-evoked single inward channel currents shown in A had a slope conductance (γ) of 2.1 pS between −120 and −40 mV and an extrapolated Er (↓) of +23 mV. C, bath application of 10 mm caffeine evoked single channel currents in a different cell-attached patch. Note the multiple single channel current openings at negative patch potentials. D, the I–V relationship of the caffeine-evoked single inward currents shown in C that had a slope conductance of 2 pS between −120 and −40 mV and an extrapolated Er (↓) of +22 mV.
Table 1.
Comparison of the properties of CPA-, caffeine-, BAPTA-AM-, W-7-evoked andspontaneous cation channels recorded in cell-attached patches with a 126 mm NaCl pipettesolution containing 1.5 mm
| γg(pS) | Er (mV) | Oτ1 (ms) | Oτ2 (ms) | n | |
|---|---|---|---|---|---|
| CPA | 2.5 ± 0.3 | +16 ± 5 | 4.6 ± 0.3 | 26 ± 2.6 | 5 |
| Caffeine | 2.1 ± 0.1 | +24 ± 7 | 4.9 ± 0.1 | 26 ± 1.8 | 5 |
| BAPTA-AM | 2.2 ± 0.4 | +22 ± 4 | 5.3 ± 0.4 | 29 ± 3.3 | 5 |
| Spontaneous | 2.5 ± 0.4 | +20 ± 5 | 4.5 ± 0.2 | 32 ± 5.4 | 8 |
| W-7 | 2.8 ± 0.6 | +25 ± 5 | 4.8 ± 0.3 | 32 ± 3.5 | 4 |
Single channel conductance (γ) was calculated from the slope of the I–V relationship between −40 and −120 mV. Er was calculated by extrapolation. Oτ1 and Oτ2 are time constants estimated from the distributionof open times at −80 mV.
Figure 2C shows a typical response of a quiescent cell-attached patch to bath application of 10 mm caffeine using the standard NaCl patch pipette solution. The channel currents evoked by caffeine were observed within 30–60 s after its application. Application of 10 mm caffeine also activated single channel currents that were only observed at negative patch potentials and had amplitudes of ∼0.2 pA at −80 mV. In this patch, multiple channel currents were observed, indicating that there was more than one channel in the patch. Figure 2D shows the I–V relationship of the channel currents stimulated by caffeine in Fig. 2C, which had similar characteristics to the CPA-induced events. In this patch, the slope conductance between −120 and −40 mV was 2 pS and the extrapolated Er was +22 mV. In five patches, application of 10 mm caffeine activated channel currents that had a mean slope conductance of 2.1 ± 0.1 pS between −120 and −40 mV and an extrapolated Er of +24 ± 7 mV (Table 1).
The CPA- and caffeine-evoked channel currents had a low relative open probability (NPo < 0.2). The fact that several channels were often recorded in the patch meant that we were unable to determine accurately the open probability (Po) for an individual cation channel.
The cell-permeant Ca2+ chelator, BAPTA-AM, activates channel currents with similar properties to the CPA- and caffeine-evoked channel currents
It is generally accepted that store-operated ion channels are activated by a reduction in [Ca2+] within the internal stores and, therefore, are not activated by a rise in cytosolic [Ca2+]. To exclude the possibility that the CPA- and caffeine-evoked cation channels observed in the present study were activated by a rise in intracellular [Ca2+], we carried out experiments using the cell-permeant Ca2+ chelator, BAPTA-AM, to deplete internal Ca2+ stores.
Figure 3A shows a typical response of a quiescent cell-attached patch to bath application of 50 μm BAPTA-AM. After ∼15–20 min, single channel currents were activated and were only observed at negative membrane potentials. The lag between application of BAPTA-AM and activation of channels is probably due to diffusion of BAPTA-AM into the cells and subsequent cleavage of the BAPTA-AM to yield free acid. Figure 3B shows the I–V relationship of the BAPTA-AM-evoked channel currents illustrated in Fig. 3A. The channel currents had a slope conductance of between −120 and −40 mV of 2.3 pS and an extrapolated Er of +25 mV. In five patches, the BAPTA-AM-evoked channel currents had a mean slope conductance of 2.2 ± 0.4 pS between −120 and −40 mV and a mean extrapolated Er of +22 ± 4 mV (Table 1). These data indicate that the cation channels are not Ca2+ activated and are therefore evoked by the depletion of the internal Ca2+ stores.
