Abstract
Lactate is formed in the coronary arterial wall and in the myocardium as a consequence of ischaemia and infarction. We combined direct measurement of coronary artery diameter and interstitial arterial wall lactate concentration ex vivo in order to ascertain the possible role of lactate in hypoxia-induced vasodilatation. The wall of porcine coronary arteries, precontracted during an intraluminal pressure of 40 mmHg by addition of prostaglandin F2α, was cannulated using a microdialysis catheter, and exposed to hypoxia for 60 min, followed by 45 min of reoxygenation. The exchange fraction of [14C]lactate over the microdialysis membrane increased from 0.38 ± 0.04 to 0.52 ± 0.05 (P < 0.001) during the study period. Coronary artery diameter increased by 15.5 ± 2.0 % (n = 20) during hypoxia (P < 0.001, compared to normoxic controls) and interstitial lactate concentration rose from 1.07 ± 0.21 to 2.50 ± 0.40 mmol l−1 during hypoxia (P < 0.01) and was unchanged in controls. The increase in coronary artery diameter correlated with the increase in interstitial lactate concentration in the period between 30 and 60 min of hypoxia (r = 0.62; P = 0.02). Dichloroacetate (10−5m), an agent that reduces lactate generation by activating pyruvate dehydrogenase, abolished hypoxia-induced lactate production, but caused a further increase in coronary arterial diameter (30.2 ± 4.4 %, n = 9; P < 0.001 vs. hypoxia and no dichloroacetate). Under control conditions, the addition of l-lactate (10−3-10−2m) increased dose-dependently coronary arterial diameter by 22.0 ± 4.2 % (n = 5) and interstitial lactate concentration from 0.52 ± 0.04 to 5.70 ± 0.66 mmol l−1 (P < 0.001). There was a correlation between the increase in coronary artery diameter and interstitial lactate concentration (r = 0.60; P = 0.02). The present observations represent the first direct measurements of metabolites by microdialysis in a blood vessel wall. The lactate concentration may affect, but is not essential for, hypoxia-induced vasodilatation in porcine coronary arteries.
Hypoxia induces vasodilatation in most systemic vascular beds but there is controversy regarding the mechanisms responsible for this important protective response. Cytosolic AMP breakdown results in increased adenosine concentrations in vascular smooth musculature during hypoxia. Because of the potent vasodilatory properties of adenosine, it has been suggested that this compound may be responsible for hypoxia-induced vasodilatation (Dart & Standen, 1993; MacLean et al. 1998). However, the extremely short half-life of adenosine in blood and the finding that the vasorelaxation induced by adenosine is virtually abolished in hypoxic porcine carotid strips (Barron & Gu, 2000) question the importance of this metabolite in sustained hypoxia-induced vasorelaxation. Alternative hypotheses for the cause of hypoxia-induced vasodilatation include accelerated prostaglandin synthesis (Kalsner, 1977), release of nitric oxide (Cable et al. 1999), increased pHi (Foy et al. 1997), decreased Ca2+ sensitivity (Shimizu et al. 2000), and decreased energy state (Leach et al. 2000).
Lactate, the major metabolite produced by anaerobic glycolysis, is formed locally in the arterial wall (Shimizu et al. 2000) and in the myocardium as a consequence of ischaemia and infarction (Bagger et al. 1981; Guth et al. 1990). Experimental studies in various species and vascular structures have demonstrated vasodilatory properties by lactate (Gaskell, 1880; Omar et al. 1993a,b; McKinnon et al. 1996; Chen et al. 1996; Mori et al. 1998) but a link between artery wall lactate concentration and vasodilatation has not been established. We have introduced a microdialysis method to assess interstitial lactate concentration in porcine coronary arteries ex vivo and describe a possible role of lactate in hypoxia-induced vasodilatation.
Part of this work was presented in abstract form at the 50th Annual Scientific Session of the American College of Cardiology, Orlando, Florida, March 18–21, 2001.
METHODS
Experimental preparation
Hearts from 70-90 kg hogs were obtained shortly after slaughter (animals were killed in the abattoir in accordance with internationally approved standards). The aorta was cannulated and the coronary circulation was perfused with 100 ml of a physiological salt solution (PSS) bubbled with 5 % CO2-95 % O2. The hearts were bathed in PSS at 5 °C for approximately 2 h until the start of the experiment. The left anterior descending coronary artery (LAD) was carefully dissected and the proximal 3–4 cm of the artery was left intact. The arterial segments usually had one to three branches in the proximal part. The branches were ligated with 5–0 silk sutures during dissection in cold PSS. The anterior surface of the LAD was marked at both ends with india ink and the in situ length of the segment was recorded.
