Abstract
The effects of the Cl− channel antagonists, niflumic acid (NFA), dichloro-diphenylamine 2-carboxylic acid (DCDPC) and diisothiocyanato-stilbene-2,2′-disulphonic acid (DIDS) on Ca2+-activated Cl− current (ICl(Ca)) evoked by adding fixed intracellular calcium concentrations ([Ca2+]i) to the pipette solution were studied in rabbit pulmonary artery myocytes. With 250 and 500 nm [Ca2+]i bath application of NFA (100 μm) increased inward current at negative potentials, but inhibited outward current at positive potentials. On wash out of NFA, ICl(Ca) was greatly enhanced at all potentials. When external Na+ ions were replaced by N-methyl-d-glucamine (NMDG+) NFA still enhanced ICl(Ca) at negative potentials but the increase of ICl(Ca) on wash out was blocked. When the mean reversal potential (Er) of ICl(Ca) was shifted to negative potentials by replacing external Cl− with SCN−, NFA increased inward current but blocked outward current suggesting that the effect of NFA is dependent on current flow. Inclusion of NFA in the pipette solution had no effect on ICl(Ca). Voltage jump experiments indicated that ICl(Ca) displayed characteristic outward current relaxations at +70 mV and inward current relaxations at −80 mV that were abolished by NFA. DCDPC (100 μm) produced similar effects to NFA but 1 mm DIDS produced inhibition of ICl(Ca) at both positive and negative potentials and there was no increase in current on wash out of DIDS. These results suggest that NFA and DCDPC, but not DIDS, simultaneously enhance and block ICl(Ca) by binding to an external site, probably close to the mouth of the chloride channel.
Calcium-activated chloride currents (ICl(Ca)) have been recorded in many smooth muscle types and have many distinctive properties including voltage-dependent kinetics, modulation by external anions and inhibition by a range of chemically disparate agents (see reviews by Large & Wang, 1996 and Greenwood & Large, 1999a). The majority of experiments studying the properties of ICl(Ca) in vascular smooth muscle cells have utilised the perforated patch configuration of the whole-cell recording technique where the resting intracellular Ca2+ concentration ([Ca2+]i) is normally below threshold for activation of ICl(Ca). The channel is then stimulated by an increase in [Ca2+]i elicited by several methods. ICl(Ca) can be activated by agonist-dependent or spontaneous intracellular Ca2+ release (the latter have been termed spontaneous transient inward currents or STICs, Wang et al. 1992) or as a consequence of Ca2+ influx through voltage-gated Ca2+ channels in response to depolarising pulses (Lamb et al. 1994; Greenwood & Large, 1996; Yuan, 1997).
Using these techniques we have studied the mechanism of action of several chloride channel antagonists (e.g. see review by Large & Wang, 1996). The most potent blocker of ICl(Ca) studied so far in smooth muscle is niflumic acid (NFA) which inhibits ICl(Ca) in micromolar concentrations (Pacaud et al. 1989; Hogg, et al. 1994b) and produces distinctive effects on the decay time course of STICs. Previously it had been proposed that the mono-exponential decay of STICs represents closure of the chloride channels (Hogg et al. 1993) but in the presence of NFA the STIC decay time course can be described by two exponentials (Hogg et al. 1994b). The data could be interpreted by a scheme in which NFA acts as an open channel blocking agent which remains in the channel for a short time relative to the mean open duration of the channel, i.e. NFA is a rapidly dissociating open channel blocker (Hogg et al. 1994b). Due to the potency of NFA and its action on the channel molecule NFA is frequently used to characterise ICl(Ca) in electrophysiological experiments and also to assess the functional role of ICl(Ca) in intact tissue preparations (e.g. see Greenwood & Large, 1999a).
In studies on ICl(Ca) using the above techniques the activating Ca2+ pulse is free to change. However, ICl(Ca) can also be activated persistently by a clamped [Ca2+]i imposed by dialysis from the patch pipette. This approach has been used to study ICl(Ca) in other cell types (e.g. rat parotid acinar cells, Arreola et al. 1996; cultured calf pulmonary artery endothelial cells, Nilius et al. 1997; Xenopus oocytes, Kuruma & Hartzell, 1999) and more recently in vascular smooth muscle cells (Greenwood et al. 2001). In the present study we used [Ca2+]i of 0.25–1 μm to activate ICl(Ca) as this is the concentration range which is likely to be achieved physiologically during stimulation of vascular smooth muscle. During the course of these experiments we observed a surprising and unexpected result in that in some experimental conditions NFA did not inhibit ICl(Ca) activated by a maintained concentration of Ca2+ but actually increased ICl(Ca). This is a novel mechanism of drug action as well as an interesting characteristic of the calcium-activated chloride conductance. Therefore we explored this effect in detail and we propose that NFA can both increase and decrease ICl(Ca) by binding to an extracellular site, probably close to the mouth of the chloride channel.
METHODS
Preparation of pulmonary artery smooth muscle myocytes
Experiments were performed on freshly dispersed rabbit pulmonary artery smooth muscle cells. Male or female New Zealand white rabbits were killed with an overdose of sodium pentobarbitone injected via an ear vein as approved under Schedule 1 of the UK Animals (Scientific Procedures) Act 1986 and the pulmonary artery excised. The artery was dissected free of fat and connective tissue and single smooth muscle cells were isolated by acute or overnight treatment with papain as described previously (Wang & Large, 1993). Briefly, strips of tissue (2–3 mm wide by 10 mm long) were placed in a solution containing (mm): NaCl, 120; KCl, 6; MgCl2, 1.2; CaCl2, 0.05; 4-(2-hydroxy-ethyl)-1-piperazine ethane sulphonic acid (Hepes), 10 and glucose, 11; pH adjusted to 7.2 with NaOH. When cells were prepared on the same day papain (2 mg ml−1), bovine serum albumin (5 mg ml−1) and dithiothreitol (1 mm) were added and the tissue strips were incubated at 37 °C for 15–20 min. For an overnight dissociation, papain (0.5 mg ml−1), bovine serum albumin (2 mg ml−1) and dithiothreitol (1 mm) were added and the tissue stored at 4 °C for 12–4 h. The next morning the tissue strips were incubated at 37 °C for 5 min. After acute or overnight enzyme treatment single cells were isolated by trituration with a fire-polished wide bore pipette. Drops of the resultant cell suspension were placed on glass coverslips and stored at 4 °C for up to 4–6 h prior to an experiment.
Solutions
In order to record Cl− currents in isolation all experiments were performed using K+-free solutions. Cells were perfused with a solution of the following composition (mm): NaCl, 126; MgCl2, 1.2; CaCl2, 1.5; Hepes, 10 and glucose, 11; pH adjusted to 7.2 with NaOH. In some experiments NaCl was replaced with an equimolar amount of NaSCN or NMDG+-Cl. The K+ channel blocker tetraethylammonium (TEA, 10 mm) and the Ca2+ channel inhibitor nicardipine (5 μm) were also added to the external solution. The pipette solution contained (mm): CsCl, 106; MgCl2, 0.4; 1,2-bis(2-aminophenoxy)ethane-tetra-acetic acid (BAPTA), 10; Hepes, 5; MgATP, 3; GTP, 0.2 and the pH was adjusted to 7.2 with CsOH. Varying amounts of CaCl2 (6.9, 7.8 or 8.8 mm) were added to the pipette solution in order to buffer free Ca2+ to either 250 nm, 500 nm or 1 μm, respectively (calculated using Eqcal for Windows). TEA (10 mm) and 4-aminopyridine (10 mm) were also added to the pipette solution in order to block K+ channels.
