Abstract
Activation of neurons in the region of the dorsomedial hypothalamus (DMH) appears to generate the sympathetically mediated tachycardia seen in experimental stress in rats. The purpose of this study was to assess the role of neurons in the area of the medullary raphe pallidus (RP) in the tachycardia caused by stimulation of the DMH. The cardiovascular response to microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI) 10 pmol (100 nl)−1 into the DMH was assessed before, and after, injection of the GABAA receptor agonist muscimol 80 pmol (100 nl)−1 or saline vehicle 100 nl into the RP in urethane-anaesthetized rats. Tachycardia evoked by microinjection of BMI into the DMH was mimicked by microinjection of BMI 30 pmol (75 nl)−1 into the RP. This DMH-induced tachycardia was markedly suppressed after injection of muscimol into the RP, but the response was unaffected by injection of saline into the same region. Thus, DMH-induced tachycardia is mediated through activation of neurons in the area of the RP, suggesting that these neurons may play a previously unrecognized role in stress-induced cardiac stimulation.
Chemical stimulation or disinhibition of neurons in the region of the dorsomedial hypothalamus (DMH) in rats evokes physiological and behavioural responses similar to those seen in emotional stress, including marked increases in heart rate and more modest increases in blood pressure (Shekhar et al. 1990; Soltis & DiMicco, 1991; Bailey & DiMicco 2001). In conscious animals, microinjection of the GABAA receptor agonist and neuronal inhibitor muscimol into the same region has been shown to inhibit the increase in heart rate evoked by experimental air stress (Stotz-Potter et al. 1996a, b). These data suggest that activation of neurons in the region of the DMH is important in eliciting stress-induced tachycardia.
Currently, the central pathway mediating the increase in heart rate caused by activation of the DMH is unknown. However, disinhibition of neurons in the region of the raphe pallidus (RP) has been reported to elicit a pattern of cardiovascular changes similar to those seen upon stimulation of the DMH (Morrison et al. 1999, 2000). Tracing studies provide evidence for a direct projection from neurons in the DMH to the RP (ter Horst & Luiten, 1986; Hosoya et al. 1987) and for projections from neurons in the RP to the region of the spinal cord where sympathetic preganglionic neurons influencing heart rate are found (Miura et al. 1983; Jansen et al. 1995). However, no experimental evidence exists that would suggest a specific role for this pathway in cardiovascular regulation. We hypothesize that activation of neurons in the region of the RP is responsible for the tachycardia evoked by activation of neurons in the DMH. If so, this would point to an important but heretofore unrecognized role for neurons in the RP in stress-induced cardiac stimulation. To test this hypothesis, we examined the increases in heart rate after microinjection of BMI (10 pmol (100 nl)−1) into the DMH prior to and following inhibition of the RP with muscimol (80 pmol (100 nl)−1).
METHODS
Animal preparation
Experiments employed adult male Sprague-Dawley rats (250–350g) that were housed individually and allowed food and water ad libitum. All procedures conformed to the guidelines set forth by NIH and were approved by the Institutional Animal Care and Use Committee. Under pentobarbital anaesthesia (50 mg (kg body wt)−1i.p., supplemented as needed), rats were implanted with a unilateral guide cannula (26 gauge, Plastics One Inc., Roanoke, VA, USA) targeted to the DMH as described previously (Bailey & DiMicco, 2001). The guide cannula was inserted at an angle of 10 deg from the saggital plane and targeted to 1.2 mm posterior, 2.1 mm lateral and 9.1 mm ventral, relative to bregma with the stereotaxic incisor bar elevated 5 mm above the interaural line. The guide cannula was fixed in place with dental acrylic anchored to one or two screws placed in the skull. Animals were allowed to recover in their home cages for at least 3 days following this surgery.
