Abstract
Short chain fatty acids, particularly butyrate, stimulate electroneutral NaCl absorption from the colon. Their effect in colonic epithelia lacking basal electroneutral NaCl absorption is unknown. Butyrate is also reported to inhibit active Cl− secretion in the colon. The present studies were undertaken to investigate the inter-relationships between the effects of butyrate on active Na+ and Cl− transport in the colon. Studies were carried out in rabbit distal colon (known to have predominant electrogenic Na+ absorption), rat distal colon (characterised by electroneutral Na+ absorption), and hyperaldosteronaemic rat distal colon (characterised by electrogenic Na+ absorption). The effect of cholera toxin (CT) was also noted. Potential difference, short-circuit current (ISC) and fluxes of Na+ and Cl− were measured in stripped mucosa under voltage-clamp conditions. Butyrate stimulated electroneutral Na+ and Cl− absorption in distal colon of normal and salt-depleted rats, and stimulated Na+ absorption in rabbit distal colon. Amiloride (10−4m) or CT did not inhibit this process. In rabbit distal colon, stimulation of Na+ absorption by butyrate was not dependent on the presence of Cl− in the medium. Butyrate significantly decreased conductance, decreased flux of sodium from serosa to mucosa (particularly in rabbit distal colon), and decreased ISC. Net Cl− secretion, induced by CT, was completely inhibited by butyrate. Stimulation of Na+ absorption was independent of exposure to CT. Bumetanide reversed net Cl− secretion to net absorption, but did not alter Na+ or Cl− fluxes in tissues exposed to butyrate. Thus butyrate stimulates active Na+ absorption in colonic epithelia, with or without expression of basal Na+-H+ exchange. Independently, butyrate inhibits active Cl− secretion induced by cAMP in these epithelia.
Short chain fatty acids (SCFA), products of bacterial fermentation of unabsorbed carbohydrate, are the major anions of the colon in health. The important SCFA in the normal human colon are acetate, propionate and butyrate, which together reach or exceed concentrations of 100 mm. Of these, acetate is the most abundant, but butyrate plays the most important role in colonic physiology. SCFA play an important role in modulating colonic electrolyte transport. The ability of SCFA to stimulate sodium absorption from the mammalian colon has been shown in vivo and in vitro (Bugaut, 1987). Flux studies in vitro have shown that SCFA stimulate an electroneutral Na+ and Cl− absorptive process in the colon (Binder & Mehta, 1989). The SCFA-linked absorption pathway appears to involve the movement of SCFA across the apical membrane into the colonic epithelial cell, through either non-ionic diffusion or SCFA-HCO3− exchange, followed by stimulation of Na+-H+, Cl−-SCFA and Cl−-HCO3− exchanges across the apical membrane of the colon (Rajendran & Binder, 1994; Charney et al. 1998). SCFA-dependent electroneutral NaCl transport is distinct from bicarbonate-dependent electroneutral NaCl transport, and is now established as one of the major transport pathways for Na+ in the mammalian colon. Cyclic AMP (cAMP) causes net secretion by inhibiting bicarbonate-dependent electroneutral NaCl absorption in surface epithelial cells and by turning on electrogenic Cl− secretion in crypt epithelial cells. In vivo studies have shown that butyrate inhibits net fluid secretion induced by cholera toxin in the rat colon (Ramakrishna et al. 1990). In studies using rat distal colon mounted in flux chambers in vitro, theophylline failed to inhibit butyrate-linked NaCl absorption (Binder & Mehta, 1990), suggesting that butyrate-dependent NaCl absorption was not sensitive to the effect of cAMP. On the other hand, studies using rat distal colon mucosa in vitro have shown that butyrate had an inhibitory effect on cAMP-induced Cl− secretion, which is the process underlying toxin-induced fluid secretion (Dagher et al. 1996).
Electrogenic Cl− secretion, via chloride channels in the apical membrane of epithelial cells, is the fundamental means by which mucosal surfaces are hydrated in health. Defective regulation of this process underlies a number of diseases of considerable importance, including secretory diarrhoea and cystic fibrosis. Multiple intracellular pathways acting through cAMP and cGMP, calcium/calmodulin and diacylglycerol regulate these chloride channels (Berger et al. 1993; Gabriel et al. 1993; Vaandrager et al. 1998; Seibert et al. 1999). Disturbance in one or more regulatory pathways leads to hypersecretion in the intestine, causing secretory diarrhoea. For electrogenic Cl− secretion to take place at the apical membrane, chloride has to be internalised into the cell across the basolateral membrane primarily via bumetanide-inhibitable Na+-K+-2Cl− cotransport. In cAMP-elicited Cl− secretion, activation of apical Cl− channels is generally viewed as the primary regulatory event. However, basolateral Na+-K+-2Cl− cotransport must also increase to maintain cell electrolyte composition, and therefore active Cl− secretion demands co-ordinated control of apical Cl− exit and basolateral Cl− entry. It has been shown that Na+-K+-2Cl− cotransport is stimulated by an increase in cytosolic cAMP, and inhibited by divalent cations and by a decrease in ATP levels (Haas, 1989; Hecht & Koutsouris, 1999; Marunaka et al. 1999). The factors responsible for'cross-talk' between apical and basolateral transport events are incompletely defined, and also the complex interaction of various transport elements with the intracellular mediators and secretagogues makes it difficult to clearly identify a specific effect of SCFA on secretion.
Na+-H+ exchange in the apical membrane of colonic epithelial cells is considered essential to the stimulation of Na+ absorption by SCFA (Rajendran & Binder, 1994; Gonda et al. 1999). Na+-H+ exchange is present in epithelia that demonstrate electroneutral Na+ absorption (e.g. rat distal colon), whereas it is absent in epithelia without electroneutral Na+ absorption (e.g. rabbit distal colon). In the latter, Na+ absorption across the epithelium is a two-step, electrogenic process involving mucosal amiloride-sensitive Na+ channels and active transport across the basolateral membrane via the Na+-K+-ATPase (McCabe et al. 1982). The rate-limiting step in this process is apical amiloride-sensitive Na+ entry (Thompson & Sellin, 1986; Turnheim et al. 1987). The epithelial Na+ channel (ENaC) complex is composed of three homologous subunits, α, β and γ (McDonald et al. 1994; Prince & Welsh, 1998; Stokes & Sigmund, 1998). Aldosterone induces electrogenic Na+ absorption in the distal colon, by increasing β- and γ-ENaC mRNA, with little or no effect on α-ENaC mRNA (Epple et al. 2000).
The present studies were performed to determine the effect of butyrate on active Na+ and Cl− transport in the colon, i.e. to determine whether it causes stimulation of Na+ absorption or inhibition of Cl− secretion, or both. The studies were also designed to determine whether SCFA-dependent Na+ absorption would occur in epithelia where electrogenic Na+ absorption was the major pathway of Na+ absorption, i.e. rabbit distal colon and salt-depleted rats. Salt-depleted rats exhibit suppression of electroneutral Na+ absorption with occurrence of electrogenic Na+ absorption. This process, i.e. secondary hyperaldosteronism, complicates acute gastroenteritis, resulting in altered transport characteristics of the colon (Rubens & Lambert, 1972), and was therefore included for study. Studies of the butyrate effect on Na+ and Cl− transport were therefore carried out in these various epithelia, and the effect on active Cl− secretion induced by cholera toxin was also examined.