Changes in liquid junction potential were minimised by the use of a 150 mm KCl-agar bridge connecting the recording chamber and a side bath containing the intracellular solution.
Chemicals were prepared as concentrated stock solutions in dimethyl sulphoxide (DMSO) and diluted to the final concentration in the external solution. Papain, bovine serum albumin, dithiothreitol, Hepes, BAPTA, TEA, 4-aminopyridine, nicardipine, niflumic acid, and 4,4-diisothiocyanato-stilbene-2,2-disulphonic acid (DIDS) were all supplied by Sigma. Dichloro-diphenyl 2-carboxylic acid (DCDPC) was a gift from Professor R. Greger, University of Freiburg.
Electrophysiological recording
All experiments were carried out at room temperature (20–25 °C). Voltage protocols were generated and whole-cell current recorded using a Heka EPC8 patch clamp amplifier and Cambridge Electronic Design (CED) hardware and voltage clamp software.
Cells were held at a holding potential of −50 mV. The voltage dependence of ICl(Ca) was studied with voltage ramps. Slow (2 s) voltage ramps from −100 to +100 mV or from −80 to +80 mV in the presence of external NaCl and from −120 to +40 mV in the presence of external NaSCN were applied at 20 s intervals. Cells were held at the most hyperpolarised voltage for 200 ms before the voltage ramp was applied to allow current to reach a steady state. The voltage-dependent kinetics of ICl(Ca) were studied by initially depolarising the cell to +70 mV for 1 s and then to −80 mV for 750 ms before returning to the holding potential of −50 mV. This step protocol was applied at 20 s intervals.
Statistical analysis
Data are expressed as means ± s.e.m. and statistical significance was assessed with Student's paired or unpaired t test as appropriate (applied using Microsoft Excel 97 software).
RESULTS
Properties of ICl(Ca) activated by adding a fixed [Ca2+] to the patch pipette solution in rabbit pulmonary artery myocytes
Rabbit pulmonary artery myocytes were dialysed with a pipette solution in which the free concentration of Ca2+ was fixed at 500 nm (see Methods) in order to activate ICl(Ca). Immediately after rupturing the membrane and achieving the whole-cell configuration with 500 nm free Ca2+ in the pipette solution there was an instantaneous inward current (IH) at the holding potential of −50 mV of −163 ± 27 pA (n = 18). Subsequently IH declined and after 3 min IH reached a steady-state level which was −60 ± 13 pA (n = 18) and after this early equilibration had taken place IH remained constant for the duration of the experiment. The majority of the inward current recorded at −50 mV was Ca2+-dependent because when pipette solutions containing 20 or 100 nm free Ca2+ were used the evoked currents at −50 mV were −9 ± 5 pA (n = 5) and −8 ± 2 pA (n = 5), respectively. These data indicate that approximately 87 % of the whole-cell current recorded at −50 mV was Ca2+ activated and furthermore that the Ca2+ threshold for activation of this current at −50 mV was greater than 100 nm.
The I–V relationship for the Ca2+-activated current was determined by applying 2 s voltage ramps from −100 to +100 mV at 20 s intervals from the holding potential of −50 mV (Fig. 1A(i)). The I– curve displayed outward rectification (Fig. 1A(ii)) and reversed close to 0 mV (mean reversal potential (Er) was 1 ± 1 mV, n = 8). The theoretical chloride equilibrium potential (ECl) in these experiments was calculated to be 0 mV, suggesting that the Ca2+-activated current recorded in rabbit pulmonary artery myocytes was ICl(Ca). In the presence of 126 mmNaSCN-containing external solution (Fig. 1A(i)) the reversal potential of the ramp currents was shifted by around 50 mV to more negative values (e.g. Fig. 1A(ii); mean Er was −46 ± 2 mV, n = 8). Previous studies have shown that equimolar replacement of external Cl− with SCN− causes a negative shift in the reversal potential of ICl(Ca) of approximately 50 mV (Amédée et al. 1990; Greenwood & Large, 1999b). These data are in accordance with Greenwood et al. (2001) and confirm that dialysing rabbit pulmonary artery myocytes with a pipette solution containing 500 nm Ca2+ activates ICl(Ca).
Figure 1. I-V relationship for ICl(Ca) activated by 500 nm[Ca2+]i in 126 mm external NaCl, 126 mm external NaSCN or 126 mm external NMDG-Cl.

A(i) a trace of current evoked by 500 nm[Ca2+]i from a typical pulmonary artery cell bathed in 126 mmNaCl-containing external solution. The external solution was changed for one containing 126 mmNaSCN as indicated by the filled bar. Depolarising voltage ramps from −100 to +100 mV were applied at 20 s intervals from the holding potential of −50 mV. A(ii), current recorded during single voltage ramps in the presence of external 126 mmNaCl (Control) and 126 mmNaSCN (SCN− ext). B(i) a trace of current recorded from a second pulmonary artery myocyte bathed in 126 mmNaCl external solution. The external solution was changed for one containing 126 mm NMDG-Cl as indicated by the filled bar. Depolarising voltage ramps from −100 to +100 mV were applied at 20 s intervals from the holding potential of −50 mV. B(ii), current recorded during single voltage ramps in the presence of external 126 mmNaCl (Control) and 126 mm NMDG-Cl (NMDG+ ext). Note that replacement of external Na+ by NMDG+ reduced the current at all potentials but did not alter the reversal potential. In both A(i) and B(i) the dotted line represents zero current.
It was also important to ascertain whether a Ca2+-activated cation conductance contributed to the recorded currents as these have been recorded previously in smooth muscle (Wang et al. 1996). In the next set of experiments we investigated the effect of replacing external Na+ by the impermeant cation NMDG+ on ICl(Ca) activated by 500 nm [Ca2+]i; Fig. 1B(i). In the presence of external NaCl in these experiments the I–V relationship displayed outward rectification and the current reversed at around 0 mV (Fig. 1B(ii); mean Er was −1 ± 1.8 mV, n = 8). Interestingly, replacement of Na+ by NMDG+ reduced the amplitude of ICl(Ca) (Fig. 1B(i) and (ii)) and the mean IH at −50 mV was reduced from −41 ± 6 pA in NaCl to −26 ± 6 pA in NMDG-Cl (n = 8, P < 0.01). However in the presence of external NMDG-Cl there was no change in reversal potential and in NMDG-Cl Er was 0 ± 2.2 mV (n = 8). The data presented above therefore indicate that the Ca2+-activated conductance recorded in rabbit pulmonary artery myocytes is indeed due to the opening of Ca2+-activated Cl− channels and that there is no significant cation current contribution, although external cations appear to modulate ICl(Ca).