After their recovery, rats were anaesthetized with urethane (1.35 g (kg body wt)−1i.p., supplemented as needed) and the femoral artery was cannulated. Throughout all experiments, body temperature was maintained at 36–38 °C with a heating blanket connected to a homeothermic temperature control unit. The arterial line was attached directly to a pressure transducer for continuous recording of blood pressure and heart rate using MacLab hardware and software (AD Instruments, Mountain View, CA, USA). Animals were then placed in a stereotaxic instrument (ASI, Inc., Warren, MI, USA) with the incisor bar lowered 11 mm below the interaural line for access to the RP as described previously by Morrison and co-workers (Morrison et al. 1999). A partial occipital craniotomy was performed and the atlanto-occipital membrane removed for direct observation of the obex. This landmark was used as a reference for targeting microinjections to the RP at the following co-ordinates: 3.0 mm anterior, 0.0 mm lateral, and 2.6 mm ventral relative to the obex.
Chemicals
Bicuculline methiodide (Sigma) was dissolved in 0.1 m phosphate buffered saline (PBS) to final concentrations of 0.1 and 0.4 mm for injections into the DMH and RP, respectively. Muscimol (Sigma) was dissolved in 0.1 m PBS to a final concentration of 0.8 mm for all microinjections. Alcian Blue was dissolved in 100 % ethanol to a final concentration of 2.5 % for marking sites of injection.
Experimental procedures
For the duration of each experiment, heart rate and blood pressure were recorded continuously. The criteria for inclusion in this study were that animals have an initial baseline heart rate of ≤ 400 beats per minute (beats min−1), and that animals in the experimental groups exhibit an increase in heart rate of at least 50 beats min−1 in response to microinjection of BMI into both the DMH and the RP. The microinjection protocol commenced only after a stable baseline heart rate and blood pressure had been established for ∼10 min. Adequate time (at least 40 min) was allowed between injections for return to a stable baseline. Microinjections of BMI (10 pmol (100 nl)−1 over 30 s) into the DMH were performed using a 33 gauge injection cannula attached to a 10 μl Hamilton syringe mounted on an infusion pump. Microinjections into the region of the RP were performed using glass micropipettes (O.D. ∼40 μm) fitted with a reticule eyepiece micrometer. The micropipettes were attached to a picospritzer and back filled with the appropriate solution using negative pressure. The pipettes were lowered into the RP and graded puffs of compressed nitrogen were used to deliver precise volumes of drug as measured by observing the movement of the meniscus relative to the eyepiece micrometer.
In experimental studies, each animal (n = 9) received an initial injection of BMI (10 pmol (100 nl)−1 into the region of the DMH, and upon returning to a stable baseline received an injection of BMI (30 pmol (75 nl)−1) into the region of the RP. This dose of BMI was selected in order to provoke sympathetically mediated tachycardia on the basis of the reported results of Morrison and coworkers (1999). Animals were then randomly assigned to receive either an injection of muscimol (80 pmol (100 nl)−1; muscimol treatment group, n = 5) or saline (100 nl; saline treatment group, n = 4) into the RP followed 5 min later by a second identical injection of BMI into the DMH. We have previously demonstrated that injection of muscimol 80 pmol (100 nl)−1 at hypothalamic sites elicits inhibitory effects with a high degree of neuroanatomical resolution (Stotz-Potter et al. 1996a, b; Morin et al. 2001).
In a parallel series of control studies, the effects of saline or muscimol treatment alone on heart rate and blood pressure were assessed. Thus, animals received only an initial injection of BMI into the DMH and into the RP, followed by an injection of muscimol (80 pmol (100 nl)−1; muscimol control group, n = 4) or saline (100 nl; saline control group, n = 4) into the RP without a second injection of BMI into the DMH.
At the end of each experiment, 100 nl of 2.5 % Alcian Blue dye in 100 % ethanol was microinjected at the injection sites in the DMH and RP for subsequent histologic confirmation. Animals were then perfused transcardially with 120 ml of 0.9 % saline followed by 180 ml of 4 % paraformaldehyde in 0.1 m PBS. The brains were removed and stored overnight at 4 °C in 4 % paraformaldehyde and then cryoprotected by immersion in 30 % sucrose for at least three days. Brains were blocked and 40 μm coronal sections were cut on a cryostat in the region of the DMH and the RP. Sections were mounted on slides and then counterstained with Neutral Red for histological confirmation of the injection sites in the DMH and RP. The atlas of Paxinos & Watson (1998) was used as a reference.