METHODS
Rat distal colon
Non-fasting male Wistar rats weighing 200–250 g were used for these experiments. Absorption of sodium from rat distal colon is electroneutral in the basal state, and due to Na+-H+ exchange coupled to Cl−-HCO3− exchange (Rajendran & Binder, 1993). Salt depletion on the other hand induces electrogenic amiloride-sensitive Na+ transport in the colonic segment with inhibition of electroneutral NaCl absorption (Foster et al. 1983; Perrone et al. 1984; Halevy et al. 1986; Sandle & Binder, 1987; Fromm et al. 1993; Stokes & Sigmund, 1998; Grotjohann et al. 1999). Two groups of animals, a control and a salt-depleted group, were studied. Rats in both groups were fed purified diets based on AIN-93M diet for maintenance of rodents (Reeves, 1997). Normal rats received 500 mg of sodium per kilogram of diet, as recommended by the American National Research Council (Reeves, 1997), while salt-depleted rats received approximately 250 mg sodium per kilogram of diet for 14 days. Plasma aldosterone levels were determined by a commercial radioimmunoassay (Coat-A-Count Aldosterone, DPC, USA), using 125I-labelled aldosterone antibody-coated tube technology for final separation of free from bound aldosterone. Levels were measured in control and dietary sodium-depleted rat serum at the time of death. The plasma aldosterone level was 12.4 ± 3.15 ng (dl serum)−1 in control and 652 ± 51.93 ng (dl serum)−1 in salt-depleted rats. Preliminary studies revealed that this serum concentration of aldosterone was reached within 8–10 days. Rats were anaesthetised with pentobarbitone (30 mg (kg body weight)−1) and the abdomen opened by a midline incision. The distal colon was flushed with cold Ringer solution. Thereafter it was ligated at both ends, and injected luminally with 20 μg of cholera toxin (Sigma, USA) in 1 ml of Ringer solution. The abdomen was then closed in layers, and the animal kept warm under a lamp. After 150 min of incubation, the abdomen was reopened and the colon removed after exsanguination of the animal by section of the inferior vena cava. Two pieces of colon were cut from distal colon, after stripping the serosa and external muscular layers, taking care to avoid any lymph node in the area.
Rabbit distal colon
Male New Zealand White rabbits weighing 2.5–3 kg were maintained on standard diet ad libitum with free access to water. Under pentobarbitone sodium (30 mg (kg body wt)−1) and ketamine (25 mg (kg body wt)−1) anaesthesia the rabbit abdomen was opened through a 5 cm long midline incision just below the xiphisternum. All experiments were done at 16:00 h, since Na+ transport in this tissue shows circadian variation due to the influence of aldosterone (Hoffmann & Clauss, 1989). For cholera toxin pretreatment, the distal 15 cm of colon was flushed with cold Ringer solution, and a loop was constructed in which 6 ml of Ringer solution, containing 100 μg of cholera toxin (or Ringer solution without cholera toxin, as control) was instilled. The abdomen was then closed in layers, and animals maintained with anaesthesia under a lamp. The abdomen was reopened after 30 min and the loop removed after the animals were killed by exsanguination. The colon was then cut longitudinally, flushed with cold, oxygenated Ringer solution, and fixed on a wax block. The serosa and outer muscular layers were stripped by blunt dissection under a dissecting microscope. The animal protocols were approved by the Institutional Animal Ethics Committee of the Christian Medical College, Vellore.
Ion flux measurements
The isolated mucosal sheet was mounted in Lucite flux chambers (World Precision Instruments, Sarasota, FL, USA) exposing 1.13 cm2 surface area. Both sides of the tissue were bathed in the same solution. All experiments were performed at 37 °C. Solution mixing, oxygenation and pH (7.2–7.4) were maintained by continuous bubbling with a mixture of 95 % O2 and 5 % CO2. Potential difference (PD) and short-circuit current (ISC) were measured using an automatic voltage/current clamp apparatus (DVC-1000, World Precision Instruments). Conductance (G) expressed as mS cm−2 was derived through application of Ohm's law. Unidirectional mucosa-to-serosa and serosa-to-mucosa fluxes of Na+ (JNa,ms and JNa,sm) and Cl− (JCl,ms and JCl,sm) were measured by adding radioactive isotopes, 22Na and 36Cl, to either the mucosal or serosal side as appropriate. Tissues were paired for ion flux studies on the basis of differences in conductance no greater than 10 %. After equilibration, the zero time PD and ISC were noted. One millilitre of solution was withdrawn from the (cold) side opposite to the hot side, and replaced with 1 ml of unlabelled solution. From then on, the tissue was continuously clamped at zero voltage, to eliminate passive flow of ions due to electrochemical drag. After two time periods of 15 min each, bumetanide (100 μg in 0.1 % DMSO final concentration, to serosal bath solution) or amiloride (10 μm in 0.1 % DMSO final concentration, to mucosal bath solution) were added in some experiments as noted. After such perturbations, 15 min was allowed for equilibration before measuring steady-state fluxes. Samples were taken from the cold side after each flux period of 15 min, and fluxes calculated using the mean of two flux periods. At the end of the experiment, 100 μl of solution was withdrawn from the hot side to estimate total radioactivity added to the side. 22Na activity was measured using a gamma counter (CompuGamma, LKB, Sweden), while 36Cl was measured using a liquid scintillation counter (RackBeta, LKB, Sweden) after appropriate correction for 22Na. Unidirectional fluxes were calculated using standard formulae, and expressed as μmol h−1 cm−2. Net flux (Jnet) was calculated as the difference between Jms and Jsm fluxes across tissue pairs. All experiments were performed under short-circuit conditions. Both sides of the rat tissue were bathed in bicarbonate (HCO3−)-free Ringer solution in the first set of experiments, and then HCO3−-free Ringer solution containing 25 mm butyrate in the second set of experiments, while rabbit tissues were bathed in Ringer solution. HCO3−-free Ringer solution contained (mmol l−1): Na+ 140, Cl− 119.8, K+ 5.2, HPO4− 2.4, H2PO4− 0.4, Mg2+ 1.2, Ca2+ 1.2, isethionate 25 and glucose 10. Butyrate Ringer solution contained (mmol l−1): Na+ 140, Cl− 119.8, K+ 5.2, HPO4− 2.4, H2PO4− 0.4, Mg2+ 1.2, Ca2+ 1.2, butyrate 25 and glucose 10. HCO3−-free Ringer solution was used in the rat in order to avoid HCO3−-stimulated Na+ and Cl+ absorption.
Statistical analysis
Results are given as means ± s.e.m. Student's two-tailed t test (unpaired or paired as appropriate) was used to determine significance of differences. P < 0.05 was considered significant.
RESULTS
Studies in rat distal colon
Basal Na+ and Cl− flux
In HCO3−-free Ringer solution, net Na+ (3.04 ± 0.2 μmol h−1 cm−2) and Cl− (2.9 ± 0.4 μmol h−1 cm−2) absorption were of similar magnitude under basal conditions (Table 1). Addition of 10 μm amiloride did not result in any significant decrease in unidirectional flux or JNa,net and JCl,net, or in ISC, indicating that amiloride-sensitive sodium channels did not contribute to this absorption.
Table 1.
Unidirectional and net fluxes of Na+ and Cl− across rat distal colon:effect of mucosal amiloride and butyrate
| G | ISC | JNa,ms | JNa,sm | JNa,net | JCl,ms | JCl,sm | JCl,net | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Basal | 7.7 ± 0.4 | 1.4 ± 0.2 | 6.8 ± 0.4 | 3.7 ± 0.2 | 3.0 ± 0.2 | 4.6 ± 0.5 | 1.7 ± 0.4 | 2.9 ± 0.4 |
| Amiloride | 7.9 ± 0.4n.s. | 1.3 ± 0.2n.s. | 6.2 ±0.3n.s. | 3.8 ± 0.3n.s. | 2.4 ± 0.3n.s. | 5.1 ± 0.5n.s. | 2.3 ± 0.5n.s. | 2.8 ± 0.3n.s. |
| Butyrate | ||||||||
| Basal | 9.3 ± 0.4† | 0.7 ± 0.1‡ | 11.0 ± 0.6§ | 5.0 ± 0.4* | 6.1 ± 0.5§ | 8.8 ± 0.7§ | 3.8 ± 0.2§ | 5.0 ± 0.6* |
| Amiloride | 9.1 ± 0.4n.s. | 0.6 ± 0.1n.s. | 11.3 ± 0.8n.s. | 4.7 ± 0.6n.s. | 6.7 ± 0.7n.s. | 9.0 ± 1.0n.s. | 3.8 ± 0.5n.s. | 5.2 ± 0.6n.s. |
G,conductance (mS cm−2);ISC,short-circuit current (μmol h−1 cm−2);JNa,ms and JCl,ms are Na+ and Cl− flux in the mucosa-to-serosa direction;JNa,sm and JCl,sm are Na+and Cl− flux in the serosa-to-mucosa direction;JNa,net and JCl,net are net flux of Na+ and Cl− (all fluxes in μmol h−1cm−2).Control:Jnet(n =8)and G (n =16);butyrate:Jnet (n =11)and G (n =22).Amiloride was always added to the mucosal bath solution after three 15 min flux periods. Significance refers to comparison of post-amiloride with the basal condition in each group,and also between the two basal conditions
P > 0.05
P > 0.01
P > 0.001
P > 0.0001
not significantly different. Values are given as means ±s.e.m.