Effect of niflumic acid on Ca2+-activated chloride current stimulated by fixed [Ca2+]i
In previous studies we and others have demonstrated that niflumic acid (NFA) inhibits ICl(Ca) in micromolar concentrations with an IC50 (concentration to reduce the amplitude by 50 %) of around 2–5 μm for inhibition of STICs (Hogg et al. 1994b; Greenwood & Large, 1996). Using the present method of activation of ICl(Ca) with 500 nm Ca2+ in the pipette solution NFA had markedly different effects. Application of NFA to rabbit pulmonary artery myocytes in concentrations of between 1–0 μm had no effect on ICl(Ca). Surprisingly, concentrations of NFA higher than 10 μm actually produced an increase in holding current. Figure 2A shows a representative current trace recorded from a single rabbit pulmonary artery myocyte dialysed with 500 nm Ca2+ pipette solution. When NFA (100 μm) was applied IH at −50 mV increased from −26 to −50 pA. Furthermore on wash out of NFA there was a large increase in IH which reached a maximum of −70 pA after around 30 s which then gradually declined and returned to control values after approximately 2 min (Fig. 2A). NFA produced a mean increase in IH at −50 mV from −55 ± 9 to −73 ± 9 pA (n = 34; P < 0.01) and on wash out of NFA IH reached a maximum of −152 ± 21 pA (n = 34). In order to determine the voltage dependence of this conductance depolarising voltage ramps were applied to cells at 20 s intervals in order to record the quasi steady-state current-voltage relationship of ICl(Ca) in the absence and presence of NFA (100 μm). A representative trace and an example of the I–V curves generated are shown in Fig. 2B and C. The control I–V relationship displayed mild outward rectification and reversed at 1 ± 1 pA (n = 8). In the presence of NFA the inward current at negative potentials was enhanced, while outward current at positive membrane potentials was inhibited (Fig. 2C). On wash out of NFA ICl(Ca) was markedly increased at all potentials (Fig. 2C). There was no change in Er during the application of NFA (2 ± 1 mV, n = 8) or on wash out (3 ± 2 mV, n = 6).
Figure 2. Effect of NFA on ICl(Ca) stimulated by 500 nm[Ca2+]i.

A, whole-cell current recorded from a typical rabbit pulmonary artery myocyte at a holding potential (Vh) of −50 mV. NFA (100 μm) was applied to the cell as indicated by the filled bar, the dotted line represents zero current. B, recording of current from a second pulmonary artery cell where depolarising voltage ramps from −80 to +80 mV were applied at 20 s intervals from Vh of −50 mV. The cell was exposed to NFA (100 μm) as indicated by the filled bar, dotted line represents zero current. C, plot of current recorded during the indicated voltage ramps from the cell shown in B against voltage before (a, Control), during the application of NFA (b, NFA) and on wash out (c, Wash). Note that NFA increased ICl(Ca) at negative potentials but decreased the current at positive potentials.
However, it was important to ascertain whether the NFA-induced increase in current was a Ca2+-activated current or whether another chloride conductance was involved. For these experiments NFA was applied to cells in which [Ca2+]i was buffered to 50 nm which is subthreshold for activation of ICl(Ca). With 50 nm [Ca2+]i the holding current at −50 mV was −9 ± 2 pA (n = 5) and in the same cells after 100 μm NFA was added to the bathing solution IH at −50 mV was −10 ± 3 pA (n = 5). Therefore the increase in current produced by NFA with 500 nm [Ca2+]i is a Ca2+-activated conductance. These data indicate that in pulmonary artery myocytes NFA increased inward ICl(Ca) at negative potentials but blocked outward current at positive membrane potentials.
Effect of niflumic acid on ICl(Ca) in the presence of the impermeant cation NMDG+
Ca2+-activated cation channels have been previously described in smooth muscle myocytes (Wang et al. 1996). In order to determine if the current activated by NFA had a significant cation component the experiment described above was repeated using solutions in which the major cations (Na+ and Cs+ in external and pipette solutions, respectively) were replaced by the impermeant cation NMDG+. A representative trace of the effect of NFA on ICl(Ca) in the presence of external NMDG+ is shown in Fig. 3A where it can be seen that application of NFA increases IH at −50 mV. Under these conditions application of NFA (100 μm) increased IH at −50 mV from −53 ± 8 to −74 ± 12 pA (n = 7; P < 0.01). This effect is similar to that seen with Cs+ and Na+ ions in the pipette and bathing solutions, respectively. In the presence of NFA inward current at negative potentials was increased while outward current at positive potentials was inhibited. The Er was not changed and the mean Er values in control, NFA and on wash out were, respectively, −1 ± 1 mV (n = 7), 2 ± 1 mV (n = 7) and 0 ± 1 mV (n = 4). These data indicate that the increase in inward current at negative potentials produced by the application of NFA to rabbit pulmonary artery myocytes is not due to the activation of an additional cation conductance but is due to an increase in ICl(Ca).
Figure 3. Effect of NFA on ICl(Ca) stimulated by 500 nm[Ca2+]i in the presence of the impermeant cation NMDG+.

A, whole-cell current recorded from a typical rabbit pulmonary artery myocyte at a Vh of −50 mV. NMDG+ replaced Cs+ and Na+ in pipette and external solutions, respectively. Depolarising voltage ramps from −100 to +100 mV were applied at 20 s intervals. The cell was exposed to NFA (100 μm) as indicated by the filled bar, dotted line represents zero current. Note that there was no increase in ICl(Ca) on wash out of NFA. B, plot of current recorded during the indicated voltage ramps from the cell shown in A against voltage before (d, Control), during the application of NFA (e, NFA) and on wash out (f, Wash).
Interestingly, upon wash out of NFA there was no further increase in holding current, in contrast to the data recorded with external Na+ and Cs+ in the pipette solution (cf. Fig. 2A and B). The I-V relationship recorded from the cell shown in Fig. 3A is shown below in Fig. 3B. The control I–V curve displayed pronounced outward rectification and reversed close to 0 mV. However, if on removal of NFA from the bathing solution simultaneously external NMDG+ was replaced by Na+ ions there was a large ‘wash out’ current. In these experiments, IH at −50 mV increased from −81 ± 10 pA (n = 5) to −123 ± 19 pA (n = 5) on wash out of NFA and replacement of external NMDG+ with Na+ ions. This result shows that the increase in ICl(Ca) on wash out of NFA is dependent on external Na+ ions.
Effect of niflumic acid on Ca2+-activated chloride current stimulated by fixed [Ca2+]i in the presence of extracellular NaSCN
As described above, in the present study NFA enhanced inward ICl(Ca) at negative potentials but inhibited ICl(Ca) at positive membrane potentials. We were interested to ascertain whether this effect of NFA was related to membrane voltage or was a consequence of the direction of current flow. We therefore repeated the experimental protocol described above in the presence of extracellular NaSCN where there is a large negative shift in Er.
Figure 4 shows the mean I–V curve for current recorded in the absence and presence of NFA with 126 mmNaSCN in the bathing solution. Current was normalised to the maximum inward current recorded at −120 mV during the control voltage ramp from −120 to +40 mV (mean control value at −120 mV was −37 ± 6 pA, n = 5). The control current-voltage relationship displayed pronounced outward rectification and reversed at −46 ± 2 mV (n = 5) and in the presence of NFA (100 μm) the Er of the current was unchanged (−45 ± 2 mV, n = 5). It was observed that NFA enhanced inward current at potentials more negative than Er (inward current at −120 mV was −70 ± 10 pA compared to control of −37 pA, see above; P < 0.05) but at potentials more positive than Er current was inhibited (Fig. 4) e.g. current at +40 mV was reduced from 260 ± 27 to 170 ± 29 pA (P < 0.05). More convincing evidence for dependence of this effect of NFA on current flow was obtained by measuring the current at −20 mV. In external NaCl, when Er was approximately 0 mV, NFA increased the current at −20 mV from −28 ± 6 to −38 ± 8 pA (n = 8; P < 0.01). In NaSCN, when Er was −46 mV, at −20 mV NFA reduced the current from 41 ± 8 to 30 ± 7 pA (n = 8; P < 0.01).