Data analysis
Baseline heart rate and blood pressure in each group as well as changes in these parameters over time were analysed using one-way or repeated measures ANOVA as appropriate. Where significant differences were detected, post-hoc comparisons were made with Student's paired or grouped t tests corrected for multiple comparisons as necessary. Level of significance was set at P < 0.05. All data are reported as means ± standard error of the mean.
RESULTS
There were no significant differences in mean baseline heart rate or blood pressure between any of the groups (one-way ANOVA). Mean baseline heart rates were 360 ± 8, 362 ± 13, 379 ± 6 and 368 ± 5 beats min−1 and mean baseline blood pressures were 102 ± 8, 91 ± 5, 90 ± 3, and 92 ± 1 mmHg in the muscimol treatment, saline treatment, muscimol control and saline control groups, respectively.
Injection of BMI (10 pmol (100 nl)−1) into the DMH elicited a marked and immediate increase in heart rate that reached its maximum (mean, +84 ± 8 and +87 ± 10 beats min−1 in muscimol and saline treatment groups, respectively) 6 to 12 min after the beginning of the injection (mean time to peak, 10 ± 0.6 and 8.5 ± 0.9 min in the muscimol and saline treatment groups, respectively) and slowly returned to a stable baseline over the next 20–30 min (Figs 1 and 2). Neither the mean increase in heart rate evoked by the initial injection of BMI nor the mean time to peak increase for this effect was different between the two treatment groups (Student's t test). The corresponding increases in blood pressure were smaller and more variable among different animals, but usually paralleled the increases in heart rate (Fig. 2).
Figure 1.
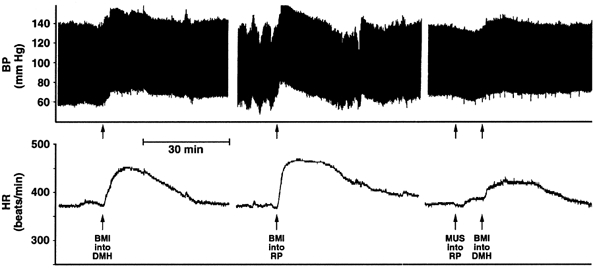
Recording of arterial pressure (BP; top) and heart rate (HR; bottom) from a typical experiment depicting effects of bicuculline methiodide (BMI) injected into the region of the raphe pallidus (RP at 30 pmol (100 nl)−1; second arrow from left) and into the dorsomedial hypothalamus (DMH at 0 pmol (100 nl)−1; first and fourth arrows) before and after injection of muscimol (MUS at 80 pmol (100 nl)−1; third arrow) into RP.
Figure 2. Effect of treatment in raphe pallidus on cardiovascular changes evoked from the dorsomedial hypothalamus.
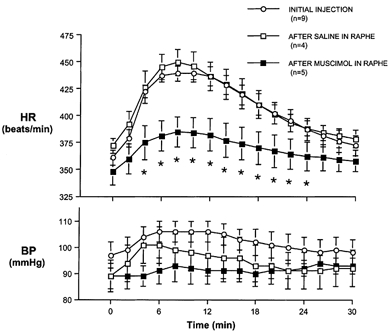
Mean heart rate (HR, top) and blood pressure (BP, bottom) after microinjection of bicuculline methiodide (BMI) 10 pmol (100 nl)−1 into the dorsomedial hypothalamus (DMH) initially (○) and after injection of saline (100 nl; □) or muscimol (80 pmol (100 nl)−1; ▪) into the region of the raphe pallidus. * Significantly different from initial response to BMI (repeated measures ANOVA and post-hoc paired t test).