Effect of butyrate on basal Na+ and Cl− fluxes
Net Na+ and Cl− absorption were significantly higher (P < 0.0001 and P < 0.01, respectively) in studies with butyrate-containing solution, compared with the absence of butyrate (Table 1). Increase in net absorption was due largely to significant increase in mucosa-to-serosa flux of both Na+ (P < 0.0001) and Cl− (P < 0.0001). ISC was significantly lower when compared with HCO3−-free Ringer solution (P < 0.001). Addition of 10 μm amiloride did not bring about any significant change in unidirectional or net fluxes of Na+ or Cl−, indicating that butyrate stimulation of Na+ and Cl− absorption was not inhibited by this concentration of amiloride.
Basal Na+ and Cl− fluxes in hyperaldosteronaemic rat distal colon
An increase in ISC was noted in hyperaldosteronaemic rat colon when compared with normal rat colon (P < 0.0001) (Table 2). Unidirectional flux and net absorption of Na+ did not show any significant difference from the control rats. Unidirectional Cl− fluxes were approximately equal, leading to nearly absent net Cl− movement across the mucosa. Thus JCl,sm was significantly increased (P < 0.0001) and JCl,net significantly decreased (P < 0.0001) compared with normal rat colon. Addition of amiloride resulted in a significant reduction of ISC (P < 0.0001), and in JNa,net (P < 0.0001), the latter largely due to reduction in JNa,ms (P < 0.017), indicating that amiloride-sensitive Na+ channels were present in hyperaldosteronaemic rats. Net Cl− absorption on the other hand did not show any significant change.
Table 2.
Unidirectional and net fluxes of Na+ and Cl− across distal colon of hyperaldosteronaemic rats:effect of amiloride and butyrate
| G | ISC | JNa,ms | JNa,sm | JNa,net | JCl,ms | JCl,sm | JCl,net | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Basal | 8.3±0.4 | 2.4± 0.2 | 7.6 ± 0.8 | 4.2 ± 0.7 | 3.4 ±0.3 | 4.8 ± 0.6 | 5.0 ± 0.5 | −0.2 ± 0.2 |
| Amiloride | 8.7 ± 0.4§ | 0.4 ±0.1§ | 5.2 ±0.9* | 5.0 ± 1.0n.s. | 0.2 ± 0.2§ | 5.9 ± 0.3§ | 5.8 ± 0.3n.s. | 0.1 ± 0.1n.s. |
| Butyrate | ||||||||
| Basal | 9.6 ± 0.5n.s. | 1.7 ± 0.3n.s. | 9.6 ± 0.5* | 3.7 ± 0.4n.s. | 5.9 ± 0.2§ | 6.1 ± 0.4n.s. | 4.0 ± 0.3n.s. | 2.1 ± 0.3§ |
| Amiloride | 11.1 ± 0.5§ | 0.1 ±0.2† | 8.0 ± 0.8n.s. | 5.4 ± 0.7n.s. | 2.7 ± 0.4§ | 7.0 ±0.8n.s. | 4.1 ± 0.5n.s. | 2.9 ± 0.5n.s. |
for both control and butyrate studies,the values shown are means ±s.e.m. of eight tissue pairs. Amiloride was always added to the mucosal bath solution after three 15 min flux periods. See Table 1 for further details and definitions.
Effect of butyrate on Na+ and Cl− fluxes in hyperaldosteronaemic rat distal colon
In butyrate solution there was a significant increase in JNa,net (P < 0.0001) and JCl,net (P < 0.0001) compared with control studies using butyrate-free solution (Table 2). This was associated with a decrease in ISC (P < 0.016). Addition of amiloride resulted in near abolition of the ISC (P < 0.002), and a significant reduction in JNa,net (P < 0.0001), but no significant change in JCl,net (P, n.s.). The reduction in ISC and JNa,net was comparable in magnitude to that seen in the absence of butyrate. However, unlike the control, there was a significant residual JNa,net in the presence of butyrate, which equalled JCl,net, indicating electroneutral NaCl absorption.
Effect of butyrate in cholera toxin-treated rat distal colon
Cholera toxin-exposed colon in butyrate-free solution demonstrated reversal of net Na+ and Cl− absorption to net Na+ and Cl− secretion (Table 3). This is consistent with inhibition of electroneutral NaCl absorption with induction of active Cl− secretion. Addition of serosal bumetanide resulted in a decreased ISC (P < 0.0001) along with markedly reduced JCl,sm (P < 0.001) and JNa,sm, due to inhibition of basolateral Na+-K+-2Cl− cotransport (Table 3). The increase in JCl,net (P < 0.0001) seen after addition of bumetanide was twice that for JNa,net (P < 0.0001), in keeping with the stoichoiometry of Na+-K+-2Cl− cotransport. Cholera toxin-exposed tissue, in butyrate-containing solution, was associated with a significantly lower ISC (P < 0.0001) and an increase in net Na+ (P < 0.0001) and Cl− (P < 0.0001) absorption compared with butyrate-free solution. Addition of bumetanide did not further change Na+ (P, n.s.) or Cl− (P, n.s.) absorption. Therefore, butyrate not only increased net Na+ and Cl− absorption compared with control, but also completely inhibited cholera toxin-induced secretion.
Table 3.
Unidirectional and net fluxes of Na* and Cl− across distal colon of rats pre-exposed to cholera toxin (CT):effect of butyrate and bumetanide
| G | ISC | JNa,ms | JNa,sm | JNa,net | JCl,ms | JCl,sm | JCl,net | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Post-CT | 10.2 ± 0.5 | 3.0 ± 0.2 | 6.2 ± 0.5 | 7.5 ± 0.4 | −1.3 ± 0.3 | 13.7 ± 1.0 | 16.7 ± 1.0 | −3.0 ± 0.4 |
| Bumetanide | 11.1 ± 0.5§ | 1.8 ± 0.1‡ | 7.4 ± 0.2* | 5.1 ± 0.3§ | 2.3 ± 0.3§ | 14.5 ± 0.5n.s. | 10.2 ± 0.5§ | 4.3 ± 0.3§ |
| Butyrate | ||||||||
| Post-CT | 8.3 ± 0.3† | 1.0 ± 0.6§ | 10.4 ± 0.7§ | 4.1 ± 0.5§ | 6.3 ± 0.6§ | 19.8 ± 0.8§ | 13.2 ± 0.6† | 6.6 ± 0.9§ |
| Bumetanide | 8.3 ±0.2n.s. | 0.5 ± 0.1§ | 9.9 ± 0.6n.s. | 3.8 ± 0.6n.s. | 6.1 ± 0.8n.s. | 20.2 ± 0.9n.s. | 14.1 ± 0.6n.s. | 6.1 ± 0.6n.s. |
for both control and butyrate studies,values shown are means ±s.e.m. of nine matched tissue pairs. Bumetanide was always added to the serosal reservoir after three 15 min flux periods. See Table 1 for further details and definitions.