Figure 4. I-V relationship for ICl(Ca) activated by 500 nm[Ca2+]i in the absence and presence of NFA (100 μm) in 126 mm external NaSCN.
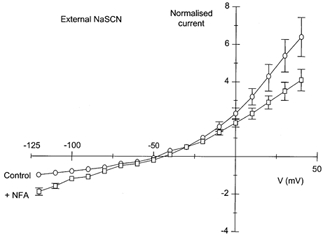
I-V curves for ICl(Ca) in the absence (Control, ○) and presence of 100 μm NFA (+NFA, □) in 126 mmNaSCN-containing external solution determined by depolarising voltage ramps from −120 to +40 mV. Currents have been normalised to the control current at −120 mV (-1). Points are means ±s.e.m. (n = 5). Standard error bars that lie within the symbols have been omitted for clarity.
Upon wash out of NFA the reversal potential of the whole cell current was unchanged (−46 ± 2.8 mV) but there was an increase in inward current at −120 mV as NFA was washed out which reached a maximum of −92 ± 10 pA (n = 5; P < 0.05) after around 30 s.
These data show that the dual potentiating and inhibitory effects of NFA on ICl(Ca) recorded in these experiments appear to be related to the direction of current flow i.e. NFA enhances inward current (outward movement of Cl−) but inhibits outward current (inward movement of anions), regardless of the reversal potential.
Effect of internal NFA on ICl(Ca) activated by 500 nm [Ca2+]i
We were interested to determine if NFA could produce the effects on ICl(Ca) described above when applied to the internal side of the channel. Therefore experiments were carried out where NFA (100 μm) was added to the pipette solution with 500 nm Ca2+.
With 100 μm NFA in the pipette solution on achieving the whole cell configuration, the instantaneous IH at −50 mV was −181 ± 54 pA (n = 5) which was not significantly different from control cells (−163 pA, see earlier). After 3 min, IH had declined to −55 ± 15 pA which was also not different to the value obtained without NFA in the pipette solution (−60 pA, see earlier) and remained constant for the duration of the experiment. The I–V relationship for ICl(Ca) as determined by depolarising ramps (from −100 to +100 mV) displayed outward rectification and reversed at 3 ± 1 mV (Fig. 5, n = 5). When NFA (100 μm) was applied to cells dialysed with 100 μm NFA it produced an increase in holding current to −80 ± 16 pA (n = 5; P < 0.05) at −50 mV which was not significantly different from the increase in IH produced by NFA in control cells (i.e. no internal NFA). Also application of 100 μm NFA in the bathing solution reduced ICl(Ca) at positive potentials with NFA in the recording pipette (Fig. 5). When external NFA was washed out there was an increase in IH at −50 mV which reached a maximum of −102 ± 24 pA (n = 5) and ICl(Ca) was increased at all voltages. The reversal potential of the whole-cell current was unchanged (4 ± 1 mV; Fig. 5).
Figure 5. Effect of internal NFA on I-V relationship for ICl(Ca) activated by 500 nm[Ca2+]i.

I-V curves for ICl(Ca) before (Control, ○), during (+external 100 μm NFA, □) and after (Wash, ▵) application of external 100 μm NFA when the pipette solution contained 100 μm NFA as determined by depolarising voltage ramps from −100 to +100 mV. Currents have been normalised to the control current at −100 mV (-1). Points are means ± s.e.m. (n = 5). Overlapping standard error bars have been omitted for clarity.
These data indicate that NFA applied intracellularly does not activate or block ICl(Ca) and the dual effects described above are due to NFA binding to an external site.
Effect of NFA on ICl(Ca) in the presence of an inhibitor of CaMKII
Since ICl(Ca) decays significantly immediately after rupture of the cell membrane it seems that a significant proportion of the channels are in the inactivated state. Recently it has been shown that phosphorylation by calcium/calmodulin-dependent protein kinase (CaMKII) is responsible for inactivation of ICl(Ca) in equine tracheal smooth muscle (Wang & Kotlikoff, 1997) and inhibitors of this kinase increase ICl(Ca) in pulmonary and coronary artery myocytes (Greenwood et al. 2001). Therefore we investigated whether the increase of ICl(Ca) by NFA at negative potentials was due in some way to an effect on Cl− channel inactivation.
When 5 μm KN93, an inhibitor of CaMKII, was applied to cells after whole cell recording had been established it produced an increase in IH at −50 mV from −48 ± 8 pA to −81 ± 13 pA (n = 11; P < 0.05) confirming the results obtained by Greenwood et al. (2001). In the continued presence of KN93 IH at −50 mV declined and after 3 min was −66 ± 9 pA (n = 11).
NFA (100 μm) was then applied to rabbit pulmonary artery myocytes in the presence of KN93 and it produced a similar effect on ICl(Ca) in the presence of KN93 compared to control cells. IH was increased in the presence of NFA from −66 to −110 ± 15 pA (n = 11, P < 0.001) and on wash out of NFA there was also a further increase in ICl(Ca) to −275 ± 37 pA (n = 8).
These data would suggest that the enhancing effect of NFA on ICl(Ca) activated by 500 nm [Ca2+]i at negative membrane potentials is not due to the prevention of CaMKII-induced channel inactivation.
Effect of NFA on relaxations of ICl(Ca) in response to voltage steps
The experiments described above have illustrated the effect of NFA on the quasi steady-state I–V relationship of ICl(Ca). However ICl(Ca) also possesses time-dependent and voltage-dependent properties that are revealed during voltage steps (Arreola et al. 1996; Nilius et al. 1997; Greenwood et al. 2001) and therefore we carried out a series of experiments to determine the effect of NFA on the time-dependent kinetics of the current.
Figure 6 shows representative traces of current recorded during the voltage step protocol before (Fig. 6A), during application of NFA (Fig. 6B) and on washing out NFA (Fig. 6C). Before NFA was applied to the cell shown in Fig. 6A IH at −50 mV was −37 pA (mean IH was −55 ± 9 pA, n = 26). Stepping to +70 mV produced an instantaneous current (Iinst+70) representing the conductance of channels open at −50 mV. Subsequently an outward current relaxation (Irelax+70) developed during the voltage step reflecting a voltage-dependent increase in conductance. When the membrane potential was repolarised to −80 mV a large instantaneous current (Iinst−80) was recorded which was larger than Iinst+70 due to the increase of ICl(Ca) which occurred during the depolarising step. Subsequently the current declined over the course of the hyperpolarising step (Irelax−0) as the channels opened by depolarisation closed (Fig. 6A, for mean data see Table 1).
Figure 6. Effect of NFA on ICl(Ca) current relaxations in response to voltage steps.

A, trace of current recorded from a single pulmonary artery myocyte. Potential was stepped from a Vh of −50 to +70 mV for 1000 ms then to −80 mV for 750 ms before returning to −50 mV. The current parameters described in Tables 1 and 2, Iinst+70, Irelax+70, Iinst-80 and Irelax-80 are shown by the arrows (see text). B, current during the voltage step protocol in the presence of NFA (100 μm). C, peak current recorded on wash out of NFA. The horizontal arrow represents zero current.
Table 1.
