Abstract
Anticipatory activity of hand and eye has been examined during oculo-manual tracking of a constant velocity visual target with a hand cursor. Both target and cursor were presented briefly (< 480 ms), but repeatedly, at regular inter-stimulus intervals (ISI). In Expt 1, the build-up of hand and eye responses was examined for target velocities varying from 10–40 deg s−1 with an ISI of 2.4 s. The velocity 100 ms after target onset (i.e. prior to visual feedback) for both hand and eye (V100) progressively increased over the first four presentations but then attained a steady state (SS). SS V100 values for eye and hand increased in proportion to target velocity and were thus predictive of forthcoming movement. Hand velocity exceeded eye velocity but both exhibited similar anticipatory trajectories. In Expt 2, target velocity was constant (40 deg s−1) but ISI varied from 0.48–3.74 s. Subjects made anticipatory eye movements for all ISIs but hand movements were often reactive at the longest ISI. If the target failed to appear as expected, subjects initiated predictive hand and eye responses with timing appropriate for the prevailing ISI. In Expt 3, predictive responses were compared with responses to randomised presentation. Peak hand velocity was greater in the randomised mode than in the predictive condition, whereas the converse was true for peak eye velocity. This difference is discussed in terms of the mechanisms of positional error correction in hand and eye. Results provide evidence of similar anticipatory mechanisms in hand and eye, using storage of velocity and timing to achieve rapid prediction of target motion.
Manual pursuit of moving visual targets has been widely investigated for many years (see Poulton, 1974) but in early experiments emphasis was placed on the recording of hand movement rather than the simultaneous recording of eye movements. Nevertheless, one of the major observations was that there is evidence of prediction in the tracking response. More recently, simultaneous eye and hand recordings have been made during manual tracking, both in normal subjects and patients with neurological disorders (Mather & Putchat, 1983; Gauthier et al. 1988; Vercher & Gauthier, 1992; Cody et al. 1993; van Donkelaar & Lee, 1994). Simultaneous recordings have revealed that both hand and eyes exhibit predictive characteristics when operating together (Xia & Barnes, 1999).
The ability to improve performance through prediction has long been known when the eye alone is used to track the motion of a target (Stark et al. 1962; Dallos & Jones, 1963; Young & Stark, 1963). One way in which we have previously attempted to investigate predictive behaviour in ocular pursuit is by examination of simple anticipatory movements and through this we have recently been able to show a possible link with predictive ocular pursuit of sinusoidal stimuli (Barnes & Wells, 1999; Barnes et al. 2000). Anticipatory eye movements provide an interesting example of a predictive motor response because it is not normally possible to initiate smooth pursuit movements at will (von Noorden & MacKensen, 1962; Heywood & Churcher, 1971; Kao & Morrow, 1994). However, it has been shown that anticipatory eye movements can be revealed by repeated presentation of identical transient target motion stimuli (Barnes & Asselman, 1991; Kao & Morrow, 1994; Barnes & Donelan, 1999). In the present experiments, we have examined whether the same type of anticipatory movements can be demonstrated for the hand. This might seem like a simple question to answer since, for hand movements, there is no problem in initiating smooth movements at will. However, there are a number of crucial questions to be answered. Are anticipatory movements of the hand, like those of the eye, scaled in proportion to target velocity? Do they exhibit a build-up in velocity with repetition similar to that seen in the eye? Is there a clear difference between responses to predictable and randomised stimuli? Do concomitant hand movements influence anticipatory eye movements? Does the timing of anticipatory hand and eye movements exhibit similar features to that of other repetitive motor responses?
A number of previous experiments have examined the co-ordination and synchrony between hand and eye movements (Gauthier & Hofferer, 1976; Vercher et al. 1995). One factor of particular importance is that hand movements appear to assist in the generation of concomitant, smooth eye movements (Steinbach, 1969; Gauthier & Hofferer, 1976; Leist et al. 1987; Koken & Erkelens, 1992). For example, during transient motion of a target moved by the subject's own hand, the latency of eye movement onset to target motion may be reduced from its normal value (>100 ms) to near zero (Vercher et al. 1995). Vercher et al. suggested that hand-related activity (efferent and/or afferent) could trigger and maintain the generation of smooth eye movements, in accord with the control co-ordination model proposed by Gauthier et al. (1988). However, it is also possible that the anticipatory smooth movements that have been observed in the absence of hand movement (Barnes & Asselman, 1991; Kao & Morrow, 1994) could make a contribution to this modification in latency of the response.
To investigate the characteristics of anticipatory hand and eye movements we conducted three experiments using the techniques of repeated intermittent stimulation that have been used before to evoke anticipatory eye movements (Ohashi & Barnes, 1996). In each experiment, anticipatory hand movements were found to be similar to those of the eye. In the first experiment, we examined stimuli with varying velocity to determine whether anticipatory hand movements exhibited velocity scaling. In the second, the interval between presentations was varied to determine changes in the timing of onset of anticipatory movements. In the final experiment, we compared the anticipatory responses in a predictable condition with responses to the randomised presentation of target motion. In addition, we compared the response of the eyes when tracking the target with both hand and eye (EH condition) or with the eye alone (EA condition).
A preliminary report of this work has been presented previously (Marsden et al. 1998).
METHODS
This study was performed in accord with the Declaration of Helsinki. Experiments were carried out with the informed and written consent of the subjects and with local ethics committee approval. Eight normal, healthy subjects (two male), without any known disorder of hand or eye mobility, participated in all experiments. Mean subject age was 36.0 years (s.d. 13.4).
Subjects were seated in a darkened room at the centre of a semicircular screen (radius 1.5 m). Horizontal eye movements were recorded during binocular viewing using an infra red limbus reflection technique (Skalar Iris, Delft, Netherlands). This allowed recordings to be made in a linear range of at least ±20 deg in the horizontal plane with a resolution of 5–10 min of arc. The head was immobilised using an adjustable head clamp and chin rest. The subject was presented with two images projected onto the screen at eye level, both of which could be moved in the horizontal axis. One was a white circle of diameter 1.2 deg with cross hairs and two small bars above and below the circle. This formed the target for pursuit eye movements. The other was a green cursor that also had a diameter of 1.2 deg and bars above and below which could be interleaved with those of the target. The cursor was coupled to the movement of the hand in the following way. The forearm of the subjects' dominant side was strapped into a padded gutter forming part of a manipulandum that was free to rotate about a vertical axis. The subject lightly gripped a vertical bar located at the end of the arm of the manipulandum. The manipulandum rotated with negligible impedance and was moved by flexion-extension movements of the wrist. The movement of the manipulandum was transduced by a potentiometer, the output of which then caused the green cursor to move on the screen in the horizontal axis. To keep wrist movement within a normal working range, the wrist-to-cursor displacement gain was set so that a movement of ±20 deg of the cursor on the screen was achieved by flexion-extension movement of the wrist of ±30 deg. This wrist-to-cursor displacement gain was held constant throughout the experiment so subjects became accustomed to the amount of hand movement required to generate a particular movement of the green cursor on the screen.
A total of three experiments will be described. In each of these, subjects were required to track the movement of the white target with the green cursor that was coupled to the hand. The objective was to align the green cursor with the white target as closely as possible throughout its movement. Prior to any recording, subjects practised tracking examples of the different stimuli.