Effect of butyrate in cholera toxin-treated hyperaldosteronaemic rat distal colon
In Ringer solution, net Na+ absorption was noted in cholera toxin-treated tissue exposed to butyrate-free solution (P < 0.01) (Table 4), and was similar in magnitude to net Na+ absorption from hyperaldosteronaemic rat colon not treated with cholera toxin (P, n.s.). Cholera toxin induced net Cl− secretion, similar in magnitude to that seen in normal rat colon. These results are consistent with the observation that Na+ absorption mediated through Na+ channels (as in hyperaldosteronism) is not inhibited by cholera toxin, whereas Cl− secretion is induced by cholera toxin. On addition of bumetanide, there was no significant increase in Na+ absorption, whereas net Cl− secretion was converted to net Cl− absorption (P < 0.001). The change in ISC following bumetanide was less than the change in JCl,net. Hyperaldosteronism induces electrogenic K+ secretion (Foster et al. 1984), and this will cause changes in ISC in a direction opposite to Cl− secretion. It is likely that bumetanide reduced K+ and Cl− secretion simultaneously, accounting for the disparity in the ISC and JCl,net response.
Table 4.
Unidirectional and net fluxes of Na* and Cl− across distal colon of hyper-aldosteronaemic rats pre-exposed to cholera toxin:effect of butyrate and bumetanide
| G | ISC | JNa,ms | JNa,sm | JNa,net | JCl,ms | JCl,sm | JCl,net | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Post-CT | 9.7 ± 0.6 | 3.2 ± 0.2 | 7.4 ± 0.7 | 4.3 ± 0.6 | 3.2 ± 0.4 | 12.2 ± 0.7 | 13.4 ± 0.7 | −1.2 ± 0.5 |
| Bumetanide | 11.5 ± 0.6§ | 1.1 ± 0.1§ | 8.2 ± 0.9n.s. | 5.3 ± 0.8* | 3.0 ± 0.9n.s. | 13.8 ± 0.9* | 10.1 ± 0.8* | 3.7 ± 1.0‡ |
| Butyrate | ||||||||
| Post-CT | 9.0 ± 0.5n.s. | 1.3 ± 0.1§ | 10.2 ± 1.2n.s. | 4.1 ± 0.5n.s. | 6.2 ± 0.9* | 19.3 ± 1.4‡ | 12.7 ± 0.7n.s. | 6.6 ± 1.0‡ |
| Bumetanide | 10.3 ± 0.5† | 0.8 ± 0.1§ | 11.1 ± 0.8n.s. | 5.6 ± 0.8† | 5.6 ± 0.8n.s. | 20.2 ± 1.1n.s. | 12.9 ± 0.8n.s. | 7.3 ± 0.6n.s. |
Values shown are means ±s.e.m. of matched tissue pairs;control,n =8 pairs and butyrate,n =9 pairs. Bumetanide was always added to the serosal reservoir after three flux periods. See Table 1 for further details and definitions.
Studies in 25 mm butyrate showed increased net absorption of both Na+ and Cl− due to increased JNa,ms and JCl,ms, while Jsm was comparable to control studies in the absence of cholera toxin. Butyrate thus stimulated both JNa,net (P < 0.01) and JCl,net (P < 0.001), while preventing the increase in serosa-to-mucosa flux brought about by cholera toxin. ISC in butyrate solution was significantly lower (P < 0.0001) than in control. There was no further increase in net Na+ or Cl− flux on addition of bumetanide.
Studies in rabbit distal colon
Basal Na+ and Cl− fluxes in rabbit distal colon
JNa,net of 1.8 ± 0.2 μmol h−1 cm−2 was observed in the basal state from Ringer solution. This was not accompanied by net Cl− absorption, and in fact a minimal net Cl− secretion (-0.7 ± 0.4 μmol h−1 cm−2) was observed (Table 5). The observed ISC (2.4 ± 0.1 μmol h−1 cm−2) could be accounted for by a combination of electrogenic Na+ absorption and minimal electrogenic Cl− secretion. The virtual absence of electroneutral Na+ absorption under basal conditions was shown by the fact that 10−4m amiloride (a concentration that inhibits Na+ channels) inhibited JNa,net by 78 % (0.4 ± 0.2 μmol h−1 cm−2 post-amiloride, compared with 1.8 ± 0.1 μmol h−1 cm−2 in the basal state). This was accompanied by a change in ISC from 2.0 ± 0.1 μmol h−1 cm−2 in the basal state to 1.0 ± 0.1 μmol h−1 cm−2 after amiloride (P < 0.001).
Table 5.
Effect of butyrate and amiloride on unidirectional and net fluxes of Na+and Cl−across rabbit distal colon
| G | ISC | JNa,ms | JNa,sm | JNa,net | JCl,ms | JCl,sm | JCl,net | |
|---|---|---|---|---|---|---|---|---|
| Control | 8.8 ± 0.4 | 2.4 ± 0.1 | 4.2 ± 0.3 | 2.4 ± 0.4 | 1.8 ± 0.2 | 5.7 ± 0.4 | 6.4 ± 0.4 | −0.7 ± 0.4 |
| Butyrate | 6.9 ± 0.3§ | 0.9 ± 0.0§ | 4.3 ± 0.4n.s. | 1.4 ± 0.3* | 2.9 ± 0.3* | 4.4 ± 0.5n.s. | 4.4 ± 0.5† | 0.0 ± 0.5n.s. |
For both control (n = 9) and butyrate (n = 12) studies, values shown are means ±s.e.m. of matched tissue pairs. See Table 1 for furtherdetails and definitions.
Effect of butyrate on basal Na+ and Cl− fluxes
As shown in Table 5, in the presence of 25 mm butyrate a significant increase was noted in JNa,net (2.9 ± 0.3 μmol h−1 cm−2) compared with the basal values (P < 0.02). This was secondary to a significant decrease (P < 0.04) in JNa,sm while JNa,ms was almost unaltered. JCl,sm decreased in the presence of butyrate, but JCl,ms and JCl,net were not significantly affected by butyrate. A decrease in conductance was noted after addition of butyrate (P < 0.0001), and this could partially account for reduced JNa,sm. The fact that this was not accompanied by a comparable decrease in JNa,ms indicates that there was active Na+ absorption. Although butyrate increased JNa,net, it decreased the ISC (0.9 ± 0.0 μmol h−1 cm−2), indicating that the stimulation of net Na+ absorption was through an electroneutral mechanism. This was confirmed by the observation that 10−4m amiloride inhibited JNa,net only partially (2.0 ± 0.4 μmol h−1 cm−2 post-amiloride, compared with 3.1 ± 0.2 μmol h−1 cm−2 in the basal state) in the presence of butyrate. This was accompanied by a change in ISC from 0.9 ± 0.1 μmol h−1 cm−2 in the basal state to −0.5±0.1 μmol h−1 cm−2 after amiloride (P < 0.0001). Fluxes in Cl−-free Ringer solution indicated that butyrate stimulation of Na+ absorption was not dependent on presence of Cl− in the medium (Fig. 1).
Figure 1. Effect of Cl− withdrawal from the bath solution on unidirectional and net Na+ fluxes in rabbit descending colon.
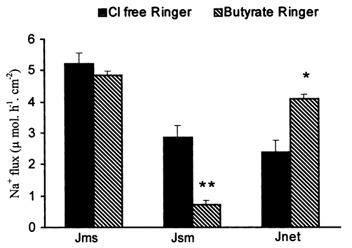
Both the solutions used for the above study contained equimolar amounts of isethionate in place of Cl−. Basal as well as butyrate-stimulated Na+ absorption took place even in the absence of Cl−. Butyrate induced a significant increase in JNa,net (*P = 0.006) and JNa,sm (**P = 0.002) compared with basal values.