Pressure myograph
Cylindrical arterial segments (1.5–2 cm) were mounted at both ends on stainless steel cannulae and fastened with sutures in a 20 ml vessel chamber (Fig. 1). The arterial segments were bathed in PSS continuously bubbled with 5 % CO2-95 % O2 (PO2, > 650 mmHg; ISO2-D oxygen analyser, WPI, Sarasota, FL, USA) and the temperature was gradually raised to 37 °C. The internal pressure was controlled by adjustment of two reservoirs containing PSS mounted on a pressure column and connected to the cannulae. Pressure transducers close to the [arterial] end of each cannula continuously measured the internal pressure. The segments were stretched to the in situ length by operating a micrometer device on one of the cannulae and a pressure of 40 mmHg was applied to the vessel during a stabilising period of 1 h. The external diameter of the arterial segment was automatically determined by video imaging at a frequency of 20 Hz. Internal arterial pressure, the outer diameter, and a video image of the arterial segment were continuously monitored and stored on computer (Vessel View software, version 1.2, JP Trading, Aarhus, Denmark). Before the start of the experiments the video dimension analyser was calibrated with a 3000 μm × 3000 μm phantom image in the horizontal and vertical directions. At the end of the experiments the calibration was verified by repeated measurements of the phantom.
Figure 1. The experimental set-up.
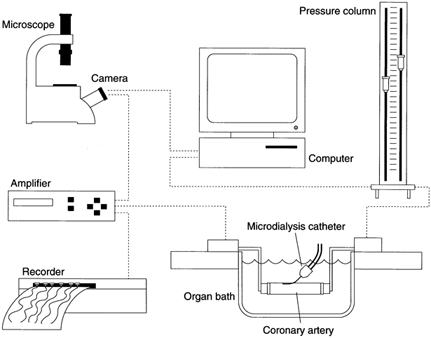
Illustration of the experimental set-up. Refer to the text for details.
Microdialysis and calculations
Microdialysis catheters (CMA/7 microdialysis probe, CMA, Stockholm, Sweden) were placed in the interstitium of the coronary artery mounted on the pressure myograph. The microdialysis catheters have a 6 kDa molecular cut-off, an outer diameter of 0.24 mm and a membrane length of 1 mm. Perfusion of the catheter was started immediately at a rate of 0.3 μl min−1 with a physiological perfusate (Perfusion fluid T1, CMA; composition (mm): Na+ 147, K+ 4, Ca2+ 2.3 and Cl− 156). The catheters were perfused for 60 min prior to the experiments. The effluent from the microdialysis catheters (dialysate) was collected in consecutive 30 min fractions (9 μl) starting before induction of hypoxia. There was a delay of 12 min between the passage of the perfusate through the microdialysis catheter and collection in vials. This time delay was considered in the calculations and all data are presented in real time.
Glucose and lactate concentrations in the dialysate were measured by an automated spectrophotometric kinetic enzymatic analyser (CMA 600). To determine the exchange fraction (i.e. relative recovery, RR) of lactate over the microdialysis membrane a small amount of [14C]lactate (0.95 μCi ml−1; 3.51 pmol l−1) was included in the perfusate as an internal reference marker. The RR was measured in six animals and calculated as perfusatedpm - dialysatedpm/perfusatedpm, where perfusatedpm and dialysatedpm are the radioactivity in the perfusate and the dialysate, respectively, measured in disintegrations per minute (d.p.m.), and interstitial lactate concentration was calculated as [lactate]dialysate/RR, where [lactate]dialysate is the concentration of lactate in the dialysate. The mean RR increased throughout the study period: before hypoxia, 0.38 ± 0.04; 0–30 min hypoxia, 0.42 ± 0.04; 30–60 min hypoxia, 0.52 ± 0.05. The values of RR for these three time periods differed significantly from each other (P < 0.001, repeated measures ANOVA). Data were incomplete for the reoxygenation period (RR = 0.58 ± 0.04, n = 2). The mean RR values were used for further calculations of lactate concentration.
Procedures
During a stabilising period of 1–2 h, contraction of the coronary arteries was induced by addition of potassium chloride (125 mm) until a reproducible response was obtained (at least twice). With the artery temporarily not pressurised, the microdialysis catheter was inserted subsequent to forming a small oblique channel in the wall with a 0.3 mm syringe. After an additional stabilising period with PSS in the organ bath arterial contraction was induced with prostaglandin F2α (PGF2α; 10−5m). When a stable contraction had been established, hypoxia was induced by gassing with 5 % CO2-95 % N2 (PO2, 34 ± 7 mmHg). Simultaneously the PSS in the lumen was exchanged with deoxygenated PSS. Oxygenation was re-established after 60 min of hypoxia by washing with PSS aerated with 5 % CO2-95 % O2 and the arterial response during the following 45 min was recorded (reoxygenation period). pH in the organ bath and the dialysate was monitored with a small pH electrode (MI-410, Microelectrodes, Inc., Bedford, NH, USA) connected to a pH meter (PHM210, Radiometer, Copenhagen, Denmark). In some experiments the pyruvate dehydrogenase complex activator dichloroacetate was added to the organ bath at the beginning of the experiment to promote the oxidation of lactate. A dichloroacetate concentration of 10−5m was chosen since pilot studies had demonstrated this to be the highest concentration devoid of intrinsic vasodilatory properties in our experimental set-up.
The coronary artery response to increasing concentrations of l-lactate (10−3, 3 × 10−3 and 10−2m), applied intraluminally and in the organ bath, were studied during microdialysis under normoxic conditions in the presence of PGF2α (10−5m). In order to obtain comparable results and sampling volumes (9 μl) from microdialysis sampling we used a fixed 30 min time period for each l-lactate concentration.