Each trial consisted of four to six consecutive series and was preceded by a calibration of the eye movement. Within each series there were 8 to 20 intermittent presentations of identical, constant velocity, target motion stimuli.
In Expt 1, the ramp stimuli had constant velocity throughout each series but alternated in direction from left to right, crossing the centre in each presentation (Fig. 1). In separate series, the velocity had values of 16, 32, 48 or 64 deg s−1, these velocities being presented in a randomised order. The interval between onset of successive presentations was constant at 1.25 s and the duration of each ramp was 480 ms. Both the target and cursor were only visible (i.e. presented) whilst the target executed this ramp trajectory. Hence, the subject was not able to see the hand movement before the target actually appeared or after it had disappeared. Between series there was a blank interval equivalent to two presentations (2.5 s), during which neither the target nor the hand cursor were presented. Within each series the number of presentations was randomly varied from 8 to 12. Since subjects were unaware of the precise number, the blank interval formed an effective catch trial, in which anticipatory movements were made in the absence of a visual target.
Figure 1.
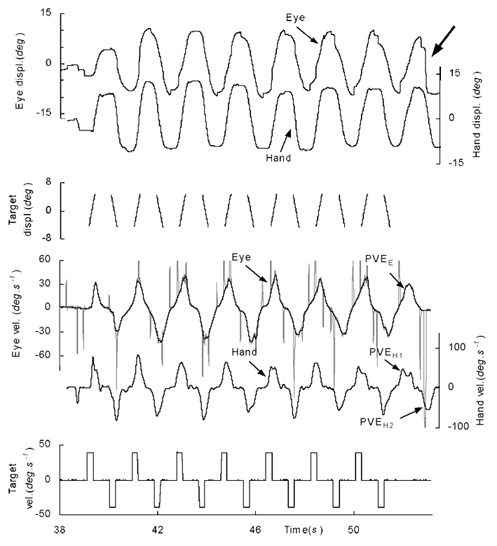
Examples of hand and eye movements of a single subject made in response to the regular, intermittent presentation of a target moving alternately left and right at a constant velocity of 40 deg s−1. Spikes in the eye velocity trace represent the small, saccadic movements evident in the eye position trace. At the end of the record both the target and hand cursor failed to appear as expected. The subject initiates one predictive velocity estimate (PVEE) in the eye, but two in the hand (PVEH1 and PVEH2). See text for an explanation of the largearrow.
In Expt 2, the stimulus presentation conditions were similar to those in Expt 1. However, target velocity remained constant throughout all series at 40 deg s−1 and the duration of the ramp was reduced to 240 ms. Target and cursor were only visible during the ramp. Each trial consisted of five series and from one series to the next there was a change in the inter-presentation interval, which took values of 0.48, 0.62, 0.91, 1.87 or 3.74 s in a randomised order. Each trial was performed twice. The number of presentations in each series was varied randomly from 8 to 16 and blank intervals were again given to provide catch trials.
In Expt 3, we compared predictable presentation with a randomised presentation of the ramp stimuli. The target always appeared at the centre and moved either to the left or the right. There were 10 presentations per series. In the predictable (PRD) mode all motion was in the same direction and had identical velocity throughout a series. Velocity ranged from 10 to 40 deg s−1 in separate series and from left to right in separate trials. The interval between presentations was constant at 2.4 s and the duration of target exposure was 480 ms. In the random (RND) condition, the direction and velocity of successive ramps was randomised and the interval between presentations was also randomised in the range of 2.7–3.7 s. In both the predictable and randomised conditions, the subjects were presented with two fixation cues which indicated the position of centre. These cues were placed vertically, 3 deg above and below the midpoint so that they did not cause interference with the movement of the target or cursor. The fixation cues remained on throughout the duration of each trial. In this experiment, there were two conditions. In the first (EH) the subject tracked the target when it appeared with hand and eyes together. In this condition, the hand cursor did not come on until the target also appeared but it remained on briefly after the target had disappeared to allow the subjects to bring the hand back to centre. In fact the cursor remained on for a randomised time of 0.4–0.8 s so that the subjects could not use the disappearance of the hand cursor to determine when the next ramp would appear. In addition to the manual tracking task, all subjects were given another condition (EA) in which they tracked the target with the eyes alone. For this purpose, only the white target was illuminated. Half of the subjects carried out the EH condition first, the other half the EA condition first.
Data analysis
Signals representing the eye and hand movements were recorded along with movement of the target. After low pass filtering at 80 Hz, they were digitized at 200 or 250 Hz and data were stored on disc for subsequent analysis. Eye movements were analysed by first identifying and removing fast phase components of the response using a technique similar to that described previously (Barnes, 1982) but based on a combination of acceleration and velocity threshold criteria. A linear interpolation routine was used to bridge the gaps produced by removal of the saccades from the eye velocity trajectory. Fast phase movements were generally of small amplitude (less than 5 deg) and brief duration, making linear interpolation a simple and adequate method of waveform restoration. Where measured variables are referred to as steady state (SS) these have been derived by averaging the velocity trajectory for the fourth and subsequent presentations of the ramp stimuli within each series. This yielded one steady-state trajectory per subject per series.
For both hand and eye in each presentation, we measured the velocity at target onset (V0) and 100 ms after onset (V100), the peak velocity (Vpk) and the latency of peak velocity with respect to target onset (Tpk). The acceleration at target onset (A0) was calculated by fitting a linear regression to a section of the velocity trace from 80 ms before, to 80 ms after, target onset. The time of onset of each individual response (T0) was determined by first estimating the time at which the velocity exceeded a threshold of 5 % of the SS peak velocity for each series. Then a linear regression was carried out on data points for 100 ms after this time and extrapolated backward to define a more precise time of response onset.
Statistical comparisons were carried out on the measured variables using SPSS software. Prior to performing repeated measures analysis of variance (ANOVA), the data were tested for normality (Shapiro-Wilk test) and no data transformation was deemed necessary. The Mauchly test was applied to determine sphericity of the data and when this was found to be significant, the Greenhouse-Geisser correction was applied to determine probability levels.
RESULTS
General observations
Figure 1 shows typical examples (from Expt 2) of the movement of the eye and hand during the intermittently presented stimulus. The responses observed in Expts 1 and 3 were similar. In the first presentation, the responses, of both hand and eye typically occurred around 100 to 150 ms after the target appeared and started to move. But, with repetition of the stimulus, anticipatory movements of both hand and eye were built up in the dark period prior to the onset of target motion. A similar pattern was observed when the eyes alone were used to track the target in Expt 3 as found previously (Ohashi & Barnes, 1996). In the example of Fig. 1, the peak velocity of the eye gradually increased over the first three presentations and appeared to stabilise thereafter. In contrast, peak velocity of the hand was high for the first three presentations, but diminished with repetition. A fairly stable response was obtained in the hand from the fourth presentation onwards but, in general, there was much more irregularity in the hand than in the eye movements. It should be noted that the saccadic components were removed from the eye velocity trace prior to further analysis whereas no such operation was carried out on the hand movements.
When the target failed to appear as expected in the blank period at the end of each series, subjects initiated anticipatory responses (the predictive velocity estimates) in both the hand (PVEH1) and eye (PVEE), which were then terminated after a short delay (Fig. 1). In the example of Fig. 1, the subject continued to make a further smooth movement with the hand (PVEH2) in the blank period. However, the attempt to continue the eye movement in this way resulted in the generation of a large saccade, rather than smooth movement, as indicated by the large arrow in this figure.