Na+ and Cl− fluxes in cholera toxin-treated rabbit distal colon
Unidirectional and JNa,net after exposure to cholera toxin were similar to fluxes in rabbit colon not exposed to cholera toxin (P, n.s.). This indicates a lack of suppression of electrogenic Na+ absorption by cholera toxin. On the other hand, cholera toxin produced a significant increase in net Cl− secretion (Table 6) (P < 0.0001), which resulted from a significant increase (P = 0.0001) in JCl,sm without any significant alteration in JCl,ms. This was accompanied by an increase in ISC to 3.2 ± 0.3 μmol h−1 cm−2 (P = 0.0001). The magnitude of the increase in ISC did not completely account for the sum of JNa,net and JCl,net. Prostaglandin E2, possibly acting through cAMP, is known to induce simultaneous, and electrogenic, K+ and Cl− secretion in rabbit descending colon (Halm & Frizzell, 1986; Roden et al. 1992), and both these are inhibited by bumetanide. It is therefore likely that the ISC response to Cl− secretion was blunted by the presence of concomitant K+ secretion induced by cAMP. Bumetanide addition to the serosal side did not significantly affect unidirectional or net JNa (P, n.s.). On the other hand, bumetanide totally inhibited net Cl− secretion (P < 0.001) and resulted in minimal net Cl− absorption. This was due to increased JCl,ms (P = 0.001) while JCl,sm was not significantly affected. Bumetanide inhibited ISC significantly, and reduced residual flux from 3.0 to 1.2 μmol h−1 cm−2, probably due to simultaneous inhibition of Cl− and K+ secretion.
Table 6.
Unidirectional and net fluxes of Na+ and Cl− across rabbit distal colon pre-incubated with cholera toxin (CT)
| G | ISC | JNa,ms | JNa,sm | JNa,net | JCl,ms | JCl,sm | JCl,net | |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| Post-CT | 8.2 ± 0.2 | 3.2 ± 0.3 | 4.3 ± 0.4 | 2.6 ± 0.3 | 1.7 ± 0.3 | 5.3 ± 0.7 | 9.8 ± 0.6 | −4.5 ± 0.5 |
| Post-bumetanide | 8.6 ± 0.3n.s. | 0.8 ± 0.1§ | 5.3 ± 0.4n.s. | 2.8 ± 0.5n.s. | 2.5 ± 0.5n.s. | 8.6 ± 0.7‡ | 7.8 ± 0.9n.s. | 0.8 ± 0.4‡ |
| Butyrate | ||||||||
| Post-CT | 5.6 ± 0.2§ | 2.0 ± 0.1§ | 3.4 ± 0.5n.s. | 0.8 ± 0.2§ | 2.6 ± 0.4† | 5.3 ± 0.6n.s. | 5.0 ± 0.6§ | 0.3 ± 0.5§ |
| Post -bumetanide | 5.0 ± 0.2§ | 0.6 ± 0.1§ | 4.3 ± 0.4n.s. | 1.4 ± 0.2n.s. | 2.9 ± 0.6n.s. | 5.6 ± 1.0n.s. | 4.1 ± 0.9n.s. | 1.5 ± 0.4n.s. |
For both control (n =7) and butyrate (n = 8), values shown are means ±s.e.m. of matched tissue pairs. Bumetanide was always added totheserosal bath solution after three flux periods. See Table 1 for further details and definitions.
Effect of butyrate on Na+ and Cl− fluxes in cholera toxin-treated rabbit distal colon
In cholera toxin-treated rabbit distal colon, the presence of butyrate increased net Na+ absorption significantly (P = 0.004) compared with control colon (Table 6). This was due to decreased JNa,sm (P < 0.0001) while JNa,ms was not significantly altered. There was a significant decrease in conductance (P < 0.0001) when compared with that in the Ringer solution. In the presence of butyrate, net Cl− absorption was noted in cholera toxin-treated colon (P < 0.0001, compared with control). This was due to significant reduction in JCl,sm (P = 0.0001), while JCl,ms remained unaltered compared with that in the absence of butyrate. Addition of bumetanide did not significantly alter unidirectional or net fluxes of either Na+ or Cl− in these experiments. These results are consistent with an inhibitory effect of butyrate on active Cl− secretion induced by cholera toxin.
DISCUSSION
Active absorption of sodium by the colon is necessary for water conservation by the body in health, and becomes critical in disease conditions characterised by excessive fluid losses from the body. The absorptive process involves either Na+ channels (electrogenic absorption) or Na+-H+ exchange (electroneutral absorption). Marked regional and species variations exist in the distribution of these transport processes. While Na+-H+ exchange predominates in rat distal colon, and Na+ channel activity predominates in rabbit distal colon, there is a mixture of the two processes in human distal colon (Binder et al. 1987; Sellin & De Soignie, 1987; Sandle, 1989). Short chain fatty acids (SCFA) stimulate active Na+ absorption from the colon of many mammalian species including man, and this has been shown to be secondary to stimulation of electroneutral NaCl absorption (Binder et al. 1989). Butyrate-dependent NaCl absorption is not inhibited by cAMP (Binder & Mehta, 1990; Krishnan et al. 1999). There is also evidence to suggest that butyrate inhibits active Cl− secretion, the basis of secretory diarrhoea, in rat colon and secretory T84 cells (Dagher et al. 1996; Matthews et al. 1998). The studies reported here suggest that augmentation of NaCl absorption and prevention of Cl− secretion are simultaneous and independent effects of butyrate.
As demonstrated earlier, butyrate-stimulated electroneutral NaCl absorption in rat distal colon was not inhibited by 10−4m amiloride. In the same tissue, electrogenic Cl− secretion induced by cholera toxin was prevented completely by butyrate, and there was no significant change in fluxes after addition of bumetanide to butyrate-exposed tissues. This observation confirms the earlier finding of the antisecretory effect of butyrate in rat colon (Dagher et al. 1996). The increase in net Na+ absorption after butyrate was also observed in cholera toxin-treated colon and after addition of bumetanide, showing that this effect was independent of the effect of butyrate on Cl− secretion. We also demonstrate here that butyrate increased electroneutral Na+ absorption in rabbit distal colon, a tissue not previously examined in this regard, and which lacks basal Na+-H+ exchange. Again, in rabbit colon, cholera toxin-induced Cl− secretion was completely prevented by addition of butyrate to the medium.
Several of our findings appear contradictory, but may be explained further. In the rat distal colon, butyrate augmented net Na+ and Cl− absorption by increasing Jms of both Na+ and Cl−, indicating that it stimulated inward fluxes of these ions. On the other hand, in the rabbit distal colon, butyrate augmented net Na+ absorption principally by reducing JNa,sm. Under basal conditions, JNa,sm in the colon is generally considered to be a reflection of paracellular permeability. Butyrate reduced tissue conductance by 22 %, and this reduction in paracellular permeability would probably account for half the reduction in JNa,sm following butyrate. Reduced paracellular permeability would have concurrently reduced the passive component of JNa,ms, and this reduction probably masked an increase in the active component of JNa,ms in the presence of butyrate. Butyrate has been shown to reduce paracellular permeability in intestinal epithelial cell monolayers (Mariadason et al. 1997). An inhibitory effect of butyrate on basal Na+ secretion in the colon would also have to be considered as a possibility. Na+ secretion has not been reported under basal conditions in the colon. However, Na+ secretion, largely coupled to HCO3− secretion, may be induced by cAMP in rabbit proximal colon in the presence of high extracellular Na+ concentrations (Hyun et al. 1994).