Histology
In all experiments correct placement of the microdialysis catheter was assured by visual inspection. Perforation of an artery by the catheter tip made application of a positive intraluminal pressure impossible and such arteries were discarded. In order to verify that the microdialysis catheters were actually placed in the media of the coronary arteries, five arteries were fixed in 4 % buffered neutral formaldehyde after the experiment and after retracting the dialysis catheter. The coronary arteries were cross-sectioned at 30–100 μm intervals. The sections were embedded in paraffin and counterstained with elastic Van Gieson's stain for collagen, before microscopy as previously described (Frøbert et al. 1997).
Solutions
The PSS had the following composition (mm): NaCl 119, NaHCO3 25, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.5, EDTA 0.0027 and glucose 11. In experiments where high K+-containing PSS was used, NaCl was exchanged for KCl on an equimolar basis to a final K+ concentration of 125 mm. All solutions were made using analytical grade chemicals and twice-distilled water. PGF2α was from Dinoprost, Upjohn, Germany, and dichloroacetate and l-lactate were from Bie & Berntsen A/S, Denmark. Lactic acid was dissolved in water and the pH of the solution was adjusted with NaOH (1 mm) to 7.4.
Statistics
Values are presented as means ± s.e.m. and number of vessels (one per pig). Because the coronary artery diameter varied between pigs the steady state diameter induced by PGF2α was used as an internal standard (0 %). Data were evaluated using Student's two-tailed unpaired and paired t tests. Bivariate associations were examined by least square linear regression. Differences in RR between time periods were evaluated using one-way repeated measures analysis of variance and a Tukey's post hoc test. P < 0.05 was regarded as significant.
RESULTS
The microdialysis catheters made imprints in the media in all five coronary arteries (Fig. 2).
Figure 2. Stained histological section of a porcine coronary artery.
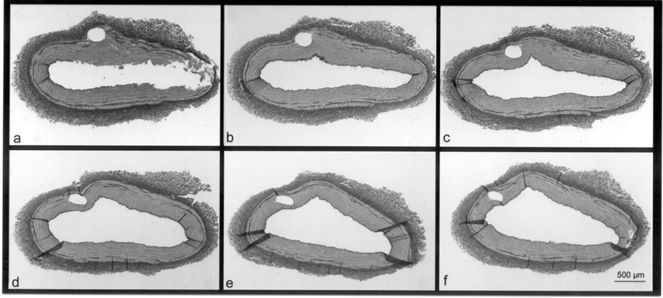
Elastic Van Gieson-stained histological section of a porcine coronary artery cross-sectioned at 50 μm intervals in a proximal (a) to distal (f) direction. A circular imprint from a microdialysis catheter is visible in the arterial media.
Hypoxia-induced coronary dilatation
Coronary artery diameter typically demonstrated a triphasic pattern during hypoxia (Fig. 3). After an initial flow-dependent artefact, caused by changing the PSS in the arterial lumen, a small contraction was seen with a peak after about 8 min. Maximal hypoxia-induced dilatation occurred after approximately 20 min and a steady state dilatation, which lasted for at least 3 h (data not shown), was present from 40 min and onwards (15.5 ± 2.0 % at 60 min, n = 20; P < 0.001 compared to baseline). After 60 min of steady state hypoxia, dilatation of those coronary arteries with a microdialysis catheter inserted into the wall did not differ from the dilatation of hypoxic arteries with no catheter (13.9 ± 3.1 %, n = 5; P = 0.9). Control arteries showed a small but significant constriction (-4.0 ± 0.5 % at 60 min, n = 5; P < 0.01 compared to baseline).
Figure 3. Effect of hypoxia and reoxygenation on coronary artery diameter.
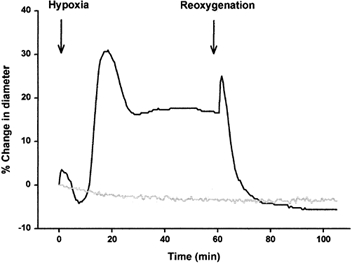
Example of the response of coronary artery diameter to hypoxia and reoxygenation (black line) and time control (grey line).
Interstitial lactate and glucose
Interstitial lactate concentrations (corrected for RR) increased 3-fold during hypoxia and fell during reoxygenation but remained significantly higher than control (normoxia, Fig. 4). There was an insignificant rise in interstitial glucose concentration (crude values, i.e. not corrected for RR) during hypoxia (30–60 min hypoxia, 4.72 ± 0.48 mmol l−1; control, 3.80 ± 0.29 mmol l−1; P = 0.16). The degree of coronary dilatation (steady state diameter after 60 min hypoxia) and interstitial lactate concentration (dialysate concentration in samples from 30–60 min hypoxia) correlated positively (n = 13; r = 0.62; P = 0.02; Fig. 5).
Figure 4. Interstitial lactate concentrations during normoxia, hypoxia and reoxygenation.
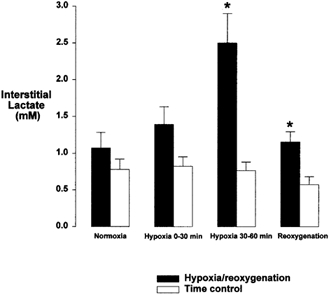
Bar graph showing interstitial lactate concentrations during normoxia, hypoxia and reoxygenation. Normoxia, hypoxia and reoxygenation (▪; n = 11–15) and time control experiments (□; n = 8–11). * Significantly different from time control; P < 0.01.