In 17 of 19 experiments, injection of BMI (30 pmol (75 nl)−1 at stereotaxic co-ordinates for the RP elicited marked and immediate tachycardia that met the criterion for further study, as described above, and closely resembled that seen after microinjection of this agent into the DMH (Figs 1 and 3). In every such case, the site of injection proved to be localized to the immediate vicinity of the RP (Fig. 4). Heart rate reached its maximum (mean +86 ± 10 and +99 ± 11 beats min−1 in muscimol and saline treatment groups, respectively) between 8 and 10 min after injection (mean time to peak, 9.2 ± 0.5 and 8.5 ± 0.8 min, in the muscimol and saline treatment groups, respectively) and returned to a stable baseline over the next 40–50 min. Again, the amplitude and time-to-peak increase was equivalent in the two treatment groups. Microinjection of BMI into the RP was followed by an immediate modest pressor response (mean maximum: +12 ± 5 and +10 ± 4 mmHg in the muscimol and saline treatment groups, respectively) that reached its peak at 3.6 ± 0.4 min in the muscimol treatment group and at 4 min in the saline treatment group, and returned to baseline after ∼45 min.
Figure 3.

Mean heart rate (HR, top) and blood pressure (BP, bottom) after microinjection of bicuculline methiodide (BMI) 30 pmol (75 nl)−1, muscimol 80 pmol (100 nl)−1, or saline 100 nl into region of the raphe pallidus. * Significant change from baseline (repeated measures ANOVA and post-hoc paired t test with Bonferroni correction.)
Figure 4. Schematic coronal sections of rat brainstem (adapted from Paxinos & Watson, 1998).
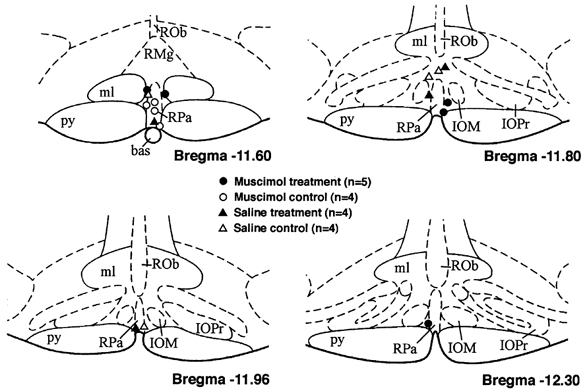
Sites of injection in region of raphe pallidus are shown for all experiments described above. Abbreviations: bas, basilar artery; IOM, inferior olive medial nucleus; IOPr, inferior olive principal nucleus; ml, medial lemniscus; py, pyramidal tract; Rob, raphe obscurus; RMg, raphe magnus; RPa, raphe pallidus.
After injection of saline vehicle (100 nl) into the RP, a second microinjection of BMI into the DMH produced increases in heart rate (maximum, +78 ± 10 beats min−1 at 8 min) and small, variable changes in blood pressure that were virtually identical to those seen after initial injection of BMI into the DMH (P > 0.05 by Student's paired t test). In contrast, the increases in heart rate produced by microinjection of BMI into the DMH after microinjection of the GABAA receptor agonist muscimol (80 pmol (100 nl)−1) into the RP were significantly attenuated relative to the initial response (mean maximum, +38 ± 3 beats min−1). Mean time-to-peak increase in heart rate remained similar at 8.0 ± 0.6 min following the beginning of the injection.
In control experiments in which cardiovascular status was monitored after microinjection of muscimol and saline into the RP without subsequent injection of BMI into the DMH, blood pressure and heart rate after muscimol did not differ significantly from that seen after microinjection of saline (Fig. 3). In both groups, heart rate increased slightly over the 30 min observation period (maximum increases of 17 ± 1 beats min−1 after muscimol and 15 ± 2 beats min−1 after saline) while blood pressure tended to decrease slightly (maximum change, −1 ± 7 and −6 ±3 mmHg after muscimol or saline, respectively).
As indicated above, injection sites targeting the RP in all experiments for which data are presented were found to be in the region of this nucleus (Fig. 4). Injection sites targeting the DMH (not shown) were all found to be in the active region as described previously (Soltis & DiMicco, 1991, 1992) in or immediately adjacent to the dorsomedial hypothalamic nucleus according to the atlas of Paxinos & Watson (1998).
