Abstract
Proteinase-activated receptor-2 (PAR-2) may participate in epithelial ion transport regulation. Here we examined the effect of mouse activating peptide (mAP), a specific activator of PAR-2, on electrogenic transport of mouse distal colon using short-circuit current (ISC) measurements. Under steady-state conditions, apical application of amiloride (100 μm) revealed a positive ISC component of 74.3 ± 6.8 μA cm−2 indicating the presence of Na+ absorption, while apical Ba2+ (10 mm) identified a negative ISC component of 26.2 ± 1.8 μA cm−2 consistent with K+ secretion. Baseline Cl− secretion was minimal. Basolateral addition of 20 μm mAP produced a biphasic ISC response with an initial transient peak increase of 11.2 ± 0.9 μA cm−2, followed by a sustained fall to a level 31.2 ± 2.6 μA cm−2 (n = 43) below resting ISC. The peak response was due to Cl− secretion as it was preserved in the presence of amiloride but was largely reduced in the presence of basolateral bumetanide (20 μm) or in the absence of extracellular Cl−. The secondary decline of ISC was also attenuated by bumetanide and by Ba2+, indicating that it is partly due to a stimulation of K+ secretion. In addition, the amiloride-sensitive ISC was slightly reduced by mAP, suggesting that inhibition of Na+ absorption also contributes to the ISC decline. Expression of PAR-2 in mouse distal colon was confirmed using RT-PCR and immunocytochemistry. We conclude that functional basolateral PAR-2 is present in mouse distal colon and that its activation stimulates Cl− and K+ secretion while inhibiting baseline Na+ absorption.
It is now widely recognised that proteolytic enzymes such as trypsin and thrombin play a role in cellular signalling via activation of distinct proteinase-activated receptors (PARs; Dery et al. 1998; MacFarlane et al. 2001). The PAR superfamily is comprised of at least four receptors, namely PAR-1 to PAR-4, which differ with respect to protease specificity. Agonists act by directly cleaving the receptor, thus exposing tethered ligand domains that bind and activate the cleaved receptor. Thrombin preferentially activates PAR-1, −3, and −4, whereas trypsin and mast cell tryptase have been shown to activate PAR-2 (Molino et al. 1997).
PARs are expressed in many tissues including the kidney, pancreas, and gastrointestinal tract (Dery et al. 1998). The endogenous proteases activating these receptors and their physiological role are still unknown. In the gastrointestinal tract, PAR-2 has been localised to the murine stomach and small intestine (Nystedt et al. 1994) as well as to the distal colon and small intestine of both human and rat (Böhm et al. 1996b; Kong et al. 1997). Since PAR-2 is found in epithelial cells of various origins (Böhm et al. 1996b; D'Andrea et al. 1998; Vergnolle et al. 1998; Nguyen et al. 1999; Bertog et al. 1999; Cuffe et al. 2000a) it may play a role in regulating epithelial transport.
Indeed, PAR-2 and PAR-2-related receptors have been shown to be involved in transepithelial ion transport regulation in canine pancreatic duct (Nguyen et al. 1999) and human bronchial (Danahay et al. 2001) epithelial cells, porcine ileum (Green et al. 2000), rat jejunum (Vergnolle et al. 1998), and mouse cortical collecting duct cells where basolateral PAR-2 induces chloride secretion (Bertog et al. 1999). Interestingly, the renal cortical collecting duct shares several characteristic transepithelial ion transport properties with the distal colon. Both epithelia absorb sodium via the amiloride-sensitive epithelial sodium channel (ENaC) and are capable of secreting potassium. Moreover, both tissues express the cystic fibrosis transmembrane conductance regulator (CFTR) which mediates cAMP-stimulated Cl− secretion (Letz & Korbmacher, 1997; Greger, 2000). These ion transport processes are electrogenic and can be assessed by using transepithelial short circuit current (ISC) measurements.
Synthetic activating peptides (APs), which constitute the tethered ligand for the PAR subtype in question, are useful experimental tools to specifically activate PARs without the use of proteases, which may have non-specific effects (Vu et al. 1991; Nystedt et al. 1994; Kahn et al. 1998; Xu et al. 1998). The aim of the present study was to examine the effect of mouse PAR-2 activating peptide (mAP) on electrogenic transepithelial ion transport in mouse distal colon. In addition, we wanted to confirm the expression of PAR-2 in mouse colon using immunocytochemistry and RT-PCR experiments.
Part of this work has been published in abstract form (Cuffe et al. 2000a).
METHODS
Tissue preparation for transepithelial measurements
Twelve- to 14-week-old male C57Bl/6J mice, fed on a regular salt diet, were killed by exposure to a rising concentration of CO2, in accordance with UK Home Office regulations. Starting from the anus, 3 cm of colon tissue was dissected with the terminal 1.5 cm termed the late distal colon and the next 1.5 cm the early distal colon. The colon was washed with ice-cold standard bicarbonate buffered solution (see below) and was cut longitudinally to expose the luminal surface. The smooth muscle layers were removed by blunt dissection and the partially stripped colon samples were glued to Perspex rings using histoacryl tissue glue (B. Braun, Medical Ltd, Sheffield) and transferred to purpose-built Ussing chambers for continuous short-circuit current (ISC) measurements.
Transepithelial measurements
Ussing experiments were performed using a CVC 6 clamp device (Fiebig, Berlin, Germany) as previously described (Bertog et al. 1999; Cuffe et al. 2000b). Transepithelial resistance (Rte) was evaluated every 2 s by measuring the voltage deflections induced by 200 ms symmetrical square current pulses of ± 50 μA cm−2. Transepithelial voltage (Vte) measured in open circuit mode and Rte were used to calculate equivalent ISC according to Ohm's law. Alternatively, in some experiments ISC was determined by clamping Vte to 0 mV using the voltage clamp mode of the amplifier. Since the two methods yielded similar ISC values, the data were pooled and are reported together. Conventionally, a lumen negative Vte corresponds to a positive ISC which may be due to electrogenic cation absorption, or anion secretion, or a combination of both. Samples were bathed in a standard solution containing (mm): 119 NaCl, 21 NaHCO3, 1.2 CaCl2, 1.2 MgCl2, 0.6 KH2PO4, 2.4 K2HPO4, 10 d-glucose, 10 d-mannose, 2.5 l-glutamine, 0.5 β-hydroxybutyric acid and azlocillin (50 mg l−1), maintained at 37 °C, and pH 7.4 by gassing with 95 % O2-5 % CO2 (Fromm et al. 1993). Chloride-free solutions were achieved by replacing Cl− by gluconate; they contained 6 mm Ca2+ gluconate to compensate for the Ca2+ buffering properties of gluconate.
RT-PCR
Colons from three mice were removed and total RNA was prepared using Trizol (Gibco-BRL, Grand Island, NY, USA). RNA (0.5 μg) was reverse transcribed using the Promega reverse transcription system (Promega, Madison, WI, USA) using random hexamers according to manufacturer's instructions. cDNA was amplified with Taq polymerase (Gibco-BRL). Primers to mouse PAR-2 (forward, 5′-TCTGGATCTTCCTTTTCCGAA-3′; reverse, 5′-TACGAG CAGAAGGTTGCTAGGAGC-3′) were chosen to amplify a 635 bp fragment. The PCR conditions were: denaturation for 9 min, 35 cycles of the iterative loop at 94 °C for 1 min, 52 °C for 2 min and 72 °C for 2 min, and a final elongation at 72 °C for 9 min. To exclude contamination with genomic DNA, control RT-PCR reactions were performed without reverse transcriptase for each RNA sample. PCR controls also included omission of cDNA. PCR products were analysed by electrophoresis on a 1 % agarose gel with ethidium bromide.