Figure 5. Correlation between coronary artery diameter and interstitial lactate concentration.
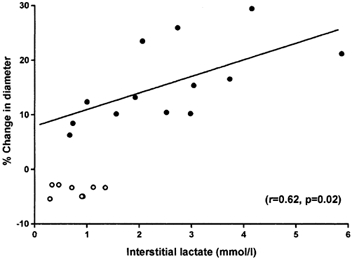
Correlation between percentage change in coronary artery diameter (baseline = 0 %) and interstitial lactate concentration. Each circle represents a separate vessel. •, lactate values from microdialysis sampling in the arteries from 30–60 min of hypoxia and steady state values of diameter after 60 min of hypoxia (r = 0.62; P = 0.02). ○, time controls.
Effect of dichloroacetate
Dichloroacetate abolished lactate production during hypoxia (0.39 ± 0.05 mmol l−1vs. 0.24 ± 0.02 mmol l−1 at baseline, n = 8; P = 0.4) while coronary diameter increased more than in control experiments without pyruvate dehydrogenase activation (30.2 ± 4.4 %, n = 9; P < 0.001 vs. hypoxia and no dichloroacetate).
l-Lactate dose-response experiments
In normoxic conditions, the maximal increase in coronary diameter in the presence of l-lactate (10−3-10−2m) was 22.0 ± 4.2 % (n = 5) and interstitial lactate concentration rose from 0.52 ± 0.04 to 5.70 ± 0.66 mmol l−1 (P < 0.001). However, because of the fixed 30 min time periods for each concentration, a cumulative dose-response curve for l-lactate could not be obtained and the dilatory response to lactate decreased over time. There was a significant positive correlation between the increase in coronary diameter and the interstitial lactate concentration (n = 5 arteries, 3 measurements per artery; r = 0.60; P = 0.02; Fig. 6).
Figure 6. Effect of addition of l-lactate on coronary artery diameter and interstitial lactate concentration.
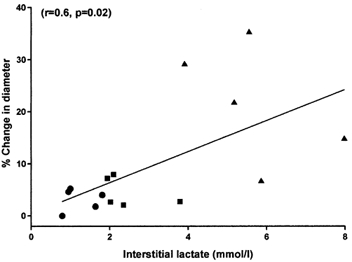
Correlation between change in coronary artery diameter and interstitial lactate concentration, in l-lactate concentration-response experiments (n = 5 arteries). Each artery is represented by values at 10−3m (•), 3 × 10−3m (▪) and 10−2m (▴). r = 0.60; P = 0.02.
pH
The pH of the solution in the organ bath remained constant at 7.7 during 60 min of hypoxia while the pH of the dialysate rose (pH at time 0 (pH0 min), 7.88 ± 0.01; pH after 60 min of hypoxia (pH60 min), 8.27 ± 0.09, n = 5; P < 0.01). However, the PSS in the lumen was not aerated with 5 % CO2-95 % N2 and time control experiments suggested that the observed increase in interstitial pH could be attributed to lack of intraluminal buffering (PSS in vials pH0 min, 7.85 ± 0; PSS in vials pH60 min, 8.19 ± 0.02, n = 3; P < 0.01).
DISCUSSION
Our main findings in isolated porcine coronary arteries were: (1) that interstitial lactate concentration increased 3-fold during hypoxia; (2) that there was a significant correlation between interstitial lactate concentration and the degree of hypoxia-induced vasodilatation between 30 and 60 min of hypoxia; (3) based on lactate dose-response experiments and results obtained with dichloroacetate, that changes in lactate concentration may contribute to, but are not essential for, the vasodilatation induced by hypoxia.
Physiological considerations
The crude interstitial lactate values in this study were somewhat lower than the crude values of about 9 mmol l−1 reported by Wikström et al. (1995) and 6 mmol l−1 found by Van Wylen (1994) in porcine myocardium following 40 and 60 min of LAD occlusion, respectively. In parallel with our observations in the vessel wall, myocardial lactate concentration increased with time to plateau at the end of the ischaemic period (Van Wylen, 1994; Wikstrom et al. 1995). The concentration of l-lactate (10−3-10−2m) needed to dilate coronary arteries in our ex vivo experiments under normoxic conditions may seem high but is in line with venous lactate levels (up to 32 mm) in healthy oarsmen after competitive rowing (Nielsen, 1999).
Lactate-induced coronary artery dilatation may be caused by a rise in blood lactate concentration and/or by lactate production within the coronary artery wall. The results from direct interstitial measurements in this study support both of these mechanisms: during dose-response experiments with the l-stereoisomer of lactate in the organ bath, interstitial lactate concentrations rose concomitantly with a proportional coronary artery dilatation; induction of hypoxia increased lactate in the coronary artery interstitium, an increase that correlated with the degree of arterial dilatation. Our finding of decreased lactate production together with further vasodilatation following addition of dichloroacetate to the organ bath seems to contradict a role for lactate in hypoxia-induced vasodilatation. Since dichloroacetate eliminated lactate production it can be inferred that the central part of the artery had sufficient oxygen for mitochondrial function (Chance, 1976). In other words, both oxidative phosphorylation and glycolysis probably take place concomitantly in the arterial wall. This is in line with previous findings in the myocardium during infarction (Opie & Thomas, 1976). Dichloroacetate stimulates activity of pyruvate dehydrogenase and thus reduces lactate production. However, dichloroacetate may exert early vasodilatation independent of the presence of lactate (Stacpoole, 1989). Such an effect may be related to a reduction in high-energy phosphates (Barron & Parrillo, 1995). We found support for this in pilot studies where dichloroacetate concentrations greater than 10−5m relaxed coronary arteries under aerobic conditions.