Changes in response magnitude as a function of target velocity (Expt 1)
The changes that occurred with repetition can be demonstrated by comparison of the first two presentations with the steady-state (SS) response derived from averaging responses from the fourth presentation onwards (Fig. 2). Note that left- and right-going responses have been averaged separately, although subsequent analysis revealed no directional difference and all comparisons were therefore carried out on combined data. In order to quantify the changes in the response, we examined the eye and hand velocity 100 ms after target onset (V100) when the effects of visual feedback would first be expected to become apparent. V100 for the first four presentations is compared in Fig. 3 with the steady-state response (SS) and the response that occurred when the target unexpectedly failed to appear (PVE). Analysis of variance indicated that there was a significant build up in V100 over the first four presentations (F4,28 = 42.32; P < 0.001), but there was no significant difference between the fourth presentation and the SS. In the first presentation, V100 was negligible because of the latency associated with the delay in visual feedback. At the highest target velocity (64 deg s−1) there was also little response in the second presentation. There was no significant difference in V100 between the hand and eye (F1,7 = 2.23; P = 0.18), but there was a significant increase in V100 with increasing target velocity (F3,28 = 16.18; P < 0.001). There was a small but significant reduction (12 % for eye; 16 % for hand) in V100 for the PVE when compared with V100 for the SS (F1,7 = 12.58; P = 0.009).
Figure 2.
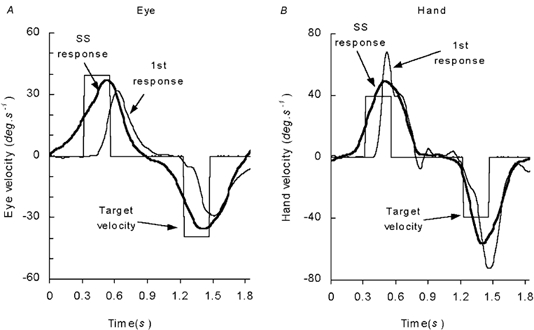
Comparison of the responses in the first presentation in each direction (first right; first left) of a new series with the steady-state responses (SS right, SS left) of (A) the eye and (B) the hand. The SS response is the average of all responses after the first three presentations. Data derived from example in Fig 1.
Figure 3.
A, eye and B, hand velocity 100 ms after target onset (V100) for the first four presentations, the steady state (SS) and the predictive velocity estimate (PVE) that occurred when the target unexpectedly failed to appear. Target velocity (V) = 16–64 deg s−1. Mean of data from eight subjects ± 1 s.e.m
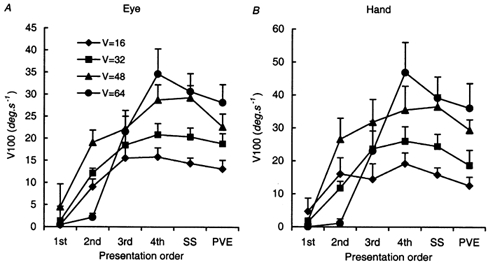
Peak velocity (Vpk) showed a rather different trend with presentation order (Fig. 4). Vpk for the eye was significantly less in the first presentation than in the SS (F1,7 = 16.89; P = 0.005), but Vpk in the second, third and fourth presentations was not significantly different from SS. By contrast, Vpk for the hand was, on average, greatest in the second presentation. Simple contrasts showed that values of Vpk in the first, second and third presentations were all significantly greater than SS (e.g. F1,7 = 22.21; P = 0.002 for third vs. SS) but there was no difference between the fourth presentation and the SS. Vpk of the PVE for both hand and eye was significantly less than SS (F1,7 = 61.92; P < 0.001) because it generally became attenuated when no visual target appeared as expected. However, Vpk of the PVE increased significantly with target velocity (F3,21 = 31.13; P < 0.001), attaining high levels at the highest target velocity (37 deg s−1 for the eye; 52 deg s−1 for the hand) even though these movements were made in the absence of a moving target.
Figure 4.
Changes in peak hand and eye velocity over the first four presentations compared with the steady state (SS) and the predictive velocity estimate (PVE). Mean of data from eight subjects ± 1 s.e.m. Responses for target velocities of 32 and 64 deg s−1 only are shown.
Changes in response as a function of inter-stimulus interval (Expt 2)
In Expt 2, each series had a different inter-stimulus interval (ISI), so that the subjects had to assess the appropriate time at which to initiate the anticipatory response. Figure 5 shows the changes in onset time (T0) with repetition for an inter-stimulus interval (ISI) of 0.91 s, corresponding to the example of Fig. 1. A similar pattern of change was observed at all ISIs. Both hand and eye initially had a latency in excess of 100 ms before onset of the first response, but the onset became significantly more anticipatory over the first four presentations (F4,28 = 30.48; P < 0.001). Simple contrasts, using the SS as the reference, showed that there was a significant difference between the first and second values and the SS (P < 0.015), but no difference between the third or fourth and the SS. T0 for the PVE was also not significantly different to T0 of the SS, even though the PVE occurred in the absence of any timing cues.
Figure 5.
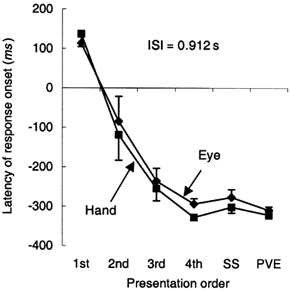
Changes in latency (T0) of anticipatory response initiation with respect to target onset for the first four presentations compared with the SS and the PVE. Mean of data from eight subjects ± 1 s.e.m. Inter-stimulus interval (ISI) = 0.91 s.
In steady-state conditions, there were significant changes in the timing of the anticipatory response as ISI increased from 0.48 to 3.74 s (F1,7 = 17.01; P = 0.004), but there was a significant interaction in the ANOVA because of the different behaviour of the hand and eye at ISIs ≥1.87 s. For both the hand and eye the (negative) latency (T0) progressively increased as ISI increased from 0.48 to 0.91 s and, for the eye, there was a further increase in T0 at ISI = 1.87 s (Fig. 6A). Eye movements were still anticipatory at ISI = 3.74 s in all subjects, but in six of the eight subjects, hand movements were no longer initiated before target onset. For this reason, T0 values for both the hand and eye at ISIs of 1.87 and 3.74 s have been plotted separately in Fig. 6A for the six subjects who did not make anticipatory hand movements (group A) and the two who did (group B). Note that the mean hand latency for the group A subjects when ISI was 3.74 s was 78 ms, so that these were not truly reactive responses. In only two of group A were the responses consistently reactive (i.e. T0 >100 ms). When ISI was 3.74 s, the eye movements in group A were initiated somewhat later than in group B, but they were still anticipatory and were initiated much earlier than hand movements. For ISIs from 0.48 to 0.91 s ANOVA on all subjects showed that hand movements occurred significantly earlier than those of the eye (F1,7 = 10.16; P = 0.015). This trend was reversed at ISI = 3.74 s (F1,7 = 14.39; P = 0.007), with mixed effects in different subjects at ISI = 1.87 s.
Figure 6.