Localisation of PAR-2 by immunohistochemistry and immunofluorescence
PAR-2 antibody 9717 was raised in rabbits to peptide corresponding to the C-terminal six residues of mouse PAR2 C-S394VKTSY399 (C- for conjugation) conjugated to keyhole limpet haemocyanin and was used at a dilution of 1:250–1:1000 (Corvera et al. 1999). Antibody B5 was raised to rat PAR-2 (30GPNSKGR↓SLIGRLDT46P-YGGC, ↓ = cleavage site) (Dr M. Hollenberg, University of Calgary, Canada) conjugated to keyhole limpet haemocyanin, and was used at a 1:1000–1:4000 dilution (Kong et al. 1997). PAR-2 antibody 95159 was raised to mouse PAR-2 (SKGR↓SLIGRLETQPP49Y, ↓ = the cleavage site) conjugated to keyhole limpet haemocyanin and was used at a 1:250–1:1600 dilution. Mice (n = 12, male and female, 20–25 g, C57Bl/6J) were anaesthetised with sodium pentabarbitone (200 mg kg−1, i.p.) and were transcardially perfused with 100 ml PBS, pH 7.4 followed by 4 % paraformaldehyde in PBS. All procedures were approved by the committee on animal research, University of California, San Francisco, CA, USA. The colons were removed and immersion fixed for 24 h at 4 °C. Tissues were washed in PBS and then cryoprotected in 25 % sucrose in PBS for 16 h at 4 °C. Tissues were embedded in Tissue-Tek OCT (optimum cutting temperature) compound (Sakuna Finetek, Torranie, CA, USA) and 10 μm frozen OCT sections were prepared. Sections were incubated in PBS containing 0.1 % Triton X-100 and 1–10 % normal goat serum. Sections were incubated with primary antibodies (16 h, 4 °C) and washed. For immunofluorescence, sections were incubated with goat anti-rabbit IgG conjugated to FITC (1:200, 2 h; Jackson Immuno-Research, PA, USA) (Corvera et al. 1999). For immunohistochemistry, sections were processed for ABC staining according to the manufacturer's directions, with diaminobenzidine as a substrate (Dako, Carpenteria, CA, USA) (Steinhoff et al. 1999). In control experiments, PAR-2 antibody was incubated with 10–100 μm of the unconjugated peptide used for immunisation for 24–48 h before staining. Tissues were observed with a Zeiss Axioplan microscope and photographed using a Sony DKC5000 digital camera or with a Zeiss Axiovert and an MRC 1000 laser scanning confocal microscope (Bio-Rad, Hercules, CA, USA) equipped with a krypton-argon laser (Grady et al. 1996).
Drugs
Synthetic mouse activating peptide (mAP) with the amino acid sequence (SLIGRL-NH2) and reverse peptide (mRP) with the sequence (LRGILS-NH2) were synthesised by solid phase methods and purified by HPLC. Trypsin, trypsin inhibitor, amiloride hydrochloride, and indomethacin were purchased from Sigma-Aldrich (Poole, Dorset, UK), while diphenylamine-2-carboxylic acid (DPC) was obtained from Fluka (Neu-Ulm, Germany). Tetrodotoxin (TTX) and barium chloride dihydrate were supplied by Tocris-Cookson Ltd (Bristol, UK) and Merck (Darmstadt, Germany), respectively. Trypsin, mAP, and mRP were dissolved in sterile water while 100 mm stock solutions of amiloride and DPC were prepared in methanol and DMSO, respectively.
Data analysis
For all groups, significance of difference was estimated using the appropriate version of Student's t test. Group data are expressed as means ± standard error of mean and a P value less than 0.05 was required to reject the null hypothesis. Unless otherwise stated in the text, experimental responses to agents were compared to time-matched control tissues run in parallel and obtained from animals of similar age; n signifies the number of experiments conducted.
RESULTS
Baseline parameters
After an equilibration period of between 40 and 60 min following transfer into Ussing chambers, Vte, Rte and ISC for the late distal colon averaged −7.3 ± 0.4 mV (lumen negative), 116 ± 4 Ω cm−2 and 65.7 ± 3.2 μA cm−2 (n = 166), respectively (Fig. 1). Apical application of 100 μm amiloride (n = 24) reversed ISC (Fig. 1A) from 58.2 ± 6.7 to −16.1 ± 2.2 μA cm−2 (P < 0.001) and changed Vte (Fig. 1B) from −5.9 ± 0.7 to 2.0 ± 0.4 mV (P < 0.001), while Rte increased (Fig. 1C) from 110 ± 8 to 117 ± 9 Ω cm−2 (P < 0.001). These data indicate that amiloride-sensitive Na+ absorption predominates under baseline conditions, while the negative ISC component revealed in the presence of amiloride is indicative of ongoing electrogenic K+ secretion. These findings are consistent with previously reported ion transport properties of mammalian colon (Greger, 2000). The presence of a K+ secretory ISC component was further confirmed by demonstrating an increase of ISC from −11.2 ± 6.8 to 15.1 ± 1.9 μA cm−2 (P < 0.001) upon application of 10 mm apical Ba2+ (Fig. 1A) which hyperpolarised Vte (Fig. 1B) from 1.3 ± 0.2 to −2.0 ± 0.3 mV (P < 0.01) and increased Rte (Fig. 1C) from 118 ± 9 to 134 ± 11 Ω cm−2 (P < 0.01; n = 19). Thus, under steady state conditions there is a dominant amiloride-sensitive Na+-absorptive ISC component averaging 74.3 ± 6.8 μA cm−2 (n = 24), while the Ba2+-sensitive K+ secretory ISC component averaged 26.2 ± 1.8 μA cm−2 (n = 19). In contrast, application of 1 mm DPC (in the presence of amiloride and Ba2+) had only a minor effect (Fig. 1) reducing ISC from 15.1 ± 1.9 to 14.4 ± 1.8 μA cm−2 (n = 11; P < 0.05). This indicates that DPC-sensitive electrogenic Cl− secretion is minimal under steady-state conditions.
Figure 1. Amiloride-sensitive ISC predominates under resting conditions in the mouse late distal colon.

Corresponding traces of ISC (A), Vte (B) and Rte (C) from an experiment on murine late distal colon where amiloride (100 μm), Ba2+ (10 mm), and DPC (1 mm) were added sequentially to the apical (ap) bath. Drugs were applied as indicated by the horizontal bars. Positive ISC corresponds to electrogenic cation absorption or anion secretion or a combination of both.
Biphasic effect of mouse activating peptide (mAP) on ISC
Figure 2A shows an experiment in which mAP was applied to the basolateral side of late distal colon to test its effect on ISC. mAP (20 μm) induced an initial transient increase in ISC with a peak reaching its maximum within ∼30 s of addition. The peak amplitude averaged 11.2 ± 0.9 μA cm−2 with ISC changing from 60.3 ± 5.6 to 71.5 ± 5.4 μA cm−2 (n = 43). The initial peak increase was followed by a subsequent ISC decline well below the current level measured prior to the addition of mAP. Within 15 min ISC declined 31.2 ± 2.6 μA cm−2 below resting ISC to a level of 29.2 ± 3.7 μA cm−2 (n = 43). Apical application of mAP (20 μm) had no significant effect on ISC which averaged 35.6 ± 9.6 before, and 37.4 ± 10.4 μA cm−2 after, application of mAP (n = 8). Similarly, basolateral addition of a peptide (mRP) with the reverse amino acid sequence to that of mAP at a concentration of 20 μm failed to significantly influence ISC, which averaged 71.2 ± 16.9 before, and 72.0 ± 18.0 μA cm−2 after, the addition of mRP, respectively (n = 8). This indicates that the effect of mAP is mediated by a specific interaction of the peptide with basolateral PAR-2.