Vasodilatation is a relevant response to decreased tissue oxygen tension in order to increase blood flow and oxygen supply to an endangered region. The dilatory response is not confined to resistance arteries (Wadsworth, 1994; Dart & Standen, 1995; Liu & Flavahan, 1997; MacLean et al. 1998) but is also present in conduit vessels (Kalsner, 1977; Wadsworth, 1994; Foy et al. 1997; Cable et al. 1999; Barron & Gu, 2000; Shimizu et al. 2000). An intraluminal rise in lactate concentration results in vasodilatation of various arteries. In porcine coronary arteries this dilatation may be mediated by the opening of Ca2+-dependent K+ channels (IK,Ca) and not ATP-dependent potassium channels (IK,ATP) (Mori et al. 1998). In other vascular beds and in other species different endothelium-independent mechanisms for lactate-induced dilatation have been suggested such as a drop in pH (Gaskell, 1880), inhibition of high affinity Ca2+ mobilisation (Hester et al. 1980), increased tissue osmolality (Miller et al. 1981), an increase in cGMP levels (Hester et al. 1980; Omar et al. 1993b), and stimulation of protein kinase A via increases in cAMP (McKinnon et al. 1996). In myocardial tissue, excess lactate may cause mitochondrial damage (Armiger et al. 1974) and decrease action potential duration (Wissner, 1974). A comprehensive review of hypoxia-induced vasodilatation has been produced by Taggart & Wray (1998). We have not specifically addressed any of the possible mechanisms of the effect of lactate in the present study. However, the relatively slow rise in interstitial lactate concentration as compared to the time course of vessel dilatation indicates that other compounds may contribute to the initial dilatory phase (e.g. adenosine, which has a short half life) and that any possible role of lactate occurs in the later steady state phase of hypoxia-induced dilatation. A rapid rise and fall of myocardial adenosine concentration has been found during hypoxia (Van Wylen, 1994) and following brain death in pigs (Halejcio-Delophont et al. 1998).
In the reoxygenation period, lactate production continued to be significantly higher than in the control situation. We interpret this as ongoing anaerobic glycolysis (a result of slow reoxygenation of the entire coronary arterial wall) and a washout effect of lactate accumulated in the 60 min hypoxic period. Studies from our laboratory and by others have previously demonstrated long-lasting lactate production from the myocardium in vivo following pacing (Bagger et al. 1981; Thomassen et al. 1991) and after coronary artery ligation (Van Wylen, 1994).
Methodological considerations
The present observations demonstrate that metabolites can be measured directly by microdialysis in a blood vessel wall. Our finding of a relative lactate recovery of 38- 58 % is in line with results from previous in vivo studies in other organs and with larger microdialysis catheters (MacLean et al. 1998; Gravholt et al. 1999; Green et al. 1999). RR depends on mass transport in three regions; the probe lumen, the dialysis membrane and the sample medium (Hansen et al. 1999). We have no reason to expect changes in the first two regions. The increase in RR could reflect the fact that a steady state with the sample medium had not been reached when sampling was initiated but an increase in RR from rest to exercise in human skeletal muscle has previously been described (Green et al. 1999) indicating that external perturbations may alter RR. We used RR to calculate accurate interstitial lactate concentrations from the crude values.
Absolute recovery (i.e. the total amount of substance removed from the tissue through microdialysis) was 0.30 nmol min−1 at baseline and 0.75 nmol min−1 during hypoxia. Given that an average relaxed coronary arterial segment weighed 250 mg, was 20 mm long, had an inner diameter of 2.5 mm and an outer diameter of 3.0 mm, and that the dialysis membrane was 1 mm long and probably drains a volume of about 1 mm around the probe (Rosdahl et al. 1993) it can be calculated that the probe drains maximally 1.8 % (3.1 mm2 out of 173 mm2) of the segment or 4.5 mg of tissue. This equals 0.067 μmol g−1 min−1 lactate at baseline and 0.167 μmol g−1 min−1 during hypoxia, which is in line with studies in porcine carotid arteries by Barron and coworkers (Barron & Parrillo, 1995; Barron et al. 1996) who found lactate production values of around 0.1 μmol g−1 min−1 lactate in resting arteries and 0.2 μmol g−1 min−1 in arteries contracted with KCl. However the figures are somewhat lower than the values found by Pettersson and Lundholm (1985) in porcine carotid artery (0.23 μmol g−1 min−1 at baseline and 0.42 μmol g−1 min−1 during hypoxia). Glucose concentrations in the arterial wall remained relatively unchanged during hypoxia indicating that probe perfusion did not deplete local substrate concentrations (Kennergren et al. 1997).
Pyruvate, the only known metabolite of lactate in vascular tissue, was not measured in this study. Some investigators have suggested that pyruvate may have vasorelaxant properties but it is generally accepted that the potency of pyruvate in this respect is much lower than that of lactate (Omar et al. 1993a,b; McKinnon et al. 1996).