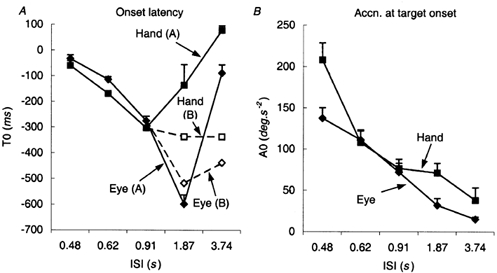
A, the latency (T0) of anticipatory response onset with respect to target onset and B the acceleration (A0) at target onset for the hand and eye as a function of the inter-stimulus interval (ISI). Mean of data from eight subjects ± 1 s.e.m.In A, data points for the hand and eye at ISI = 1.87 and 3.74 s have been split into two groups; six subjects who did not make anticipatory hand responses at ISI = 3.74 s (group A) and two subjects who did (group B).
The magnitude and form of the anticipatory responses in the steady state (SS) also changed with ISI and in order to describe this we have considered the changes in T0 together with the acceleration at target onset (A0, Fig. 6B) and the velocity 100 ms after onset (SS V100, Fig. 7). SS V100 values for the hand and eye remained relatively constant as ISI increased from 0.48 to 0.91 s, but then decreased significantly at ISIs of 1.87 s (F1,7 = 37.32; P < 0.001) and 3.74 s (F1,7 = 40.0; P < 0.001) as revealed by simple contrasts (Fig. 7). For the lower ISIs (0.48–0.91 s) it would be expected that, since T0 increased with increasing ISI whilst V100 remained constant, A0 would show a decrease as ISI increased. This was indeed observed, as shown in Fig. 6B (NB; values of A0 are means of all subjects, since groups A and B differed very little). A0 for both the hand and eye decreased progressively in a significant manner (F4,28 = 57.06; P < 0.001) as ISI increased. On this basis, it appeared that the goal of the anticipatory activity was probably to achieve a certain value of V100, irrespective of the starting time, by controlling anticipatory acceleration. However, as ISI increased beyond 0.91 s, this mechanism appeared to break down. Thus, at ISI = 3.74 s, the response of the eye was less anticipatory than at ISI = 1.87 s, but A0 continued to decrease, resulting in SS V100 being considerably less for ISI = 3.74 s than for ISI = 1.87 s. This result therefore suggests that the general level of anticipatory activity actually diminished as ISI increased beyond 0.91 s.
Figure 7.
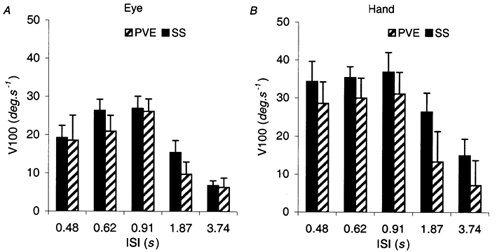
Comparison of V100 for the predictive velocity estimate (PVE) and the steady state (SS) responses of (A) the eye and (B) the hand as a function of the inter-stimulus interval (ISI). Mean of data from eight subjects ± 1 s.e.m
In the catch trials, when subjects initiated a PVE in expectation of target appearance, V100 values were not significantly different to the SS V100 values (Fig. 7) for either hand or eye at ISI values of 0.48–0.91 s. At the longer ISI values, mean V100 for the PVE was less than SS V100 because there were a few poorly initiated responses. Basically, when the ISI was longer, there was a less automatic response and a small proportion of responses initiated had very low V100 levels.
An important implication of the emergence of the responses in the catch trials is that there is an underlying timing mechanism that causes the PVE to be released at widely different times for the differing ISIs. A clear indication of this can be obtained by inspection of the inter-response interval (IRIPVE) between onset of the PVE and onset of the immediately preceding response. As indicated in Fig. 8A, the timing of the PVE release was quite precisely controlled over the range from an ISI of 0.48 to 1.87 s, although accuracy and inter-subject variability increased considerably for the longest ISI (3.74 s).
Figure 8.
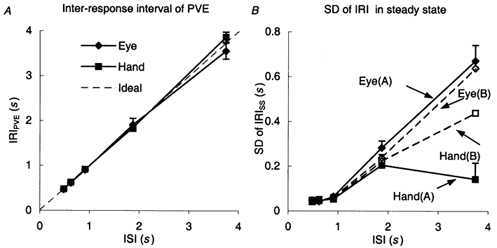
A, inter-response interval (IRIPVE) as a function of inter-stimulus interval (ISI). IRIPVE was defined as the time between the initiation of the PVE and the initiation of the previous anticipatory response. Mean of eight SSs ± 1 s.e.m. B, standard deviation (s.d.) of the inter-response interval in the steady state (IRISS) as a function of inter-stimulus interval (ISI). Mean of data from eight subjects ± 1 s.e.m. Data for ISI = 1.87 and 3.74 s split into two groups as defined in Fig. 6A
An assessment of timing variability was made by calculating the within-subject standard deviation (s.d.) of the IRI in the steady-state conditions (s.d. of IRISS, Fig. 8B). Note that when ISI was between 0.48 and 1.87 s, s.d. was derived from the last eight responses of each series in two trials (i.e. n = 16). For ISI = 3.74 s only five values were available in each series (i.e. n = 10) for this calculation, so that s.d. values are less reliable. Of particular note was the finding that s.d. of the IRISS for the eye increased significantly (F3,21 = 5.40; P = 0.006) as ISI increased over the range from 0.62 to 3.74 s. Data for groups A and B (defined above) have been plotted separately in Fig. 8B, but for the eye they were very similar. For the hand, a similar increase was observed for ISIs from 0.62 to 1.87 s, but when ISI was 3.74 s different values were obtained for groups A and B. For the subjects in group B, who made anticipatory movements at ISI = 3.74 s, s.d. of the IRISS continued to increase in the same way as for the eye (open squares, Fig. 8B). In the six subjects of group A, however, there was a considerable reduction in s.d. of the IRISS. ANOVA on all subjects revealed that, when data for ISI = 3.74 s were excluded, s.d. of IRISS for the hand was significantly less than that for the eye (F1,7 = 15.35; P = 0.006). Thus, timing of the hand was less variable than that of the eye. The variability of the difference in IRI for hand and eye (s.d. of IRIDIFF) was also calculated. Standard deviation of IRIDIFF was not significantly different from s.d. of IRISS of the eye (F1,7 = 0.14; P = 0.72). The fact that s.d. of IRIDIFF was not significantly less than s.d. of IRISS for either eye or hand indicates that there was no strong covariance of hand and eye timing. The preceding analysis was based on onset time for the anticipatory response (T0), which is sometimes difficult to define, but an additional analysis based on the time at which the anticipatory velocity reached 25 % of the mean SS peak velocity confirmed the validity of these findings.
Predictable vs. randomised responses (Expt 3)
Figure 9 shows representative examples of the hand and eye responses to the PRD and RND conditions at a stage where steady-state conditions had developed. Examination of the averaged hand and smooth component eye velocity profiles showed that they were similarly affected by the predictability of the stimulus. In the randomised (RND) mode, hand and eye movements both exhibited a delay before onset of any smooth movement and anticipatory movements were largely eliminated (Fig. 9A). Latencies of response in the RND mode did not change significantly with target velocity and had means of 142.9 ms (s.e.m. ± 8.1 ms) for the eye and 171.1 ms (s.e.m. ± 23.1 ms) for the hand in the EH condition and 110.3 ms (s.e.m. ± 11.2 ms) for the eye in the EA condition. In the predictive (PRD) mode, hand and eye movements were also very similar, exhibiting slowly rising anticipatory velocity profiles (Fig. 9B). This occurred even though the subjects were presented with fixation cues throughout each trial and had to bring the eye and hand to rest in the central position between presentations. Mean latencies for the onset of the anticipatory response were −158.7 ms (s.e.m. ± 20.0 ms) for the eye and −123.0 ms (s.e.m. ± 23.4 ms) for the hand in the EH condition and −124.0 ms (s.e.m. ± 35.1 ms) for the eye in the EA condition. In both the PRD and RND modes, the eye returned to centre with a large saccadic movement that has been removed from the traces in Fig. 9. By contrast, the hand returned with a smooth movement and a peak velocity that was similar to that of the outward movement (Fig. 9).