DISCUSSION
The results of the present study clearly indicate that the tachycardia seen after disinhibition of the DMH in rats is mediated, at least in large part, through activation of neurons in the region of the RP. As reported by Morrison and colleagues (1999), microinjection of the GABAA receptor antagonist BMI into the RP provoked marked and immediate increases in heart rate with modest increases in blood pressure, closely resembling the cardiovascular changes seen after similar disinhibition of neurons in the DMH (Fig. 2). In this study, microinjection of muscimol, an agent thought to be inhibitory to virtually all mammalian neurons by virtue of its GABAA receptor agonist properties (Johnston, 1996), into the same sites in the RP markedly suppressed the tachycardic response to subsequent microinjection of BMI into the DMH. These findings suggest that the same neurons in the region of the RP that mediate tachycardia when disinhibited by local injection of BMI are excited by activation of the DMH to produce similar cardiovascular changes.
The precise location of the neurons that are relevant to these changes is not clear from our results but seems likely to be in the immediate vicinity of the injection sites in the RP. In our previous studies, the same dose and volume of muscimol injected into hypothalamic sites was shown to produce effects that are highly localized (Stotz-Potter et al. 1996a). Furthermore, identical injections of muscimol alone into the region of the RP provoked changes in blood pressure and heart rate that were not different from those seen after similar injection of saline vehicle. Thus, this treatment failed to influence the activity of sympathetic premotor neurons that are responsible for maintaining sympathetic vasomotor tone and located lateral to the RP in the rostral ventrolateral medulla (RVLM). Recently, Fontes and colleagues (2001) reported that microinjection of a much larger dose of muscimol (1 nmol (100 nl)−1) into the RVLM effectively antagonized the pressor response elicited by injection of a slightly higher concentration of BMI into the DMH (20 pmol (40 nl)−1). However, the accompanying increase in heart rate was unaffected, suggesting that neurons in the RVLM were responsible for the pressor response but not the tachycardia evoked from the DMH. Our data indicate that cardiac effects of disinhibition of neurons in the region of the DMH are mediated through a distinct pathway involving activation of neurons in the immediate vicinity of the midline RP.
Tracing studies provide evidence for a relay in the RP in the pathway from the DMH to sympathetic preganglionic motor neurons influencing the heart. In a retrograde tracing study in cats, the RP contained the most labelled cells of any brain region after tracer was injected into the spinal cardioacceleratory centre in the intermediolateral cell column at T3–T4 (Miura et al. 1983). Neurons in the RP were also heavily retrogradely labelled after microinjection of a transynaptic viral tracer into the stellate ganglion (Jansen et al. 1995). Neurons in the region of the DMH send direct projections to the RP (ter Horst & Luiten, 1986) and these neurons seem to be concentrated specifically in the area dorsally adjacent to the dorsomedial hypothalamic nucleus itself (Hosoya et al. 1987; Hermann et al. 1997). Thus, anatomical and functional evidence support the notion that DMH-induced tachycardia is mediated through a direct excitatory projection to spinally projecting neurons in the RP.
Fontes and colleagues (2001) speculated that neurons in the region of the midline raphe might mediate the cardiac effects of stimulation of the DMH by means of an additional synaptic relay in the periaqueductal grey (PAG). This conjecture was based upon evidence that the PAG, a structure implicated in the integration of defence-like reactions, both receives synaptic input from the DMH and sends projections to neurons in the ventral medullary raphe that are trans-synaptically labelled from the stellate ganglion (Farkas et al. 1998). However, this pathway to which Fontes and coworkers alluded appears to involve primarily neurons in the raphe magnus. Unlike the raphe pallidus, stimulation of neurons in the raphe magnus has never been reported to provoke tachycardia. Nevertheless, because of the relative proximity of the raphe magnus and raphe pallidus and the limited anatomical resolution afforded by current microinjection techniques, the effects reported here may be mediated by neurons in either region. Therefore, the alternative pathway suggested by Fontes and coworkers (2001) cannot be excluded on the basis of our findings.