Figure 3. Application of the cell-permeant Ca2+ chelator, BAPTA-AM, activates inward channel currents that have similar properties to the CPA- and caffeine-evoked channel currents.
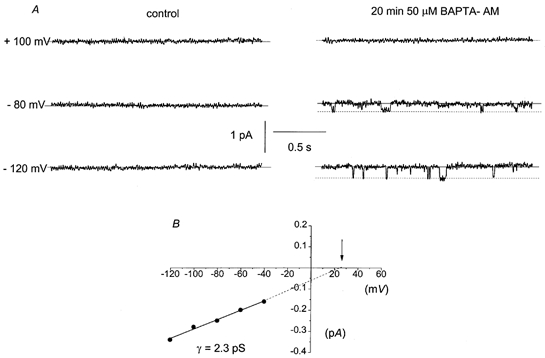
A, activation of inward channel currents at negative membrane potentials after bath application of BAPTA-AM for ∼20 min. Note that no channel currents could be observed at positive membrane potentials. B, the I–V relationship of the channel currents shown in A. The channel currents had a slope conductance of 2.3 pS between −120 and −40 mV and an extrapolated Er (↓) of +25 mV.
CPA-evoked cation channels are highly permeable to Ca2+ ions
The shift in Er of the CPA-evoked whole-cell currents after removing (Fig. 1B) and the positive extrapolated Er values of the CPA-, caffeine- and BAPTA-AM-evoked cation channel currents indicate that these ion channels may be significantly permeable to Ca2+ ions. To investigate this possibility, we studied the properties of CPA-evoked single inward cation currents using a 110 mm CaCl2 patch pipette solution.
Figure 4A illustrates a typical experiment in which bath application of 10 μm CPA activated single cation channel currents recorded with a 110 mm CaCl2 patch pipette solution. In 110 mm CaCl2, the channel currents had properties similar to the CPA-evoked events recorded in a 126 mm NaCl patch pipette solution and channel currents were observed only at negative patch potentials. Figure 4B illustrates the pooled I–V relationship of the CPA-evoked channel currents. The CPA-evoked channel currents recorded in 110 mm CaCl2 had a slope conductance of 1.5 pS between −120 and −40 mV and the extrapolated Er was shifted to much more positive potentials, about +86 mV. The degree of extrapolation was large, but nevertheless there appeared to be a large positive shift in the extrapolated Er compared to that recorded in standard (126 mm NaCl, 1.5 mm Ca2+) pipette solution, suggesting that the cation channels are highly permeable to Ca2+ ions.
Figure 4. Application of CPA activates single cation channels that are permeable to Ca2+ ions.
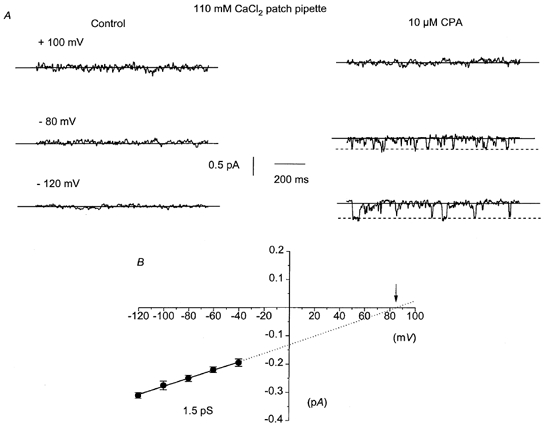
A, with a 110 mm CaCl2 patch pipette solution, bath application of 10 μm CPA activated single cation currents in a previously quiescent patch. The CPA-evoked single cation currents were only observed at negative patch potentials. B, the pooled I–V relationships of CPA-evoked single cation currents recorded with 110 mm CaCl2 patch pipette solution. The I–V relationship had a slope conductance of 1.5 pS between −120 and −40 mV and an extrapolated Er (↓)of +86 mV. Each point is the mean of at least 4 patches.