In view of the fact that interstitial lactate concentration increased, it was somewhat surprising that the pH of the dialysate rose during hypoxia. However, in vitro experiments measuring pH in non-aerated PSS showed similar increases. Others have also found a trend towards alkalinisation with hypoxia in porcine coronary artery smooth muscle (Shimizu et al. 2000) and porcine coronary endothelial cells (Foy et al. 1997). These observations do not support the previous notion that a fall in pH is the reason for a possible vasodilatory effect of lactate (Gaskell, 1880). The pH in the organ bath was 7.7 rather than the intended 7.4 which may be due to insufficient buffering capacity of the bicarbonate in the PSS. By increasing the aeration of the organ bath, pH becomes closer to 7.4, but at the expense of image quality with the video dimension system. Although our pilot studies showed that this slight alkalinisation had no influence on vasoreactivity, others (Nagesetty & Paul, 1994) have demonstrated an increase in isometric force with increasing pH in unstimulated porcine coronary artery smooth muscle. pH changes between 6.2 and 7.8 have also been reported to have minor effects on ion fluxes and metabolism (Arheden et al.1989) and our findings must be interpreted with this in mind. We did not measure oxygen tension in the different layers of the coronary artery and it could be argued that the region where the microdialysis catheter was located represents both oxygenated areas and an anoxic core in the middle of the wall (Pittman & Duling, 1973).
The transitory nature of l-lactate-induced vasodilatation in this study contrasts with findings by others (McKinnon et al. 1996; Chen et al. 1996; Mori et al. 1998) and is likely to be related to various aspects of our methodology, including the fixed 30 min time periods, the relatively large size of the coronary artery, the pressure myograph system and the type of precontraction. Compared with the study by Mori et al. (1998) our maximal relaxation induced by 10−2m l-lactate (22 % of PGF2α-induced contraction) was comparable (about 35 % relaxation of 30 mm KCl-induced contraction). We used an intravascular steady state pressure of 40 mmHg because in previous experiments we demonstrated that this pressure was optimal in terms of the coronary arterial response to an agonist recorded using a pressure myograph (Frøbert et al. 1999).
Histological examination of a random sample of coronary arteries demonstrated that the microdialysis catheters were positioned in the media layer. Since catheter placement was not examined by histology in all arteries, the possibility that some catheters were positioned in the adventitia cannot be excluded. However, free movement of ions and small molecules have been reported between the different layers of the arterial wall (Nordestgaard et al. 1995). Thus, a minor misplacement of the catheter is probably of no practical importance.
In conclusion, the correlation between vascular reactivity and interstitial lactate may suggest a role for lactate in hypoxia-induced vasodilatation. Dichloroacetate abolished lactate production despite an even greater increase in vessel diameter indicating that lactate is not essential for hypoxia-induced dilatation in porcine coronary arteries. Measurement of interstitial substances in a blood vessel wall by microdialysis provides new information. If methodological barriers can be overcome and the technique refined regarding temporal resolution it should be possible in future studies to gain further insight into the processes involved in vasomotion.
Acknowledgments
We thank Margit Nielsen and Anette Mengel for expert technical help and Dr Ulrik Baandrup, of the Institute of Pathology, University Hospital Aarhus, for the histological evaluations and microphotographs. The study was supported by Direktør Ib Henriksens Fond, Fabrikant G. Andersens Fond, Fonden af 1870, The Danish Heart Foundation (no. 00-1-2-18-22790), Lundbeckfonden, Løvens kemiske Fabriks Forskningsfond, the Novo Nordisk Foundation and the Danish Medical Research Council.
REFERENCES
- Arheden H, Arner A, Hellstrand P. Calcium sensitivity and energetics of contraction in skinned smooth muscle of the guinea pig taenia coli at altered pH. Pflügers Archiv. 1989;413:476–481. doi: 10.1007/BF00594176. [DOI] [PubMed] [Google Scholar]
- Armiger LC, Gavin JB, Herdson PB. Mitochondrial changes in dog myocardium induced by neutral lactate in vitro. Laboratory Investigation. 1974;31:29–33. [PubMed] [Google Scholar]
- Bagger JP, Toftegaard Nielsen T, Henningsen P, Thomsen PEB, Eyjolfsson K. Myocardial release of citrate and lactate during atrial pacing-induced angina pectoris. Scandinavian Journal of Clinical Laboratory Investigation. 1981;41:431–439. doi: 10.3109/00365518109090480. [DOI] [PubMed] [Google Scholar]