Regardless of which of these pathways is relevant to our results, its importance is highlighted by its potential role as the principal mediator of stress-induced cardiac stimulation. Previous findings indicate that neurons in the DMH are responsible for signalling a variety of autonomic, neuroendocrine, and behavioural alterations typically seen under conditions of emotional stress. Thus, the cardiovascular response to activation of the DMH not only closely resembles that typically seen in such a setting in rats and other mammals, but is accompanied by other physiological and behavioural changes appropriate to this context (Shekhar et al. 1987, 1990; Bailey & DiMicco, 2001). Conversely, microinjection of muscimol or of antagonists of excitatory ionotropic glutamate receptors into the same region of the DMH suppresses the increases in heart rate seen in experimental air jet stress (Soltis & DiMicco, 1992, Stotz-Potter et al. 1996a, b). It is therefore likely that the same neurons in the DMH are responsible for the tachycardia seen after local disinhibition and under conditions of experimental stress. Accordingly, the same efferent pathway involving a synaptic relay through neurons in the RP probably mediates stress induced cardiac stimulation.
To summarize, activity of neurons in the region of the medullary RP appears to be required for the generation of sympathetically mediated tachycardia resulting from activation of the DMH. As neurons in the DMH are likely to be responsible for the generation of the similar marked tachycardia typically occurring in experimental emotional stress, this finding points to a new role for neurons in the RP as key players in the cardiac stimulation seen under this condition. The key test of this hypothesis will rely on future studies that can only be pursued in conscious animals. Nonetheless, together with previous findings, these results point to a putative pathway for emotional stress-induced tachycardia extending from hypothalamic command neurons in the DMH through a medullary relay in the RP to sympathetic preganglionic neurons in the spinal cord that give rise to cardiac sympathetic pathways.
Acknowledgments
This work was supported by NIH Grant NS 19883. The authors wish to thank Dr Clyde Killian for his assistance with the statistical analysis of the data.
REFERENCES
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. American Journal of Physiology — Regulatory, Integrative and Comparative Physiology. 2001;280:R8–15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Research. 1998;792:179–192. doi: 10.1016/s0006-8993(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Fontes MAP, Tagawa T, Polson JW, Cavanagh S -J, Dampney RAL. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H2891–2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi P -H, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) Journal of Chemical Neuroanatomy. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Ito R, Kohno K. The topographical organization of neurons in the dorsal hypothalamic area that project to the spinal cord or to the nucleus raphe pallidus in the rat. Experimental Brain Research. 1987;66:500–506. doi: 10.1007/BF00270682. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- Johnston GAR. GABAA receptor pharmacology. Pharmacology and Therapeutics. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Miura M, Onai T, Takayama K. Projections of upper structure to the spinal cardioacceleratory center in cats: an HRP study using a new microinjection method. Journal of the Autonomic Nervous System. 1983;7:119–139. doi: 10.1016/0165-1838(83)90041-3. [DOI] [PubMed] [Google Scholar]
- Morin SM, Stotz-Potter EH, Dimicco JA. Injection of muscimol into dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. American Journal of Physiology — Regulatory, Integrative and Comparative Physiology. 2001;280:R1276–1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [DOI] [PubMed] [Google Scholar]
- Morrison S, Sved A, Passerin A. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. American Journal of Physiology. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Morrison S, Ramamurthy S, Young J. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. Journal of Neuroscience. 2000;20:9264–9271. doi: 10.1523/JNEUROSCI.20-24-09264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Shekhar A, Hingtgen JN, Dimicco JA. Selective enhancement of shock avoidance responding elicited by GABA blockade in the posterior hypothalamus of rats. Brain Research. 1987;420:118–128. doi: 10.1016/0006-8993(87)90246-0. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Hingtgen JN, Dimicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Research. 1990;512:81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- Soltis RP, Dimicco JA. Interaction of hypothalamic GABAA and excitatory amino acids receptors controlling heart rate in rats. American Journal of Physiology. 1991;30:R527–433. doi: 10.1152/ajpregu.1991.261.2.R427. [DOI] [PubMed] [Google Scholar]
- Soltis RP, Dimicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia. American Journal of Physiology. 1992;262:R689–697. doi: 10.1152/ajpregu.1992.262.4.R689. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, Dimicco JA. Muscimol acts in the dorsomedial hypothalamus but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. Journal of Neuroscience. 1996a;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, Dimicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Research. 1996b;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- ter Horstter GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Research Bulletin. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]