Effect of NFA (100 μm) on the kinetic properties of ICl(Ca)activated by 500μm or 1μm[Ca2+]i in voltage jump experiments in rabbit pulmonary artery smooth muscle cells
| +70mV | −80mV | |||||||
|---|---|---|---|---|---|---|---|---|
| Iinst+70(pA) | (n) | Irelax+70(pA) | (n) | Iinst−80(pA) | (n) | Irelax−80(pA) | (n) | |
| 500nm[Ca2+]i | ||||||||
| Control | 153 ± 21 | (26) | 121 ± 21 | (26) | 470 ± 69 | (26) | 173 ± 25 | (26) |
| +NFA | 159 ± 15 | (26) | 12 ± 2 | (26)* | 259 ± 23 | (26)* | 24 ± 3 | (26)* |
| Wash | 327 ± 49 | (17)* | 55 ± 9 | (17)* | 555 ± 62 | (17) | 80 ± 18 | (17)* |
| 1 μ[Ca2+]i | ||||||||
| Control | 296 ± 24 | (11)† | 76 ± 16 | (11)† | 492 ± 46 | (11) | 92 ± 15 | (11)† |
| +NFA | 158 ± 45 | (11)* | 9 ± 2 | (11)* | 246 ± 56 | (11)* | 18 ± 5 | (11)* |
| Wash | 272 ± 28 | (6) | 33 ± 9 | (6)* | 324 ± 67 | (6) | 36 ± 10 | (6)* |
The definitions of the current parameters are shown in Fig.6A and n values are shown in parentheses.
Indicates significant difference (P < 0.05) from control as determined by Student's paired t test
indicates significant difference (P < 0.05) from 500 μm[Ca2+]i as determined by Student's unpaired ttest.
In the presence of NFA in this cell the holding current at −50 mV was increased from −37 to −50 pA (in NFA mean IH was −73 ± 9 pA, n = 26, P < 0.01). There was no significant difference in instantaneous current on stepping to +70 mV in the presence of NFA but the outward current relaxation was virtually abolished (Fig. 6B). Interestingly there was no evidence of developing channel block at +70 mV as the current immediately settled at the blocked level indicating that the block of ICl(Ca) by NFA must occur very rapidly. On stepping back to −80 mV from +70 mV Iinst-80 was much smaller than the control value and Irelax−80 was almost totally blocked. (Fig. 6B, for mean data see Table 1). However, there was a marked increase in the amplitude of the current at the end of the pulse at −80 mV in NFA consistent with the results obtained with voltage ramps i.e. in the presence of NFA steady-state outward current at positive potentials was inhibited while inward current at negative potentials was enhanced.
On wash out of NFA there was an increase in holding current which reached a maximum (mean IH of −146.9 ± 27 pA, n = 17; P < 0.001 compared to control) after approximately 1 min. During the wash out of NFA the instantaneous currents Iinst+70 and Iinst-80 were greatly increased while the current relaxations recorded at +70 and −80 mV (Irelax+70 and Irelax-80) were much smaller than the control values reflecting an overall increase in conductance due to most of the channels being open (Fig. 6C, Table 1). This reflects the marked increase in ICl(Ca) at all potentials seen with voltage ramps on wash out of NFA. After continued wash out of NFA the holding current returned to the control level concomitant with the amplitudes of the voltage-step parameters.
These voltage-jump experiments show that NFA inhibited the voltage-dependent characteristics of ICl(Ca).
Effect of NFA on ICl(Ca) activated by varying fixed [Ca2+]i
In a further set of experiments we investigated the effects of NFA on ICl(Ca) activated by different concentrations of free Ca2+ in the pipette solution.
With a pipette solution containing 250 nm free Ca2+ immediately after rupturing the membrane and achieving the whole-cell configuration there was an instantaneous inward current at the holding potential of −50 mV of −60 ± 10 pA (n = 5) which was significantly less than that recorded with 500 nm [Ca2+]i (−63 ± 27 pA, see earlier; P < 0.05). Subsequently the current declined and after 3 min with 250 nm [Ca2+]iIH was −42 ± 10 pA (n = 6) which was not significantly different from the steady-state holding current recorded with 500 nm free Ca2+ in the pipette solution (-60 ± 13 pA, see earlier). When 100 μm NFA was applied the results obtained were very similar to those recorded with 500 nm Ca2+ and the holding current at −50 mV increased to −64 ± 9 pA (n = 6, P < 0.05). After wash out of NFA there was a further increase in IH to a maximum of −83 ± 15 pA (n = 6). Application of the voltage-step protocol described above showed that NFA had a similar effect on ICl(Ca) activated by 250 nm [Ca2+]i compared to 500 nm [Ca2+]i. The instantaneous current on stepping to +70 mV was unchanged by NFA and the outward current relaxation was abolished while the instantaneous current on stepping to −80 mV was reduced and the inward current relaxation was abolished (data not shown).
With a pipette solution of 1 μm free [Ca2+] on rupturing the membrane and achieving the whole-cell configuration there was an instantaneous inward current at −50 mV of −341 ± 42 pA (n = 20). Subsequently the current declined and after 3 min IH was −89 ± 20 pA (n = 20). Both these values were significantly greater than the currents recorded with 500 nm [Ca2+]i (P < 0.01 and P < 0.05, respectively).
Figure 7A shows the effect of 100 μm NFA on current recorded from a pulmonary artery myocyte dialysed with 1 μm Ca2+ and the same trace is shown on an expanded time scale in Fig. 7B. In this cell NFA produced a reduction in IH from −74 pA to −57 pA. IH was reduced in the presence of NFA in six out of eleven cells tested and was unchanged in the remaining five cells. Overall there was no significant change in IH which was −102 ± 25 and −74 ± 19 pA (n = 11) in the absence and presence of NFA, respectively. Upon washing out NFA there was an apparent increase in holding current within 1–2 min in four of six cells tested, but overall there was no significant difference in IH (−17 ± 30 pA, n = 6) after 2 min compared to IH recorded before NFA was applied.
Figure 7. Effect of NFA on ICl(Ca) stimulated by 1 μm[Ca2+]i.
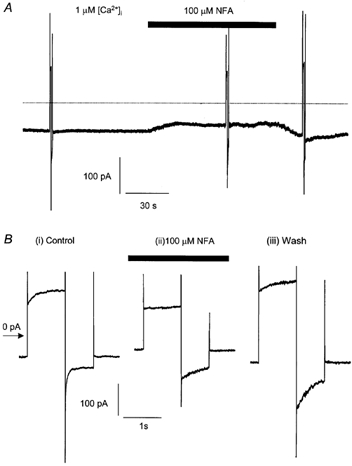
A, recording of ICl(Ca) evoked by 1 μm[Ca2+] in the pipette solution in a single pulmonary artery cell. The cell was stepped from −50 to +70 mV for 1000 ms and then to −80 mV for 750 ms before returning to −50 mV and this voltage jump protocol was repeated before, during and after application of NFA. The cell was exposed to NFA (100 μm) as indicated by the filled bar, dotted line represents zero current. B, current recorded during the voltage- jump protocol from the cell shown in A on an expanded time scale before (i, Control), during the application of NFA (ii, NFA) and on wash out (iii, Wash).
As can be seen in Fig. 7B(i) step depolarisations to +70 and then to −80 mV in control conditions produced characteristic outward and inward Cl− currents. However with 1 μm [Ca2+]i instantaneous currents (Iinst+70, Iinst-80) were larger and current relaxations (Irelax+70, Irelax−80) were smaller than those recorded with 500 nm [Ca2+]i, consistent with an increased open probability with the higher [Ca2+]i (Arreola et al. 1996). These data are consistent with 1 μm [Ca2+]i producing a significantly larger conductance than 500 nm [Ca2+]i.