Cell-attached patches contain spontaneous single channel currents with properties similar to CPA- and caffeine-evoked single cation channel currents
In experiments using the standard patch pipette solution (126 mm NaCl, 1.5 mm Ca2+), approximately 40 % of cell-attached patches exhibited spontaneous single cation channel currents. Figure 5A illustrates a typical experiment in which spontaneous channel currents were observed in a cell-attached patch. As in the other experiments, spontaneous channel currents were observed only at negative patch potentials. In patches where spontaneous channel activity was recorded, the channel currents were observed immediately upon obtaining a giga-ohm seal at −40 mV, after which activity was usually sustained for the duration of the recording. The amplitude of the spontaneous channel currents was about 0.2 pA at −80 mV and multiple spontaneous channel openings were observed, indicating the presence of more than one channel in the patch (e.g. at −120 mV in Fig. 5A).
Figure 5. Cell-attached patches contain spontaneous single channel currents with characteristics similar to the CPA- and caffeine-evoked cation channels.
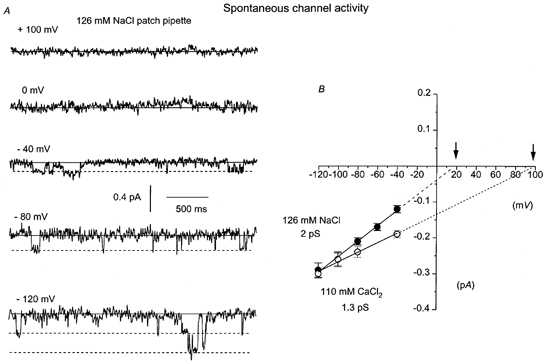
A, spontaneous channel activity between −40 and −120 mV in one patch recorded with 126 mm NaCl patch pipette solution. B, pooled I–V relationship of spontaneous non-selective cation channels recorded with 126 mm NaCl with 1.5 mm (•) or 110 mm CaCl2 (○) in the patch pipette solution. In 126 mm NaCl, the I–V relationship had a slope conductance of 2 pS between −120 and −40 mV and an extrapolated Er (↓) of +18 mV. In 110 mm CaCl2, the I–V relationship had a slope conductance of 1.3 pS and an extrapolated Er (↓) of + 98 mV. Each point is the mean of at least 4 patches.
To investigate whether the spontaneous channel currents were permeable to Ca2+ ions, we recorded spontaneous channel activity using a 110 mm CaCl2 patch pipette solution. A comparison of the pooled I–V relationships of spontaneous single inward channels currents recorded from different patches with either 126 mm NaCl or 110 mm CaCl2 in the pipette solution is presented in Fig. 5B. In the 126 mm NaCl solution, the slope conductance was 2 pS and the extrapolated Er was +18 mV, which are similar to values calculated from individual patches (Table 1). In the 110 mm CaCl2 solution, 37 % of cell-attached patches exhibited spontaneous channel currents that had properties similar to the CPA-evoked cation channel currents recorded in the 110 mm CaCl2 solution. Figure 5B shows that the pooled I–V relationship of the spontaneous channel currents had a slope conductance of 1.3 pS and an extrapolated Er of +98 mV. The shifts in the extrapolated Er, by the replacement of external Na+ with Ca2+ (Fig. 5B), suggest that the spontaneous channels are also Ca2+-permeable non-selective cation channels.
Open lifetime distributions of CPA-, caffeine- and BAPTA-AM-evoked and spontaneous cation channel currents
Figure 6 illustrates representative open lifetime distributions of CPA-, caffeine-, BAPTA-AM-evoked and spontaneous cation channels recorded in different patches at −80 mV. The open lifetime distributions were similar in that the majority of openings were < 10 ms in duration, although a substantial number of channels opened for longer than 20 ms. The open lifetime distributions in each experimental condition could be fitted by the sum of at least two exponentials using the maximum likelihood method, with time constants (Oτ1 and Oτ2) of approximately 5 and 30 ms, respectively (Table 1).
Figure 6. Open lifetime distributions of the CPA-, caffeine-, BAPTA-AM-evoked and spontaneous cation channel currents recorded with a 126 mm NaCl patch pipette solution.
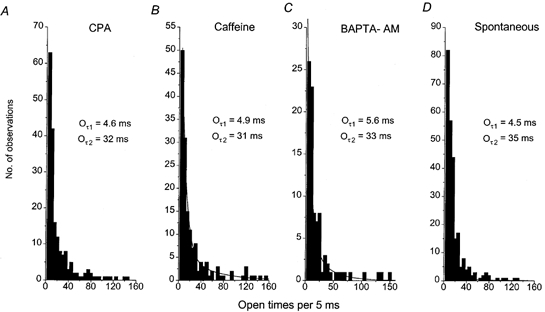
The open lifetime distributions of CPA- (A), caffeine- (B), BAPTA-AM-evoked (C) and spontaneous (D) cation channels could all be fitted with (continuous lines) the sum of two exponentials with time constants of approximately 5 ms (Oτ1) and 30 ms (Oτ2). The holding potential was −80 mV in all cases.