Figure 9.
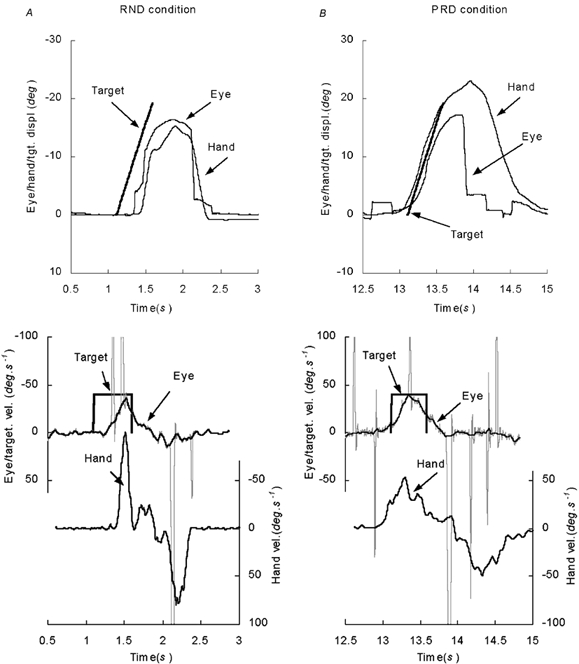
Examples of the responses of the eye and hand in A the randomised (RND) and B, the predictable (PRD) presentation conditions of Expt 3. Spikes on the eye velocity trace represent saccadic eye movements.
An important aspect of the PRD responses was the scaling of the anticipatory velocity (V100, Fig. 10A) which was similar to that in Expt 1, even though hand and eye were stationary prior to each target presentation. Analysis of variance indicated a significant increase in V0 and V100 with target velocity for the eye alone in ocular pursuit (EA condition) and for both the hand and eye in manual tracking (EH condition) (F7,49 = 162.1; P < 0.001). Although mean values of V0 and V100 for the eye were 32 and 35 % higher, respectively, in the EH condition than in the EA condition, this difference was not significant. In the EH condition, V100 for the hand was 15 % greater than V100 for the eye, but this difference was also not significant.
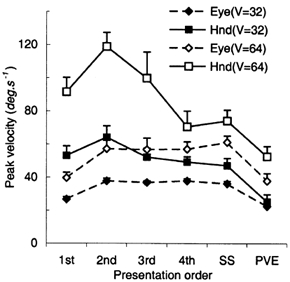
Figure 10.
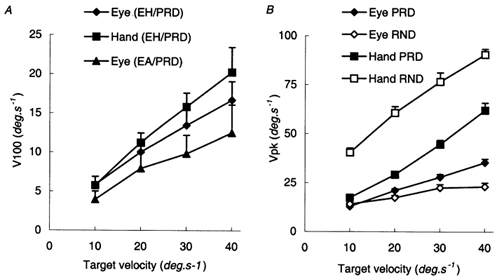
A, comparison of V100 in the predictable (PRD) mode for EH (combined hand and eye tracking) and EA (eye alone tracking) conditions. B, peak velocity (Vpk) in the PRD and RND conditions for the EH condition only. Vpk for the EA condition was very similar to the EH condition. Mean of data from eight subjects ± 1 s.e.m
In the EH and EA conditions, peak velocity of the eye (Vpk) was significantly greater in the PRD than in the RND mode (F1,7 = 23.92; P = 0.002; Fig. 10B), but there was no significant difference between the EH and EA conditions. In contrast, peak hand velocity was greater in the RND mode than in the PRD mode at all target velocities (F = 86.147; P < 0.001; Fig. 10B). These effects parallel the response in the first and SS presentations shown in Fig. 2. In effect, each RND response was like the first response of a PRD stimulus. In the PRD mode, the latency at which peak velocity of the eye and hand occurred (Tpk) was significantly less (289.4 ± 14.9 ms for the hand; 267.4 ± 11.5 ms for the eye) than in the RND mode (373.5 ± 12.5 ms for the hand; 365.8 ± 9.1 ms for the eye). This temporal advantage conferred by prediction is similar to that observed previously for the eye alone (Ohashi & Barnes, 1996).
DISCUSSION
Similarities of anticipatory movements in hand and eye
The results of these experiments show that human subjects naturally make anticipatory movements of the hand prior to the expected appearance of a regularly repeated moving target. These anticipatory movements were similar to those observed previously in the control of the eyes (Barnes & Asselman, 1991; Kao & Morrow, 1994), exhibiting a steady increase in velocity prior to target onset that is quite different to the abrupt rise in velocity that occurs after target onset in the randomised condition (Fig. 9). In some conditions the timing of hand and eyes appeared to be very similar (e.g. Fig. 5, ISI = 0.91 s), whereas in other conditions they could be quite disparate in timing (e.g. at long ISIs; Fig. 6A). The hand movements exhibited four particular properties that have been noted previously for eye movements (Barnes & Asselman, 1991). Firstly, there was a progressive increase in anticipatory velocity (V100) with repetition of the stimulus that we have previously suggested may represent the build-up of an internal store of pre-motor drive (Barnes & Asselman, 1991; Barnes & Grealy, 1992). As with eye movements, this occurred after only a few repetitions, reaching a steady state after three to four presentations. Secondly, there was a decrease in the magnitude of the anticipatory response (V100) as the ISI increased beyond 0.91 s, a feature that may be associated with the inability to build up or retain the store with long ISIs (Wells & Barnes, 1998). Thirdly, there was evidence that the anticipatory velocity (V100) was scaled in proportion to target velocity, indicating that it could function as a predictive estimate of the expected target velocity, even when the hand movement was brought to a halt between presentations (Expt 3). This scaling was present in the hand movement even though the hand cursor could not be seen before the target also appeared. Finally, in the catch trials, when the target unexpectedly failed to appear, the appropriately timed emergence of the predictive velocity estimate (PVE, Fig. 1) revealed that both the timing and velocity of the anticipatory hand movements had been pre-programmed.
The timing of the initiation of anticipatory activity showed characteristics compatible with those observed in other motor synchronisation tasks such as finger tapping, in which it has often been observed that variability of timing (s.d. of IRISS) is positively correlated with mean IRI (Bartlett & Bartlett, 1959; Michon, 1967; Wing & Kristofferson, 1973). It is of particular note that the extension of this effect in the hand to the longest ISI (3.74 s) applied only to the two subjects who reliably produced anticipatory movements at this ISI. The considerably reduced variability in the other six subjects could be easily explained if they had made reactive responses with similar latency, but this was not the case. It suggests that the responses were fully prepared and could be released with shorter latency than normal when cued by stimulus onset. A breakdown in anticipation for ISIs over 2 s, with a proportion of reactive responses, was also reported by Mates et al. (1994) during finger tapping.