In the presence of NFA, Iinst+70 and Iinst−80 were reduced and Irelax+70 and Irelax−80 were virtually abolished (Fig. 7B(ii), Table 1). As NFA was washed out the holding current returned to control values. After 2 min the instantaneous current on stepping to +70 mV was not significantly different from that recorded before NFA was applied. However, both the outward current relaxation at +70 mV and the inward current relaxation at −80 mV were reduced (Fig. 7B(iii), for mean data see Table 1).
These experiments indicate that with 1 μm [Ca2+]i, NFA did not increase ICl(Ca) at negative potentials but blocked the current at positive potentials.
Effect of DCDPC on ICl(Ca) activated by 500 nm[Ca2+]i
In the light of our experiments with NFA we decided to investigate the effects of other agents that have been shown to inhibit ICl(Ca). We investigated the effects of dichloro-diphenylamine 2-carboxylic acid (DCDPC), a compound structurally related to NFA, that inhibits STICs in a similar manner to NFA (Greenwood & Large, 1998).
Figure 8A shows that 100 μm DCDPC increased IH at −50 mV (mean increase was from control of −41 ± 6 to −55 ± 8 pA, n = 14; P < 0.05). Moreover there was a large enhancement of IH on washout of DCDPC (mean value of IH = −162 ± 15 pA, n = 10; P < 0.01).
Figure 8. Effect of DCDPC on ICl(Ca) activated by 500 nm[Ca2+]i.

A, whole-cell current recorded from a typical rabbit pulmonary artery myocyte at a holding potential of −50 mV. The cell was stepped from the holding potential of −50 to +70 mV for 1000 ms and then to −80 mV for 750 ms before returning to −50 mV and this voltage-jump protocol was repeated at 20 s intervals. DCDPC (100 μm) was applied to the cell as indicated by the filled bar, the dotted line represents zero current. B, recording of current during depolarising voltage steps from the cell shown in A on an expanded time base. Current was recorded before (i, Control), during (ii, DCDPC) and after (iii, Wash) the cell was exposed to 100 μm DCDPC (filled bar).
As can be seen from the representative trace in Fig. 8B and Table 2, DCDPC produced essentially similar results to NFA in voltage-jump experiments. In the presence of DCDPC both inward and outward current relaxations were abolished. As with NFA, block of outward current occurred instantaneously and there was no sign of a transition from a facilitated to blocked state at +70 mV and vice versa when the cell was returned to −80 mV. The increase in holding current produced on washing out DCDPC resulted in a large instantaneous current associated with small outward and inward current relaxations (Fig. 8B(iii)).
Table 2.
Effect of Cl−channel blockers on the kinetic properties of ICl(Ca) activated by 500nm[Ca2+]i involtagejumpexperiments in rabbit pulmonary artery smooth muscle cells
| +70 mV | −80 mV | |||||||
|---|---|---|---|---|---|---|---|---|
| IinstA+70(pA) | (n) | Irelax+70(pA) | (n) | Iinst−80(pA) | (n) | Irelax−80(pA) | (n) | |
| DCDPC (100 μm) | ||||||||
| Control | 117 ± 12 | (14) | 89 ± 12 | (14) | 368 ± 39 | (14) | 144 ± 16 | (14) |
| +DCDPC | 138 ± 15 | (14) | 12 ± 4 | (14)* | 209 ± 23 | (14)* | 14 ± 2 | (14)* |
| Wash | 370 ± 38 | (10)* | 26 ± 5 | (10)* | 543 ± 49 | (10)* | 40 ± 9 | (10)* |
| DIDS (1mm) | ||||||||
| Control | 188 ± 39 | (5) | 90 ± 31 | (5) | 449 ± 49 | (5) | 139 ± 31 | (5) |
| +DIDS | 137 ± 27 | (5)* | 13 ± 6 | (5)* | 272 ± 67 | (5)* | 84 ± 40 | (5)* |
| Wash | 133 ± 25 | (4) | 56 ± 13 | (4) | 350 ± 64 | (4) | 140 ± 25 | (4) |
Values in parentheses indicate number of cells.
Indicates significant difference (P < 0.05) from control as determined by Student's paired t test.
These data suggest that DCDPC is similar to NFA in that it increases current at negative membrane potentials and inhibits outward current at positive potentials while causing a large increase in conductance on washout.
Effect of DIDS on ICl(Ca)) activated by 500 nm [Ca2+]i
Diisothiocyanato-stilbene−2,2′-disulphonic acid (DIDS) is an agent, structurally dissimilar to NFA and DCDPC, that has been shown to inhibit STICs in rabbit portal vein smooth muscle cells (Hogg et al. 1994a). The action of DIDS differed from that of NFA and DCDPC in that DIDS produced little change in STIC decay which suggests that DIDS might not be a channel blocking agent.
An example of the effect of DIDS (1 mm) on ICl(Ca) recorded from a single rabbit pulmonary artery myocyte is shown in Fig. 9A and it can be seen that DIDS reduced IH at −50 mV. The holding current at −50 mV was reduced from a mean control value of −76 ± 14 to −59 ± 9 pA (n = 5; P < 0.05) in the presence of DIDS. The reduction in holding current produced by DIDS was poorly reversible and after 2 min washing IH at −50 mV was −59 ± 8 pA.
Figure 9. Effect of DIDS on ICl(Ca) current relaxations.
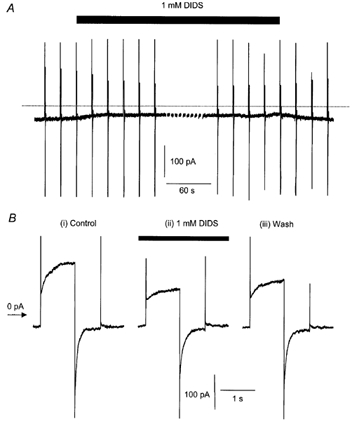
A, whole-cell ICl(Ca) evoked by 500 nm[Ca2+]i in a typical rabbit pulmonary artery myocyte at a holding potential of −50 mV. The holding potential was stepped from −50 to +70 mV for 1000 ms and then to −80 mV for 750 ms before returning to −50 mV and this voltage-jump protocol was repeated at 20 s intervals. DIDS (1 mm) was applied to the cell as indicated by the filled bar, the dotted line represents zero current. Breaks in the current trace indicate the point at which a voltage step protocol was applied and which has been omitted for clarity. B, current recorded during depolarising voltage steps from the cell shown in A on an expanded time base. Current was recorded before (i, Control), during (ii, DIDS) and after (iii, Wash) the cell was exposed to 1 mm DIDS (filled bar).
As can be seen in Fig. 9B(i) application of the voltage step protocol described above produced characteristic time-dependent outward and inward currents at +70 mV and −80 mV, respectively. DIDS produced a reduction in both the instantaneous current recorded on stepping to +70 and −80 mV and the outward and inward current relaxations but the relaxations were clearly observed (see Fig. 9, Table 2).
These data show that the effect of DIDS on ICl(Ca) activated by a persistent level of Ca2+ is markedly different from the effect of the putative open channel blocking agents NFA and DCDPC.