Characteristics of CPA-evoked and spontaneous single cation channel currents in the absence of
In the whole-cell recording experiments (see above and Fig. 1), we showed that CPA-evoked cation currents recorded in 0 mm had larger amplitudes than in 1.5 mm . To investigate this observation at the single channel level, we recorded CPA-evoked and spontaneous channel currents in cell-attached patches using a -free patch pipette solution.
Figure 7A shows typical spontaneous channel currents recorded using a -free patch pipette solution. In comparison to the channel currents recorded using the 1.5 mm Ca2+ solution, channel events in the absence of were larger with amplitudes of 0.6–0.7 pA at −80 mV and also reversed in an outward direction (upward deflections in Fig. 7A) at positive membrane potentials. Similar channel currents were activated by bath applications of 10 μm CPA. Figure 7B shows the I–V relationship of the channel currents presented in Fig. 7A. The I–V relationship was linear between −110 and +80 mV with a slope conductance of 8 pS and an Er of −4 mV. In five patches (3 spontaneous, 2 CPA evoked), the channel currents had a unitary conductance of 7.4 ± 0.5 pS and Er was −4 ± 1 mV. Figure 7C shows the open time distribution of the channel events presented in Fig. 7A at −110 mV. The open time distribution could be described by the sum of two exponentials with time constants (Oτ1 and Oτ2) of approximately 5.4 and 32 ms, respectively.
Figure 7. Characteristics of non-selective cation channel currents in the absence of .
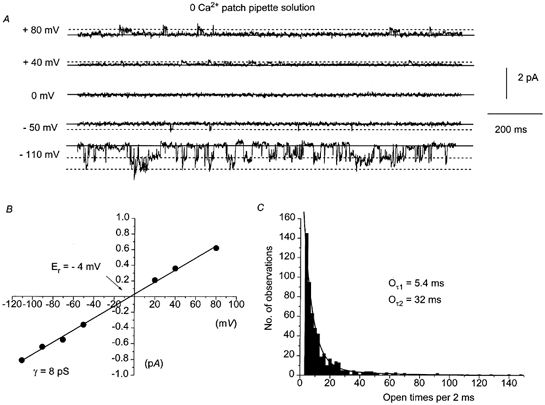
A, spontaneous channel currents recorded at different membrane potentials with a 0 mm patch pipette solution. Note that outward channel currents (denoted by upward deflections) can be observed at positive membrane potentials. B, the I–V relationship of the channel currents shown in A. The I–V relationship was linear between −110 and +80 mV with a slope conductance of 8 pS and an Er of −4 mV. C, open time distribution of the channel currents shown in A at −110 mV. The open times could be described by the sum of two exponentials with time constants of 5.4 ms (Oτ1) and 32 ms (Oτ2).
The NPo of the channel currents recorded in 0 mm was between 0.3 and 0.4 at negative membrane potentials, which was higher than the NPo of the events recorded in 1.5 mm . However, because most patches contained more than one channel, we were not able to confirm whether the CPA-evoked and spontaneous cation channel currents have an increased Po in 0 mm .
Agents that inhibit the action of calmodulin also activate single inward channel currents with properties similar to those activated by CPA and caffeine
There is evidence that the Ca2+-binding protein, calmodulin, reduces the rate of activation, peak amplitude and sustained current level of a store-operated Ca2+ conductance in cultured bovine aortic endothelial cells (Vaca, 1996). To investigate whether calmodulin may modulate the cation channels described in this study, we studied the effect of the calmodulin antagonists, W-7 and calmidazolium, on quiescent patches.