Figure 2. Effects of basolateral mouse activating peptide (mAP) on late distal colon ISC.

A, continuous ISC recording from a late distal colonic sample depicting the effects of basolateral (bl) mAP (20 μm) application. Amiloride (100 μm) and Ba2+ (10 mm) were added apically in the continuous presence of mAP. B, the effects of basolateral mAP (20 μm) in the presence of apical amiloride (100 μm).
Effect of amiloride on mAP response
Stimulation or inhibition of electrogenic Na+ absorption will result in an increase or decrease of ISC, respectively. Basolateral mAP applied in the presence of apical amiloride (100 μm) induced an initial 19.4 ± 2.8 μA cm−2 increase in ISC from −20.8 ± 1.9 to −1.5 ± 3.5 μA cm−2, followed by a 8.8 ± 2.8 μA cm−2 decline below baseline to −29.6 ± 2.7 μA cm−2 within 10 to 15 min of application of mAP (n = 5; Fig. 2B). The lack of an inhibitory effect of amiloride on the transient initial ISC increase rules out the possibility that this component of the response is due to stimulation of electrogenic Na+ absorption by mAP.
To test the possibility that inhibition of electrogenic Na+ absorption contributes to the secondary ISC decline, we investigated the effect of mAP on the amiloride-sensitive ISC component using matched colon tissues studied in parallel experiments (Fig. 3). Both groups of tissues displayed similar baseline ISC values averaging 56.8 ± 5.5 and 48.2 ± 5.8 μA cm−2 for test and control groups, respectively. The amiloride-sensitive ISC was determined 15 min after application of basolateral mAP (Fig. 3B) and was significantly reduced by about 15 μA cm−2 averaging 43.2 ± 3.7 μA cm−2 (n = 19; Fig. 3B) compared to 58.2 ± 5.8 μA cm−2 in controls (n = 19; Fig. 3A; P < 0.05). These data are consistent with the interpretation that a modest inhibition of Na+ absorption contributes to the secondary ISC decline induced by mAP. However, the data also indicate that an inhibition of Na+ absorption is unlikely to account fully for the sizeable negative ISC component elicited by mAP. This is also supported by the finding that the secondary ISC decline was at least partially preserved in the presence of amiloride (Fig. 2B).
Figure 3. The secondary decline phase is partially due to a decrease in amiloride-sensitive ISC.

A, continuous ISC trace in a control experiment demonstrating long-term stability of ISC and the effect of apical amiloride (100 μm) on resting ISC. B, effects of basolateral mAP (20 μm) over the same time period as shown in A and subsequent apical amiloride (100 μm) addition.
Moreover, application of mAP caused a sustained decline of 20 ± 3 (n = 43) or 20 ± 10 Ω cm−2 (n = 5) in the absence or presence of amiloride, respectively. Thus, an overall increase of transepithelial conductance rather than an inhibition of an apical sodium conductance is associated with the sustained negative ISC component elicited by mAP. Interestingly, the sustained mAP-induced Rte reduction was preceded by a small transient Rte increase averaging 8 ± 1 Ω cm−2 (n = 43) or 10 ± 3 Ω cm−2 (n = 5) in the absence or presence of amiloride, respectively. The nature of this transient Rte increase is unclear and it may be due to either transient inhibition of cellular conductances or to a transient increase of tight junctional resistance. In any case, the lack of effect of amiloride on this transient Rte response confirms that a change of sodium transport is not involved in the initial mAP response.
Chloride dependence of the mAP response
A positive ISC may be due to either electrogenic cation absorption, or anion secretion, or a combination of both. To examine a possible Cl− dependence of the mAP response, we incubated samples of late distal colon on both sides in Cl−-free solutions (Cl− replaced by gluconate). Under nominally Cl−-free conditions (Fig. 4A) the initial peak response and the subsequent decrease in ISC averaged 1.9 ± 0.5 and 8.8 ± 1.7 μA cm−2 (n = 6), respectively. These responses were significantly smaller than those in the presence of Cl− (see above; Fig. 2A) with an average peak increase of 11.2 ± 0.9 μA cm−2 (n = 43; P < 0.001) and a secondary ISC decrease of 31.2 ± 2.6 μA cm−2 (n = 43; P < 0.001). Indeed, as shown in Fig. 4A, re-addition of Cl− to the bath solutions partially restored both components of the mAP response with a peak increase averaging 7.6 ± 3.2 μA cm−2 and a secondary decline by 14.0 ± 3.8 μA cm−2 (n = 5). These findings demonstrate that the effects of mAP on ISC are largely Cl− dependent and that the initial peak ISC increase is due to mAP-induced electrogenic Cl− secretion.
Figure 4. Both phases of the mAP response are largely dependent on an intact Na+-K+-2Cl− cotransport system.

A, mouse late-distal colonic tissues were exposed to basolateral mAP (20 μm) in the nominal absence and presence of extracellular Cl− (ap + bl). The effect of apical amiloride (100 μm) is also shown. The noisy ISC trace after the first application of mAP is due to washout of mAP. B and C, effects of basolateral mAP (20 μm) in the presence (B) and absence (C) of basolateral bumetanide (20 μm), a Na+-K+-2Cl− cotransport inhibitor. Amiloride (100 μm) was applied apically, followed by sequential additions of 1 mm (hatched bar) and 10 mm (open bar) Ba2+ apically.
Bumetanide largely inhibits both phases of the mAP response
Chloride ion secretion via apical Cl− channels may only occur if Cl− is accumulated intracellularly above electrochemical equilibrium. In mammalian colon, a bumetanide-sensitive basolateral Na+-K+-2Cl− cotransport has been shown to exist, and both Cl− and K+ secretion are thought be driven by basolateral Cl− and K+ uptake via this co-transport (Greger, 2000). Therefore, we examined the effect of basolateral bumetanide on the mAP-mediated response.
Basolateral application of bumetanide (20 μm) induced an immediate increase in steady-state ISC to a level of 20.5 ± 2.0 μA cm−2 (n = 6) above baseline (Fig. 4B). This is consistent with the finding that under baseline conditions a considerable K+ secretion (negative ISC component) predominates while Cl− secretion is negligible (Fig. 1). Thus, inhibition of the negative K+ secretory ISC component by basolateral bumetanide increases ISC (Fig. 4B). In the presence of basolateral bumetanide the ISC response to mAP (20 μm) was largely reduced with an initial peak averaging 1.2 ± 0.5 μA cm−2 and a subsequent ISC decline of 5.8 ± 2.0 μA cm−2 below the ISC level prior to mAP application (n = 6; Fig. 4B). In contrast, in time-matched control tissues (Fig. 4C) mAP induced a significantly larger initial ISC increase averaging 5.4 ± 0.5 μA cm−2 (n = 6; P < 0.001) as well as a more pronounced ISC decrease of 11.9 ± 1.0 μA cm−2 below baseline (n = 6; P < 0.05).