DISCUSSION
The main finding of the present work is that in rabbit pulmonary artery smooth muscle cells the chloride channel antagonist NFA has a dual effect on ICl(Ca) when activated by 250 and 500 nm [Ca2+]i imposed by dialysis from the pipette solution. At negative potentials NFA increased the amplitude of ICl(Ca) but at positive potentials NFA inhibited the current, i.e. NFA has a dual effect on ICl(Ca). This is a surprising result because in all previous reports it has been shown that NFA behaves only as a blocker of ICl(Ca) not only in smooth muscle but also in other cell types (e.g. see Large & Wang, 1996). However, using similar techniques of activating ICl(Ca) NFA increases ICl(Ca) in rabbit portal vein myocytes (I. A. Greenwood & N. Leblanc, unpublished results). The most likely explanation for the discrepancy in the results with NFA is a difference in the experimental conditions used. In previous studies where only inhibitory effects of NFA have been recorded in single cells NFA has been added to the cells when [Ca2+]i was at resting levels, probably around 50–100 nm, which is below the threshold for activation of the chloride channels (see Large & Wang, 1996). In our experiments with 50 nm [Ca2+]i there was no activation of ICl(Ca) and NFA did not activate ICl(Ca) at −50 mV in these conditions. When STICs are recorded there are sporadic transient increases of Ca2+ at the sarcolemma but for most of the time [Ca2+]i is at resting levels. In the present work NFA is added to cells in which ICl(Ca) has been persistently activated by a relatively high [Ca2+]i (e.g. 500 nm) imposed by the patch pipette solution. Therefore, it seems that the effect of NFA is altered when the chloride channels are tonically activated by constantly elevated [Ca2+] ions in single cells.
The increase in current observed at negative potentials produced by external application of NFA appeared to be due to an increase solely in Cl− conductance since the effect of NFA was similar when the impermeant cation NMDG+ replaced Na+ ions in the pipette and bathing solutions. Since NFA produced no increase in membrane conductance when [Ca2+]i was 50 nm this is good evidence that the increased current observed at −50 mV is ICl(Ca). It is worth noting that with inside-out patches there is only one conductance state activated by 500 nm Ca2+ (A. S. Piper & W. A. Large, unpublished results) suggesting that there is only one class of Ca2+-activated chloride channel in rabbit pulmonary artery myocytes. Therefore it can be concluded that in these conditions NFA can both increase and decrease ICl(Ca) depending on the membrane potential.
Voltage-dependence of the effect of NFA
The effect of NFA was potential sensitive and depended on the direction of the net current flow. It was found that NFA increased ICl(Ca) at potentials negative to Er but decreased the current at potentials positive to Er even when Er was changed by about −50 mV by substituting external NaCl with NaSCN. Therefore, when the net flux of anions was outward, NFA produced an overall increase of the conductance, but when there was net inward movement of anions, NFA produced an overall decrease of ICl(Ca) irrespective of Er.
In voltage-jump experiments on stepping the potential from negative to positive potentials in control conditions the instantaneous current was followed by an outward relaxation reflecting an increase in conductance. It has been suggested that this increase in conductance is due to a smaller channel closing rate (Hogg et al. 1993) and an increased affinity of Ca2+ ions for the intracellular binding site on the channel (Arreola et al. 1996) at positive, compared to, negative membrane potentials. On returning to negative potentials there was a much larger instantaneous current, representing the increased conductance caused by the depolarising step to positive potentials, followed by an outward relaxation which represents closing of the channels opened by the depolarising step. In the presence of NFA the relaxations were abolished indicating that NFA had altered these voltage-dependent properties of the conductance. A salient observation was that on stepping from negative potentials, where ICl(Ca) was enhanced by NFA, to positive potentials, where ICl(Ca) was decreased, there was no discernible inward relaxation corresponding to the transition from the increased to the decreased state of the conductance. Equally on returning to negative potentials it was not possible to resolve an inward relaxation representing the transition from the decreased to the increased state of the conductance. It is possible that the rate of these transitions were faster than the capabilities of our recording techniques. However we favour the possibility that NFA simultaneously increases ICl(Ca) and blocks the conductance and the net effect observed depends on the direction of ion flux.
Large increase of ICl(Ca) on wash out of NFA
Another notable feature of the effect of NFA in the present study was that on wash out of NFA there was a large increase of ICl(Ca). Measurement of Er in the presence of external NaSCN confirmed that the increase in current at −50 mV after wash out of NFA was indeed ICl(Ca) and moreover the conductance was increased at positive, as well as, negative membrane potentials. In the voltage-jump experiments the large washout current was associated with marked increases in the instantaneous currents to voltage steps but the amplitude of the relaxations at positive and negative membrane potentials were greatly reduced. Similar effects were observed with the chemically related DCDPC. These observations are consistent with the idea that the NFA-induced wash out current is due to a large increase in the probability of chloride channel opening and suggest that reversal of the inhibitory effect of NFA occurs more rapidly than the reversal of the potentiating effects of this agent.
It seems possible that the enhancement of ICl(Ca) on wash out of NFA is linked to the increase of the current produced in the presence of NFA. With the structurally related compound DCDPC which increased ICl(Ca) at −50 mV there was a large current on wash out of the agent. In contrast with DIDS, which simply inhibited ICl(Ca) at all potentials in its presence, there was no increase in ICl(Ca) when the agent was removed from the bathing solution. An argument against the above hypothesis is that when NMDG+ was used in the pipette and bathing solutions NFA increased ICl(Ca) at −50 mV but there was no increase in conductance on removal of NFA. This would imply that the NFA induced, and ‘wash out’ increase, in current amplitude are not related or that they are related but external Na+ ions are necessary for the development of the washout current. This interpretation is supported by the observation that in the experiments with NMDG+ a washout current was observed when NMDG+ was replaced by Na+ on wash out of NFA.
It is interesting that replacement of external Na+ ions with NMDG+ significantly reduced ICl(Ca) which was also observed by Arreola et al. (1996) in rat parotid acinar cells which suggests that external (and possibly internal) cations modulate ICl(Ca). After achieving the whole-cell conformation ICl(Ca) was approximately −160 pA but then declined to approximately one third of this value after a few minutes. Therefore many channels are in the inactivated state and it is possible that the equilibrium between open and inactivated states is sensitive to external cations. Some of the inactivation of ICl(Ca) appears to be due to the activation of CaMKII as KN93, a CaMKII inhibitor, was able to increase ICl(Ca) activated by 500 nm [Ca2+]i both in the present and in previous studies (Greenwood et al. 2001). However it is apparent that the majority of inactivation of ICl(Ca) recorded in the present study is not mediated by CaMKII. It is possible that another Ca2+-dependent inactivation process is occurring, or alternatively, that inactivation is an inherent property of the channel, as is the case with other ion channels e.g. voltage-dependent Ca2+ channels (Stotz & Zamponi, 2001) or voltage-gated potassium channels (Martens et al. 1999).