Figure 8 shows a typical response of a cell-attached patch to bath application of 50 μm W-7 using a 126 mm NaCl patch pipette solution. This treatment activated single channel currents at patch potentials at, or more negative than, −40 mV (Fig. 8A). The W-7-evoked channel currents had properties similar to the CPA-, caffeine-evoked and spontaneous cation channel currents, with an amplitude of ∼0.2 pA at −80 mV and similar gating behaviour. Figure 8B illustrates the I/V relationship of the W-7-evoked channel currents presented in Fig. 8A. The slope conductance was 2.3 pS between −120 and −40 mV and the extrapolated Er was +17 mV. In four cell-attached patches, the mean I–V relationships of the W-7-activated channel currents had a slope conductance of 2.8 ± 0.6 pS between −120 and −40 mV and an Er of +25 ± 5 mV (Table 1). In all patches tested, the W-7-evoked channel currents had low activity with NPo < 0.2. Patches frequently contained more than one channel. Figure 8C illustrates that the open time distribution of the W-7-evoked single channels at −80 mV shown in Fig. 6A could be described by the sum of two exponentials with time constants of 5 and 36 ms. In four cell-attached patches, the open time distributions could all be described by the sum of two exponentials with time constants (Oτ1 and Oτ2) of 4.8 and 32 ms, respectively (Table 1). Similar results were obtained with bath application of another calmodulin antagonist, calmidazolium (1 μm, data not shown).
Figure 8. Application of the calmodulin inhibitor, W-7, activates single channel currents with properties that are similar to CPA-evoked cation channel currents.
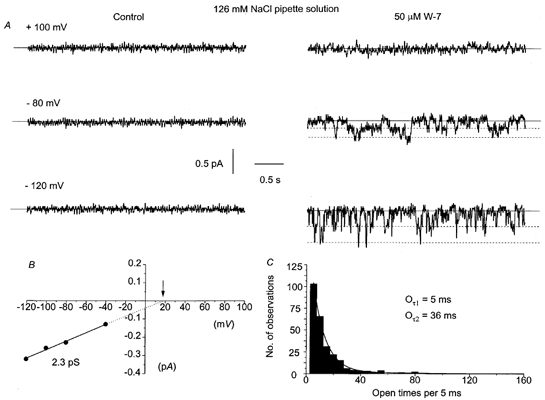
A, with a 126 mm NaCl pipette solution, bath application of 50 μm W-7 evoked single channel currents at negative patch potentials. B, the I–V relationship of the W-7-evoked single channels currents shown in A. C, at −80 mV, the open time distribution of the W-7-evoked single channels shown in A could be described by the sum of two exponentials with time constants of 5 ms (Oτ1) and 36 ms (Oτ2).
DISCUSSION
A non-selective cation conductance activated by Ca2+ store depletion in rabbit portal vein smooth muscle cells
The present study describes the properties of a Ca2+-permeable non-selective cation conductance in freshly dispersed rabbit portal vein smooth muscle cells that is activated by agents that deplete internal Ca2+ stores.
Several lines of evidence suggest that the single channel currents monitored with cell-attached recording underlie the whole-cell current. First, both whole-cell and single channel currents activated by CPA had a similar latency (∼120 s), suggesting that they are activated by the same mechanism. Greenwood et al. (1997) showed that CPA abolished Ca2+-activated chloride currents after about 150 s in the same type of cell. Since these latter responses are mediated by Ca2+ ions released from internal stores, the results of the present study suggest that the whole-cell and single channel currents are both activated by depletion of internal Ca2+ stores. Second, the properties of both the store-operated whole-cell and single channel currents were altered in 0 mm . Specifically, in 0 mm , the amplitude of the whole-cell currents was much larger (∼10-fold at −50 mV) than in 1.5 mm and Er was shifted from +50 to −5 mV. With cell-attached recording in 0 mm , the unitary conductance of the single channel currents increased 3- to 4-fold, from approximately 2 to 7 pS, which contributed (at least partially) to the larger amplitude whole-cell currents. Furthermore, channel currents had an Er of −4 mV in 0 mm , which is a similar value to the Er of the store-operated whole-cell currents in 0 mm .
In the present study, the I–V relationship of the store-operated whole-cell currents in 1.5 mm exhibited marked rectification with inward rectification at potentials < −50 mV, outward rectification at potentials > +60 mV and an Er of +50 mV, whereas the single channel currents were resolved only at negative membrane potentials and had an extrapolated Er of ∼+20 mV. Single channel currents might not have been resolved at very positive potentials due to a reduction in conductance and/or a change in open probability. The difference in the Er of the whole-cell currents and the extrapolated Er for single channel currents may be caused by an underestimation of the extrapolated Er of the channel currents if the I–V relationship of the single channel currents deviated from linearity at potentials positive to −40 mV.