Anticipatory movements are ballistic
One of the major features of anticipatory smooth eye movements is that they are ballistic, in the same sense as saccadic eye movements. The evidence for this lies in the fact that the velocity of the anticipatory movement is clearly pre-programmed (Barnes et al. 1995, 2000; Barnes & Schmid, 2000) and initiated well before target appearance. In addition, when the duration of the target motion stimulus is known, the termination of the drive for pursuit is also predetermined (Robinson et al. 1986; Boman & Hotson, 1988), inducing an anticipatory slowing of eye movement before the end of the stimulus. Whereas the goal of the saccadic system is to produce a fixed displacement, that of the smooth anticipatory system is probably to produce a fixed acceleration of hand or eye motion for a brief period in order to attain a fixed velocity level. The implication is that there is an internally generated burst of neural activity that results in a period of constant eye acceleration prior to target onset (Kao & Morrow, 1994). Evidence in support of this has been obtained from experiments in the monkey, where microstimulation of the frontal eye field leads to an initial period of constant eye acceleration (Gottlieb et al. 1993). Given the similarity of the anticipatory velocity profiles for the hand and eye, it seems probable that this internal acceleration drive is a common feature for each of these motor systems.
It was recently shown that concatenation of anticipatory eye movements can account for the generation of predictive responses to sinusoidal target motion (Barnes et al. 2000) as well as more complex stimuli (Barnes & Schmid, 2000). Oculo-manual tracking of a sinusoidal stimulus evokes predictive behaviour similar to that observed in ocular pursuit (Bock, 1987; Vercher et al. 1993). It seems likely that this ability can also be attributed to the concatenation of bursts of anticipatory activity of the type demonstrated here in both hand and eye.
Differences in positional error correction between hand and eye movements
Although hand and eye exhibited similar anticipatory velocity profiles, there were other aspects of the response that were quite different, notably, the manner in which positional error corrections were made. In the RND response of Fig. 9A, for example, both hand and eye started well after target onset, so that initially there was a large positional error. The subject responded by making two catch-up saccades that brought the eye closer to the target. When the target disappeared, the return to centre was mostly achieved with one large saccadic movement, combined with a small proportion of smooth movement. In contrast, the movement of the hand appeared wholly smooth and the return movement in particular was made with a trajectory that was very similar to that of the initial outward movement (Fig. 9). The subject responded to the initial positional error by making a catch-up movement of the hand that resulted in the peak hand velocity being much greater than that of the target. In the RND condition of Expt 3, peak hand velocity remained higher because of this positional correction, whereas in the PRD condition, the earlier start of hand movement resulted in less need for a positional error correction (Fig. 9B). The opposite effect (Vpk less in the RND mode than the PRD mode) was observed in the smooth eye movement because the positional error correction (the saccade) had been removed. Although positional error correction in the hand serves a similar purpose to the catch-up saccade of the eye, peak velocity of these hand corrections was never as high as saccadic eye movements.
Given these apparent differences in hand and eye responses, what is the evidence for a similar underlying mechanism of positional error correction? In the eye, random positional errors are normally corrected with saccades, although there is some evidence that they sometimes result in smooth eye movements when the positional error is small (<∼4 deg) (Pola & Wyatt, 1980; Carl & Gellman, 1987). The most important feature of saccadic eye movements for the argument that follows is that they exhibit a stereotypic relationship between peak-eye velocity and saccade displacement (the main sequence (Bahill et al. 1975)) that cannot be modified voluntarily. Positional error correction also plays a major role in manual tracking tasks (Navas & Stark, 1968; Miall et al. 1988) and the corrections often appear saccade-like. In particular, the corrections tend to be intermittent, in a similar way to the saccades that occur in ocular pursuit, with a frequency of between 0.5 and 1.8 Hz (Miall et al. 1993). It is often possible, during continuous oculo-manual pursuit, to see spikes of activity on the hand velocity trace that represent these intermittent positional corrections (Xia & Barnes, 1999). Other evidence suggests that limb movements exhibit many of the properties associated with saccades. In particular, transient movements of the arm, such as in goal-directed pointing, exhibit a bell-shaped profile that is not dissimilar to that seen in eye saccades, although they tend to be slower. Such movements exhibit a main sequence relationship for the arm (Vercher et al. 1994) that is similar to that of the eye, peak velocity increasing with displacement. However, the critical difference is that although such limb movements are usually executed at a particular velocity for a particular displacement, they can be made at any velocity at will if required and the relationship between velocity and displacement can be different for different experimental conditions (Hoffman & Strick, 1986).
Thus there does appear to be a fundamental difference between the eye and hand in the way in which positional errors are corrected. The hand does not seem to have access to the mechanism that is responsible for generating the very rapid, fixed velocity saccades that the eye can make. This difference is evident in a goal-directed reaching task, where the eye normally attains its goal well in advance of the hand (Vercher et al. 1994; Land et al. 1999), thus facilitating the use of visual feedback to guide the more slowly moving hand to its goal.
Effects of hand movement on the initiation of smooth eye movements
In the absence of a moving, visual target smooth eye movements can only be initiated if certain conditions are met. These conditions are illustrated by the behaviour of the eye at the end of the record shown in Fig. 1. When the target failed to appear as expected the subject was able to use the stored information about velocity and timing to initiate one smooth movement (PVE) in the absence of the target, but a second attempt to do so resulted in the generation of a large saccadic movement. The ability to initiate any further smooth, anticipatory movement was previously shown to be dependent on the expectancy that the target would reappear at a particular time (Barnes et al. 1997) and in the example of Fig. 1 the subject had no such expectancy. However, expectancy is not the only factor that is important in allowing the release of smooth eye movements; they can also be released when subjects track a self-moved target (Vercher et al. 1995). Although Vercher et al. indicated that efferent information associated with the hand is important for initiation of this response, it is also possible that the eye movements may be generated by the same mechanisms that generate anticipatory smooth pursuit without hand movement. Scarchilli & Vercher (1999) have argued against this on the grounds that smooth eye movements associated with hand movements are immediate and do not require the build-up of a store. Although the build-up of a store of information is important in the scaling of the anticipatory movement, it is not a necessary requirement for the release of the movement. In an experiment using a stabilised image, Barnes et al. (1995) showed that subjects can initiate smooth eye movements at will simply by transferring attention to either side of the stabilised image.
We suggest that central initiation of a hand movement effectively does two things. First, it creates a high level of expectancy, which opens the gate for the release of smooth movement. Second, it provides immediate information prior to the initiation of hand movement about the magnitude and duration of the intended hand movement which may obviate the need to build up internally stored information to scale the anticipatory eye movement. This does not necessarily imply that hand and eye commands would be released simultaneously; indeed the evidence (Fig. 6A) is that they may be released at quite disparate times. However, it does imply that there is an exchange of information between hand and eye systems in accord with the general concept of the control co-ordination model (Gauthier et al. 1988; Lazzari et al. 1997).