- Barron JT, Gu L. Energetic effects of adenosine on vascular smooth muscle. American Journal of Physiology - Heart and Circulatory Physiology. 2000;278:H26–32. doi: 10.1152/ajpheart.2000.278.1.H26. [DOI] [PubMed] [Google Scholar]
- Barron JT, Kopp SJ, Tow J, Parrillo JE. Substrate-dependent alteration in O2 consumption and energy metabolism in vascular smooth muscle. American Journal of Physiology. 1996;270:H1869–1877. doi: 10.1152/ajpheart.1996.270.6.H1869. [DOI] [PubMed] [Google Scholar]
- Barron JT, Parrillo JE. Production of lactic acid and energy metabolism in vascular smooth muscle: effect of dichloroacetate. American Journal of Physiology. 1995;268:H713–719. doi: 10.1152/ajpheart.1995.268.2.H713. [DOI] [PubMed] [Google Scholar]
- Cable DG, Pompili VJ, O'Brien T, Schaff HV. Recombinant gene transfer of endothelial nitric oxide synthase augments coronary artery relaxations during hypoxia. Circulation. 1999;100:II335–II339. doi: 10.1161/01.cir.100.suppl_2.ii-335. [DOI] [PubMed] [Google Scholar]
- Chance B. Pyridine nucleotide as an indicator of the oxygen requirements for energy-linked functions of mitochondria. Circulation Research. 1976;38:I31–I38. [PubMed] [Google Scholar]
- Chen YL, Wolin MS, Messina EJ. Evidence for cGMP mediation of skeletal muscle arteriolar dilatation to lactate. Journal of Applied Physiology. 1996;81:349–354. doi: 10.1152/jappl.1996.81.1.349. [DOI] [PubMed] [Google Scholar]
- Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. Journal of Physiology. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart C, Standen NB. Activation of ATP-dependent K+ channels by hypoxia in smooth muscle cells isolated from the pig coronary artery. Journal of Physiology. 1995;483:29–39. doi: 10.1113/jphysiol.1995.sp020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy RA, Shimizu S, Paul RJ. The effects of hypoxia on pHi in porcine coronary artery endothelium and smooth muscle. A novel method for measurements in endothelial cells in situ. Circulation Research. 1997;80:21–27. doi: 10.1161/01.res.80.1.21. [DOI] [PubMed] [Google Scholar]
- Frøbert O, Mikkelsen EO, Bagger JP. The influence of transmural pressure and longitudinal stretch on K+- and Ca2+-induced coronary artery constriction. Acta Physiologica Scandinavica. 1999;165:379–385. doi: 10.1046/j.1365-201x.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- Frøbert O, Schiønning J, Gregersen H, Baandrup U, Petersen JAK, Bagger JP. Impaired human coronary artery distensibility by atherosclerotic lesions: a mechanical and histological investigation. International Journal of Experimental Pathology. 1997;78:421–428. doi: 10.1046/j.1365-2613.1997.470374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell WH. On the tonicity of the heart and blood vessels. Journal of Physiology. 1880;3:48–75. doi: 10.1113/jphysiol.1880.sp000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravholt CH, Schmitz O, Simonsen L, Bülow J, Christiansen JS, Møller N. Effects of a physiological GH pulse on interstitial glycerol in abdominal and femoral adipose tissue. American Journal of Physiology. 1999;277:E848–854. doi: 10.1152/ajpendo.1999.277.5.E848. [DOI] [PubMed] [Google Scholar]
- Green S, Bulow J, Saltin B. Microdialysis and the measurement of muscle interstitial K+ during rest and exercise in humans. Journal of Applied Physiology. 1999;87:460–464. doi: 10.1152/jappl.1999.87.1.460. [DOI] [PubMed] [Google Scholar]
- Guth BD, Wisneski JA, Neese RA, White FC, Heusch G, Mazer CD, Gertz EW. Myocardial lactate release during ischaemia in swine. Relation to regional blood flow. Circulation. 1990;81:1948–1958. doi: 10.1161/01.cir.81.6.1948. [DOI] [PubMed] [Google Scholar]
- Halejcio-Delophont P, Siaghy EM, Devaux Y, Ungureanu-Longrois D, Richoux JP, Beck B, Burlet C, Villemot JP, Mertes PM. Increase in myocardial interstitial adenosine and net lactate production in brain-dead pigs. An in vivo microdialysis study. Transplantation. 1998;66:1278–1284. doi: 10.1097/00007890-199811270-00003. [DOI] [PubMed] [Google Scholar]
- Hansen DK, Davies MI, Lunte SM, Lunte CE. Pharmacokinetic and metabolism studies using microdialysis sampling. Journal of Pharmaceutical Sciences. 1999;88:14–27. doi: 10.1021/js9801485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RK, Weiss GB, Willerson JT. Basis of pH-independent inhibitory effects of lactate on 45Ca movements and responses to KCl and PGF2 alpha in canine coronary arteries. Circulation Research. 1980;46:771–779. doi: 10.1161/01.res.46.6.771. [DOI] [PubMed] [Google Scholar]
- Kalsner S. The effect of hypoxia on prostaglandin output and on tone in isolated coronary arteries. Canadian Journal of Physiology and Pharmacology. 1977;55:882–887. doi: 10.1139/y77-117. [DOI] [PubMed] [Google Scholar]
- Kennergren C, Nystrom B, Nystrom U, Berglin E, Larsson G, Mantovani V, Lonnroth P, Hamberger A. In situ detection of myocardial infarction in pig by measurements of aspartate aminotransferase (ASAT) activity in the interstitial fluid. Scandinavian Cardiovascular Journal. 1997;31:343–349. doi: 10.3109/14017439709075951. [DOI] [PubMed] [Google Scholar]