It is generally accepted that store-operated conductances are activated by depletion of Ca2+ within internal stores, rather than by a rise in the intracellular Ca2+ concentration. Therefore, an important similarity between the whole-cell and single channel currents is that they were not activated by a rise in intracellular Ca2+ concentration. Whole-cell currents were activated with a patch pipette solution that contained 10 mm BAPTA to chelate the cytosolic Ca2+ concentration to approximately 14 nm. Single channel currents were activated by the cell-permeable Ca2+ chelator, BAPTA-AM, which depletes internal Ca2+ stores by chelating Ca2+ which is passively moving out of the stores. In addition, application of Ca2+ (2 μm) to the intracellular surface of inside-out patches does not activate single cation channel currents (A. P. Albert & W. A. Large, unpublished results). Therefore, both the store-operated whole-cell and single channel currents described in this paper are not Ca2+ activated.
Comparison to store-operated conductances in other vascular smooth muscle preparations
The store-operated cation conductance described here has some similarities with, but also a number of important differences to, store-operated conductances described recently for other vascular smooth muscle preparations. In particular, there are differences in unitary conductance, permeability to Ca2+ ions, effect of 0 mm Ca2+, I–V relationships and gating properties. The store-operated channel currents described in the present study have a unitary conductance of ∼2 pS, which is smaller than the unitary conductances of store-operated cation channels described in aortic myocytes (∼3 pS, Trepakova et al. 2000, 2001) and pulmonary artery myocytes (∼5 pS, Golovina et al. 2001). Moreover, in our experiments in 0 mm the unitary conductance was greater than in 110 mm (7 vs. 1 pS, respectively). By contrast, in aortic myocytes, the unitary conductance was the same (in ∼3 pS) in 0, 1.5 and 90 mm (Trepakova et al. 2001).
In the present study, the store-operated whole-cell and single channel currents were shown to be highly permeable to Ca2+ ions. The Er of the whole-cell currents was shifted from +50 mV in 1.5 mm to −5 mV in 0 mm . The extrapolated Er of the single channel currents was shifted from about +20 mV in 1.5 mm to about −4 and +90 mV in 0 and 110 mm , respectively. A rigorous study of the relative permeability of monovalent and divalent ions through the cation channel was not carried out, due to the large degree of extrapolation needed to estimate Er. However, using the equation of Hille (1992), from measurement of changes in Er, the relative permeability ratio of Ca ions to Na ions (PCa/PNa) was approximately 50 in our study. In aortic myocytes, the Er of the store-operated single channel currents was not altered in 0 and 90 mm , indicating that the channels are not highly permeable to Ca ions (PCa/PNa = 1, Trepakova et al. 2001). In choroidal arteriole myocytes, the Er of caffeine-activated cation currents was shifted from about +29 to +7 mV upon changing the external Ca2+ concentration from 2 to 0 mm, indicating that this store-operated conductance has a high permeability to Ca ions (Curtis & Scholfield, 2001). In pulmonary artery myocytes, the relative permeability of Na+ ions to Ca ions of CPA-evoked single channel currents was not measured, although the extrapolated Er in 20 mm was approximately +20 mV, suggesting that the store-operated channels are also permeable to Ca ions (Golovina et al. 2001).
We have demonstrated in 1.5 mm that the I–V relationship of the store-operated whole-cell currents has marked rectification properties. In aortic myocytes, the I–V relationship of the store-operated whole-cell currents exhibited outward rectification that was not affected by (Trepakova et al. 2001). These differences in the I–V relationships might reflect differences either in the behaviour of single channel currents or in experimental conditions. However, there were significant differences in the gating properties of the single channel currents. In the present study, the store-operated single channel currents in 1.5 mm could only be resolved at negative potentials, whereas single channel currents in aortic myocytes could be resolved at negative and positive potentials, with the single channel activity having a higher open probability at positive potentials (Trepakova et al. 2001). We have also shown in portal vein myocytes that store-operated single channel currents in inside-out patches have similar properties to those recorded in cell-attached patches. Therefore, these gating properties might be an intrinsic characteristic of the channel. In addition, with cell-attached recordings in 0 mm , single channel currents could be resolved at positive potentials, suggesting that has a profound effect on the gating properties of store-operated channels. By contrast, in aortic myocytes, the gating properties were not altered in 0 mm (Trepakova et al. 2001). Therefore, these gating properties represent another important difference in the store-operated conductances observed in different vascular smooth cell types.