Mechanism of action of NFA
Previously it has been proposed that NFA is a channel blocker due to its voltage dependence and effect on the decay of STICs (see Hogg et al. 1994b for details). This implies that the binding site for the blocking effect of NFA is in the conducting pathway. However, there have been no previous reports on its ability to increase ICl(Ca) and it is evident that the potentiating effect of NFA is revealed with a sustained level of [Ca2+]i. We have not found any precedent in the literature for a channel blocker increasing a conductance but nevertheless it is worth considering putative mechanisms. It is possible that NFA and DCDPC might activate ICl(Ca) in addition to blocking the conductance. It has been shown that NFA and DCDPC evoke tetraethylammonium-sensitive Ca2+-activated potassium current (IK(Ca)) in rabbit portal vein myocytes (Greenwood & Large, 1995, 1998). This effect was not due to an action on internal Ca2+ stores and we proposed that this was an action on the channel protein (Greenwood & Large, 1995). This property is shared by other Cl− channel antagonists (Toma et al. 1996). At present we do not understand the molecular mechanism for this action but it is possible that these compounds might directly open the K+ channels. It is possible that NFA may also have a similar effect on the Ca2+-activated Cl− conductance and increase the probability of chloride channel opening by a mechanism similar to that with IK(Ca). Thus the observed data result from a net effect of NFA increasing ICl(Ca) as well as simultaneously inhibiting the conductance by a channel blocking mechanism. This model explains why at negative potentials NFA increases ICl(Ca) when low [Ca2+]i was used to activate ICl(Ca) but there was a trend towards inhibition when relatively high (1 μm) [Ca2+]i was used to evoke the current. In the former conditions the probability of channel opening will be considerably smaller and therefore the facilitatory effect of NFA on ICl(Ca) predominates. With 1 μm [Ca2+]i the probability of channel opening is much higher and therefore the blocking effect of NFA is predominant. The large increase in the amplitude of ICl(Ca) on wash out of NFA may be explained by a faster reversal of the inhibitory, compared to the facilitatory effect. This might occur, for example, if the facilitatory and blocking effects of NFA were mediated by an action on two distinct binding sites. However the present data do not show whether the dual effects of NFA are produced via one or two sites and further work is needed to solve this problem. Nevertheless it is clear that NFA must be applied extracellularly to produce both inhibitory and facilitatory effects and it is probably that the binding site(s) is close to the external mouth of the conducting pore. This is in contrast to Xenopus oocytes where NFA produced a significant block of ICl(Ca) when applied to the internal surface of inside-out patches (Qu & Hartzell, 2001).
Physiological implication of present results
NFA has often been used to probe the role of ICl(Ca) in producing smooth muscle contraction (e.g. Large & Wang, 1996; Greenwood & Large, 1999). This approach was based on the observation that in previous studies NFA was shown to be potent compared to other blockers of ICl(Ca) as well as relatively selective. However the present work shows that under conditions where [Ca2+]i is maintained at a sustained level at physiological resting membrane potentials (about −50 to −60 mV) NFA produced an increase in ICl(Ca) with [Ca2+]i at 250 and 500 nm and only a small decrease of ICl(Ca) when [Ca2+]i was 1 μm. These [Ca2+]i are readily achieved physiologically during smooth muscle contraction, for example to agonists. Therefore, if the present results observed in single cells also occur in intact preparations it is possible that there may be physiological responses involving ICl(Ca) which may not be sensitive to NFA, notably in cells where [Ca2+]i is elevated to around 500 nm before NFA is applied, i.e. the lack of effect of NFA on vasoconstrictor responses may not necessarily mean that ICl(Ca) is not involved in that response.
Acknowledgments
This work was supported by the Wellcome Trust.
REFERENCES
- Amédée T, Large WA, Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. Journal of Physiology. 1990;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. Journal of General Physiology. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca2+-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. British Journal of Pharmacology. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Analysis of the time course of calcium-activated chloride ‘tail’ currents in rabbit portal vein smooth muscle cells. Pflügers Archiv. 1996;432:970–979. doi: 10.1007/s004240050224. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Inhibition of Ca2+-activated Cl− currents in smooth muscle cells by compounds structurally similar to niflumic acid. British Journal of Pharmacology. 1998;123:324P. [Google Scholar]
- Greenwood IA, Large WA. Properties and role of chloride channels in smooth muscle. In: Kozlowski R, editor. Chloride Channels. Oxford: Isis Medical Media; 1999a. pp. 121–135. [Google Scholar]
- Greenwood IA, Large WA. Modulation of the decay of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle cells by external anions. Journal of Physiology. 1999b;516:365–376. doi: 10.1111/j.1469-7793.1999.0365v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. Journal of Physiology. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Time course of spontaneous calcium-activated chloride currents in smooth muscle cells from the rabbit portal vein. Journal of Physiology. 1993;464:15–31. doi: 10.1113/jphysiol.1993.sp019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Effects of Cl channel blockers on Ca-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. British Journal of Pharmacology. 1994a;111:1333–1341. doi: 10.1111/j.1476-5381.1994.tb14891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. British Journal of Pharmacology. 1994b;112:977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruma A, Hartzell HC. Dynamics of calcium regulation of chloride currents in Xenopus oocytes. American Journal of Physiology. 1999;276:C161–175. doi: 10.1152/ajpcell.1999.276.1.C161. [DOI] [PubMed] [Google Scholar]
- Lamb FS, Volk KA, Shibata EF. Calcium-activated chloride current in rabbit coronary artery myocytes. Circulation Research. 1994;75:742–750. doi: 10.1161/01.res.75.4.742. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;268:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Martens JR, Kwak YG, Tamkun MM. Modulation of Kv channel alpha/beta subunit interactions. Trends in Cardiovascular Medicine. 1999;8:253–258. doi: 10.1016/s1050-1738(00)00037-2. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Voets T, Van Den Bremt K, Eggermont J, Droogmans G. Kinetic and pharmacological properties of the calcium-activated chloride-current in macrovascular endothelial cells. Cell Calcium. 1997;22:53–63. doi: 10.1016/s0143-4160(97)90089-0. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Loirand G, Lavie LJ, Mironneau C, Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short term primary culture. Pflügers Archiv. 1989;413:629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Qu Z, Hartzell HC. Functional geometry of the permeation pathway of Ca2+-activated Cl− channels inferred from analysis of voltage-dependent block. Journal of Biological Chemistry. 2001;276:18423–18429. doi: 10.1074/jbc.M101264200. [DOI] [PubMed] [Google Scholar]
- Stotz SC, Zamponi GW. Structural determinants of fast inactivation of high voltage-activated Ca2+ channels. Trends in Neurosciences. 2001;24:176–181. doi: 10.1016/s0166-2236(00)01738-0. [DOI] [PubMed] [Google Scholar]
- Toma C, Greenwood IA, Helliwell RM, Large WA. Activation of potassium currents by inhibitors of calcium-activated chloride conductance in rabbit portal vein smooth muscle cells. British Journal of Pharmacology. 1996;118:513–520. doi: 10.1111/j.1476-5381.1996.tb15432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Akbarali HI, Hatakeyama N, Goyal RK. Caffeine- and carbachol-induced Cl− and cation currents in single opossum esophageal circular smooth muscle cells. American Journal of Physiology. 1996;271:C1725–1734. doi: 10.1152/ajpcell.1996.271.5.C1725. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hogg RC, Large WA. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. Journal of Physiology. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Large WA. Action of histamine on single smooth muscle cells dispersed from the rabbit pulmonary artery. Journal of Physiology. 1993;468:125–139. doi: 10.1113/jphysiol.1993.sp019763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-X, Kotlikoff Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proceedings of the National Academy of Sciences of the USA. 1997;94:14918–14923. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XJ. Role of calcium-activated chloride current in regulating pulmonary vasomotor tone. American Journal of Physiology. 1997;272:L959–968. doi: 10.1152/ajplung.1997.272.5.L959. [DOI] [PubMed] [Google Scholar]