The inhibition of the mAP-induced ISC peak increase by bumetanide confirms that this ISC component is due to Cl− secretion. The additional inhibitory effect of bumetanide on the mAP-induced subsequent ISC decline indicates that the second phase of the mAP response is also dependent on basolateral Na+-K+-2Cl− cotransport activity and is probably due to a stimulation of K+ secretion. Indeed, in tissues exposed to basolateral mAP and apical amiloride (Fig. 4C), sequential application of 1 and 10 mm apical BaCl2 (a K+ channel inhibitor) induced a 5.8 ± 0.6 and 12.7 ± 0.8 μA cm−2 (n = 6) increase in ISC, respectively, confirming the presence of a substantial K+ secretory ISC component. In contrast, in parallel experiments in which mAP was applied in the presence of basolateral bumetanide (Fig. 4B), the steady-state ISC increase in response to sequential addition of 1 and 10 mm BaCl2 was reduced to 0.6 ± 0.1 and 2.7 ± 0.4 μA cm−2 (n = 6), respectively. Taken together, these data suggest that part of the mAP-induced ISC decline is mediated by stimulation of electrogenic K+ secretion which depends on K+ uptake via basolateral Na+-K+-2Cl− cotransport.
Interestingly, the amiloride-sensitive ISC component averaged 45.1 ± 2.9 μA cm−2 in the presence of bumetanide whereas it averaged 29.9 ± 3.9 μA cm−2 (n = 6; P < 0.05) in control tissues (Fig. 4B and C). This apparent increase in electrogenic Na+ absorption may be due to an expected fall in [Na+]i in the presence of basolateral bumetanide which would increase the driving force for apical Na+ entry via ENaC.
Ba2+ inhibits the secondary phase of the mAP response
To examine further the possibility that the secondary mAP-mediated ISC decline is caused by a stimulation of K+ secretion via apical K+ channels, experiments were performed in which mAP was applied in the presence of apical Ba2+ (Fig. 5A). Application of 10 mm Ba2+ (in the presence of 100 μm apical amiloride) increased steady-state ISC by 22.7 ± 2.5 from −13.7 ± 5.3 to 9.0 ± 4.0 μA cm−2, consistent with the presence of a sizeable baseline K+ secretory ISC component. In the presence of 10 mm apical Ba2+, the initial mAP response remained largely unaltered averaging 11.0 ± 1.9 μA cm−2 (n = 6) compared to 9.5 ± 2.7 μA cm−2 (n = 7) in controls. This lack of effect of Ba2+ on the peak response is consistent with the finding that it is due to Cl− secretion (see above). In contrast, Ba2+ largely prevented the mAP-mediated secondary ISC decline (Fig. 5A). In the presence of apical Ba2+, ISC remained 1.3 ± 1.5 μA cm−2 (n = 6) above its level prior to the addition of mAP (Fig. 5A) compared to an ISC decline of 4.1 ± 1.4 μA cm−2 below baseline in control tissues (P < 0.05; n = 6; Fig. 5B). These data confirm that stimulation of K+ secretion contributes to the mAP-induced secondary decrease in ISC. Moreover, in this set of experiments the Ba2+-induced ISC increase averaged 26.8 ± 1.4 μA cm−2 in the presence of mAP (n = 6; Fig. 5B) compared to 22.7 ± 2.5 μA cm−2 in the absence of mAP (n = 6; Fig. 5A). This trend did not reach statistical significance but is consistent with the interpretation that mAP stimulates K+ secretion.
Figure 5. The mAP-induced secondary decline is partially due to a stimulation of Ba2+-sensitive K+ transport.
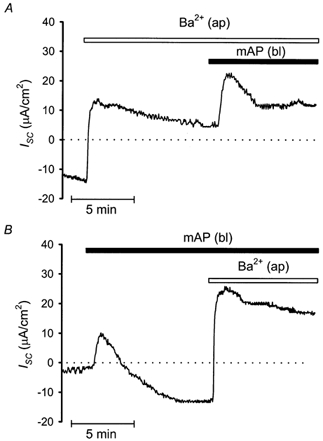
A, effects of apical Ba2+ (10 mm), in the continuous presence of 100 μm apical amiloride, on mAP-induced changes in ISC in late distal colon. B, a control tissue displaying the response to basolateral mAP (20 μm) prior to application of 10 mm apical Ba2+, again conducted in the presence of apical amiloride (100 μm).
Concentration dependence of the mAP response
To investigate the concentration dependence of the mAP response, we performed experiments similar to that shown in Fig. 2A using mAP in concentrations ranging from 200 nm to 200 μm. While no peak response was detected with 200 nm or 2 μm (n = 3), the peak increase in ISC induced by 7, 20, 70 or 200 μm mAP averaged 12.2 ± 3.7 (n = 3), 15.3 ± 1.3 (n = 5), 12.4 ± 7.1 (n = 3) and 14.4 ± 5.0 μA cm−2 (n = 4), respectively, with a threshold value between 2 and 7 μm (Fig. 6A). In the same set of experiments, the decrease of ISC below baseline induced by 2, 7, 20, 70 or 200 μm mAP averaged 10.2 ± 3.7 (n = 4), 23.7 ± 8.1 (n = 3), 30.1 ± 4.4 (n = 5), 45.8 ± 4.7 (n = 3) and 34.6 ± 10.9 μA cm−2 (n = 4), respectively (Fig. 6B). Using a Michaelis-Menten fit of the data shown in Fig. 6B we estimated an EC50 value for mAP of about 5 μm.
Figure 6. The initial peak increase and subsequent secondary decline in ISC induced by mAP were concentration dependent.

A, summary of the initial mAP-induced peak increase in ISC. Data are taken from 3–5 experiments where varying concentrations of mAP (0.2–200 μm) were applied basolaterally to tissues run in parallel, similar to experiments shown in Fig. 2A. B, summary of the subsequent mAP-induced secondary decline in ISC, again data are taken from the same experiments as described in A, where n = 3–5 for mAP concentrations over the range 0.2–200 μm. A Michaelis-Menten fit of the data points representing the mean ISC in B, reveals an EC50 value of 5 μm for the secondary decline.
Effect of trypsin on ISC in the mouse colon
To date, the proteolytic enzyme trypsin constitutes the most potent agonist for PAR-2. Therefore we compared its effects to that of mAP in late distal colonic segments. Apical trypsin (20 μg ml−1) failed to influence resting ISC (n = 7), while basolateral trypsin elicited a prolonged ISC response after a lag period of approximately 2 min, with an average increase of 28.3 ± 8.0 μA cm−2 changing from 61.8 ± 7.4 to 90.1 ± 10.7 μA cm−2 (n = 21). The response displayed a somewhat variable shape and time course (Fig. 7A). In 19 out of 21 preparations, trypsin induced an increase in ISC while in 2 out of 21 tissues a decrease was observed.
Figure 7. Basolateral trypsin induces a concentration-dependent increase in ISC which is not amiloride sensitive.
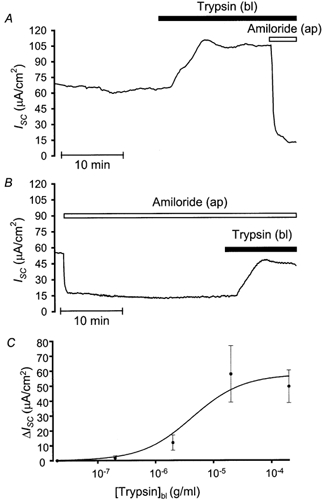
A, a continuous recording showing the effects of basolateral trypsin (20 μg ml−1) on resting ISC in the late distal colon; amiloride (100 μm) was added apically in the presence of trypsin. B, depicts the effects of basolateral trypsin (20 μg ml−1) on ISC in the presence of apical amiloride (100 μm). C, summary of the trypsin-induced plateau increase in ISC, data are taken from 4–6 experiments where varying concentrations of trypsin (0.2–200 μg ml−1) were applied basolaterally to tissues run in parallel, similar to experiments shown in A. A Michaelis-Menten fit of the data points representing the mean values reveals an EC50 of about 4 μg ml−1.