The store-operated cation channel currents can be spontaneously active
In the present study, there were spontaneously active single channel currents with similar properties to store-operated channel currents in 40 % of cell-attached patches. In vascular smooth muscle cells, localised release of Ca2+ (or Ca2+ sparks) has been observed in many preparations, including rabbit portal vein (Jagger et al. 2000; Gordienko et al. 2001). Ca2+ sparks generate spontaneous outward K+ and inward Cl− currents (STOCs and STICs, respectively; Gordienko et al. 1999; Jagger et al. 2000), which have both been found in rabbit portal vein myocytes (Benham & Bolton, 1986; Wang et al. 1992) and represent sporadic but continuous release of Ca2+ from internal stores. Therefore, the activity of spontaneous store-operated channel currents seen in some cells may be triggered by this spontaneous release of Ca2+ ions.
Agents that inhibit calmodulin activate cation channel currents that have properties similar to store-operated cation channel currents
There are a number of proposed mechanisms which may link the depletion of internal Ca2+ stores to ion channels in the plasmalemma. For example, it has been suggested that diffusible intracellular factors and IP3 receptors activate Ca2+ store-operated plasmalemmal channels (Parekh & Penner, 1997). An important finding in the present study was that application of calmodulin inhibitors activated cation channel currents with characteristics similar to currents evoked by depletion of internal Ca2+ stores. This implies that in an intact resting cell, the Ca2+-binding protein, calmodulin, is involved in inhibiting the activity of store-operated cation channels. Zhang et al. (2001) showed that human Trp3 cation channels, which are proposed to be store-operated channels, can be activated by calmidazolium and a Trp-binding peptide (produced from a portion of the IP3 receptor gene sequence), suggesting that an interaction between calmodulin and IP3 receptors plays a pivotol role in linking depletion of internal Ca2+ stores with the opening of plasmalemmal ion channels.
In conclusion, the present study describes a Ca2+-permeable cation channel in rabbit portal vein smooth muscle cells that is activated by depletion of intracellular Ca2+ stores. This channel has marked differences from store-operated channels in other types of vascular myocytes. We propose that this channel is important for refilling internal Ca2+ stores.
Acknowledgments
The work was supported by the Wellcome Trust and the British Heart Foundation. We are grateful to Dr D. C. Ellershaw for assistance in preparing the manuscript.
REFERENCES
- Albert AP, Large WA. Store-operated inward ion channels in rabbit portal vein myocytes. Journal of Physiology. 2001;531P:80P. doi: 10.1113/jphysiol.2002.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. Journal of Physiology. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Capacititive calcium entry. Biochemical Journal. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. Journal of Physiology. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX-J. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. American Journal of Physiology - Heart and Circulatory Physiology. 2001;H280:746–755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Greenwood IA, Bolton TB. Direct visualization of sacroplasmic reticulum regions discharging Ca2+ sparks in vascular myocytes. Cell Calcium. 2001;29:13–28. doi: 10.1054/ceca.2000.0180. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Helliwell RM, Large WA. Modulation of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle by an inhibitor of mitrochrondrial Ca2+ uptake. Journal of Physiology. 1997;505:53–64. doi: 10.1111/j.1469-7793.1997.053bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. MA, USA: Sinauer Associates; 1992. pp. 337–361. [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular stores activates a calicum current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jagger JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. American Journal of Physiology — Cell Physiology. 2000;278C:235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the calcium-activated chloride conductance in smooth muscle. American Journal of Physiology. 1996;271C:435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiological Reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Csutora P, Hunton DL, Marchase RB, Cohen RA, Boltina VM. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. Journal of Biological Chemistry. 2000;34:26158–26163. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Boltina VM. The properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. Journal of Biological Chemistry. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Vaca L. Calmodulin inhibits calcium influx current in vascular endothelium. Federation of European Biomedical Societies. 1996;390:289–293. doi: 10.1016/0014-5793(96)00675-8. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hogg RC, Large WA. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. Jounal of Physiology. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. British Journal of Pharmacology. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, Wallace P, Gibson A, McFadzean I. Correlation between store-operated cation current and capacitative Ca2+ influx in smooth muscle cells from mouse anococcygeus. European Journal of Pharmacology. 1999;376:325–329. doi: 10.1016/s0014-2999(99)00400-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson D, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding site. Proceedings of the National Academy of Sciences of the USA. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]