In rabbit distal colon, the presence of butyrate was associated with a decrease in ISC while net Na+ flux increased significantly, indicating that inhibition of Na+ channels (Abriel & Horisberger, 1999; Chalfant et al. 1999) may have occurred alongside stimulation of Na+-H+ exchange. As rabbit distal colon expresses only NHE2 and not NHE3 (Hoogerwerf et al. 1996), it is possible that the butyrate-dependent Na+-H+ exchange occurred via NHE2. In rabbit distal colon, cholera toxin did not affect net Na+ absorption, demonstrating that this process was not sensitive to cAMP, unlike Na+-H+ exchange (McSwine et al. 1998; Zizak et al. 1999). Cholera toxin did induce net Cl− secretion by increasing JCl,sm, which could be completely inhibited by bumetanide, which blocks Na+-K+-2Cl− cotransporter activity. Inhibition of Na+- K+-2Cl− cotransport by bumetanide also resulted in an increased JNa,net, which possibly reflects compensation of reduced intracellular Na+ concentrations by increased apical Na+ entry. However, for reasons that are not clear, the enhanced Na+ and Cl− absorption values did not exactly coincide with the known stoichiometry for Na+-K+-2Cl− cotransport. In rabbit distal colon the magnitude of the increase in ISC after cholera toxin did not correspond to the sum of Na+ absorption and Cl− secretion. Active electrogenic K+ secretion in the colon is stimulated by PGE2 and cAMP (Halm & Frizzell, 1986; Roden et al. 1992; Merlin et al. 1995; Grotjohann et al. 1998). This could explain the high basal residual ion flux of 3.0 μmol h−1 cm−2 seen in rabbit colon after exposure to cholera toxin.
In rabbit colon, substitution of Cl− with isethionate (an unabsorbed anion) did not affect either basal or butyrate-dependent Na+ absorption. This is not surprising since Na+ absorption from rabbit descending colon is predominantly through Na+ channels. The lack of dependence of butyrate-dependent Na+ absorption on Cl− suggests that there is no necessity for linkage with Cl− absorption of butyrate-stimulated Na+-H+ exchange. It is likely that butyrate entry into the cell (either via SCFA−-HCO3− exchange or by diffusion of unionized butyrate) reduces intracellular pH and that this secondarily stimulates Na+-H+ exchange (Gonda et al. 1999).
The pattern of Na+ and Cl− absorption was also studied in hyperaldosteronaemic rat distal colon, a condition that resulted in significant increase in amiloride-inhibitable Na+ current and flux. In Ringer solution, amiloride almost totally inhibited the ISC and JNa,net, indicating the presence of amiloride-inhibitable Na+ channels in hyperaldosteronaemic rat distal colon. This colon resembled rabbit distal colon in demonstrating electrogenic Na+ absorption and lacking Cl− absorption. In fact, minimal net Cl− secretion was observed. Butyrate increased Na+ and Cl− absorption from salt-depleted rat distal colon, and this was associated with a significant decrease in ISC (P < 0.02). Amiloride almost completely abolished the ISC and produced a significant decrease in JNa,net, but did not totally inhibit it, unlike the rabbit distal colon. This could be attributable to residual electroneutral Na+ absorption in the salt-depleted animals, or may reflect different sensitivity of the Na+-H+ exchanger isoforms to amiloride. Studies have shown that rat distal colon has both NHE2 and NHE3 isoforms, while rabbit distal colon has only the NHE2 isoform (Hoogerwerf et al. 1996). NHE2 is inhibited at 10−4m amiloride concentration while NHE3 is not (Counillon et al. 1993). Downregulation of NHE3, and to a lesser extent NHE2, has been reported in the distal colon of salt-depleted rats (Ikuma et al. 1999). It is possible that butyrate stimulated NHE2 or NHE3 expression (Musch et al. 2001), since butyrate is known to have an effect on gene expression.
Cholera toxin treatment of the rat distal colon inhibited electroneutral sodium chloride absorption, and induced net Cl− secretion. Minimal net secretion of Na+ was also observed, and this may have been related to the extracellular Na+ concentration (Hyun et al. 1994). This was associated with a significant increase in ISC (P < 0.0001). Electrogenic Cl− secretion requires the co-ordinated activity of several ion transporters including the apical Cl− channel and basolateral Na+-K+-2Cl− cotransport, Na+-K+-ATPase and K+ channels (Hecht & Koutsouris, 1999). Of these, Na+-K+-2Cl− cotransport contributes directly to the chloride exit at the apical end (Matthews et al. 1998; Marunaka et al. 1999). The role of Na+-K+-2Cl− cotransport was systematically evaluated by addition of bumetanide to the serosal side. This resulted in total inhibition of the electrogenic Cl− secretion and also made it absorptive with a mean difference of 7.3 μmol h−1 cm−2 (P < 0.0001). Unidirectional fluxes of chloride showed a decrease in JCl,sm with a mean difference of 6.0 μmol h−1 cm−2 (P < 0.0001) with little or no change in JCl,ms. Addition of bumetanide also inhibited net Na+ secretion with a mean difference of 3.6 μmol h−1 cm−2. Unlike in rabbit distal colon, this inhibition of Na+ and Cl− secretion agreed well with the stoichiometry of Na+-K+-2Cl− cotransport. As expected, along with the inhibition of the electrogenic Cl− secretion there was a significant decrease in ISC (P < 0.001).
Exposure of cholera toxin-treated rat distal colon to butyrate stimulated JNa,net and JCl,net absorption with a mean difference of 7.7 μmol h−1 cm−2 (P < 0.0001) and 9.5 μmol h−1 cm−2 (P < 0.0001). From unidirectional fluxes a selective increase of JNa,ms (P < 0.0001) and JCl,ms (P < 0.0001) were noted. This could be explained by stimulation of electroneutral Na+-H+ exchange and SCFA−-linked Cl−-HCO3− exchange at the apical membrane. A decrease in JNa,sm and JCl,sm could be due to the effect of butyrate on tight junctions. Addition of bumetanide was without any further increase in JNa,net and JCl,net. It indicates that the increase in JNa,net and JCl,net occurred not because of a decrease in flux at the basolateral end, but because of a definite increase of an electroneutral absorptive process at the apical end. The current model (Fig. 2) for SCFA-dependent Na+ and Cl− absorption could explain this increased absorption. It predicts an increase in Na+-H+ and Cl+-SCFA− exchange, secondary to intracellular acidosis by SCFA−-HCO3− exchange at the brush-border membrane of the colonic epithelial cell.
Figure 2. Conceptual diagram of active Na+ and Cl− transport in mammalian colonic epithelium.
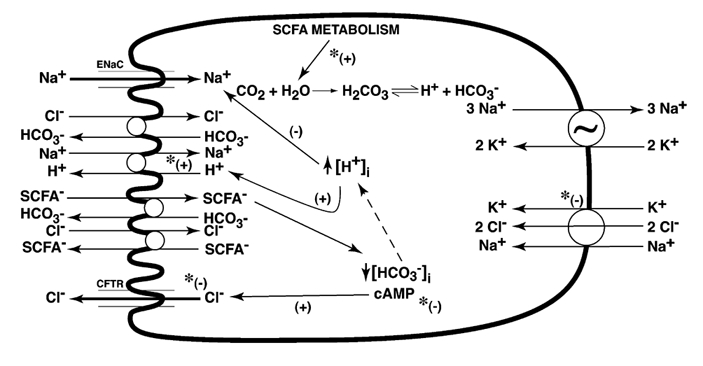
Possible transporters involved are shown, but not all are present in the same cell (e.g. rabbit distal colon lacks Na+-H+ exchange and Cl−-HCO3− exchange, while rat distal colon lacks Na+ channel). Na+-H+ exchange may be coupled to Cl−-HCO3− exchange, or independently coupled to SCFA−-HCO3− and Cl−-SCFA− exchanges (Rajendran & Binder, 1994). Butyrate absorption into the cell may occur via non-ionic diffusion, and dissociation of butyrate within the cell may increase intracellular H+ ([H+]i) with stimulation of Na+-H+ exchange. Butyrate−-HCO3− exchange (Mascolo et al. 1991) across the apical membrane will also increase [H+]i and stimulate Na+-H− exchange across the apical membrane. Increased [H+]i will also inhibit Na+ absorption via Na+ channels (Chalfant et al. 1999). Cl− secretion involves Cl− channels at the apical membrane, and Na+-K+-2Cl− cotransporter activity and sodium pump activity in the basolateral mambrane. Inhibition of Cl− secretion by butyrate may occur at any of these levels. Butyrate also inhibits cAMP generation by colonic epithelium (Krishnan et al. 1999). *Potential levels at which butyrate may potentiate (+) or inhibit (-) processes that eventually affect net Na+ transport.