The stimulatory ISC response to basolateral trypsin was preserved in the presence of apical amiloride (100 μm; P = 0.42) averaging 19.8 ± 5.3 μA cm−2 changing from −15.8 ± 3.5 to 4.0 ± 5.7 μA cm−2 (n = 15), implying that the trypsin-induced increase in ISC is not due to activation of Na+ absorption (Fig. 7B). Interestingly, the response to basolateral mAP was completely abolished by basolateral trypsin pretreatment (n = 2) but not vice versa (n = 2). However, the effect of trypsin was abolished by basolateral addition of 20 μg ml−1 trypsin inhibitor (n = 2). This indicates that the protease activity of trypsin is needed to mediate its effect.
The response to trypsin differs considerably from that of mAP, possibly due to differences in agonist potency or affinity, therefore we examined the effects of different trypsin concentrations. Trypsin increased ISC in a concentration-dependent manner over the range of 200 ng ml−1 to 200 μg ml−1, with a threshold of 2 μg ml−1 and a maximal increase occurring at 20 μg ml−1. The increase in ISC induced by 0.2, 2, 20 or 200 μg ml−1 trypsin averaged 1.6 ± 1.6 (n = 4), 11.9 ± 5.1 (n = 5), 57.9 ± 18.9 (n = 6) and 49.6 ± 10.9 μA cm−2 (n = 6), respectively. The approximate EC50 for the response was 4 μg ml−1 (Fig. 7C). The overall shape and time course of the trypsin response was consistently different from that of mAP over the range of concentrations tested, implying that trypsin induces changes in ISC which are not exclusively mediated by PAR-2. The trypsin effect may involve the activation of additional PAR subtypes or may be mediated through completely unrelated mechanisms, however changes in tight junctional integrity can be eliminated as trypsin failed to influence Rte (data not shown).
Thrombin fails to influence baseline ISC
While PAR-1 and PAR-3 respond to thrombin but not to trypsin, it has been reported that PAR-4 may respond to both thrombin and trypsin. In this study, apical or basolateral application of 100 nm thrombin failed to influence baseline ISC (n = 6). These data suggest that thrombin-sensitive receptors are not involved in the regulation of electrogenic transepithelial ion transport in the mouse late distal colon.
Are enteric nerves involved in the response?
The effects of basolateral addition of the neuronal Na+ channel inhibitor tetrodotoxin (TTX; 1 μm) were examined on the mAP-induced changes in ISC. The response to mAP was preserved in the presence of TTX with initial ISC peak and subsequent ISC decline averaging 4.5 ± 2.1 and 59.0 ± 22.0 μA cm−2 (n = 4), respectively, compared to 10.9 ± 5.0 and 53.7 ± 17.6 μA cm−2 (n = 4) in parallel controls. In contrast, the stimulatory response to trypsin was completely abolished by preincubation with basolateral TTX (1 μm; Fig. 8A) with ISC even declining in the presence of trypsin by 9.2 ± 3.2 μA cm−2 within 15 min (n = 4). These data indicate that while the response to mAP occurs independently of the enteric nervous system, the effect of trypsin appears to require intact neuronal function.
Figure 8. The trypsin-induced increase in ISC is largely prevented by pretreatment with tetrodotoxin or indomethacin.

A, effects of basolateral trypsin (20 μg ml−1) on ISC in the continuous presence of 1 μm basolateral tetrodotoxin, a neuronal inhibitor. B, effects of indomethacin (10 μm) applied to both baths on the trypsin-induced changes in ISC in the late distal colon. Amiloride (100 μm) and Ba2+ (10 mm) were applied apically where indicated.
Are prostaglandins involved in the response to PAR-2 agonists?
Preincubation with the general cyclo-oxygenase inhibitor indomethacin (10 μm) on both sides of the membrane, failed to alter the response to mAP when compared to controls, with initial and subsequent ISC changes averaging 11.4 ± 4.8 and 50.5 ± 9.4 μA cm−2 (n = 4), respectively. However, the response to trypsin was significantly reduced by indomethacin pretreatment (P < 0.05) to a transient increase of 5.4 ± 4.2 μA cm−2 (Fig. 8B) compared to the sustained increase of 27.2 ± 5.7 μA cm−2 (n = 4) in controls (Fig. 7A). Again, mAP appears to function independently of prostaglandin synthesis while the sustained stimulatory effect of trypsin is dependent on normal cyclo-oxygenase activity.
mAP induces a similar response in the early distal colon
Under baseline conditions, early distal colonic segments displayed average Vte, Rte, and ISC values of −1.8 ± 0.3 mV, 73 ± 7 Ω cm−2, and 28.0 ± 4.4 μA cm−2 (n = 38), respectively. The amiloride-sensitive ISC in the early distal colon averaged 21.1 ± 6.7 μA cm−2 (n = 14) which was significantly smaller than that observed in the late segment, averaging 74.3 ± 6.8 μA cm−2 (n = 24; P < 0.001). This finding is consistent with previous findings on segmental heterogeneity in the literature (Fromm & Hegel, 1978; Sandle, 1989). Basolateral mAP (20 μm) transiently increased ISC by 12.0 ± 2.5 μA cm−2, followed by a decrease of 18.0 ± 3.2 μA cm−2 below basal (n = 9). In the presence of apical amiloride, the response to mAP was largely preserved with an average initial ISC peak increase of 12.7 ± 3.7 μA cm−2 and a subsequent decrease by 16.8 ± 2.6 μA cm−2 (n = 5). The lack of amiloride effect suggests that the peak ISC is likely to be mediated by Cl− secretion. While an inhibition of the amiloride-sensitive ISC probably contributes to the mAP-induced secondary ISC decline in the late distal colon (see above), this was not apparent in the early distal colon, possibly due to the lower baseline expression of electrogenic Na+ transport in this segment. Thus, the mAP-induced ISC decline in early distal colon is probably largely due to a stimulation of K+ secretion, consistent with the effect of apical Ba2+ which increased ISC by 10.6 ± 1.5 μA cm−2 (n = 2) in early distal colon exposed to mAP. Taken together, these data demonstrate that functional PAR-2 are also located in the basolateral membrane of the early distal segments of mouse colon and elicit similar changes in electrogenic ion transport with a transient stimulation of Cl− secretion and a subsequent increase in K+ secretion.
RT-PCR evidence for PAR-2 expression in mouse colon
We assessed expression of PAR-2 in the mouse colon using RT-PCR with specific primers based on the sequence of mouse PAR-2. A PCR product of the predicted size of 635 bp was amplified from RNA prepared from the colon of three mice (Fig. 9). No PCR product was amplified in control reactions where either reverse transcriptase or cDNA were omitted (not shown). These results indicate that PAR-2 is expressed in the mouse colon.
Figure 9. Detection of PAR-2 in mouse colon by RT-PCR.

RNA from three different mouse colons was reverse transcribed and the expression of PAR-2 was assessed by the amplification of a specific 635 bp PCR fragment. M1, M2 and M3 correspond to the three different mice. PCR products were analysed by electrophoresis on a 1 % agarose gel with ethidium bromide and DNA size markers.