- Leach RM, Sheehan DW, Chacko VP, Sylvester JT. Energy state, pH, and vasomotor tone during hypoxia in precontracted pulmonary and femoral arteries. American Journal of Physiology. 2000;278:L294–304. doi: 10.1152/ajplung.2000.278.2.L294. [DOI] [PubMed] [Google Scholar]
- Liu Q, Flavahan NA. Hypoxic dilatation of porcine small coronary arteries: role of endothelium and KATP-channels. British Journal of Pharmacology. 1997;120:728–734. doi: 10.1038/sj.bjp.0700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon W, Aaronson PI, Knock G, Graves J, Poston L. Mechanism of lactate-induced relaxation of isolated rat mesenteric resistance arteries. Journal of Physiology. 1996;490:783–792. doi: 10.1113/jphysiol.1996.sp021186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation. 1998;98:1990–1992. doi: 10.1161/01.cir.98.19.1990. [DOI] [PubMed] [Google Scholar]
- Miller FN, Nolph KD, Joshua IG, Wiegman DL, Harris PD, Andersen DB. Hyperosmolality, acetate, and lactate: dilatory factors during peritoneal dialysis. Kidney International. 1981;20:397–402. doi: 10.1038/ki.1981.152. [DOI] [PubMed] [Google Scholar]
- Mori K, Nakaya Y, Sakamoto S, Hayabuchi Y, Matsuoka S, Kuroda Y. Lactate-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Journal of Molecular and Cellular Cardiology. 1998;30:349–356. doi: 10.1006/jmcc.1997.0598. [DOI] [PubMed] [Google Scholar]
- Nagesetty R, Paul RJ. Effects of pHi on isometric force and [Ca2+]i in porcine coronary artery smooth muscle. Circulation Research. 1994;75:990–998. doi: 10.1161/01.res.75.6.990. [DOI] [PubMed] [Google Scholar]
- Nielsen HB. pH after competitive rowing: the lower physiological range? Acta Physiologica Scandinavica. 1999;165:113–114. doi: 10.1046/j.1365-201x.1999.00485.x. [DOI] [PubMed] [Google Scholar]
- Omar HA, Figueroa R, Tejani N, Wolin MS. Properties of a lactate-induced relaxation in human placental arteries and veins. American Journal of Obstetrics and Gynecology. 1993a;169:912–918. doi: 10.1016/0002-9378(93)90026-f. [DOI] [PubMed] [Google Scholar]
- Omar HA, Mohazzab KM, Mortelliti MP, Wolin MS. O2-dependent modulation of calf pulmonary artery tone by lactate: potential role of H2O2 and cGMP. American Journal of Physiology. 1993b;264:L141–145. doi: 10.1152/ajplung.1993.264.2.L141. [DOI] [PubMed] [Google Scholar]
- Opie LH, Thomas M. Propranolol and experimental myocardial infarction: substrate effects. Postgraduate Medical Journal. 1976;52(suppl. 4):124–132. [PubMed] [Google Scholar]
- Pettersson G, Lundholm L. Pasteur effect in vascular and intestinal smooth muscle. Artery. 1985;12:312–323. [PubMed] [Google Scholar]
- Pittman RN, Duling BR. Oxygen sensitivity of vascular smooth muscle. I. In vitro studies. Microvascular Research. 1973;6:202–211. doi: 10.1016/0026-2862(73)90020-4. [DOI] [PubMed] [Google Scholar]
- Rosdahl H, Ungerstedt U, Jorfeldt L, Henriksson J. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. Journal of Physiology. 1993;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Bowman PS, Thorne G, III, Paul RJ. Effects of hypoxia on isometric force, intracellular Ca(2+), pH, and energetics in porcine coronary artery. Circulation Research. 2000;86:862–870. doi: 10.1161/01.res.86.8.862. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW. The pharmacology of dichloroacetate. Metabolism. 1989;38:1124–1144. doi: 10.1016/0026-0495(89)90051-6. [DOI] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Hypoxia and smooth muscle function: key regulatory events during metabolic stress. Journal of Physiology. 1998;509:315–325. doi: 10.1111/j.1469-7793.1998.315bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen A, Nielsen TT, Bagger JP, Pedersen AK, Henningsen P. Antiischemic and metabolic effects of glutamate during pacing in patients with stable angina pectoris secondary to either coronary artery disease or syndrome X. American Journal of Cardiology. 1991;68:291–295. doi: 10.1016/0002-9149(91)90821-2. [DOI] [PubMed] [Google Scholar]
- Van Wylen DG. Effect of ischemic preconditioning on interstitial purine metabolite and lactate accumulation during myocardial ischaemia. Circulation. 1994;89:2283–2289. doi: 10.1161/01.cir.89.5.2283. [DOI] [PubMed] [Google Scholar]
- Wadsworth RM. Vasoconstrictor and vasodilator effects of hypoxia. Trends in Pharmacological Sciences. 1994;15:47–53. doi: 10.1016/0165-6147(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Wikstrom BG, Ronquist G, Waldenstrom A. Dynamics of myocardial metabolism in the preconditioned porcine heart studied using continuous microdialysis. European Heart Journal. 1995;16:563–569. doi: 10.1093/oxfordjournals.eurheartj.a060951. [DOI] [PubMed] [Google Scholar]
- Wissner SB. The effect of excess lactate upon the excitability of the sheep Purkinje fiber. Journal of Electrocardiology. 1974;7:17–26. doi: 10.1016/s0022-0736(74)80004-x. [DOI] [PubMed] [Google Scholar]