Cholera toxin treatment of hyperaldosteronaemic rats showed again that electrogenic Na+ absorption was not inhibited by cAMP. There was no significant difference in JCl,net between cholera toxin-treated control and cholera toxin-treated hyperaldosteronaemic rats. Cl− secretion was therefore not associated with aldosterone status of the animal. Thus Na+ and Cl− absorption are not coupled and take place independently of each other. Inhibition of Na+-K+-2Cl− cotransport resulted in an increase in JCl,net (P < 0.001), with no significant difference in JNa,net. ISC showed an even greater decrease and could be attributed to inhibition of electrogenic Cl− secretion and decreased K+ secretion at the apical end. Butyrate significantly increased JNa,net (P < 0.01) and JCl,net (P < 0.001) with a significant decrease in ISC (P < 0.0001), indicating that Na+ and Cl− absorption was electrically neutral. Addition of bumetanide to the butyrate-exposed tissues did not produce further change in Na+ and Cl− absorption, excluding a role for Na+-K+-2Cl− cotransport. Thus, as in control, butyrate stimulation of JNa,net and JCl,net in hyperaldosteronaemic rat distal colon occurred because of an increase of electroneutral absorption at the apical membrane.
Figure 2 depicts a pictorial model of active transport processes for Na+ and Cl− in the mammalian colon, which is based on that suggested by Binder and co-workers. A number of points are shown (in Fig. 2) at which butyrate can modulate these transport processes, and these can exist in a particular tissue to a greater or lesser extent depending on the basal transport processes characteristic of each epithelium. In addition to direct interactions with transport processes, butyrate may also have effects on expression of one or more transporters, which would further modify the overall transport characteristics. The effect of butyrate on Cl− secretion is of particular clinical interest, since this may reduce colonic secretion in enterotoxin-induced diarrhoea such as cholera.
Acknowledgments
Dr Vidyasagar was the recipient of a Senior Research Fellowship from the Council for Scientific and Industrial Research, New Delhi, India.
REFERENCES
- Abriel H, Horisberger JD. Feedback inhibition of rat amiloride-sensitive epithelial sodium channels expressed in Xenopus laevis oocytes. Journal of Physiology. 1999;516:31–43. doi: 10.1111/j.1469-7793.1999.031aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger HA, Travis SM, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by specific protein kinases and protein phosphatases. Journal of Biological Chemistry. 1993;268:2037–2047. [PubMed] [Google Scholar]
- Binder HJ, Foster ES, Budinger ME, Hayslett JP. Mechanism of electroneutral sodium chloride absorption in distal colon of the rat. Gastroenterology. 1987;93:449–455. doi: 10.1016/0016-5085(87)90905-x. [DOI] [PubMed] [Google Scholar]
- Binder H, McGlone F, Sandle G. Effects of corticosteroid hormones on the electrophysiology of rat distal colon: implications for Na+ and K+ transport. Journal of Physiology. 1989;410:425–441. doi: 10.1113/jphysiol.1989.sp017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989;96:989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- Binder HJ, Mehta P. Characterization of butyrate-dependent electroneutral Na-Cl absorption in the rat distal colon. Pflügers Archiv. 1990;417:365–369. doi: 10.1007/BF00370654. [DOI] [PubMed] [Google Scholar]
- Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comparative Biochemistry and Physiology B. 1987;86:439–472. doi: 10.1016/0305-0491(87)90433-0. [DOI] [PubMed] [Google Scholar]
- Chalfant ML, Denton JS, Berdiev BK, Ismailov II, Benos DJ, Stanton BA. Intracellular H+ regulates the α-subunit of ENaC, the epithelial Na+ channel. American Journal of Physiology. 1999;276:C477–486. doi: 10.1152/ajpcell.1999.276.2.C477. [DOI] [PubMed] [Google Scholar]
- Charney AN, Micic L, Egnor RW. Nonionic diffusion of short-chain fatty acids across rat colon. American Journal of Physiology. 1998;274:G518–524. doi: 10.1152/ajpgi.1998.274.3.G518. [DOI] [PubMed] [Google Scholar]
- Counillon L, Scholz W, Lang HJ, Pouyssegur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Molecular Pharmacology. 1993;44:1041–1045. [PubMed] [Google Scholar]
- Dagher PC, Egnor RW, Taglietta-Kohlbrecher A, Charney AN. Short-chain fatty acids inhibit cAMP-mediated chloride secretion in rat colon. American Journal of Physiology. 1996;271:C1853–1860. doi: 10.1152/ajpcell.1996.271.6.C1853. [DOI] [PubMed] [Google Scholar]
- Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. American Journal of Physiology. 2000;278:G718–724. doi: 10.1152/ajpgi.2000.278.5.G718. [DOI] [PubMed] [Google Scholar]
- Foster ES, Hayslett JP, Binder HJ. Mechanism of active potassium absorption and secretion in the rat colon. American Journal of Physiology. 1984;246:G611–617. doi: 10.1152/ajpgi.1984.246.5.G611. [DOI] [PubMed] [Google Scholar]
- Foster ES, Zimmerman TW, Hayslett JP, Binder HJ. Corticosteroid alteration of active electrolyte transport in rat distal colon. American Journal of Physiology. 1983;245:G668–675. doi: 10.1152/ajpgi.1983.245.5.G668. [DOI] [PubMed] [Google Scholar]
- Fromm M, Schulzke JD, Hegel U. Control of electrogenic Na+ absorption in rat late distal colon by nanomolar aldosterone added in vitro. American Journal of Physiology. 1993;264:E68–73. doi: 10.1152/ajpendo.1993.264.1.E68. [DOI] [PubMed] [Google Scholar]
- Gabriel SE, Clarke LL, Boucher RC, Stutts MJ. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature. 1993;363:263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- Gonda T, Maouyo D, Rees SE, Montrose MH. Regulation of intracellular pH gradients by identified Na/H exchanger isoforms and a short-chain fatty acid. American Journal of Physiology. 1999;276:G259–270. doi: 10.1152/ajpgi.1999.276.1.G259. [DOI] [PubMed] [Google Scholar]
- Grotjohann I, Gitter AH, Kockerling A, Bertog M, Schulzke JD, Fromm M. Localization of cAMP- and aldosterone-induced K+ secretion in rat distal colon by conductance scanning. Journal of Physiology. 1998;507:561–570. doi: 10.1111/j.1469-7793.1998.561bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotjohann I, Schulzke JD, Fromm M. Electrogenic Na+ transport in rat late distal colon by natural and synthetic glucocorticosteroids. American Journal of Physiology. 1999;276:G491–498. doi: 10.1152/ajpgi.1999.276.2.G491. [DOI] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annual Review of Physiology. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Halevy J, Budinger ME, Hayslett JP, Binder HJ. Role of aldosterone in the regulation of sodium and chloride transport in the distal colon of sodium-depleted rats. Gastroenterology. 1986;91:1227–1233. doi: 10.1016/s0016-5085(86)80021-x. [DOI] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Active K transport across rabbit distal colon: relation to Na absorption and K secretion. American Journal of Physiology. 1986;251:C252–267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Hecht G, Koutsouris A. Enteropathogenic E. coli attenuates secretagogue-induced net intestinal ion transport but not Cl− secretion. American Journal of Physiology. 1999;276:G781–788. doi: 10.1152/ajpgi.1999.276.3.G781. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Clauss W. Time-dependent effects of aldosterone on sodium transport and cell membrane resistances in rabbit distal colon. Pflügers Archiv. 1989;415:156–164. doi: 10.1007/BF00370587. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. American Journal of Physiology. 1996;270:G29–41. doi: 10.1152/ajpgi.1996.270.1.G29. [DOI] [PubMed] [Google Scholar]