Immunochemical localisation of PAR-2 in mouse colon
We examined the localisation of PAR-2 in the colon by immunohistochemistry and immunofluorescence, using three region-specific antibodies. Antibodies 9717 and 95159 strongly stained enterocytes of the villi and crypts. These antibodies also strongly stained myenteric and submucosal neurones, and 9171 also stained fibres in the musculature and mucosa. Antibodies 9717 and 95159 also stained myocytes of the circular and longitudinal muscle layers. Antibody B5 stained enterocytes and myocytes more strongly than neurones. Examination by confocal microscopy indicated the presence of PAR-2 at the apical and basolateral membranes of enterocytes, in the plasma membrane of neurones, and also in intracellular locations. Staining by all antibodies was abolished by preabsorption of the primary antibodies with the peptides used for immunisation, confirming specificity (Fig. 10).
Figure 10. Localisation of PAR-2 in mouse colon by immunohistochemistry (A, B) and immunofluorescence and confocal microscopy (C-G).

A, staining of mucosal epithelial cells, longitudinal and circular muscle, and neurones of the myenteric and submucosal plexuses using antibody 9717 (arrows). B, a preabsorption control using 9717. C, staining of the mucosa using antiserum B5. D and E, staining of nerve fibres (arrow heads) in the mucosa in proximity to colonocytes. F, strong staining of myenteric neurones and weaker staining of myocytes using 9717. G, staining of a myenteric neurone and of nerve fibres in the circular muscle using 9717. Scale bar, 10 μm applies to all panels. muc, mucosa; lm, longitudinal muscle; cm, circular muscle; mp, myenteric plexus; sp, submucosal plexus.
DISCUSSION
The present study demonstrates that functional PAR-2 are expressed in mouse colon where they mediate complex changes in electrogenic ion transport resulting in parallel stimulation of Cl− and K+ secretion as well as inhibition of amiloride-sensitive Na+ absorption.
Evidence for functional basolateral PAR-2 in murine distal colon
In this study we have shown that specific activation of basolateral PAR-2 with mAP induces a concentration-dependent biphasic ISC response in the murine distal colon. The initial transient response induced by mAP was due to activation of Cl− secretion as pretreatment with the Na+-K+-2Cl−-cotransport inhibitor bumetanide or removal of extracellular Cl− almost abolished the response, while the Na+ channel inhibitor amiloride was without effect. Similar transient increases in ISC in response to APs have been observed in other epithelia, e.g. in M-1 mouse cortical collecting duct (Bertog et al. 1999), canine pancreatic acinar (Nguyen et al. 1999) and human bronchial (Danahay et al. 2001) cells, porcine ileum (Green et al. 2000), and rat jejunum (Vergnolle et al. 1998). For Cl− secretion to occur, Cl− must be distributed above its electrochemical equilibrium, e.g. through the action of basolateral, bumetanide-sensitive Na+-K+-2Cl− cotransporters, while Cl− exits the cell into the gut lumen via apical Cl− channels. In mammalian colonic epithelia, apical Cl− secretion appears to be predominantly mediated by the cAMP-activated cystic fibrosis transmembrane conductance regulator (CFTR; Greger, 2000). However, so far, PAR-2 activation has not been shown to mediate an increase in [cAMP]i. Instead, it is thought that activation of PAR-2 results in a G-protein-mediated increase in intracellular [Ca2+] and PKC activity via PLC liberation of 1,2-diacylglycerol and IP3 (Böhm et al. 1996a; Dery et al. 1998; MacFarlane et al. 2001). A rise in [Ca2+]i is unlikely to result in a direct stimulation of CFTR. However, another candidate for mediating the Cl− secretory response observed in our system is the calcium-activated chloride channel (mCaCC) that has been shown to be present in the apical membrane of murine colonic crypt cells (Gruber et al. 1998). A PAR-2-mediated increase in [Ca2+]i may also indirectly stimulate Cl− secretion via activation of Ca2+-regulated basolateral K+ channels, such as KVLQT or SK4 (Warth et al. 1999; Greger, 2000), resulting in an increased driving force for apical Cl− exit.
The prolonged secondary phase of the mAP response characterised by an ISC decline is due to parallel activation of K+ secretion and partially due to an inhibition of amiloride-sensitive Na+ absorption. The secondary decline was attenuated by pretreatment with basolateral bumetanide or apical Ba2+, indicating that apical K+ secretion maintained by basolateral Na+-K+-2Cl− cotransport contributes to the response consistent with previously reported findings (Greger, 2000). The molecular nature of the apical K+ channel(s) involved in K+ secretory responses in mammalian colon remains unresolved. However, in rat distal colon, apical Ca2+-sensitive K+ channels were confirmed (Butterfield et al. 1997; Sandle & Butterfield, 1999) and may be stimulated by a PAR-2-mediated increase in [Ca2+]i. A large body of data is available regarding the various K+ conductance pathways in the basolateral membrane of colonic epithelia, including cAMP- and Ca2+-dependent channels in rat (Warth et al. 1999; Kunzelmann et al. 2001) and mouse (MacVinish et al. 1998; Cuthbert et al. 1999) colon, respectively.
In the mouse late distal colon, Na+ absorption, via the amiloride-sensitive epithelial Na+ channel (ENaC), is driven by basolaterally located Na+-K+-ATPase, thus generating a lumen negative potential, while Na+ exits across the basolateral membrane in exchange for K+ which in turn exits via basolateral K+ channels (Greger, 2000). In our study, inhibition of amiloride-sensitive Na+ absorption partially contributes to the mAP-induced secondary decline in ISC since the amiloride-sensitive ISC was smaller in the presence than in the absence of mAP. ENaC is sensitive to increases in [Ca2+]i and PKC activity, therefore an increase in [Ca2+]i could easily explain an inhibition of amiloride-sensitive ISC (Cuffe et al. 2000b). Indeed, in human bronchial epithelial cells, a similar PAR-2-mediated secondary ISC decrease was recently attributed to inhibition of the amiloride-sensitive ISC component (Danahay et al. 2001) consistent with our findings in colon.
Differential effects of mAP and trypsin on ISC
Trypsin is known to activate PAR-2 in a wide range of preparations. Interestingly, there was a qualitative difference between the ISC response elicited by mAP compared to that of trypsin. Amiloride failed to influence the trypsin response, indicating that changes in Na+ absorption are not involved. This contrasts with experiments in Xenopus oocytes and A6 cells where trypsin or channel-activating protease (CAP1) increased, and protease inhibitors decreased, ENaC activity (Vallet et al. 1997; Chraibi et al. 1998). In mouse colon, trypsin induced a more sustained increase in electrogenic ion transport when compared to mAP consistent with ongoing Cl− secretion.
Our results suggest that the mAP and trypsin responses are mediated through different mechanisms. While the absence of an mAP effect after trypsin suggests a common pathway, the failure of AP to prevent the response to trypsin argues for a divergent mechanism. In contrast, pretreatment with AP completely abolished the trypsin-induced relaxation in prostaglandin F2α-contracted porcine coronary arteries indicating a common pathway for the action of AP and trypsin in this tissue. Moreover, the finding suggests that AP was not digested by trypsin (Hwa et al. 1996). Thus, the failure of AP to prevent a response to trypsin in colon is unlikely to be due to a proteolytic degradation of AP but indicates that trypsin has additional effects to those of AP.