Effects of hand movement on the maintenance of smooth eye movement
Even if anticipatory smooth eye movements are successfully initiated (in the absence of hand movement) they cannot be sustained for very long if the target disappears (Barnes & Asselman, 1991), whereas smooth hand movements can continue, as shown by the example in Fig. 1. On the basis of similar observations for the head and eye (Barnes & Grealy, 1992), it was suggested previously that the major difference between the eye and other motor systems might lie in the relative contributions of proprioceptive feedback. The assumption that underlies this suggestion is that the mode of operation of predictive motor control is one in which an initial anticipatory estimate of the required motor drive must be confirmed by sensory feedback indicating that the prediction was appropriate. In oculo-motor control, the feedback is normally provided by visual feedback alone (Lewis et al. 1994), whereas in the hand and head, proprioception from muscles and joints supplements any visual information. We hypothesise that the failure to provide feedback in the eye by removing visual input results in an inability to sustain the smooth control of movement, whereas in the hand, the continuous availability of proprioceptive feedback normally allows smooth movements to continue at will. In support of this hypothesis, it has been shown that hand movements also break down into a saccade-like pattern in monkeys deprived of proprioceptive feedback (Gauthier & Mussa Ivaldi, 1988).
Concomitant hand movement has an effect not only on initiation of smooth eye movement, but also on its maintenance. A sustained response can be evoked by instructing subjects to follow an imagined target moved by their own hand (Gauthier & Hofferer, 1976) and, in addition, pursuit of a real self-moved target elicits a higher gain than for a passively moved target (Gauthier et al. 1988). Evidence indicates that it is afferent feedback from the limb that is necessary and sufficient to sustain this smooth eye movement (Gauthier & Hofferer, 1976). In terms of the hypothesis outlined above, this implies that there is cross-modality transfer allowing proprioceptive information from the hand to confirm the appropriateness of an internally generated drive for the eye. Given this association between hand and eye, one might ask why it was not possible to generate a second smooth eye movement, in concert with the smooth hand movement, at the end of the record shown in Fig. 1. The answer could be that this effect only occurs if attention is directed to the hand movement, so as to specifically associate hand and eye. In the experiments presented here, subjects were instructed to match the movement of the hand cursor to that of the target, not to follow the hand movement itself.
In addition to the effects associated with tracking of the hand itself, it has also been reported that ocular pursuit is improved when tracking with the hand and eye together (EH condition) as compared with the eye alone (EA condition) (Leist et al. 1987; Gauthier et al. 1988). Although we found anticipatory velocities (V0 and V100) to be higher in the EH condition than the EA condition of Expt 3, the difference was not significant, nor was the difference in peak eye velocity (Vpk). In a previous study, we were also unable to demonstrate any difference between EA and EH conditions (Xia & Barnes, 1999). It seems most likely that such differences are only clearly observed when hand and eye track higher velocity stimuli (Leist et al. 1987; Gauthier et al. 1988) than those we have used.
Conclusions
There are many similarities in the anticipatory movements of hand and eye. They exhibit a similar velocity profile, with a gradual increase in velocity which contrasts with the abrupt increase in velocity associated with reactive responses to non-predictive conditions. In both hand and eye, there is evidence of the build-up and storage of timing and velocity-coded information that is appropriately scaled for expected target motion. However, there are also important differences between hand and eye. Stored information for hand and eye may be released at different times, suggesting separate stores or separate access to a single store. Anticipatory smooth eye movements only occur when there is a high level of expectancy of target motion; otherwise saccadic movements become dominant. In contrast, smooth hand movements can be made at will at any velocity and, it is argued, there is no true equivalent of a saccadic eye movement in the hand. Given the previous demonstration that anticipatory smooth eye movements may be concatenated to produce predictive responses to periodic stimuli (Barnes et al. 2000), it is probable that anticipatory movements play a similar role in predictive oculo-manual tracking tasks.
Acknowledgments
The authors would like to thank Dr P. Brown and Dr C. J. S. Collins for helpful support and advice. This work was supported by the Medical Research Council. The experimental work was carried out in the former MRC Human Movement and Balance Unit, Institute of Neurology, London.
REFERENCES
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Bioscience. 1975;24:191–204. [Google Scholar]
- Barnes GR. A procedure for the analysis of nystagmus and other eye movements. Aviation Space and Environmental Medicine. 1982;53:676–682. [PubMed] [Google Scholar]
- Barnes GR, Asselman PT. The mechanism of prediction in human smooth pursuit eye movements. Journal of Physiology. 1991;439:439–461. doi: 10.1113/jphysiol.1991.sp018675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Barnes DM, Chakraborti SR. Ocular pursuit responses to repeated, single-cycle sinusoids reveal behavior compatible with predictive pursuit. Journal of Neurophysiology. 2000;84:2340–2355. doi: 10.1152/jn.2000.84.5.2340. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Donelan AS. The remembered pursuit task: evidence for segregation of timing and velocity storage in predictive oculomotor control. Experimental Brain Research. 1999;129:57–67. doi: 10.1007/s002210050936. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Goodbody SJ, Collins S. Volitional control of anticipatory ocular pursuit responses under stabilized image conditions in humans. Experimental Brain Research. 1995;106:301–317. doi: 10.1007/BF00241126. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Grealy MA. Predictive mechanisms of head-eye coordination and vestibulo-ocular reflex suppression in humans. Journal of Vestibular Research. 1992;2:193–212. [PubMed] [Google Scholar]
- Barnes GR, Grealy MA, Collins S. Volitional control of anticipatory ocular smooth pursuit after viewing, but not pursuing, a moving target: evidence for a re-afferent velocity store. Experimental Brain Research. 1997;116:445–455. doi: 10.1007/pl00005772. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Schmid AM. Pre-programming of anticipatory ocular pursuit responses to double-ramp target motion sequences in humans. Journal of Physiology. 2000;527:138P. [Google Scholar]
- Barnes GR, Wells SG. Modelling prediction in ocular pursuit: the importance of short-term storage. In: Becker W, Deubel H, Mergner T, editors. Current Oculomotor Research: Physiological and Psychological Aspects. New York: Plenum Press; 1999. pp. 97–107. [Google Scholar]
- Bartlett NR, Bartlett SC. Synchronization of a motor response with an anticipated sensory event. Psychological Review. 1959;66:203–218. doi: 10.1037/h0046490. [DOI] [PubMed] [Google Scholar]
- Bock O. Coordination of arm and eye movements in tracking of sinusoidally moving targets. Behavioural Brain Research. 1987;24:93–100. doi: 10.1016/0166-4328(87)90247-6. [DOI] [PubMed] [Google Scholar]
- Boman DK, Hotson JR. Stimulus conditions that enhance anticipatory slow eye movements. Vision Research. 1988;28:1157–1165. doi: 10.1016/0042-6989(88)90142-3. [DOI] [PubMed] [Google Scholar]