- Hyun CS, Ahn J, Minhas BS, Cragoe EJ, Field M. Ion transport in rabbit proximal colon: effects of sodium, amiloride, cAMP, and epinephrine. American Journal of Physiology. 1994;266:G1071–1082. doi: 10.1152/ajpgi.1994.266.6.G1071. [DOI] [PubMed] [Google Scholar]
- Ikuma M, Kashgarian M, Binder HJ, Rajendran VM. Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. American Journal of Physiology. 1999;276:G539–549. doi: 10.1152/ajpgi.1999.276.2.G539. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chloride absorption from secreting rat colon by short-chain fatty acids. Digestive Diseases and Sciences. 1999;44:1924–1930. doi: 10.1023/a:1018871412748. [DOI] [PubMed] [Google Scholar]
- McCabe R, Cooke HJ, Sullivan LP. Potassium transport by rabbit descending colon. American Journal of Physiology. 1982;242:C81–86. doi: 10.1152/ajpcell.1982.242.1.C81. [DOI] [PubMed] [Google Scholar]
- McDonald FJ, Snyder PM, McCray PBJ, Welsh MJ. Cloning, expression, and tissue distribution of a human amiloride-sensitive Na+ channel. American Journal of Physiology. 1994;266:L728–734. doi: 10.1152/ajplung.1994.266.6.L728. [DOI] [PubMed] [Google Scholar]
- McSwine RL, Musch MW, Bookstein C, Xie Y, Rao M, Chang EB. Regulation of apical membrane Na+/H+ exchangers NHE2 and NHE3 in intestinal epithelial cell line C2/bbe. American Journal of Physiology. 1998;275:C693–701. doi: 10.1152/ajpcell.1998.275.3.C693. [DOI] [PubMed] [Google Scholar]
- Mariadason JM, Barkla DH, Gibson PR. Effect of short chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. American Journal of Physiology. 1997;272:G705–712. doi: 10.1152/ajpgi.1997.272.4.G705. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Niisato N, O'Brodovich H, Post M, Tanswell AK. Roles of Ca2+ and protein tyrosine kinase in insulin action on cell volume via Na+ and K+ channels and Na+/K+/2Cl− cotransporter in fetal rat alveolar type II pneumocyte. Journal of Membrane Biology. 1999;168:91–101. doi: 10.1007/s002329900500. [DOI] [PubMed] [Google Scholar]
- Mascolo N, Rajendran VM, Binder HJ. Mechanism of short-chain fatty acid uptake by apical membranevesicles of rat distal colon. Gastroenterology. 1991;101:331, 338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation. Modulation of Cl− secretory capacity by butyrate. Journal of Clinical Investigation. 1998;101:2072–2079. doi: 10.1172/JCI1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin D, Guo X, Laboisse CL, Hopfer U. Ca2+ and cAMP activate different K+ conductances in the human intestinal goblet cell line HT29-Cl. 16E. American Journal of Physiology. 1995;268:C1503–1511. doi: 10.1152/ajpcell.1995.268.6.C1503. [DOI] [PubMed] [Google Scholar]
- Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. American Journal of Physiology. 2001;280:G687–693. doi: 10.1152/ajpgi.2001.280.4.G687. [DOI] [PubMed] [Google Scholar]
- Perrone RD, Alexander EA, Bengele HH, Schwartz JH. Effect of aldosterone and dexamethasone pretreatment on sodium transport in rat distal colon in vitro. Pflügers Archiv. 1984;400:257–261. doi: 10.1007/BF00581556. [DOI] [PubMed] [Google Scholar]
- Prince LS, Welsh MJ. Cell surface expression and biosynthesis of epithelial Na+ channels. Biochemical Journal. 1998;336:705–710. doi: 10.1042/bj3360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran VM, Binder HJ. Cl-HCO3 and Cl-OH exchanges mediate Cl uptake in apical membrane vesicles of rat distal colon. American Journal of Physiology. 1993;264:G874–879. doi: 10.1152/ajpgi.1993.264.5.G874. [DOI] [PubMed] [Google Scholar]
- Rajendran VM, Binder HJ. Apical membrane Cl− butyrate exchange: mechanism of short chain fatty acid stimulation of active chloride absorption in rat distal colon. Journal of Membrane Biology. 1994;141:51–58. doi: 10.1007/BF00232873. [DOI] [PubMed] [Google Scholar]
- Ramakrishna BS, Nance SH, Roberts-Thomson IC, Roediger WE. The effects of enterotoxins and short-chain fatty acids on water and electrolyte fluxes in ileal and colonic loops in vivo in the rat. Digestion. 1990;45:93–101. doi: 10.1159/000200229. [DOI] [PubMed] [Google Scholar]
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. Journal of Nutrition. 1997;127:838–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- Roden M, Turnheim K. Sodium pump quantity and turnover in rabbit descending colon at different rates of sodium absorption. Pflügers Archiv. 1988;413:181–189. doi: 10.1007/BF00582529. [DOI] [PubMed] [Google Scholar]
- Roden M, Plass H, Vierhapper H, Turnheim K. Endothelin-1 stimulates chloride and potassium secretion in rabbit descending colon. Pflügers Archiv. 1992;421:163–167. doi: 10.1007/BF00374823. [DOI] [PubMed] [Google Scholar]
- Rubens RD, Lambert HP. The homeostatic function of the colon in acute gastroenteritis. Gut. 1972;13:915–919. doi: 10.1136/gut.13.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle GI. Segmental heterogeneity of basal and aldosterone-induced electrogenic Na transport in human colon. Pflügers Archiv. 1989;414:706–712. doi: 10.1007/BF00582139. [DOI] [PubMed] [Google Scholar]
- Sandle GI, Binder HJ. Corticosteroids and intestinal ion transport. Gastroenterology. 1987;93:188–196. doi: 10.1016/0016-5085(87)90333-7. [DOI] [PubMed] [Google Scholar]
- Seibert FS, Chang XB, Aleksandrov AA, Clarke DM, Hanrahan JW, Riordan JR. Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum. Biochimica et Biophysica Acta. 1999;1461:275–283. doi: 10.1016/s0005-2736(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Sellin JH, De Soignie R. Ion transport in human colon in vitro. Gastroenterology. 1987;93:441–448. doi: 10.1016/0016-5085(87)90904-8. [DOI] [PubMed] [Google Scholar]
- Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. American Journal of Physiology. 1998;274:C1699–1707. doi: 10.1152/ajpcell.1998.274.6.C1699. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Sellin JH. Relationships among sodium current, permeability, and Na activities in control and glucocorticoid-stimulated rabbit descending colon. Journal of Membrane Biology. 1986;92:121–134. doi: 10.1007/BF01870702. [DOI] [PubMed] [Google Scholar]
- Turnheim K, Hudson RL, Schultz SG. Cell Na+ activities and transcellular Na+ absorption by descending colon from normal and Na+-deprived rabbits. Pflügers Archiv. 1987;410:279–283. doi: 10.1007/BF00580277. [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, Smolenski A, Tilly BC, Houtsmuller AB, Ehlert EM, Bot AG, Edixhoven M, Boomaars WE, Lohmann SM, De Jonge HR. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activation. Proceedings of the National Academy of Sciences of the USA. 1998;95:1466–1471. doi: 10.1073/pnas.95.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizak M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun CH, Weinman EJ. cAMP-induced phosphorylation and inhibition of Na+/H+ exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor. Journal of Biological Chemistry. 1999;274:24753–24758. doi: 10.1074/jbc.274.35.24753. [DOI] [PubMed] [Google Scholar]