Indeed, we demonstrate that the mAP response was unaffected by the general cyclo-oxygenase inhibitor indomethacin, whereas the trypsin response was largely indomethacin sensitive. It is well established that activation of PAR-2 can result in an increase in prostaglandin synthesis via PLA2 (Lan et al. 2001). Similarly, the response to mAP was insensitive to the neuronal inhibitor TTX while the trypsin response was abolished by TTX. These data imply that while mAP may influence epithelial ion transport by acting directly on basolateral PAR-2, trypsin indirectly influences epithelial transport via activation of enteric nerves and downstream prostanoid synthesis. Due to the non-specific nature of trypsin it is possible that basolaterally applied trypsin may activate other PAR subtypes located on enteric nerves or on mast cells of the lamina propria leading to prostaglandin release from different cell types such as epithelial cells or fibroblasts (Vergnolle et al. 1998). Clearly the role, physiological stimulus, and effects of the colonic PAR-2 require further investigation.
Possible mediation by different PAR subtypes
Trypsin appears to activate different members of the PAR family (Kawabata et al. 1999), whereas PAR-2 APs have been shown to be selective tools in different cell systems (Hollenberg et al. 1997; Kawabata et al. 1999). In addition to PAR-2, high levels of PAR-4 were observed in the small intestine and moderate levels in the distal colon of humans (Xu et al. 1998). A receptor similar, but distinct from, PAR-2 has been identified in rat jejunum (Vergnolle et al. 1998) which suggests the existence of different PAR-2 subtypes. We can demonstrate that basolateral thrombin fails to influence baseline ISC thus excluding PAR-1, −3 and −4 as mediators of the trypsin response. Furthermore, in human umbilical vein and rat artery ring smooth muscle preparations, trypsin induced muscle relaxation via PAR-2 whereas AP induced both relaxation via PAR-2 and contraction of the vasculature via an, as yet, unidentified receptor, possibly a subtype of PAR-2 (Roy et al. 1998; Saifeddine et al. 1998). Moreover, trypsin induced a contraction of guinea-pig bronchial smooth muscle via a non-PAR-2-mediated mechanism (Carr et al. 2000). On the other hand, the effects of mAP on cardiac parameters were absent in PAR-2-deficient mice indicating that the cardiac effects of mAP were indeed PAR-2 specific (Damiano et al. 1999). Taken together, our findings do not rule out the existence of PAR-2 subtypes in the colon and future experiments in PAR-2-deficient mice may shed more light on the specific functional role of PAR-2 in the colon.
Apical versus basolateral localisation of PARs
Our immunocytochemical experiments demonstrate that PAR-2 are located in both the apical and basolateral membranes of mouse distal colon, as well as in the smooth muscle layers and in the neurones of the myenteric and submucosal plexuses. This is consistent with immunocytochemical evidence suggesting an apical as well as a basolateral PAR-2 localisation in native and cultured rat enterocytes (Kong et al. 1997), and mouse in situ hybridization studies demonstrating strong PAR-2 expression in surface epithelial cells of the stomach, entire small intestine, and colon (Böhm et al. 1996b). The signal was strongest in epithelial cells lining the upper two-thirds of the intestinal villi, and was weaker in the crypt region.
Our data show that PAR-2 agonists only induce changes in transport when applied basolaterally to our preparation implying that under our experimental conditions functional PAR-2 that alter transepithelial ion transport can only be stimulated from the basolateral membrane, consistent with previous findings in renal and pancreatic epithelia (Bertog et al. 1999; Nguyen et al. 1999). An explanation for the lack of effect of apical mAP or trypsin would be the presence of proteases or trypsin inhibitors in the unstirred layer of the mucosal surface which may prevent mAP or trypsin from reaching and activating apical PARs on enterocytes. Release of pancreatic secretory trypsin inhibitor (PSTI) from mucus secreting cells has been reported (Marchbank et al. 1998) and may explain the lack of effect of apical trypsin in the mouse colon preparation. Indeed, trypsin appears to induce prostaglandin synthesis which in turn can stimulate PSTI release, possibly constituting an autoinhibitory regulation of trypsin action. The PAR-2 signal is terminated by different mechanisms, including irreversible receptor cleavage, PKC phosphorylation, and receptor endocytosis (Böhm et al. 1996a). Proteases such as mast cell tryptase, coagulation factors VIIa and Xa, and acrosin also activate PAR-2 (Fox et al. 1997; Dery et al. 1998; Camerer et al. 2000), therefore other, as yet unassociated, proteases or peptidases may irreversibly activate PAR-2 in the gut lumen or inactivate mAP by peptide cleavage. Pancreatic proteases such as trypsin, chymotrypsin, kallikrein, or carboxypeptidase may be candidates for PAR-2 desensitization in more proximal portions of the intestine, while proteases released from normal gut flora might act locally throughout the intestine. At present, we cannot distinguish between these two possibilities to explain the absence of an effect of mAP or trypsin applied apically.
Physiological relevance of PAR-2 in the colon
The nature and tissue distribution of the physiological protease(s) acting at PAR-2 in the native colon has yet to be clarified. The relatively ubiquitous distribution of the PARs implies that trypsin, limited to the pancreas and the upper GIT with access only to the apical membrane, is not the primary agonist under normal conditions. However, luminal trypsin could penetrate the inflamed mucosa when there are alterations in the intestinal barrier. Under such circumstances, trypsin may activate PAR-2 on a variety of cell types. Mast cell tryptase may be a better candidate as it is released by mast cell degranulation allowing access to the basolateral membrane and thus may constitute the physiological activator of PAR-2 in certain tissues. The lack of an apical protease effect may prevent unwanted PAR-2 activation under physiological or pathophysiological conditions, thus preventing excessive loss of water and electrolytes since PAR-2-mediated K+ and Cl− secretion may cause diarrhoea. In PAR-2-deficient mice, the onset of inflammatory responses induced by mAP were delayed (Lindner et al. 2000) underlining the important role played by PARs during inflammation. Furthermore, PAR-2 agonists have been identified as potent mediators of the inflammatory response and recently PARs have been implicated in inflammatory diseases such as inflammatory bowel disease and coeliac disease (Vergnolle, 2000; MacFarlane et al. 2001; Vergnolle et al. 2001). Modification of ion transport during inflammatory bowel disease would tend to result in diarrhoea. Overall, the wide tissue distribution of PAR-2 may indicate an important role in the colonic signalling cascade integrating smooth muscle function with that of transepithelial ion transport, possibly involving elements of the enteric nervous system and mast cells of the lamina propria (Vergnolle et al. 2001).
In summary, we have provided functional and molecular evidence for the presence of PAR-2 in the late and early mouse distal colon. Activation of basolateral PAR-2 induced a complex biphasic response due to KCl secretion and inhibition of amiloride-sensitive Na+ absorption. Our findings also suggest that different PARs or subtypes of PAR-2 may be present in our preparation, influencing epithelial cell transport either directly via basolateral PARs or indirectly via elements of the enteric nervous system or COX-dependent prostanoid synthesis.
Acknowledgments
We thank A. Fromm and Professor M. Fromm for their helpful advice regarding the preparation of the mouse colon for transepithelial measurements. We also thank Dr E. F. Grady for her expert help with the microscopy. This work was supported by the Wellcome Trust (C.K.), the Deutsche Akademische Austauschdienst (M. B.), the EPA Cephalosporin Fund (M. B.) and NIH grants DK43207 and DK57840 (N.W.B.).