- Carl JR, Gellman RS. Human smooth pursuit: stimulus-dependent responses. Journal of Neurophysiology. 1987;57:1446–1463. doi: 10.1152/jn.1987.57.5.1446. [DOI] [PubMed] [Google Scholar]
- Cody FWJ, Lovgreen B, Schady W. Increased dependence upon visual information of movement performance during visuo-motor tracking in cerebellar disorders. Electroencephalography and Clinical Neurophysiology: Electromyography and Motor Control. 1993;89:399–407. doi: 10.1016/0168-5597(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Dallos PJ, Jones RW. Learning behaviour of the eye fixation control system. IEEE Transactions. 1963;AC-8:218–227. [Google Scholar]
- Gauthier GM, Hofferer JM. Eye tracking of self-moved targets in the absence of vision. Experimental Brain Research. 1976;26:121–139. doi: 10.1007/BF00238277. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Mussa Ivaldi F. Ocular-manual tracking of visual targets in monkey: role of the arm afferent information in the control of arm and eye movements. Experimental Brain Research. 1988;73:138–154. doi: 10.1007/BF00279668. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Vercher J-L, Mussa Ivaldi F, Marchetti E. Ocular-manual tracking of visual targets:control learning, coordination control and coordination model. Experimental Brain Research. 1988;73:127–137. doi: 10.1007/BF00279667. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Bruce CJ, Macavoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. Journal of Neurophysiology. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- Heywood S, Churcher J. Eye movements and the afterimage-1. Tracking the afterimage. Vision Research. 1971;11:1163–1168. doi: 10.1016/0042-6989(71)90120-9. [DOI] [PubMed] [Google Scholar]
- Hoffman DS, Strick PL. Step-tracking movements of the wrist in humans. I. Kinematic analysis. Journal of Neuroscience. 1986;6:3309–3318. doi: 10.1523/JNEUROSCI.06-11-03309.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao GW, Morrow MJ. The relationship of anticipatory smooth eye movement to smooth pursuit initiation. Vision Research. 1994;34:3027–3036. doi: 10.1016/0042-6989(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Koken PW, Erkelens CJ. Influences of hand movements on eye movements in tracking tasks in man. Experimental Brain Research. 1992;88:657–664. doi: 10.1007/BF00228195. [DOI] [PubMed] [Google Scholar]
- Land MF, Mennie N, Rusted J. The roles of vision and eye movements in the control of activities of daily living. Perception. 1999;28:1311–1328. doi: 10.1068/p2935. [DOI] [PubMed] [Google Scholar]
- Lazzari S, Vercher J-L, Buizza A. Manuo-ocular coordination in target tracking. I. A model simulating human performance. Biological Cyberbetics. 1997;77:1–10. doi: 10.1007/s004220050386. [DOI] [PubMed] [Google Scholar]
- Leist A, Freund H-J, Cohen B. Comparative characteristics of predictive eye-hand tracking. Human Neurobiology. 1987;6:19–26. [PubMed] [Google Scholar]
- Lewis RF, Zee DS, Gaymard BM, Guthrie BL. Extraocular muscle proprioception functions in the control of ocular alignment and eye movement conjugacy. Journal of Neurophysiology. 1994;72:1028–1031. doi: 10.1152/jn.1994.72.2.1028. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Barnes GR, Brown P. Anticipatory hand and smooth eye movements during oculo-manual tracking in humans. Journal of Physiology. 1998;506:112P–113P. doi: 10.1113/jphysiol.2001.012979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates J, Muller U, Radil T, Poppel E. Temporal integration in sensorimotor synchronization. Journal of Cognitive Neuroscience. 1994;6:332–340. doi: 10.1162/jocn.1994.6.4.332. [DOI] [PubMed] [Google Scholar]
- Mather JA, Putchat C. Parallel ocular and manual tracking responses to a continuously moving visual target. Journal of Motor Behavior. 1983;15:29–38. doi: 10.1080/00222895.1983.10735287. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Planning of movement parameters in a visuo-motor tracking task. Behavioural Brain Research. 1988;27:1–8. doi: 10.1016/0166-4328(88)90104-0. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. Journal of Motor Behavior. 1993;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Michon JA. Timing in Temporal Tracking. Soesterberg, The Netherlands: Institute for Perception RVO-TNO; 1967. pp. 1–127. [Google Scholar]
- Navas F, Stark L. Sampling or intermittency in hand control system dynamics. Biophysical Journal. 1968;8:252–302. doi: 10.1016/S0006-3495(68)86488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N, Barnes GR. A comparison of predictive and non-predictive ocular pursuit under active and passive stimulation conditions in humans. Journal of Vestibular Research. 1996;6:261–276. [PubMed] [Google Scholar]
- Pola J, Wyatt HJ. Target position and velocity: the stimuli for smooth pursuit eye movements. Vision Research. 1980;20:523–534. doi: 10.1016/0042-6989(80)90127-3. [DOI] [PubMed] [Google Scholar]
- Poulton EC. Tracking Skill and Manual Control. London: Academic Press; 1974. [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biological Cybernetics. 1986;55:43–57. doi: 10.1007/BF00363977. [DOI] [PubMed] [Google Scholar]
- Scarchilli K, Vercher JL. The oculomanual coordination control center takes into account the mechanical properties of the arm. Experimental Brain Research. 1999;124:42–52. doi: 10.1007/s002210050598. [DOI] [PubMed] [Google Scholar]
- Stark L, Vossius G, Young LR. Predictive control of eye tracking movements. IRE Transactions on Human Factors in Electronics. 1962;3:52–56. [Google Scholar]
- Steinbach MJ. Eye tracking of self-moved targets: the role of efference. Journal of Experimental Psychology. 1969;82:366–376. doi: 10.1037/h0028115. [DOI] [PubMed] [Google Scholar]
- Van Donkelaar P, Lee RG. Interactions between the eye and hand motor systems: disruptions due to cerebellar dysfunction. Journal of Neurophysiology. 1994;72:1674–1685. doi: 10.1152/jn.1994.72.4.1674. [DOI] [PubMed] [Google Scholar]
- Vercher J-L, Gauthier GM. Oculo-manual coordination control: Ocular and manual tracking of visual targets with delayed visual feedback of the hand motion. Experimental Brain Research. 1992;90:599–609. doi: 10.1007/BF00230944. [DOI] [PubMed] [Google Scholar]
- Vercher J-L, Magenes G, Prablanc C, Gauthier GM. Eye-head-hand coordination in pointing at visual targets: Spatial and temporal analysis. Experimental Brain Research. 1994;99:507–523. doi: 10.1007/BF00228987. [DOI] [PubMed] [Google Scholar]
- Vercher J-L, Quaccia D, Gauthier GM. Oculo-manual coordination control: Respective role of visual and non-visual information in ocular tracking of self-moved targets. Experimental Brain Research. 1995;103:311–322. doi: 10.1007/BF00231717. [DOI] [PubMed] [Google Scholar]
- Vercher J-L, Volle M, Gauthier GM. Dynamic analysis of human visuo-oculo-manual coordination control in target tracking tasks. Aviation Space and Environmental Medicine. 1993;64:500–506. [PubMed] [Google Scholar]
- Von Noorden GK, MacKensen G. Pursuit movements of normal and amblyopic eyes. American Journal of Ophthalmology. 1962;53:325–336. doi: 10.1016/0002-9394(62)91183-2. [DOI] [PubMed] [Google Scholar]
- Wells SG, Barnes GR. Fast, anticipatory smooth-pursuit eye movements appear to depend on a short-term store. Experimental Brain Research. 1998;120:129–133. doi: 10.1007/s002210050385. [DOI] [PubMed] [Google Scholar]
- Wing AM, Kristofferson AB. The timing of interresponse intervals. Perception and Psychophysics. 1973;13:455–460. [Google Scholar]
- Xia R, Barnes GR. Oculo-manual co-ordination in tracking of pseudo-random target motion stimuli. Journal of Motor Behavior. 1999;31:21–38. doi: 10.1080/00222899909601889. [DOI] [PubMed] [Google Scholar]
- Young LR, Stark L. Variable feedback experiments testing a sampled data model for eye tracking movements. IEEE Transactions. 1963;HFE-4:38–51. [Google Scholar]