REFERENCES
- Bertog M, Letz B, Kong W, Steinhoff M, Higgins MA, Bielfeld Ackermann A, Fromter E, Bunnett NW, Korbmacher C. Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. Journal of Physiology. 1999;521:3–17. doi: 10.1111/j.1469-7793.1999.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. Journal of Biological Chemistry. 1996a;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- Böhm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, Kahn M, Nelken NA, Coughlin SR, Payan DG, Bunnett NW. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochemical Journal. 1996b;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. Journal of Physiology. 1997;501:537–547. doi: 10.1111/j.1469-7793.1997.537bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proceedings of the National Academy of Sciences of the USA. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ, Schechter NM, Undem BJ. Trypsin-induced, neurokinin-mediated contraction of guinea pig bronchus. American Journal of Respiratory and Critical Care Medicine. 2000;162:1662–1667. doi: 10.1164/ajrccm.162.5.9912099. [DOI] [PubMed] [Google Scholar]
- Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. Journal of General Physiology. 1998;111:127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera CU, Dery O, McConalogue K, Gamp P, Thoma M, Al Ani B, Caughey GH, Hollenberg MD, Bunnett NW. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and −2. Journal of Physiology. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffe JE, Bertog M, Bunnett NW, Korbmacher C. Basolateral proteinase-activated receptor-2 modifies transepithelial ion transport in mouse distal colon. Journal of Physiology. 2000a;527P:34P–35P. [Google Scholar]
- Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. Journal of Physiology. 2000b;524:77–90. doi: 10.1111/j.1469-7793.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AW, Hickman ME, Thorn P, MacVinish LJ. Activation of Ca2+- and cAMP-sensitive K+ channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone. American Journal of Physiology. 1999;277:C111–120. doi: 10.1152/ajpcell.1999.277.1.C111. [DOI] [PubMed] [Google Scholar]
- Damiano BP, Cheung WM, Santulli RJ, Fung-Leung WP, Ngo K, Ye RD, Darrow AL, Derian CK, De Garavilla L, Andrade-Gordon P. Cardiovascular responses mediated by protease-activated receptor-2 (PAR- 2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. Journal of Pharmacology and Experimental Therapeutics. 1999;288:671–678. [PubMed] [Google Scholar]
- Danahay H, Withey L, Poll CT, Van De Graaf SF, Bridges RJ. Protease-activated receptor-2-mediated inhibition of ion transport in human bronchial epithelial cells. American Journal of Physiology - Cell Physiology. 2001;280:C1455–1464. doi: 10.1152/ajpcell.2001.280.6.C1455. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. Journal of Histochemistry and Cytochemistry. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. American Journal of Physiology. 1998;274:C1429–1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Fox MT, Harriott P, Walker B, Stone SR. Identification of potential activators of proteinase-activated receptor-2. FEBS Letters. 1997;417:267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- Fromm M, Hegel U. Segmental heterogeneity of epithelial transport in rat large intestine. Pflügers Archiv. 1978;378:71–83. doi: 10.1007/BF00581960. [DOI] [PubMed] [Google Scholar]
- Fromm M, Schulzke JD, Hegel U. Control of electrogenic Na+ absorption in rat late distal colon by nanomolar aldosterone added in vitro. American Journal of Physiology. 1993;264:E68–73. doi: 10.1152/ajpendo.1993.264.1.E68. [DOI] [PubMed] [Google Scholar]
- Grady EF, Gamp PD, Jones E, Baluk P, McDonald DM, Payan DG, Bunnett NW. Endocytosis and recycling of neurokinin 1 receptors in enteric neurons. Journal of Neuroscience. 1996;75:1239–1254. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- Green BT, Bunnett NW, Kulkarni-Narla A, Steinhoff M, Brown DR. Intestinal type 2 proteinase-activated receptors: expression in opioid- sensitive secretomotor neural circuits that mediate epithelial ion transport. Journal of Pharmacology and Experimental Therapeutics. 2000;295:410–416. [PubMed] [Google Scholar]
- Greger R. Role of CFTR in the colon. Annual Review of Physiology. 2000;62:467–491. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- Gruber AD, Gandhi R, Pauli BU. The murine calcium-sensitive chloride channel (mCaCC) is widely expressed in secretory epithelia and in other select tissues. Histochemistry and Cell Biology. 1998;110:43–49. doi: 10.1007/s004180050263. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor- activating peptides. Canadian Journal of Physiology and Pharmacology. 1997;75:832–841. [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E. Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circulation Research. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Saifeddine M, Al-Ani B, Leblond L, Hollenberg MD. Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1-targeted ligands. Journal of Pharmacology and Experimental Therapeutics. 1999;288:358–570. [PubMed] [Google Scholar]
- Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Böhm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proceedings of the National Academy of Sciences of the USA. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Hubner M, Schreiber R, Levy-Holzman R, Garty H, Bleich M, Warth R, Slavik M, von Hahn T, Greger R. Cloning and function of the rat colonic epithelial K+ channel KVLQT1. Journal of Membrane Biology. 2001;179:155–164. doi: 10.1007/s002320010045. [DOI] [PubMed] [Google Scholar]
- Lan RS, Knight DA, Stewart GA, Henry PJ. Role of PGE2 in protease-activated receptor-1, −2 and −4 mediated relaxation in the mouse isolated trachea. British Journal of Pharmacology. 2001;132:93–100. doi: 10.1038/sj.bjp.0703776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letz B, Korbmacher C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. American Journal of Physiology. 1997;272:C657–666. doi: 10.1152/ajpcell.1997.272.2.C657. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. Journal of Immunology. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- MacFarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacological Reviews. 2001;53:245–282. [PubMed] [Google Scholar]
- MacVinish LJ, Hickman ME, Mufti DA, Durrington HJ, Cuthbert AW. Importance of basolateral K+ conductance in maintaining Cl− secretion in murine nasal and colonic epithelia. Journal of Physiology. 1998;510:237–247. doi: 10.1111/j.1469-7793.1998.237bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbank T, Freeman TC, Playford RJ. Human pancreatic secretory trypsin inhibitor. Distribution, actions and possible role in mucosal integrity and repair. Digestion. 1998;59:167–174. doi: 10.1159/000007485. [DOI] [PubMed] [Google Scholar]
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. Journal of Biological Chemistry. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh DS, Bunnett NW. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. Journal of Clinical Investigation. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proceedings of the National Academy of Sciences of the USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Saifeddine M, Loutzenhiser R, Triggle CR, Hollenberg MD. Dual endothelium-dependent vascular activities of proteinase-activated receptor-2-activating peptides: evidence for receptor heterogeneity. British Journal of Pharmacology. 1998;123:1434–1440. doi: 10.1038/sj.bjp.0701726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifeddine M, Roy SS, Al-Ani B, Triggle CR, Hollenberg MD. Endothelium-dependent contractile actions of proteinase-activated receptor-2-activating peptides in human umbilical vein: release of a contracting factor via a novel receptor. British Journal of Pharmacology. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle GI. Segmental heterogeneity of basal and aldosterone-induced electrogenic Na transport in human colon. Pflügers Archiv. 1989;414:706–712. doi: 10.1007/BF00582139. [DOI] [PubMed] [Google Scholar]
- Sandle GI, Butterfield I. Potassium secretion in rat distal colon during dietary potassium loading: role of pH regulated apical potassium channels. Gut. 1999;44:40–46. doi: 10.1136/gut.44.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, Ansel JC, Bunnett NW. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Journal of Experimental Dermatology. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Review article: proteinase-activated receptors - novel signals for gastrointestinal pathophysiology. Alimentary Pharmacology and Therapeutics. 2000;14:257–266. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, MacNaughton WK, Al Ani B, Saifeddine M, Wallace JL, Hollenberg MD. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proceedings of the National Academy of Sciences of the USA. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends in Pharmacological Sciences. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Warth R, Hamm K, Bleich M, Kunzelmann K, Von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Archiv. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC. Cloning and characterization of human protease-activated receptor 4. Proceedings of the National Academy of Sciences of the USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


